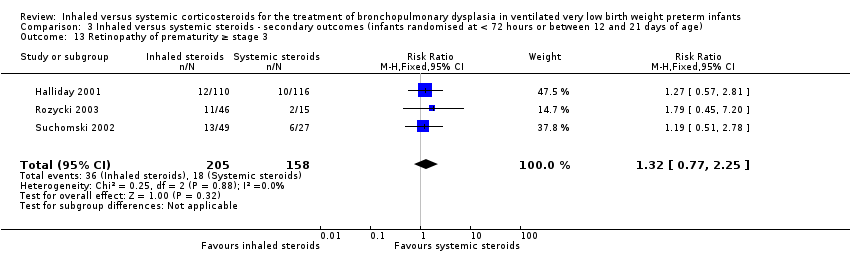
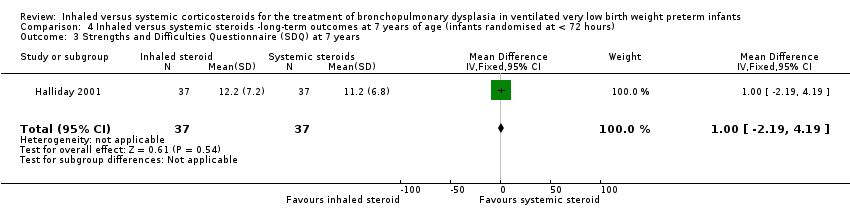
Contenido relacionado
Revisiones y protocolos relacionados
Sachin S Shah, Arne Ohlssona, Henry L Halliday, Vibhuti S Shah | 17 octubre 2017
Vibhuti S Shah, Arne Ohlssona, Henry L Halliday, Michael Dunn | 4 enero 2017
Keith J Barrington, Neil Finer, Thomas Pennaforte | 3 enero 2017
Keith J Barrington, Etienne Fortin‐Pellerin, Thomas Pennaforte | 8 febrero 2017
Luca Morescoa, Alice Sjögrena, Keri A Marques, Roger Soll, Matteo Bruschettini | 4 octubre 2023
Gautham Suresh, Jonathan M Davis, Roger Soll | 22 enero 2001
Peter Evans, Deirdre O'Reilly, Jonathan N Flyer, Roger Soll, Souvik Mitra | 15 enero 2021
Lisa M Askie, David J Henderson‐Smart | 23 octubre 2001
David A Osborn, Nicholas J Evans | 23 abril 2001
Edward F Bell, Michael J Acarregui | 4 diciembre 2014