Vacunas acelulares para prevenir la tos ferina en niños
Referencias
References to studies included in this review
References to studies excluded from this review
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Site: Ghana | |
| Participants | Included: age 6 weeks | |
| Interventions | Primary series (aP versus wP)
Number randomised: 266 aP, 137 wP | |
| Outcomes |
| |
| Notes | Local adverse events excluded (results only for all local adverse event types combined). No statement on antipyretic/analgesic use. In this review, results are combined for the 2 aP vaccine formulations | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Using a computer program (EPI Info) |
| Allocation concealment (selection bias) | Unclear risk | On‐site computer assignment but file locking not reported. Vaccines visually distinguishable |
| Blinding (performance bias and detection bias) | Unclear risk | Parents and nurses collecting efficacy and adverse event data were blinded but blinding details not stated |
| Blinding of participants and personnel (performance bias) | Unclear risk | Parents and nurses collecting efficacy and adverse event data were blinded but blinding details not stated |
| Blinding of outcome assessment (detection bias) | Unclear risk | Parents and nurses collecting efficacy and adverse event data were blinded but blinding details not stated |
| Methods | Site: Sweden Active case ascertainment (monthly telephone). Case incidence adjusted for follow‐up duration by actuarial method. Parents recorded adverse events in diary | |
| Participants | Included: age 5 to 11 months Excluded: suspected progressive neurological disease; failure to thrive; renal failure; cardiac failure; prior pertussis or pertussis immunisation | |
| Interventions | Primary series (aP versus placebo)
Number randomised: 2837 aP, 954 placebo | |
| Outcomes |
| |
| Notes | Macrolide prophylaxis not used (not recommended in Sweden for age > 6 months and efficacy follow‐up in this study started at minimum age of 7 months). Reactive antipyretic/analgesic use allowed. Blinding of study nurses confirmed by questionnaire | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Centrally generated random sequence |
| Allocation concealment (selection bias) | Low risk | Vaccines in identical, coded vials |
| Blinding (performance bias and detection bias) | Low risk | Double‐blind |
| Blinding of participants and personnel (performance bias) | Low risk | |
| Blinding of outcome assessment (detection bias) | Low risk | |
| Methods | Site: Sweden Passive case ascertainment (based on laboratory reports of pertussis and questionnaire of parents at 18‐month visit). Case incidence adjusted for follow‐up duration by Cox proportional hazard regression. Follow‐up of serious adverse events by active weekly surveillance in study area hospitals, reports from participating physicians and child health nurses, plus questioning of parents at each trial dose and when the child was 18 months old | |
| Participants | Included: age 2 months | |
| Interventions | Primary series (aP versus wP)
Number randomised: 62172 aP, 2072 wP | |
| Outcomes |
| |
| Notes | There was good evidence that the passive case ascertainment led to significant under‐reporting of cases. It is possible (not discussed in study report) that under‐reporting of serious adverse events may have occurred but there are no grounds to suspect that under‐reporting would affect the acellular and whole‐cell groups differently | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Centrally generated random sequence |
| Allocation concealment (selection bias) | Low risk | Vaccines in identical, coded vials |
| Blinding (performance bias and detection bias) | Low risk | Double‐blind |
| Blinding of participants and personnel (performance bias) | Low risk | |
| Blinding of outcome assessment (detection bias) | Low risk | |
| Methods | Site: USA Design: DB parallel‐group RCT | |
| Participants | Included: age 2 months; healthy | |
| Interventions | Primary series (aP versus wP) | |
| Outcomes |
| |
| Notes | Reactive analgesic/antipyretic use allowed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central randomisation |
| Allocation concealment (selection bias) | Low risk | Vaccines in coded vials |
| Blinding (performance bias and detection bias) | Low risk | Double‐blind |
| Blinding of participants and personnel (performance bias) | Low risk | |
| Blinding of outcome assessment (detection bias) | Low risk | |
| Methods | Site: USA Parents recorded adverse events on forms | |
| Participants | Included: age 4 to 6 years; healthy; completed DTwP primary series and 15‐ to 24‐month booster | |
| Interventions | Booster (wP.wP.aP versus wP.wP.wP)
Number randomised: 240 aP, 76 wP | |
| Outcomes |
| |
| Notes | Prophylactic antipyretic/analgesic use not allowed. Reactive antipyretic/analgesic use allowed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation at study site, but details not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment details not reported |
| Selective reporting (reporting bias) | High risk | Data for vomiting collected but not reported |
| Methods | Site: USA Parents recorded adverse events in diary | |
| Participants | Age: 15 to 20 months; healthy; completed DTwP primary series | |
| Interventions | BOOSTER (wP.aP versus wP.wP)
Number randomised: 110 aP, 22 wP | |
| Outcomes |
| |
| Notes | Reactive antipyretic/analgesic use allowed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Details not reported |
| Allocation concealment (selection bias) | Unclear risk | Details not reported |
| Selective reporting (reporting bias) | High risk | Data for vomiting collected but not reported (stated to be "infrequent" and "not significantly different") |
| Methods | Site: USA | |
| Participants | Included: age 2 months | |
| Interventions | Primary series (aP versus wP)
Number randomised: 2000 aP, 498 wP | |
| Outcomes |
| |
| Notes | Booster dose of Chiron/Biocine[3] or Lederle/Takeda[4] at age 15 to 18 months. Data for this dose are not included as there was no DTwP or placebo control | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Details not reported |
| Allocation concealment (selection bias) | Unclear risk | Details not reported |
| Methods | Site: Sweden | |
| Participants | Included: age 6 months | |
| Interventions | Primary series (aP versus wP ‐ see notes)
Number randomised: 121 aP, 119 P, 79 wP | |
| Outcomes |
| |
| Notes | The whole‐cell series was given as 3 doses of whole‐cell vaccine. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random sequence key concealed from study personnel and parents |
| Allocation concealment (selection bias) | Low risk | Vaccines indistinguishable |
| Blinding (performance bias and detection bias) | Low risk | Double‐blind |
| Blinding of participants and personnel (performance bias) | Low risk | |
| Blinding of outcome assessment (detection bias) | Low risk | |
| Selective reporting (reporting bias) | High risk | Data for anorexia and vomiting collected but not reported |
| Methods | Site: USA Parents recorded adverse events in diary | |
| Participants | Included: age 17 to 24 months; healthy; completed DTwP primary series | |
| Interventions | Booster (wP.aP versus wP.wP)
Number randomised: 38 aP, 37 wP | |
| Outcomes |
| |
| Notes | Reactive antipyretic/analgesic use allowed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Details not reported |
| Allocation concealment (selection bias) | Unclear risk | Details not reported |
| Methods | Site: USA Parents recorded adverse events in diary | |
| Participants | Included: age 2 months; healthy | |
| Interventions | Primary series (aP versus wP)
Number randomised: 245 aP, 252 wP | |
| Outcomes |
| |
| Notes | 1 death in DTwP arm due to accident (strangulation by pacifier cord) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Details not reported |
| Methods | Site: USA Parents recorded adverse events on forms | |
| Participants | Included: age 6 to 12 weeks; healthy | |
| Interventions | Primary series (aP versus wP)
Number randomised: 1827 aP, 373 wP | |
| Outcomes |
| |
| Notes | In this review, safety results are combined for all acellular vaccines. Irritability and pain were reported only for the moderate/severe category | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Details not reported |
| Blinding (performance bias and detection bias) | Unclear risk | Vaccine vials labelled with letter codes instead of type/manufacturer details but unable to ascertain from study report whether vaccinators remained unaware of what each code represented |
| Blinding of outcome assessment (detection bias) | Low risk | Vaccinators took no further part in the study and did not participate in follow‐up data collection, so outcome assessment was double‐blind |
| Methods | Site: USA Design: parallel‐group RCT Parents recorded adverse events on forms | |
| Participants | Included: age 18 to 24 months; completed DTwP primary series | |
| Interventions | Booster (wP.aP versus wP.wP)
Number randomised: 20 aP, 20 wP | |
| Outcomes |
| |
| Notes | No statement on antipyretic/analgesic use | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Details not reported |
| Selective reporting (reporting bias) | High risk | Data for drowsiness and vomiting collected but not reported (stated to be "rare"). Data for irritability, pain, redness and induration only reported for moderate/severe category |
| Methods | Site: USA Parents recorded adverse events on forms | |
| Participants | Included: age 4 to 6 years; completed DTwP primary series and 15‐ to 24‐month booster | |
| Interventions | Booster (wP.wP.aP versus wP.wP.wP)
Number randomised: 20 aP, 20 wP | |
| Outcomes |
| |
| Notes | No statement on antipyretic/analgesic use | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Details not reported |
| Selective reporting (reporting bias) | High risk | Data for drowsiness and vomiting collected but not reported (stated to be "rare’'). Data for irritability, pain, redness and induration only reported for moderate/severe category |
| Methods | Site: USA Parents recorded adverse events on forms | |
| Participants | Included: infants | |
| Interventions | Primary series (aP versus wP)
Number randomised: 23 aP, 27 wP | |
| Outcomes |
| |
| Notes | Follow‐up for deaths probably longer than until 2 weeks after dose 3 but not clearly stated to be so | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Details not reported |
| Methods | Site: USA Parents recorded adverse events on forms | |
| Participants | Included: age 18 to 24 months; completed DTwP primary series | |
| Interventions | Booster (wP.aP versus wP.wP)
Number randomised: 19 aP, 21 wP | |
| Outcomes |
| |
| Notes | No statement on antipyretic/analgesic use | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Details not reported |
| Methods | Site: USA Parents recorded adverse events on forms | |
| Participants | Included: age 4 to 6 years; completed DTwP primary series and 18‐ to 24‐month booster | |
| Interventions | Booster (wP.wP.aP versus wP.wP.wP)
Number randomised: 20 aP, 20 wP | |
| Outcomes |
| |
| Notes | No statement on antipyretic/analgesic use | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Details not reported |
| Methods | Site: Houston, USA Parents were interviewed by telephone. Parents recorded adverse events in diary for 14 days after immunisations | |
| Participants | Included: ages 16 to 20 months, who had received primary immunisation with DTwP | |
| Interventions | Booster (aP versus wP)
Number randomised: 28 aP, 13 wP | |
| Outcomes |
| |
| Notes | Prolonged crying was studied as increased crying. Use of analgesic/antipyretic was allowed during the study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Details not reported |
| Methods | Site: USA Parents recorded adverse events on forms | |
| Participants | Included: age 16 to 21 months; healthy; completed DTwP primary series | |
| Interventions | Booster (wP.aP versus wP.wP)
Number studied: 102 aP, 29 wP | |
| Outcomes |
| |
| Notes | The number of 16‐ to 24‐month old children randomised to aP or wP was not stated. A total of 258 children aged 16 to 24 months or 4 to 6 years were randomised in Englund 1994a and Englund 1994b combined: 192 to aP and 66 to wP. 240 of these contributed safety data | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Details not reported |
| Selective reporting (reporting bias) | High risk | Data for irritability collected but not reported |
| Methods | Site: USA Parents recorded adverse events on forms | |
| Participants | Included: age 4 to 6 years; healthy; completed DTwP primary series and 15‐ to 24‐month booster | |
| Interventions | Booster (wP.wP.aP versus wP.wP.wP)
Number studied: 80 aP, 29 wP | |
| Outcomes |
| |
| Notes | Number of 4 to 6‐year old children randomised to aP or wP not stated. A total of 258 children aged 16 to 24 months or 4 to 6 years were randomised in Englund 1994a and Englund 1994b combined: 192 to aP and 66 to wP. 240 of these contributed safety data. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Details not reported |
| Selective reporting (reporting bias) | Low risk | Data for irritability collected but not reported |
| Methods | Site: USA Parents recorded adverse events in diary | |
| Participants | Included: age 15 to 24 months; healthy; completed DTwP primary series | |
| Interventions | Booster (wP.aP versus wP.wP)
Number randomised: 84 aP, 78 wP | |
| Outcomes |
| |
| Notes | Reactive antipyretic/analgesic use allowed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Details not reported |
| Selective reporting (reporting bias) | High risk | Data for vomiting collected but not reported |
| Methods | Site: USA Parents recorded adverse events in diary | |
| Participants | Included: age 2 months; healthy | |
| Interventions | Primary series (aP versus wP)
Number randomised: 109 aP, 36 wP | |
| Outcomes |
| |
| Notes | 1 infant did not complete the primary series due to an adverse event (high pitched cry) but the report does not state whether this infant received aP or wP. Deaths were not specifically reported but all infants either completed the study up to the final dose or withdrew due to reasons other than death | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Details not reported |
| Selective reporting (reporting bias) | High risk | Data for vomiting collected but not reported |
| Methods | Site: USA Parents recorded adverse events in diary | |
| Participants | Included: age 17 to 24 months; healthy; full‐term; completed DTwP primary series | |
| Interventions | Booster (wP.aP versus wP.wP)
Number randomised: 345 aP, 52 wP | |
| Outcomes |
| |
| Notes | Results for DTaP lots combined | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | On‐site randomisation |
| Allocation concealment (selection bias) | Unclear risk | Details not reported |
| Selective reporting (reporting bias) | High risk | Data for prolonged crying collected but not reported |
| Methods | Site: Italy Design: DB parallel‐group RCT | |
| Participants | Included: age 6 to 12 weeks; weight > 3rd percentile | |
| Interventions | Primary series (aP versus wP versus DT)
Number randomised: 9368 aP, 4678 wP, 1555 DT | |
| Outcomes |
| |
| Notes | No statement on macrolide prophylaxis. Possible partial unblinding of DTwP (not discussed in article but study used same DTwP as Gustafsson96) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Details not reported |
| Allocation concealment (selection bias) | Low risk | Vaccines in identical, coded vials |
| Blinding (performance bias and detection bias) | Low risk | Double‐blind |
| Blinding of participants and personnel (performance bias) | Low risk | |
| Blinding of outcome assessment (detection bias) | Low risk | |
| Methods | Site: Sweden Passive and active case ascertainment (parent report and telephone every 6 to 8 weeks). Case incidence adjusted for follow‐up duration by use of Cox proportional hazards regression. Parents recorded adverse events in diary | |
| Participants | Included: age 2 to 3 months | |
| Interventions | Primary series (aP versus wP versus DT)
Number randomised: 5153 aP, 2102 wP, 2574 DT | |
| Outcomes |
| |
| Notes | Macrolide prophylaxis not used (not recommended in Sweden for age > 6 months and main efficacy follow‐up in this study started at age 6 months) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Centrally generated randomisation in blocks of 12 or 16 |
| Allocation concealment (selection bias) | Low risk | Vaccines in identical vials. At each study site, vaccines administered in numbered order |
| Blinding (performance bias and detection bias) | Low risk | Double‐blind |
| Blinding of participants and personnel (performance bias) | Low risk | |
| Blinding of outcome assessment (detection bias) | Low risk | |
| Methods | Site: Canada Adverse events recorded by structured telephone interview of parents | |
| Participants | Included: age 2 months; healthy | |
| Interventions | Primary series (aP versus wP)
Number randomised: 67 aP, 33 wP | |
| Outcomes |
| |
| Notes | The 2 acellular vaccines had differing amounts of PT and FH. In this review, results for the 2 acellular vaccine formulations are combined Antipyretic/analgesic prophylaxis was discouraged but not prohibited | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation list kept in a locked file |
| Allocation concealment (selection bias) | Low risk | Vaccines in identical, coded vials |
| Blinding (performance bias and detection bias) | Low risk | Double‐blind |
| Blinding of participants and personnel (performance bias) | Low risk | |
| Blinding of outcome assessment (detection bias) | Low risk | |
| Methods | Site: Canada Adverse events recorded by structured telephone interview of parents | |
| Participants | Included: age 17 to 19 months; healthy | |
| Interventions | Booster (wP.aP versus wP.wP)
Number randomised: 61 aP, 30 wP | |
| Outcomes |
| |
| Notes | The 2 acellular vaccines had differing amounts of PT, FH, Prn and Fim2,3. In this review, results for the 2 acellular vaccine formulations are combined | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation list kept in a locked file |
| Allocation concealment (selection bias) | Low risk | Vaccines in identical, coded vials |
| Blinding (performance bias and detection bias) | Low risk | Double‐blind |
| Blinding of participants and personnel (performance bias) | Low risk | |
| Blinding of outcome assessment (detection bias) | Low risk | |
| Methods | Site: Canada Adverse events recorded by structured telephone interview of parents | |
| Participants | Included: age 17 to 19 months; healthy; primary series DTaP or DTwP given in Halperin 1994a | |
| Interventions | Booster (aP.aP versus wP.wP)
Number studied: 56 aP, 30 wP | |
| Outcomes |
| |
| Notes | The 2 acellular vaccines had differing amounts of PT and FH. In this review, results for the 2 acellular vaccine formulations are combined. DTaP or DTwP primary series was given in Halperin 1994a. Randomisation took place in that study, parents and investigators remained blinded and children received the same vaccine in Halperin 1995 as they had received in Halperin 1994a | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation list kept in a locked file |
| Allocation concealment (selection bias) | Low risk | Vaccines in identical, coded vials |
| Blinding (performance bias and detection bias) | Low risk | Double‐blind |
| Blinding of participants and personnel (performance bias) | Low risk | |
| Blinding of outcome assessment (detection bias) | Low risk | |
| Methods | Site: Canada Adverse events recorded by structured telephone interview of parents | |
| Participants | Included: age 2 to 3 months; healthy | |
| Interventions | Primary series + booster (aP.aP versus wP.wP)
Number randomised: 324 aP, 108 wP | |
| Outcomes |
| |
| Notes | Antipyretic/analgesic prophylaxis was discouraged but not prohibited | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation list kept in a locked file |
| Allocation concealment (selection bias) | Low risk | Vaccines in identical, coded vials |
| Blinding (performance bias and detection bias) | Low risk | Double‐blind |
| Blinding of participants and personnel (performance bias) | Low risk | |
| Blinding of outcome assessment (detection bias) | Low risk | |
| Methods | Site: Canada Adverse events recorded by structured telephone interview of parents | |
| Participants | Included: age 4 to 6 years; healthy; primary series DTaP or DTwP given in Halperin 1994 or Halperin 1996 | |
| Interventions | Booster (aP versus wP)
Number studied: 178 aP, 178 wP | |
| Outcomes |
| |
| Notes | Antipyretic/analgesic prophylaxis was discouraged but not prohibited | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation list kept in a locked file |
| Allocation concealment (selection bias) | Low risk | Vaccines in identical, coded vials |
| Blinding (performance bias and detection bias) | Low risk | Double‐blind |
| Blinding of participants and personnel (performance bias) | Low risk | |
| Blinding of outcome assessment (detection bias) | Low risk | |
| Methods | Site: Canada Computer‐generated randomisation list kept in a locked file. Study personnel collecting telephone reactogenicity data were blinded as to which vaccine the subject had received | |
| Participants | Included: age 4 to 6 years; healthy | |
| Interventions | Booster (aP versus wP)
Number randomised: 408 aP, 97 wP Concurrent vaccines: Hib; IPV | |
| Outcomes |
| |
| Notes | Only participants who had received 4 previous doses of DTwP were blinded to which vaccine they received for the fifth dose, because of different vaccine container formats. Antipyretic/analgesic prophylaxis was discouraged but not prohibited | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation list kept in a locked file |
| Allocation concealment (selection bias) | High risk | Different vaccine container formats |
| Blinding (performance bias and detection bias) | High risk | Study personnel collecting telephone reactogenicity data were blinded as to which vaccine the subject had received. Only participants who had received 4 previous doses of DTwP were blinded to which vaccine they received for the fifth dose |
| Blinding of participants and personnel (performance bias) | High risk | Only participants who had received 4 previous doses of DTwP were blinded to which vaccine they received for the fifth dose |
| Blinding of outcome assessment (detection bias) | High risk | Only participants who had received 4 previous doses of DTwP were blinded to which vaccine they received for the fifth dose |
| Methods | Site: Germany Parents recorded adverse events in diary | |
| Participants | Included: age 2 to 4 months; healthy | |
| Interventions | Primary series (aP versus wP)
Number randomised: 75 aP, 74 wP | |
| Outcomes |
| |
| Notes | Excluded for adverse events after 2nd and 3rd doses (number vaccinated/studied at these doses was uncertain due to inconsistencies in tabulated data). Redness and induration were reported only for the moderate/severe category | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Details not reported |
| Methods | Site: Turkey Parents recorded adverse events in diary | |
| Participants | Included: age 15 to 20 months; completed DTwP primary series | |
| Interventions | Booster (wP.aP versus wP.wP)
Number randomised: 55 aP, 55 wP | |
| Outcomes |
| |
| Notes | Data for drowsiness, irritability (restlessness), "unusual" crying and "gastrointestinal symptoms" collected but not reported. Report states "no serious events" but serious events not defined | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Details not reported |
| Methods | Site: Turkey Parents recorded adverse events in diary | |
| Participants | Included: age 4 to 6 years; completed DTwP primary series and 15‐ to 24‐month booster | |
| Interventions | Booster (wP.wP.aP versus wP.wP.wP)
Number studied: 53 aP, 52 wP | |
| Outcomes |
| |
| Notes | Total 108 randomised, data for 105 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Details not reported |
| Selective reporting (reporting bias) | High risk | Data for drowsiness, irritability (restlessness), "unusual" crying and "gastrointestinal symptoms" collected but not reported |
| Methods | Site: Thailand Parents recorded adverse events in diary | |
| Participants | Included: age 4 to 6 years old; healthy, who had received 4 doses of DTwP at 2, 4, 6 and 18 months | |
| Interventions | Booster (aP versus wP)
Number studied: 165 aP, 165 wP | |
| Outcomes |
| |
| Notes | All symptoms (solicited or unsolicited) were classified by the investigators as not related, unlikely, suspected or probably related. But not a clear temporal definition of this criterion (before or after data collection) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Unclear risk | Details not reported |
| Methods | Site: USA Parents recorded adverse events in diary | |
| Participants | Included: age 18 to 24 months; healthy; completed DTwP primary series | |
| Interventions | Booster (wP.aP versus wP.wP)
Number randomised: 40 aP, 20 wP | |
| Outcomes |
| |
| Notes | Reactive antipyretic/analgesic use allowed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Details not reported |
| Methods | Site: USA Parents recorded adverse events in diary | |
| Participants | Included: age 15 to 20 months; completed DTwP primary series | |
| Interventions | Booster (wP.aP versus wP.wP)
Number randomised: 164 aP, 82 wP | |
| Outcomes |
| |
| Notes | Study report states that only adverse events for which there was a significant difference between vaccines were reported. Not clear whether adverse event types were omitted or just certain time points for individual adverse events | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation but no statement regarding file locking |
| Allocation concealment (selection bias) | Unclear risk | Details not reported |
| Selective reporting (reporting bias) | Low risk | Vomiting is the only target adverse event without data. Redness reported only for moderate/severe category |
| Methods | Site: UK Design: parallel‐group RCT Parents and study nurses recorded adverse events on forms | |
| Participants | Included: infants attending clinics for 1st dose DTP (due at age 3 months) | |
| Interventions | Primary series (aP versus wP)
Number randomised: 94 aP, 94 wP | |
| Outcomes |
| |
| Notes | Minor adverse event data for fever only. Anorexia, drowsiness, irritability, prolonged crying and vomiting combined as "any systemic symptom" | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random sequence |
| Allocation concealment (selection bias) | Unclear risk | Details not reported |
| Selective reporting (reporting bias) | High risk | Data for redness, swelling collected but not reported (stated not to differ in frequency) |
| Methods | Site: USA Parents recorded adverse events in diary | |
| Participants | Included: age 4 to 6 years; healthy; completed DTwP primary series and 15‐ to 24‐month booster | |
| Interventions | Booster (wP.wP.aP versus wP.wP.wP)
Number randomised: 41 aP, 42 wP | |
| Outcomes |
| |
| Notes | No statement on antipyretic/analgesic use | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Details not reported |
| Methods | Site: USA Parents recorded adverse events on questionnaires and measured their child's rectal temperature | |
| Participants | Included: infants due to receive 1st dose DTP (mean age = 2 months), 2nd dose (mean age = 4 months) and 3rd dose (mean age = 6 months) | |
| Interventions | Primary series (aP versus wP)
Number randomised:
Dose schedule: 3 doses (2, 4, 6 months) | |
| Outcomes |
| |
| Notes | The pattern of crying is not clear, considered unusual, or high‐pitched cries. 7 children left the study for severe reactions not described and 2 experienced hypotonic/hyporesponsive episodes following the second vaccination. Reactive antipyretic/analgesic use allowed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Details not reported |
| Methods | Site: USA Parents recorded adverse events on forms | |
| Participants | Included: infants due to receive 1st dose DTP (mean age = 2 months) | |
| Interventions | Primary series (aP versus wP)
Number randomised: 88 aP, 22 wP | |
| Outcomes |
| |
| Notes | The choice of reported adverse event types was not limited to those showing a significant difference between vaccines but was based on the preliminary results of Decker 1995 (reported in Pichichero 1995), which identified the chosen adverse events as sufficient to differentiate between DTP vaccines in regard to reactogenicity. Irritability and pain were reported only for the moderate/severe category. Reactive antipyretic/analgesic use allowed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Details not reported |
| Selective reporting (reporting bias) | High risk | Data on anorexia, drowsiness and vomiting were collected but not reported |
| Methods | Site: USA Parents recorded adverse events on forms | |
| Participants | Included: age 2 months (6 to 12 weeks); healthy | |
| Interventions | Primary series (aP versus wP)
Number randomised: 62 aP, 18 wP | |
| Outcomes |
| |
| Notes | Redness and pain were reported only for the moderate/severe category | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Details not reported |
| Blinding (performance bias and detection bias) | Low risk | Parents and investigators are unaware of the type of vaccination |
| Blinding of participants and personnel (performance bias) | Low risk | |
| Blinding of outcome assessment (detection bias) | Low risk | |
| Methods | Site: USA Parents recorded adverse events on forms | |
| Participants | Included: age 15 to 20 months; primary series DTaP or DTwP given in Pichichero 1993 or Pichichero 1994 | |
| Interventions | Booster (aP.aP versus wP.wP)
Number studied: 124 aP, 34 wP | |
| Outcomes |
| |
| Notes | Possible bias due to parental self selection of 83% subset who continued in the study. Number of subjects studied for redness and pain in the DTwP group is unclear due to discrepancies in the data table. Irritability and pain were reported only for the moderate/severe category | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Details not reported |
| Methods | Site: USA Parents recorded adverse events on forms | |
| Participants | Included: completed DTaP or DTwP primary series in Decker 1995 | |
| Interventions | Booster (aP.aP versus wP.aP versus wP.wP) Children who had received DTaP primary series received the same DTaP as a booster, except for those who had received Lederle[3P] (no longer available) who were boosted with Lederle/Takeda[4F2]. Children who had received DTwP primary series were re‐randomised to receive 1 of the 12 DTaP vaccines or DTwP
Number studied: 1079 aP.aP, 187 wP.aP, 16 wP.wP | |
| Outcomes |
| |
| Notes | Most failures to proceed from primary series to booster study were due to prior receipt of booster dose or reluctance regarding venipuncture. Possible selection bias exists due to loss of 39% of subjects after primary series, although authors state that DTwP primary series recipients with severe reactions were not less likely to proceed to the booster study. Safety results are combined for all acellular vaccines. Data for drowsiness and vomiting were collected but not reported (stated to be not significantly different between groups) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Details not reported |
| Allocation concealment (selection bias) | Unclear risk | Vaccine vials labelled with letter codes instead of type/manufacturer details but unable to ascertain from study report whether vaccinators remained unaware of what each code represented |
| Blinding (performance bias and detection bias) | Low risk | Vaccinators took no further part in the study and did not participate in follow‐up data collection, so outcome assessment was double‐blind |
| Blinding of participants and personnel (performance bias) | Low risk | |
| Blinding of outcome assessment (detection bias) | Low risk | |
| Selective reporting (reporting bias) | High risk | Data for drowsiness and vomiting were collected but not reported (stated to be not significantly different between groups) |
| Methods | Site: USA | |
| Participants | Included: age 4 to 6 years of age who had completed earlier National Institute of Allergy and Infectious Diseases (NIAID) multicentre acellular pertussis vaccine trials in Decker 1995, Pichichero 1997 Excluded: subjects with contraindications or precautions to immunisations as specified in the Report of the Committee on Infectious Diseases of the American Academy of Pediatrics | |
| Interventions | Booster (same aPaPaP, mixed aPaPaP, wPaPaP versus wPwPwP)
Number of studies: 316 aP, 10 wP Dose schedule: 1 dose (4 to 6 years) Concurrent vaccine: OPV | |
| Outcomes |
| |
| Notes | Possible bias due to parental self selection of 83% subset who continued in the study Reactive antipyretic/analgesic use allowed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Details not reported |
| Methods | Site: Italy Design: parallel‐group RCT Parents recorded adverse events in diary | |
| Participants | Included: age 2 months; healthy | |
| Interventions | Primary series (aP versus wP)
Number randomised: 240 aP, 240 wP | |
| Outcomes |
| |
| Notes | No statement on antipyretic/analgesic use | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Details not reported |
| Methods | Site: Germany Active case ascertainment (bi‐weekly telephone). Case incidence adjusted for follow‐up duration by use of incidence density. Parents recorded adverse events in diary | |
| Participants | Included: age 2 to 4 months; healthy | |
| Interventions | Primary series + booster (aP.aP versus wP.wP)
A third non‐randomised group received DT (n = 1739) | |
| Outcomes |
| |
| Notes | Macrolide prophylaxis was not documented | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Details not reported |
| Methods | Site: USA Parents recorded adverse events on forms | |
| Participants | Included: age 15 to 16 months; healthy; completed DTwP primary series | |
| Interventions | Booster (wP.aP versus wP.wP)
Number randomised: 48 aP, 49 wP | |
| Outcomes |
| |
| Notes | No "severe" or contraindicating adverse events but these not defined in the study report. DTaP or DTwP administered at same time as OPV and MMR (but MMR at a different injection site) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Details not reported |
| Methods | Site: Senegal Study physicians visited homes, recorded adverse events and measured temperature at 2 to 3 days after each dose. Parents interviewed regarding deaths | |
| Participants | Included: age 2 months | |
| Interventions | Primary series (aP versus wP)
Number randomised: 141 aP, 145 wP | |
| Outcomes |
| |
| Notes | Study conducted in Senegal in an area with high background infant mortality. High loss to follow‐up (see text). Data included for 1st dose only. Second and third doses excluded because follow‐up for those doses was less than 80% in both vaccine groups. Irritability and pain were reported only for the moderate/severe category | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Details not reported |
| Methods | Site: Senegal Active case ascertainment (weekly visit by field workers). Case incidence adjusted for follow‐up duration by use of incidence density. Field workers recorded adverse events during 2 weekly visits after each dose | |
| Participants | Included: age 2 months | |
| Interventions | Primary series (aP versus wP)
Number studied (efficacy): 1847 aP, 1772 wP | |
| Outcomes |
| |
| Notes | Number randomised not stated. A total of 4821 were studied for safety. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Details not reported |
| Methods | Site: China Adverse event recording method not stated | |
| Participants | Included: age 3 to 6 months | |
| Interventions | Primary series (aP versus wP versus P)
Number studied: 105 aP, 101 wP, 100 P | |
| Outcomes |
| |
| Notes | Number randomised not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Details not reported |
| Methods | Site: Sweden Passive and active case ascertainment (parent report and monthly telephone). Case incidence adjusted for follow‐up duration by use of incidence density. Parents recorded adverse events in diary | |
| Participants | Included: full‐term, healthy infants | |
| Interventions | Primary series (aP versus DT)
Number randomised: 1724 aP, 1726 DT | |
| Outcomes |
| |
| Notes | Macrolide prophylaxis not used (not recommended in Sweden for age > 6 months and main efficacy follow‐up in this study started at age 10 months) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random sequence |
| Allocation concealment (selection bias) | Low risk | Vaccines indistinguishable |
| Blinding (performance bias and detection bias) | Unclear risk | Double‐blind |
| Blinding of participants and personnel (performance bias) | Low risk | |
| Blinding of outcome assessment (detection bias) | Low risk | |
| Selective reporting (reporting bias) | High risk | Data for anorexia and irritability were collected but not reported (stated to be equally frequent with both vaccines) |
| Methods | Site: Austria and Switzerland Parents recorded adverse events in diary | |
| Participants | Included: age 10 to 16 weeks; healthy | |
| Interventions | Primary series (aP versus wP)
Number studied: 200 aP, 101 wP | |
| Outcomes |
| |
| Notes | The 2 acellular vaccine formulations contained differing amounts of PT. In this review, data for the 2 acellular vaccine formulations are combined | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Details not reported |
Interventions
Primary series: primary series pertussis immunisation performed in the study.
Booster: pertussis booster immunisation performed in the study.
The pertussis immunisation history of study participants (including doses received in the study under consideration) is indicated in the form (X.Y.Z). The first element (X) indicates the type of pertussis vaccine received in the primary series (aP = acellular, wP = whole‐cell, P = placebo, DT = diphtheria‐tetanus toxoids). The second element (Y) indicates the vaccine type received at the 15‐ to 24‐month booster and the third element (Z) indicates the vaccine type received at the 4‐ to 6‐year booster. For example: aP = acellular primary series; wP.wP.aP = acellular 4‐ to 6‐year booster after whole‐cell primary series and whole‐cell 18‐ to 24‐month booster.
The vaccines used in each study are further identified by type, manufacturer and number, and type of pertussis components, as follows:
Vaccine types:
aP: acellular pertussis vaccine
DTaP: diphtheria‐tetanus‐acellular pertussis vaccine
wP: whole‐cell pertussis vaccine
DTwP: diphtheria‐tetanus‐whole‐cell pertussis vaccine
DT: diptheria‐tetanus (toxoids) vaccine
DTP: diptheria‐tetanus (toxoids) pertussis vaccine
P: placebo
Manufacturer abbreviations:
CAMR: Centre for Applied Microbiology and Research
Canton: Canton Department of Health
JNIH: Japanese National Institute of Health
Massachusetts: Massachusetts Public Health Laboratory
Michigan: Michigan Department of Health
Porton: Porton Products (later Speywood Pharmaceuticals)
SKB: SmithKline Beecham
SSVI: Swedish Serum and Vaccine Institute
Wuhan: Wuhan Department of Health
Pertussis components (in square brackets after manufacturer: [1] = PT (inactivated pertussis toxin); [2] = PT+FH (filamentous haemagglutinin); [3] = PT+FH+Prn (pertactin); [4] = PT+FH+Fim2&3 (fimbrial antigen serotypes 2 and 3) or PT+FH+Prn+Fim2 or PT+FH+Prn+Fim (serotype unspecified); [5] = PT+FH+Prn+Fim2&3; [W] = killed whole Bordetella pertussis organisms.
Outcomes
Non‐completion of the primary series due to adverse events, defined as withdrawal from the study due to an adverse event or events before completion of all scheduled doses of the primary series.
Deaths, encephalopathy, convulsions, hypotonic‐hyporesponsive episodes (HT‐HR): these events generally led to withdrawal from the study and were reported and analysed across all doses in a study.
Minor adverse events: fever, irritability, drowsiness, anorexia, vomiting, redness, pain/tenderness and swelling/induration. These events usually did not lead to withdrawal and data were reported and analysed separately for each dose within a study. Data for these events were not combined across doses (see 'Methods' section of the review for reasons).
Other abbreviations
BCG: Bacille Calmette‐Guérin
DB: double‐blind (claim made in study report)
Hib: Haemophilus influenzae type B vaccine
IPV: inactivated polio vaccine
MMR: measles‐mumps‐rubella vaccine
OPV: oral polio vaccine
RCT: randomised controlled trial
SIDS: sudden infant death syndrome
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Booster | |
| Booster | |
| Primary series | |
| Primary series | |
| Booster | |
| Primary series | |
| Primary series | |
| Primary series | |
| Booster | |
| Primary series |
AEs: adverse events
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Primary series non‐completion due to adverse events Show forest plot | 14 | 108909 | Risk Ratio (M‐H, Random, 95% CI) | 0.23 [0.12, 0.43] |
| Analysis 1.1 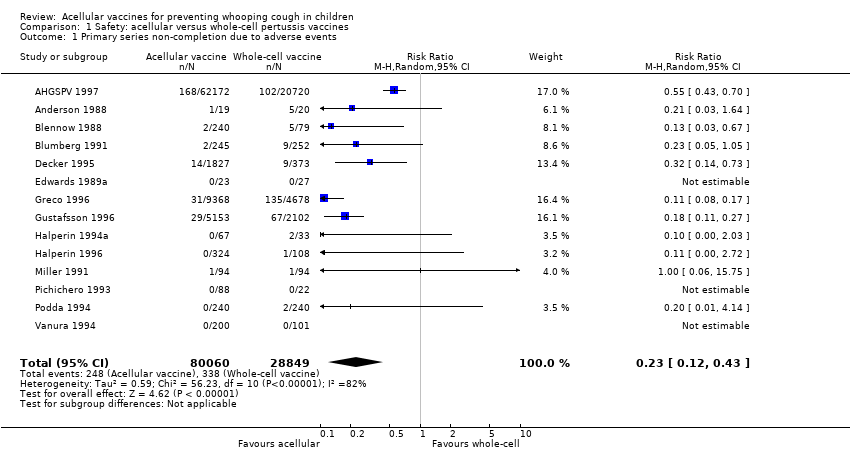 Comparison 1 Safety: acellular versus whole‐cell pertussis vaccines, Outcome 1 Primary series non‐completion due to adverse events. | ||||
| 2 Death (all causes) Show forest plot | 16 | 122451 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.62, 1.22] |
| Analysis 1.2  Comparison 1 Safety: acellular versus whole‐cell pertussis vaccines, Outcome 2 Death (all causes). | ||||
| 2.1 Primary series | 16 | 122451 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.62, 1.22] |
| 3 Death (infection) Show forest plot | 13 | 34498 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.23, 4.16] |
| Analysis 1.3  Comparison 1 Safety: acellular versus whole‐cell pertussis vaccines, Outcome 3 Death (infection). | ||||
| 3.1 Primary series | 13 | 34498 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.23, 4.16] |
| 4 Encephalopathy Show forest plot | 9 | 113762 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| Analysis 1.4 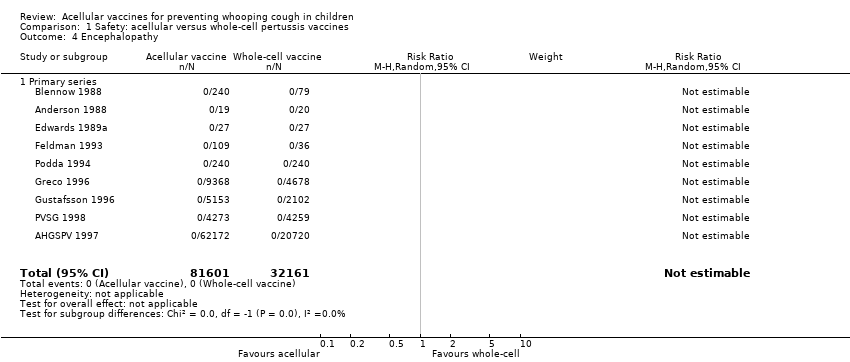 Comparison 1 Safety: acellular versus whole‐cell pertussis vaccines, Outcome 4 Encephalopathy. | ||||
| 4.1 Primary series | 9 | 113762 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Convulsions Show forest plot | 26 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.5  Comparison 1 Safety: acellular versus whole‐cell pertussis vaccines, Outcome 5 Convulsions. | ||||
| 5.1 Primary series | 15 | 124387 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.31, 0.73] |
| 5.2 Booster | 11 | 2647 | Risk Ratio (M‐H, Random, 95% CI) | 0.46 [0.02, 11.20] |
| 6 Hypotonic hyporesponsive episodes Show forest plot | 18 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.6  Comparison 1 Safety: acellular versus whole‐cell pertussis vaccines, Outcome 6 Hypotonic hyporesponsive episodes. | ||||
| 6.1 Primary series | 11 | 121573 | Risk Ratio (M‐H, Random, 95% CI) | 0.26 [0.08, 0.81] |
| 6.2 Booster | 7 | 2487 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Anorexia Show forest plot | 26 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.7 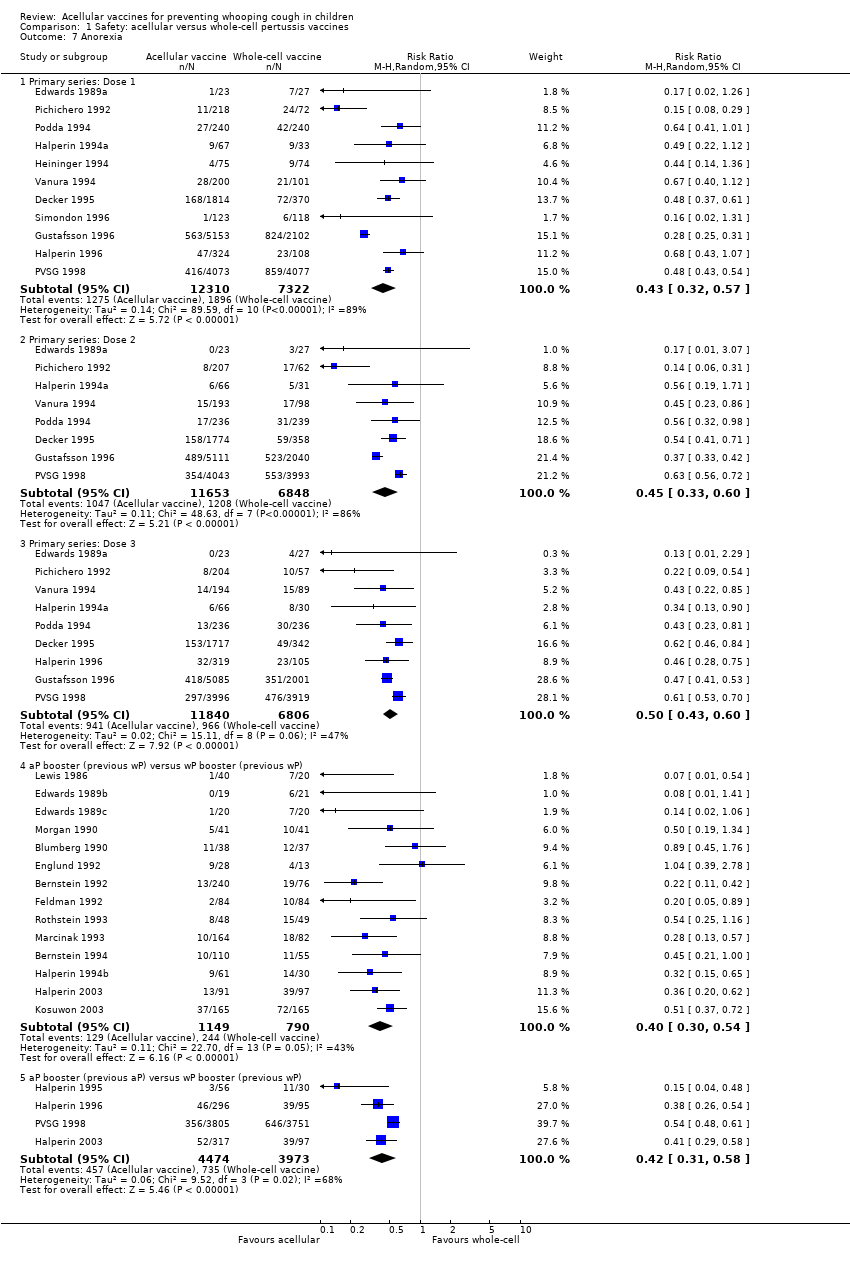 Comparison 1 Safety: acellular versus whole‐cell pertussis vaccines, Outcome 7 Anorexia. | ||||
| 7.1 Primary series: Dose 1 | 11 | 19632 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.32, 0.57] |
| 7.2 Primary series: Dose 2 | 8 | 18501 | Risk Ratio (M‐H, Random, 95% CI) | 0.45 [0.33, 0.60] |
| 7.3 Primary series: Dose 3 | 9 | 18646 | Risk Ratio (M‐H, Random, 95% CI) | 0.50 [0.43, 0.60] |
| 7.4 aP booster (previous wP) versus wP booster (previous wP) | 14 | 1939 | Risk Ratio (M‐H, Random, 95% CI) | 0.40 [0.30, 0.54] |
| 7.5 aP booster (previous aP) versus wP booster (previous wP) | 4 | 8447 | Risk Ratio (M‐H, Random, 95% CI) | 0.42 [0.31, 0.58] |
| 8 Drowsiness Show forest plot | 25 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.8  Comparison 1 Safety: acellular versus whole‐cell pertussis vaccines, Outcome 8 Drowsiness. | ||||
| 8.1 Primary series: Dose 1 | 12 | 20490 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.45, 0.68] |
| 8.2 Primary series: Dose 2 | 9 | 19308 | Risk Ratio (M‐H, Random, 95% CI) | 0.46 [0.35, 0.60] |
| 8.3 Primary series: Dose 3 | 10 | 19430 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.40, 0.77] |
| 8.4 aP booster (previous wP) versus wP booster (previous wP) | 13 | 2254 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.41, 0.56] |
| 8.5 aP booster (previous aP) versus wP booster (previous wP) | 3 | 8367 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.44, 0.54] |
| 9 Fever Show forest plot | 46 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.9 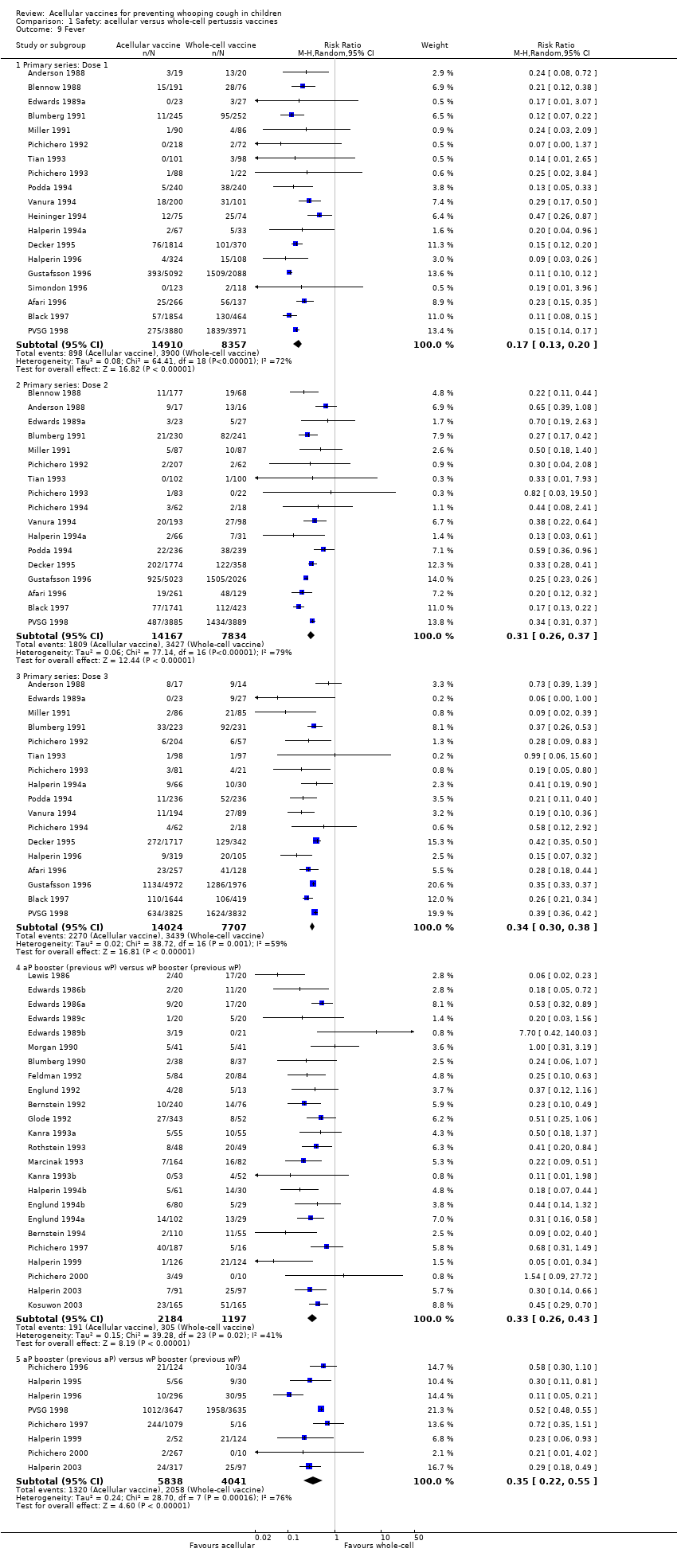 Comparison 1 Safety: acellular versus whole‐cell pertussis vaccines, Outcome 9 Fever. | ||||
| 9.1 Primary series: Dose 1 | 19 | 23267 | Risk Ratio (M‐H, Random, 95% CI) | 0.17 [0.13, 0.20] |
| 9.2 Primary series: Dose 2 | 17 | 22001 | Risk Ratio (M‐H, Random, 95% CI) | 0.31 [0.26, 0.37] |
| 9.3 Primary series: Dose 3 | 17 | 21731 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.30, 0.38] |
| 9.4 aP booster (previous wP) versus wP booster (previous wP) | 24 | 3381 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.26, 0.43] |
| 9.5 aP booster (previous aP) versus wP booster (previous wP) | 8 | 9879 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.22, 0.55] |
| 10 Irritability/fretfulness Show forest plot | 33 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.10 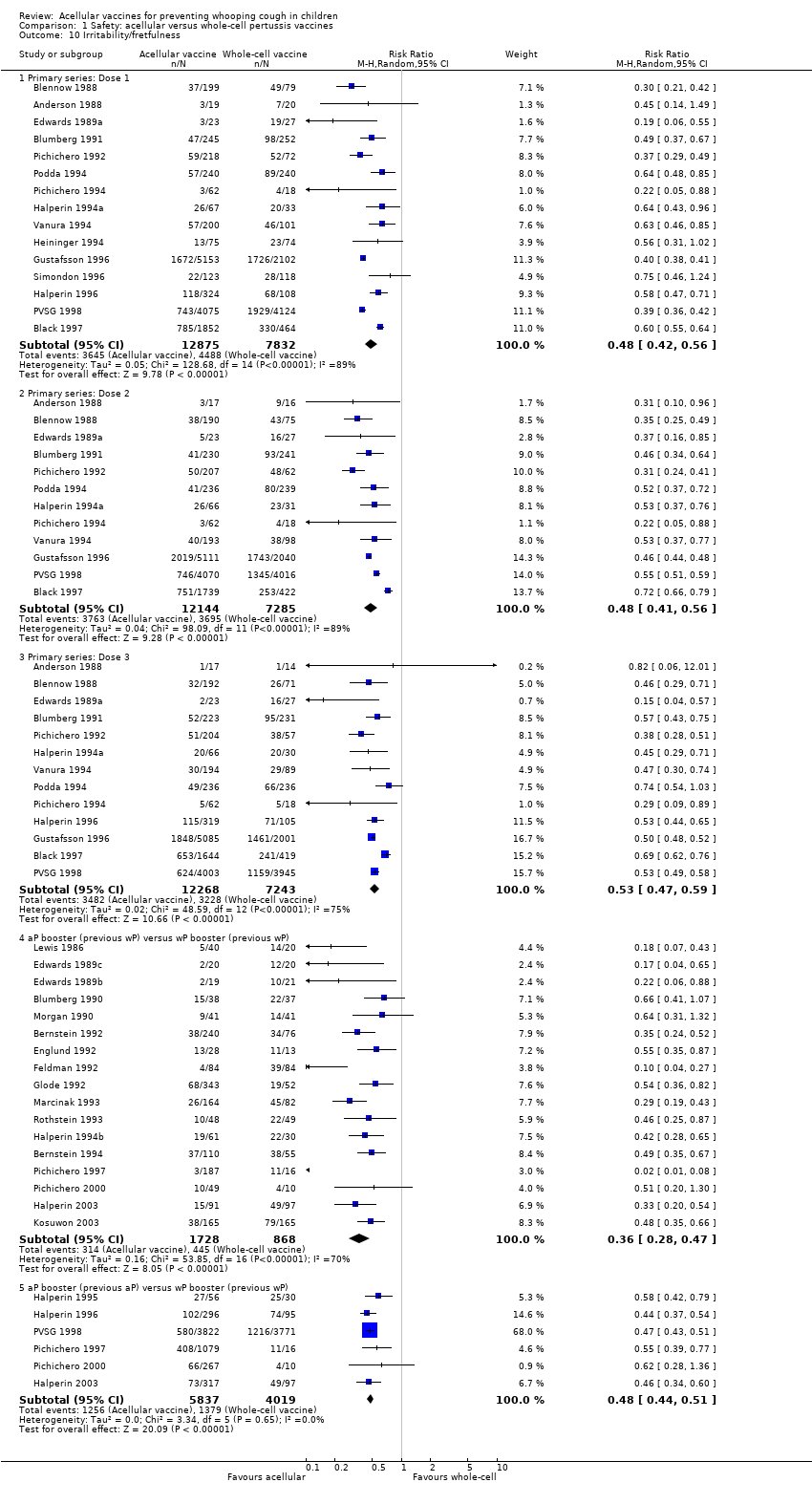 Comparison 1 Safety: acellular versus whole‐cell pertussis vaccines, Outcome 10 Irritability/fretfulness. | ||||
| 10.1 Primary series: Dose 1 | 15 | 20707 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.42, 0.56] |
| 10.2 Primary series: Dose 2 | 12 | 19429 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.41, 0.56] |
| 10.3 Primary series: Dose 3 | 13 | 19511 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.47, 0.59] |
| 10.4 aP booster (previous wP) versus wP booster (previous wP) | 17 | 2596 | Risk Ratio (M‐H, Random, 95% CI) | 0.36 [0.28, 0.47] |
| 10.5 aP booster (previous aP) versus wP booster (previous wP) | 6 | 9856 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.44, 0.51] |
| 11 Prolonged crying Show forest plot | 14 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.11  Comparison 1 Safety: acellular versus whole‐cell pertussis vaccines, Outcome 11 Prolonged crying. | ||||
| 11.1 Primary series: Dose 1 | 8 | 17184 | Risk Ratio (M‐H, Random, 95% CI) | 0.15 [0.11, 0.19] |
| 11.2 Primary series: Dose 2 | 6 | 16347 | Risk Ratio (M‐H, Random, 95% CI) | 0.29 [0.24, 0.35] |
| 11.3 Primary series: Dose 3 | 7 | 16545 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.24, 0.46] |
| 11.4 aP booster (previous wP) versus wP booster (previous wP) | 6 | 996 | Risk Ratio (M‐H, Random, 95% CI) | 0.21 [0.10, 0.48] |
| 11.5 aP booster (previous aP) versus wP booster (previous wP) | 2 | 7943 | Risk Ratio (M‐H, Random, 95% CI) | 0.27 [0.02, 3.12] |
| 12 Vomiting Show forest plot | 15 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.12 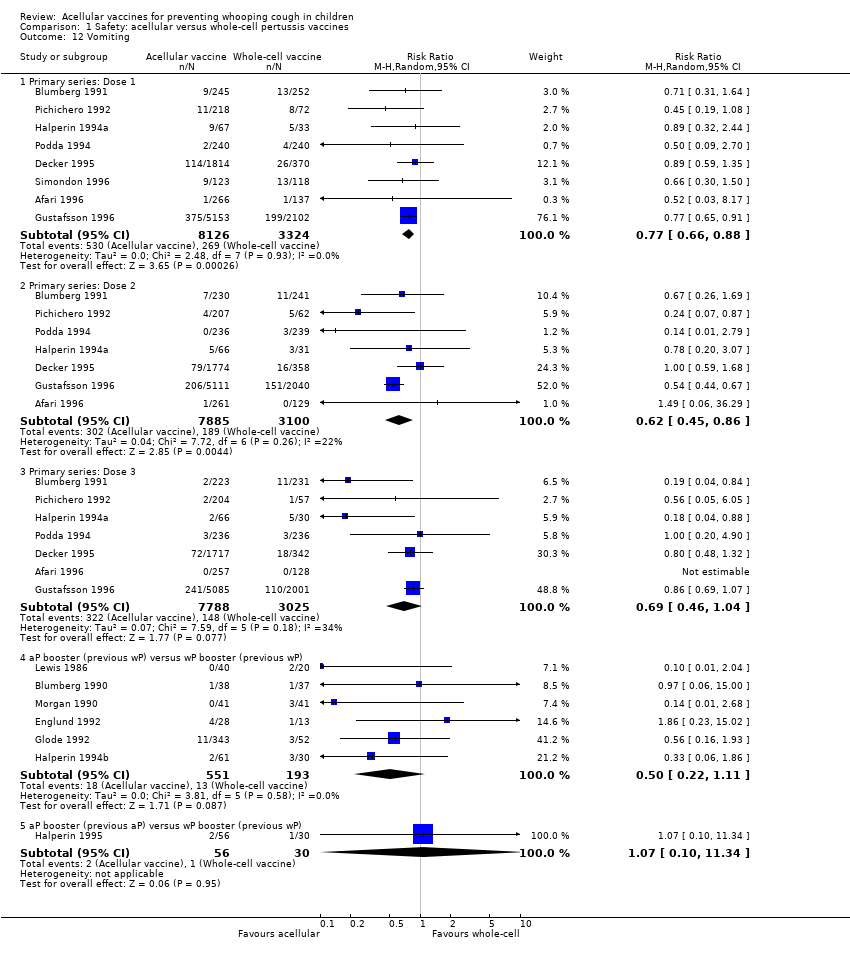 Comparison 1 Safety: acellular versus whole‐cell pertussis vaccines, Outcome 12 Vomiting. | ||||
| 12.1 Primary series: Dose 1 | 8 | 11450 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.66, 0.88] |
| 12.2 Primary series: Dose 2 | 7 | 10985 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.45, 0.86] |
| 12.3 Primary series: Dose 3 | 7 | 10813 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.46, 1.04] |
| 12.4 aP booster (previous wP) versus wP booster (previous wP) | 6 | 744 | Risk Ratio (M‐H, Random, 95% CI) | 0.50 [0.22, 1.11] |
| 12.5 aP booster (previous aP) versus wP booster (previous wP) | 1 | 86 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.10, 11.34] |
| 13 Pain/tenderness Show forest plot | 35 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.13  Comparison 1 Safety: acellular versus whole‐cell pertussis vaccines, Outcome 13 Pain/tenderness. | ||||
| 13.1 Primary series: Dose 1 | 13 | 14180 | Risk Ratio (M‐H, Random, 95% CI) | 0.20 [0.16, 0.25] |
| 13.2 Primary series: Dose 2 | 11 | 13186 | Risk Ratio (M‐H, Random, 95% CI) | 0.18 [0.15, 0.22] |
| 13.3 Primary series: Dose 3 | 12 | 13333 | Risk Ratio (M‐H, Random, 95% CI) | 0.20 [0.17, 0.24] |
| 13.4 aP booster (previous wP) versus wP booster (previous wP) | 21 | 3051 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.36, 0.53] |
| 13.5 aP booster (previous aP) versus wP booster (previous wP) | 5 | 2263 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.32, 0.58] |
| 14 Redness Show forest plot | 35 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.14  Comparison 1 Safety: acellular versus whole‐cell pertussis vaccines, Outcome 14 Redness. | ||||
| 14.1 Primary series: Dose 1 | 13 | 7153 | Risk Ratio (M‐H, Random, 95% CI) | 0.30 [0.23, 0.39] |
| 14.2 Primary series: Dose 2 | 12 | 6427 | Risk Ratio (M‐H, Random, 95% CI) | 0.39 [0.29, 0.51] |
| 14.3 Primary series: Dose 3 | 13 | 6632 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.41, 0.54] |
| 14.4 aP booster (previous wP) versus wP booster (previous wP) | 21 | 3055 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.44, 0.59] |
| 14.5 aP booster (previous aP) versus wP booster (previous wP) | 5 | 2263 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.52, 0.80] |
| 15 Swelling/induration Show forest plot | 39 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.15  Comparison 1 Safety: acellular versus whole‐cell pertussis vaccines, Outcome 15 Swelling/induration. | ||||
| 15.1 Primary series: Dose 1 | 15 | 14612 | Risk Ratio (M‐H, Random, 95% CI) | 0.24 [0.19, 0.31] |
| 15.2 Primary series: Dose 2 | 14 | 13779 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.28, 0.45] |
| 15.3 Primary series: Dose 3 | 15 | 13916 | Risk Ratio (M‐H, Random, 95% CI) | 0.40 [0.29, 0.54] |
| 15.4 aP booster (previous wP) versus wP booster (previous wP) | 22 | 3301 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.46, 0.57] |
| 15.5 aP booster (previous aP) versus wP booster (previous wP) | 6 | 2421 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.58, 0.80] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Primary series non‐completion due to adverse events Show forest plot | 4 | 25901 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.38, 1.29] |
| Analysis 2.1  Comparison 2 Safety: acellular vaccines versus placebo/DT, Outcome 1 Primary series non‐completion due to adverse events. | ||||
| 2 Death (all causes) Show forest plot | 4 | 25901 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.26, 4.42] |
| Analysis 2.2  Comparison 2 Safety: acellular vaccines versus placebo/DT, Outcome 2 Death (all causes). | ||||
| 2.1 Primary series | 4 | 25901 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.26, 4.42] |
| 3 Death (infection) Show forest plot | 4 | 25902 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [0.19, 7.80] |
| Analysis 2.3  Comparison 2 Safety: acellular vaccines versus placebo/DT, Outcome 3 Death (infection). | ||||
| 3.1 Primary series | 4 | 25902 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [0.19, 7.80] |
| 4 Encephalopathy Show forest plot | 2 | 18650 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| Analysis 2.4  Comparison 2 Safety: acellular vaccines versus placebo/DT, Outcome 4 Encephalopathy. | ||||
| 4.1 Primary series | 2 | 18650 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Convulsions Show forest plot | 4 | 25901 | Risk Ratio (M‐H, Random, 95% CI) | 0.44 [0.12, 1.69] |
| Analysis 2.5  Comparison 2 Safety: acellular vaccines versus placebo/DT, Outcome 5 Convulsions. | ||||
| 5.1 Primary series | 4 | 25901 | Risk Ratio (M‐H, Random, 95% CI) | 0.44 [0.12, 1.69] |
| 6 Hypotonic hyporesponsive episodes Show forest plot | 4 | 25901 | Risk Ratio (M‐H, Random, 95% CI) | 0.29 [0.02, 5.13] |
| Analysis 2.6  Comparison 2 Safety: acellular vaccines versus placebo/DT, Outcome 6 Hypotonic hyporesponsive episodes. | ||||
| 6.1 Primary series | 4 | 25901 | Risk Ratio (M‐H, Random, 95% CI) | 0.29 [0.02, 5.13] |
| 7 Anorexia Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 2.7  Comparison 2 Safety: acellular vaccines versus placebo/DT, Outcome 7 Anorexia. | ||||
| 7.1 Primary series: Dose 1 | 2 | 11526 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.93, 1.20] |
| 7.2 Primary series: Dose 2 | 2 | 11386 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.66, 1.46] |
| 7.3 Primary series: Dose 3 | 1 | 7623 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.91, 1.26] |
| 8 Drowsiness Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 2.8  Comparison 2 Safety: acellular vaccines versus placebo/DT, Outcome 8 Drowsiness. | ||||
| 8.1 Primary series: Dose 1 | 2 | 10954 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.96, 1.11] |
| 8.2 Primary series: Dose 2 | 2 | 10620 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.91, 1.09] |
| 8.3 Primary series: Dose 3 | 1 | 7623 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.91, 1.15] |
| 9 Fever Show forest plot | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 2.9  Comparison 2 Safety: acellular vaccines versus placebo/DT, Outcome 9 Fever. | ||||
| 9.1 Primary series: Dose 1 | 3 | 11255 | Risk Ratio (M‐H, Random, 95% CI) | 1.18 [0.73, 1.90] |
| 9.2 Primary series: Dose 2 | 3 | 10853 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.91, 1.11] |
| 9.3 Primary series: Dose 3 | 2 | 7654 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.94, 1.13] |
| 10 Irritability/fretfulness Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 2.10  Comparison 2 Safety: acellular vaccines versus placebo/DT, Outcome 10 Irritability/fretfulness. | ||||
| 10.1 Primary series: Dose 1 | 2 | 11526 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.93, 1.05] |
| 10.2 Primary series: Dose 2 | 2 | 11386 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.93, 1.03] |
| 10.3 Primary series: Dose 3 | 1 | 7623 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.91, 1.02] |
| 11 Prolonged crying Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 2.11 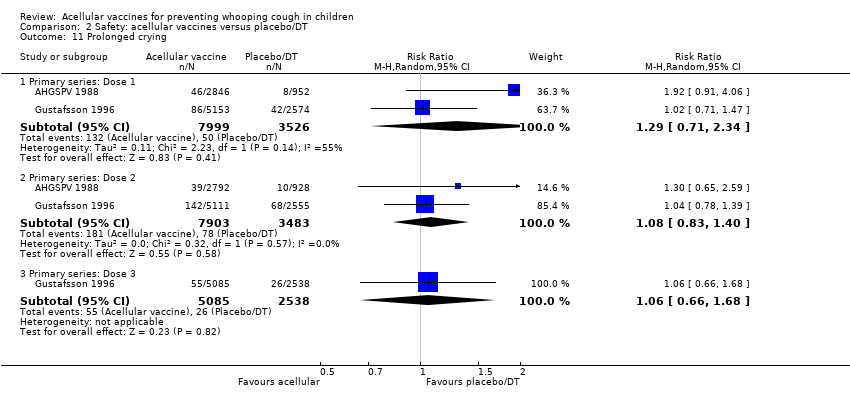 Comparison 2 Safety: acellular vaccines versus placebo/DT, Outcome 11 Prolonged crying. | ||||
| 11.1 Primary series: Dose 1 | 2 | 11525 | Risk Ratio (M‐H, Random, 95% CI) | 1.29 [0.71, 2.34] |
| 11.2 Primary series: Dose 2 | 2 | 11386 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.83, 1.40] |
| 11.3 Primary series: Dose 3 | 1 | 7623 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.66, 1.68] |
| 12 Vomiting Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 2.12  Comparison 2 Safety: acellular vaccines versus placebo/DT, Outcome 12 Vomiting. | ||||
| 12.1 Primary series: Dose 1 | 2 | 11526 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.94, 1.30] |
| 12.2 Primary series: Dose 2 | 2 | 11386 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.76, 1.07] |
| 12.3 Primary series: Dose 3 | 1 | 7623 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.75, 1.13] |
| 13 Pain/tenderness Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 2.13  Comparison 2 Safety: acellular vaccines versus placebo/DT, Outcome 13 Pain/tenderness. | ||||
| 13.1 Primary series: Dose 1 | 2 | 11451 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.78, 1.32] |
| 13.2 Primary series: Dose 2 | 2 | 11202 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.81, 1.51] |
| 13.3 Primary series: Dose 3 | 1 | 7623 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.87, 1.16] |
| 14 Swelling/induration Show forest plot | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 2.14 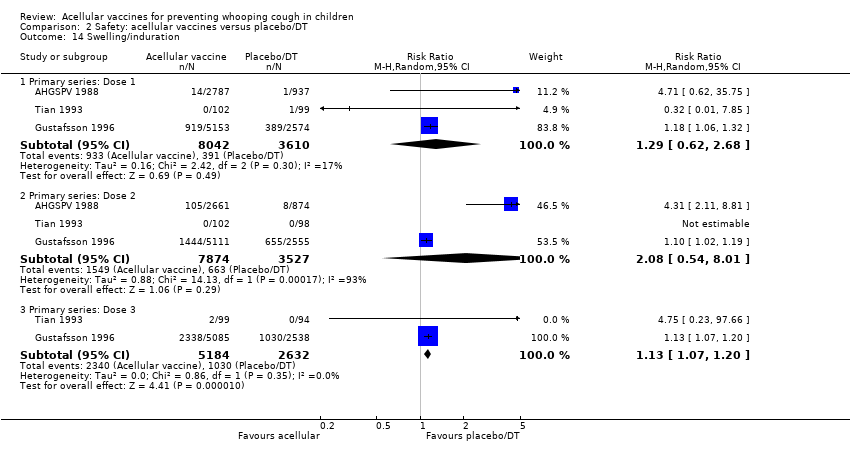 Comparison 2 Safety: acellular vaccines versus placebo/DT, Outcome 14 Swelling/induration. | ||||
| 14.1 Primary series: Dose 1 | 3 | 11652 | Risk Ratio (M‐H, Random, 95% CI) | 1.29 [0.62, 2.68] |
| 14.2 Primary series: Dose 2 | 3 | 11401 | Risk Ratio (M‐H, Random, 95% CI) | 2.08 [0.54, 8.01] |
| 14.3 Primary series: Dose 3 | 2 | 7816 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [1.07, 1.20] |
| 15 Redness Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 2.15  Comparison 2 Safety: acellular vaccines versus placebo/DT, Outcome 15 Redness. | ||||
| 15.1 Primary series: Dose 1 | 1 | 3724 | Risk Ratio (M‐H, Random, 95% CI) | 3.03 [0.38, 23.85] |
| 15.2 Primary series: Dose 2 | 1 | 3535 | Risk Ratio (M‐H, Random, 95% CI) | 8.76 [3.89, 19.72] |
| 15.3 Primary series: Dose 3 | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
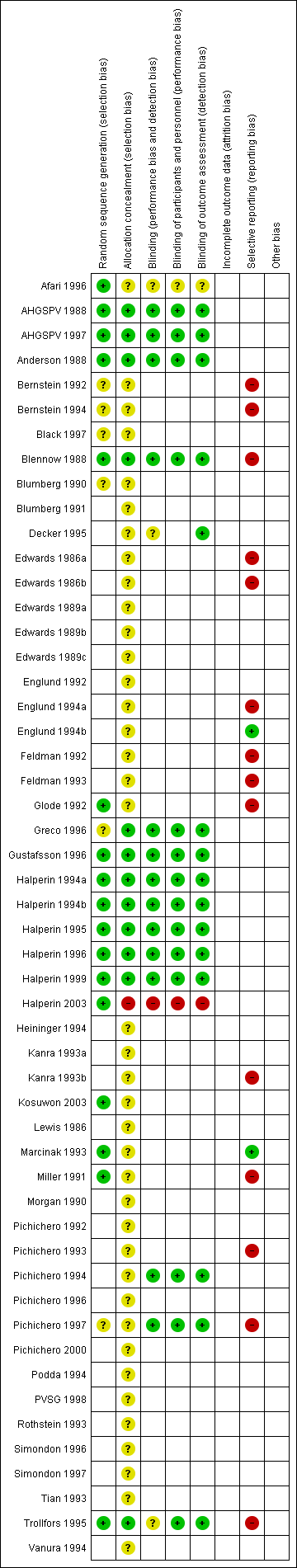
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Safety: acellular versus whole‐cell pertussis vaccines, Outcome 1 Primary series non‐completion due to adverse events.

Comparison 1 Safety: acellular versus whole‐cell pertussis vaccines, Outcome 2 Death (all causes).
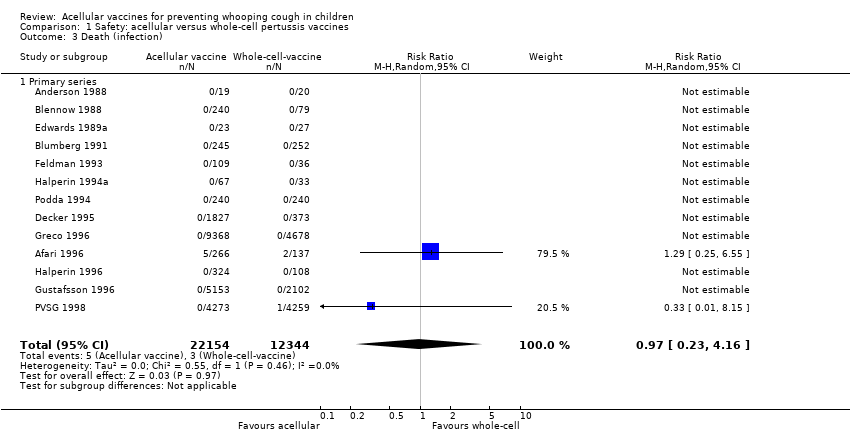
Comparison 1 Safety: acellular versus whole‐cell pertussis vaccines, Outcome 3 Death (infection).
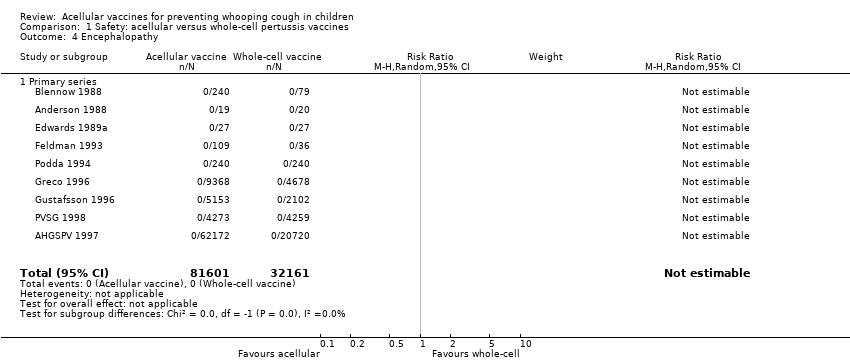
Comparison 1 Safety: acellular versus whole‐cell pertussis vaccines, Outcome 4 Encephalopathy.
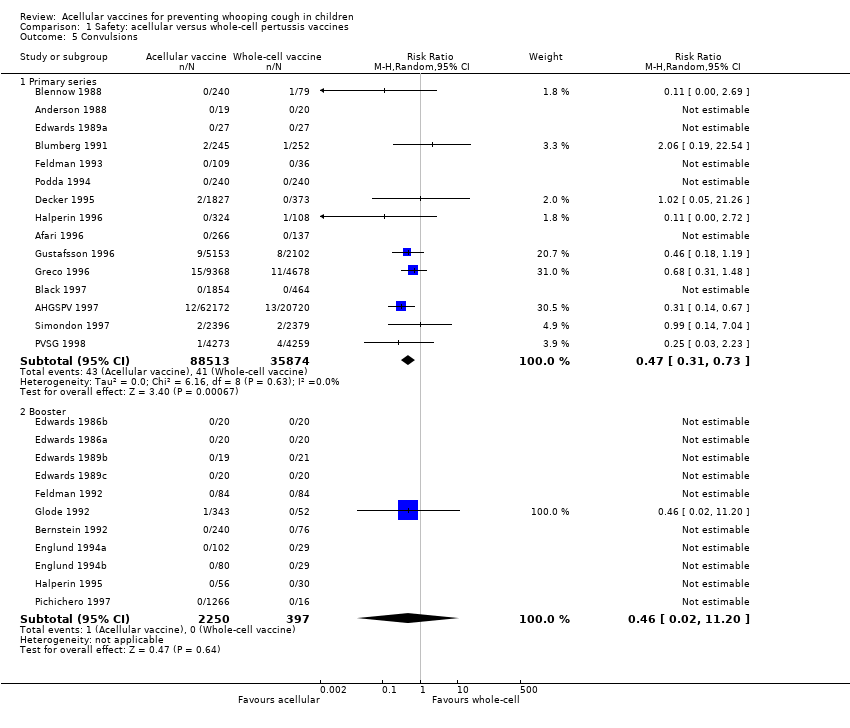
Comparison 1 Safety: acellular versus whole‐cell pertussis vaccines, Outcome 5 Convulsions.

Comparison 1 Safety: acellular versus whole‐cell pertussis vaccines, Outcome 6 Hypotonic hyporesponsive episodes.

Comparison 1 Safety: acellular versus whole‐cell pertussis vaccines, Outcome 7 Anorexia.
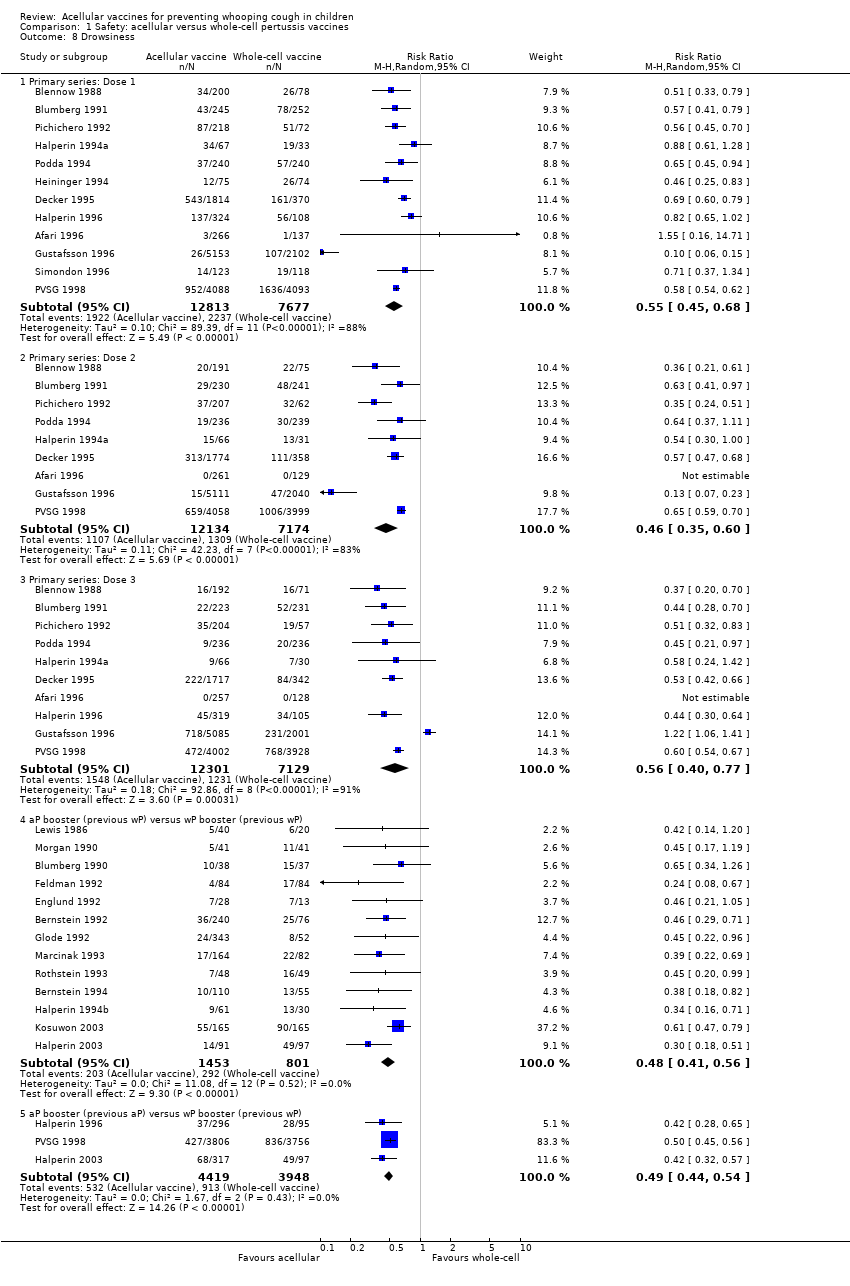
Comparison 1 Safety: acellular versus whole‐cell pertussis vaccines, Outcome 8 Drowsiness.

Comparison 1 Safety: acellular versus whole‐cell pertussis vaccines, Outcome 9 Fever.
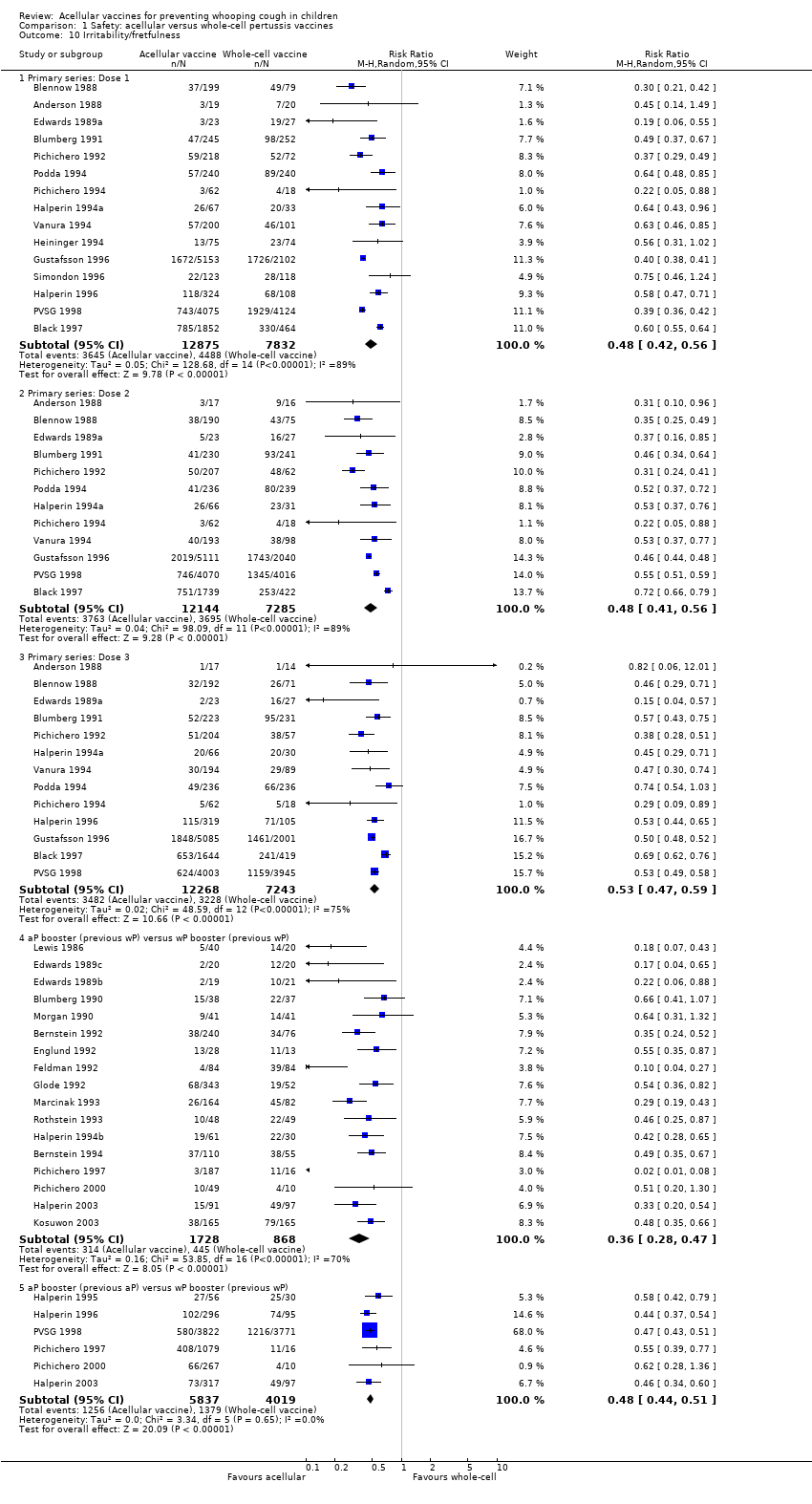
Comparison 1 Safety: acellular versus whole‐cell pertussis vaccines, Outcome 10 Irritability/fretfulness.
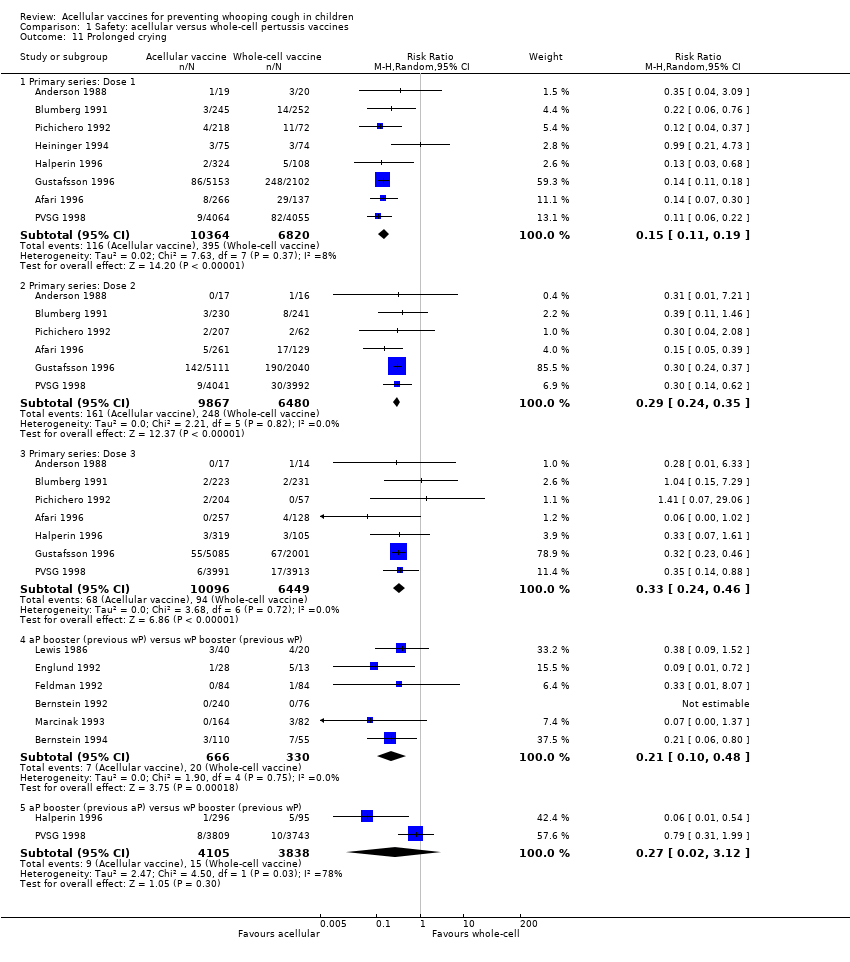
Comparison 1 Safety: acellular versus whole‐cell pertussis vaccines, Outcome 11 Prolonged crying.

Comparison 1 Safety: acellular versus whole‐cell pertussis vaccines, Outcome 12 Vomiting.

Comparison 1 Safety: acellular versus whole‐cell pertussis vaccines, Outcome 13 Pain/tenderness.
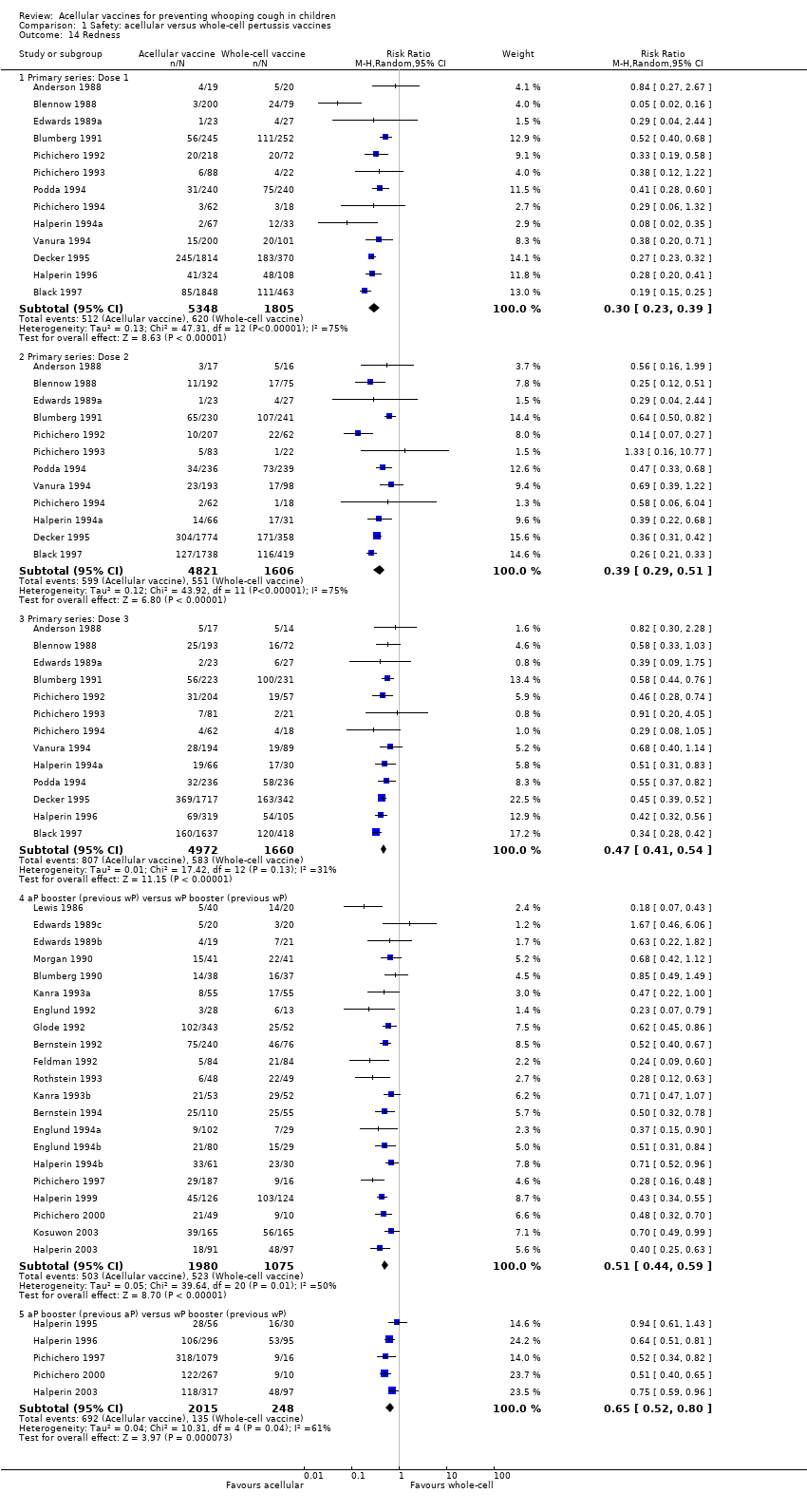
Comparison 1 Safety: acellular versus whole‐cell pertussis vaccines, Outcome 14 Redness.
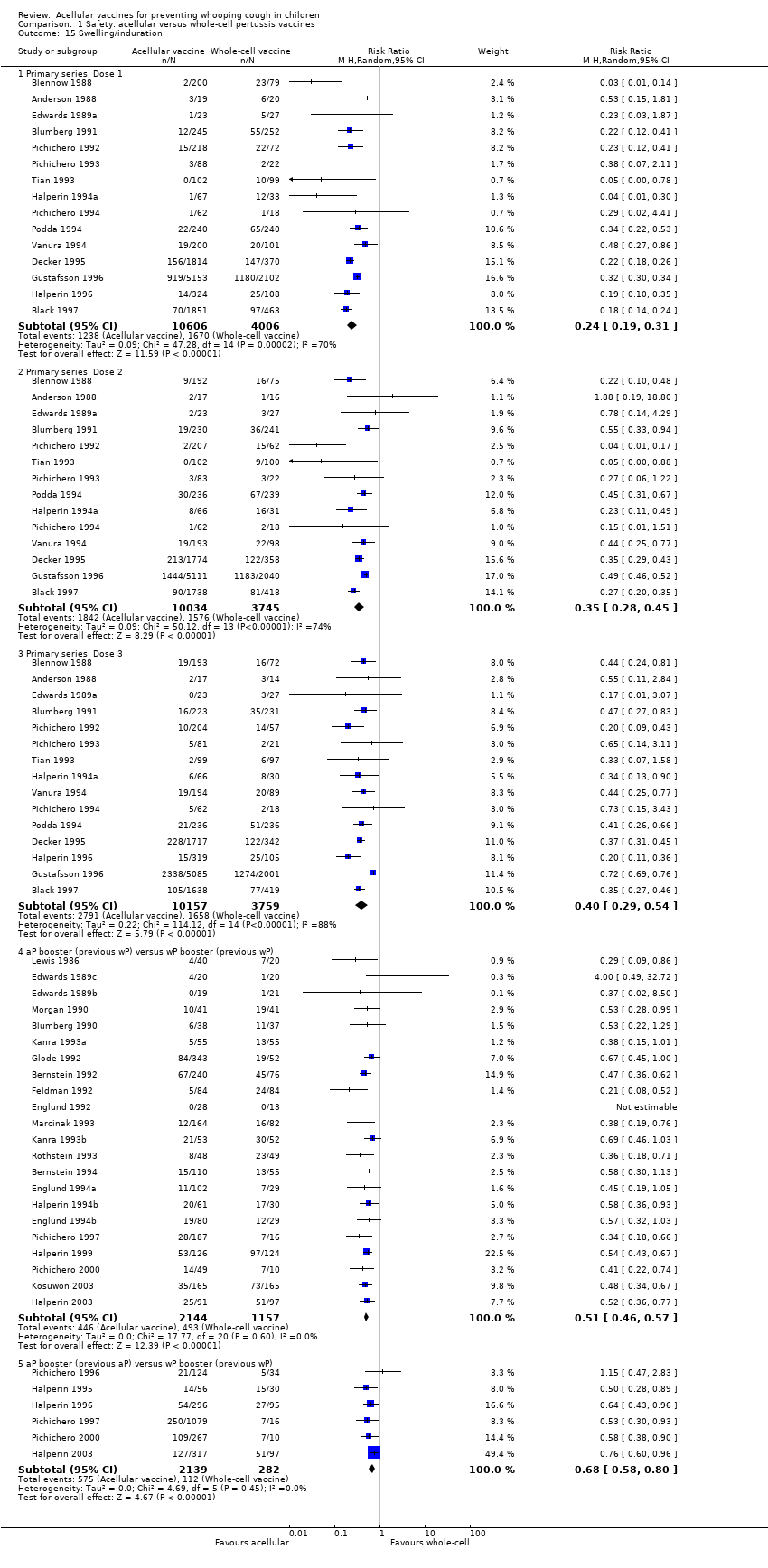
Comparison 1 Safety: acellular versus whole‐cell pertussis vaccines, Outcome 15 Swelling/induration.

Comparison 2 Safety: acellular vaccines versus placebo/DT, Outcome 1 Primary series non‐completion due to adverse events.

Comparison 2 Safety: acellular vaccines versus placebo/DT, Outcome 2 Death (all causes).

Comparison 2 Safety: acellular vaccines versus placebo/DT, Outcome 3 Death (infection).

Comparison 2 Safety: acellular vaccines versus placebo/DT, Outcome 4 Encephalopathy.
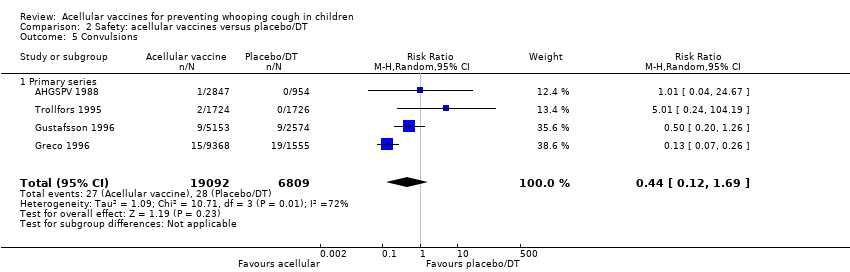
Comparison 2 Safety: acellular vaccines versus placebo/DT, Outcome 5 Convulsions.

Comparison 2 Safety: acellular vaccines versus placebo/DT, Outcome 6 Hypotonic hyporesponsive episodes.

Comparison 2 Safety: acellular vaccines versus placebo/DT, Outcome 7 Anorexia.
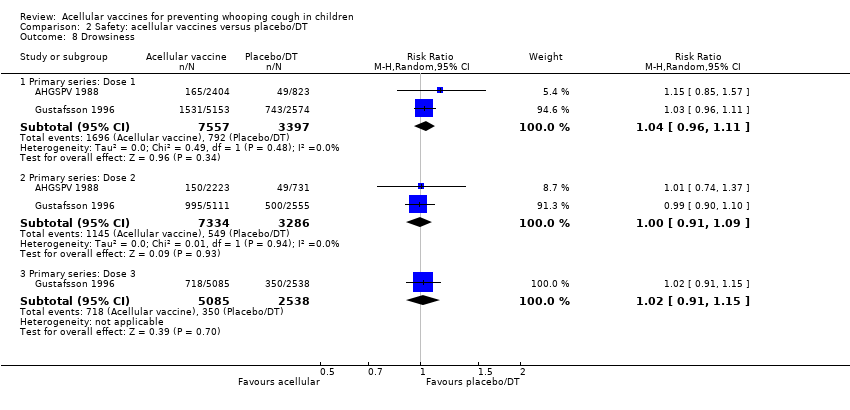
Comparison 2 Safety: acellular vaccines versus placebo/DT, Outcome 8 Drowsiness.
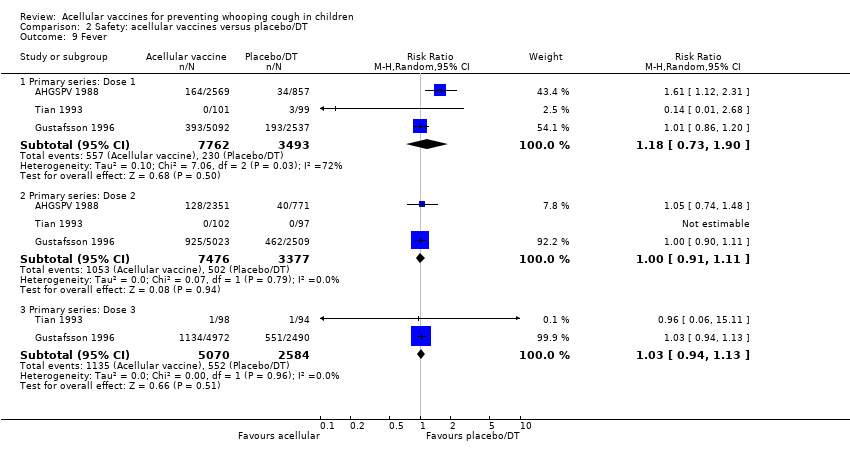
Comparison 2 Safety: acellular vaccines versus placebo/DT, Outcome 9 Fever.
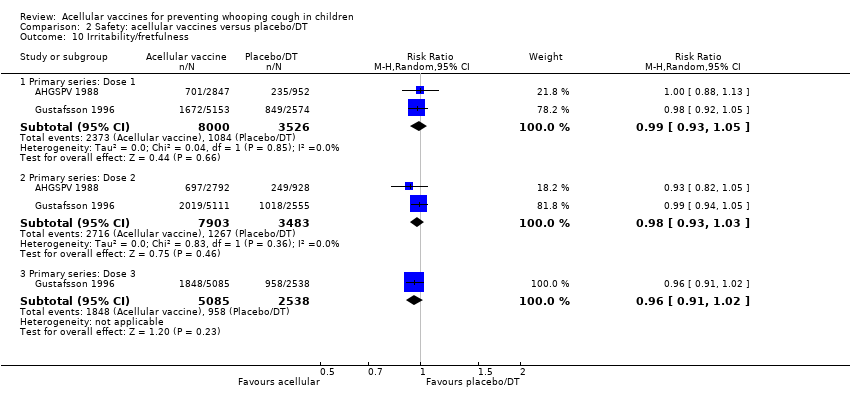
Comparison 2 Safety: acellular vaccines versus placebo/DT, Outcome 10 Irritability/fretfulness.

Comparison 2 Safety: acellular vaccines versus placebo/DT, Outcome 11 Prolonged crying.
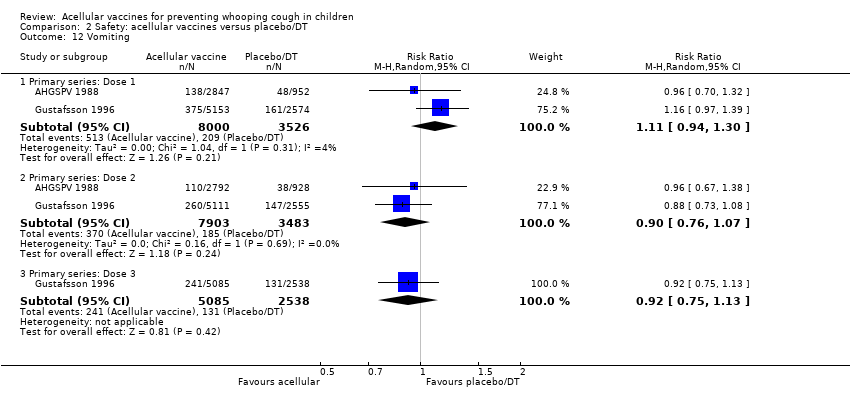
Comparison 2 Safety: acellular vaccines versus placebo/DT, Outcome 12 Vomiting.
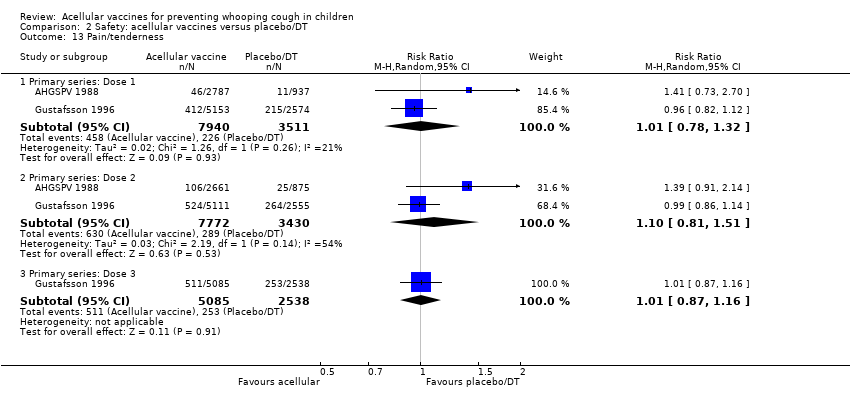
Comparison 2 Safety: acellular vaccines versus placebo/DT, Outcome 13 Pain/tenderness.

Comparison 2 Safety: acellular vaccines versus placebo/DT, Outcome 14 Swelling/induration.
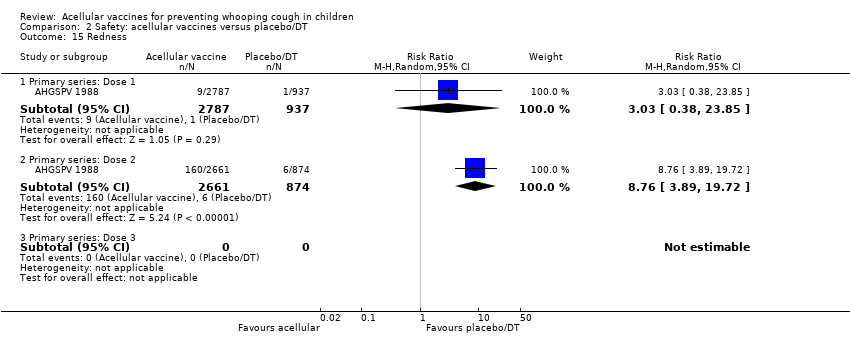
Comparison 2 Safety: acellular vaccines versus placebo/DT, Outcome 15 Redness.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Primary series non‐completion due to adverse events Show forest plot | 14 | 108909 | Risk Ratio (M‐H, Random, 95% CI) | 0.23 [0.12, 0.43] |
| 2 Death (all causes) Show forest plot | 16 | 122451 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.62, 1.22] |
| 2.1 Primary series | 16 | 122451 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.62, 1.22] |
| 3 Death (infection) Show forest plot | 13 | 34498 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.23, 4.16] |
| 3.1 Primary series | 13 | 34498 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.23, 4.16] |
| 4 Encephalopathy Show forest plot | 9 | 113762 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.1 Primary series | 9 | 113762 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Convulsions Show forest plot | 26 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 Primary series | 15 | 124387 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.31, 0.73] |
| 5.2 Booster | 11 | 2647 | Risk Ratio (M‐H, Random, 95% CI) | 0.46 [0.02, 11.20] |
| 6 Hypotonic hyporesponsive episodes Show forest plot | 18 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6.1 Primary series | 11 | 121573 | Risk Ratio (M‐H, Random, 95% CI) | 0.26 [0.08, 0.81] |
| 6.2 Booster | 7 | 2487 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Anorexia Show forest plot | 26 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 7.1 Primary series: Dose 1 | 11 | 19632 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.32, 0.57] |
| 7.2 Primary series: Dose 2 | 8 | 18501 | Risk Ratio (M‐H, Random, 95% CI) | 0.45 [0.33, 0.60] |
| 7.3 Primary series: Dose 3 | 9 | 18646 | Risk Ratio (M‐H, Random, 95% CI) | 0.50 [0.43, 0.60] |
| 7.4 aP booster (previous wP) versus wP booster (previous wP) | 14 | 1939 | Risk Ratio (M‐H, Random, 95% CI) | 0.40 [0.30, 0.54] |
| 7.5 aP booster (previous aP) versus wP booster (previous wP) | 4 | 8447 | Risk Ratio (M‐H, Random, 95% CI) | 0.42 [0.31, 0.58] |
| 8 Drowsiness Show forest plot | 25 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 8.1 Primary series: Dose 1 | 12 | 20490 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.45, 0.68] |
| 8.2 Primary series: Dose 2 | 9 | 19308 | Risk Ratio (M‐H, Random, 95% CI) | 0.46 [0.35, 0.60] |
| 8.3 Primary series: Dose 3 | 10 | 19430 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.40, 0.77] |
| 8.4 aP booster (previous wP) versus wP booster (previous wP) | 13 | 2254 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.41, 0.56] |
| 8.5 aP booster (previous aP) versus wP booster (previous wP) | 3 | 8367 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.44, 0.54] |
| 9 Fever Show forest plot | 46 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 9.1 Primary series: Dose 1 | 19 | 23267 | Risk Ratio (M‐H, Random, 95% CI) | 0.17 [0.13, 0.20] |
| 9.2 Primary series: Dose 2 | 17 | 22001 | Risk Ratio (M‐H, Random, 95% CI) | 0.31 [0.26, 0.37] |
| 9.3 Primary series: Dose 3 | 17 | 21731 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.30, 0.38] |
| 9.4 aP booster (previous wP) versus wP booster (previous wP) | 24 | 3381 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.26, 0.43] |
| 9.5 aP booster (previous aP) versus wP booster (previous wP) | 8 | 9879 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.22, 0.55] |
| 10 Irritability/fretfulness Show forest plot | 33 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 10.1 Primary series: Dose 1 | 15 | 20707 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.42, 0.56] |
| 10.2 Primary series: Dose 2 | 12 | 19429 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.41, 0.56] |
| 10.3 Primary series: Dose 3 | 13 | 19511 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.47, 0.59] |
| 10.4 aP booster (previous wP) versus wP booster (previous wP) | 17 | 2596 | Risk Ratio (M‐H, Random, 95% CI) | 0.36 [0.28, 0.47] |
| 10.5 aP booster (previous aP) versus wP booster (previous wP) | 6 | 9856 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.44, 0.51] |
| 11 Prolonged crying Show forest plot | 14 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 11.1 Primary series: Dose 1 | 8 | 17184 | Risk Ratio (M‐H, Random, 95% CI) | 0.15 [0.11, 0.19] |
| 11.2 Primary series: Dose 2 | 6 | 16347 | Risk Ratio (M‐H, Random, 95% CI) | 0.29 [0.24, 0.35] |
| 11.3 Primary series: Dose 3 | 7 | 16545 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.24, 0.46] |
| 11.4 aP booster (previous wP) versus wP booster (previous wP) | 6 | 996 | Risk Ratio (M‐H, Random, 95% CI) | 0.21 [0.10, 0.48] |
| 11.5 aP booster (previous aP) versus wP booster (previous wP) | 2 | 7943 | Risk Ratio (M‐H, Random, 95% CI) | 0.27 [0.02, 3.12] |
| 12 Vomiting Show forest plot | 15 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 12.1 Primary series: Dose 1 | 8 | 11450 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.66, 0.88] |
| 12.2 Primary series: Dose 2 | 7 | 10985 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.45, 0.86] |
| 12.3 Primary series: Dose 3 | 7 | 10813 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.46, 1.04] |
| 12.4 aP booster (previous wP) versus wP booster (previous wP) | 6 | 744 | Risk Ratio (M‐H, Random, 95% CI) | 0.50 [0.22, 1.11] |
| 12.5 aP booster (previous aP) versus wP booster (previous wP) | 1 | 86 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.10, 11.34] |
| 13 Pain/tenderness Show forest plot | 35 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 13.1 Primary series: Dose 1 | 13 | 14180 | Risk Ratio (M‐H, Random, 95% CI) | 0.20 [0.16, 0.25] |
| 13.2 Primary series: Dose 2 | 11 | 13186 | Risk Ratio (M‐H, Random, 95% CI) | 0.18 [0.15, 0.22] |
| 13.3 Primary series: Dose 3 | 12 | 13333 | Risk Ratio (M‐H, Random, 95% CI) | 0.20 [0.17, 0.24] |
| 13.4 aP booster (previous wP) versus wP booster (previous wP) | 21 | 3051 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.36, 0.53] |
| 13.5 aP booster (previous aP) versus wP booster (previous wP) | 5 | 2263 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.32, 0.58] |
| 14 Redness Show forest plot | 35 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 14.1 Primary series: Dose 1 | 13 | 7153 | Risk Ratio (M‐H, Random, 95% CI) | 0.30 [0.23, 0.39] |
| 14.2 Primary series: Dose 2 | 12 | 6427 | Risk Ratio (M‐H, Random, 95% CI) | 0.39 [0.29, 0.51] |
| 14.3 Primary series: Dose 3 | 13 | 6632 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.41, 0.54] |
| 14.4 aP booster (previous wP) versus wP booster (previous wP) | 21 | 3055 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.44, 0.59] |
| 14.5 aP booster (previous aP) versus wP booster (previous wP) | 5 | 2263 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.52, 0.80] |
| 15 Swelling/induration Show forest plot | 39 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 15.1 Primary series: Dose 1 | 15 | 14612 | Risk Ratio (M‐H, Random, 95% CI) | 0.24 [0.19, 0.31] |
| 15.2 Primary series: Dose 2 | 14 | 13779 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.28, 0.45] |
| 15.3 Primary series: Dose 3 | 15 | 13916 | Risk Ratio (M‐H, Random, 95% CI) | 0.40 [0.29, 0.54] |
| 15.4 aP booster (previous wP) versus wP booster (previous wP) | 22 | 3301 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.46, 0.57] |
| 15.5 aP booster (previous aP) versus wP booster (previous wP) | 6 | 2421 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.58, 0.80] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Primary series non‐completion due to adverse events Show forest plot | 4 | 25901 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.38, 1.29] |
| 2 Death (all causes) Show forest plot | 4 | 25901 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.26, 4.42] |
| 2.1 Primary series | 4 | 25901 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.26, 4.42] |
| 3 Death (infection) Show forest plot | 4 | 25902 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [0.19, 7.80] |
| 3.1 Primary series | 4 | 25902 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [0.19, 7.80] |
| 4 Encephalopathy Show forest plot | 2 | 18650 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.1 Primary series | 2 | 18650 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Convulsions Show forest plot | 4 | 25901 | Risk Ratio (M‐H, Random, 95% CI) | 0.44 [0.12, 1.69] |
| 5.1 Primary series | 4 | 25901 | Risk Ratio (M‐H, Random, 95% CI) | 0.44 [0.12, 1.69] |
| 6 Hypotonic hyporesponsive episodes Show forest plot | 4 | 25901 | Risk Ratio (M‐H, Random, 95% CI) | 0.29 [0.02, 5.13] |
| 6.1 Primary series | 4 | 25901 | Risk Ratio (M‐H, Random, 95% CI) | 0.29 [0.02, 5.13] |
| 7 Anorexia Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 7.1 Primary series: Dose 1 | 2 | 11526 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.93, 1.20] |
| 7.2 Primary series: Dose 2 | 2 | 11386 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.66, 1.46] |
| 7.3 Primary series: Dose 3 | 1 | 7623 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.91, 1.26] |
| 8 Drowsiness Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 8.1 Primary series: Dose 1 | 2 | 10954 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.96, 1.11] |
| 8.2 Primary series: Dose 2 | 2 | 10620 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.91, 1.09] |
| 8.3 Primary series: Dose 3 | 1 | 7623 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.91, 1.15] |
| 9 Fever Show forest plot | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 9.1 Primary series: Dose 1 | 3 | 11255 | Risk Ratio (M‐H, Random, 95% CI) | 1.18 [0.73, 1.90] |
| 9.2 Primary series: Dose 2 | 3 | 10853 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.91, 1.11] |
| 9.3 Primary series: Dose 3 | 2 | 7654 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.94, 1.13] |
| 10 Irritability/fretfulness Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 10.1 Primary series: Dose 1 | 2 | 11526 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.93, 1.05] |
| 10.2 Primary series: Dose 2 | 2 | 11386 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.93, 1.03] |
| 10.3 Primary series: Dose 3 | 1 | 7623 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.91, 1.02] |
| 11 Prolonged crying Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 11.1 Primary series: Dose 1 | 2 | 11525 | Risk Ratio (M‐H, Random, 95% CI) | 1.29 [0.71, 2.34] |
| 11.2 Primary series: Dose 2 | 2 | 11386 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.83, 1.40] |
| 11.3 Primary series: Dose 3 | 1 | 7623 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.66, 1.68] |
| 12 Vomiting Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 12.1 Primary series: Dose 1 | 2 | 11526 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.94, 1.30] |
| 12.2 Primary series: Dose 2 | 2 | 11386 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.76, 1.07] |
| 12.3 Primary series: Dose 3 | 1 | 7623 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.75, 1.13] |
| 13 Pain/tenderness Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 13.1 Primary series: Dose 1 | 2 | 11451 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.78, 1.32] |
| 13.2 Primary series: Dose 2 | 2 | 11202 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.81, 1.51] |
| 13.3 Primary series: Dose 3 | 1 | 7623 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.87, 1.16] |
| 14 Swelling/induration Show forest plot | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 14.1 Primary series: Dose 1 | 3 | 11652 | Risk Ratio (M‐H, Random, 95% CI) | 1.29 [0.62, 2.68] |
| 14.2 Primary series: Dose 2 | 3 | 11401 | Risk Ratio (M‐H, Random, 95% CI) | 2.08 [0.54, 8.01] |
| 14.3 Primary series: Dose 3 | 2 | 7816 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [1.07, 1.20] |
| 15 Redness Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 15.1 Primary series: Dose 1 | 1 | 3724 | Risk Ratio (M‐H, Random, 95% CI) | 3.03 [0.38, 23.85] |
| 15.2 Primary series: Dose 2 | 1 | 3535 | Risk Ratio (M‐H, Random, 95% CI) | 8.76 [3.89, 19.72] |
| 15.3 Primary series: Dose 3 | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |

