| 1 Primary series non‐completion due to adverse events Show forest plot | 14 | 108909 | Risk Ratio (M‐H, Random, 95% CI) | 0.23 [0.12, 0.43] |
|
| 2 Death (all causes) Show forest plot | 16 | 122451 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.62, 1.22] |
|
| 2.1 Primary series | 16 | 122451 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.62, 1.22] |
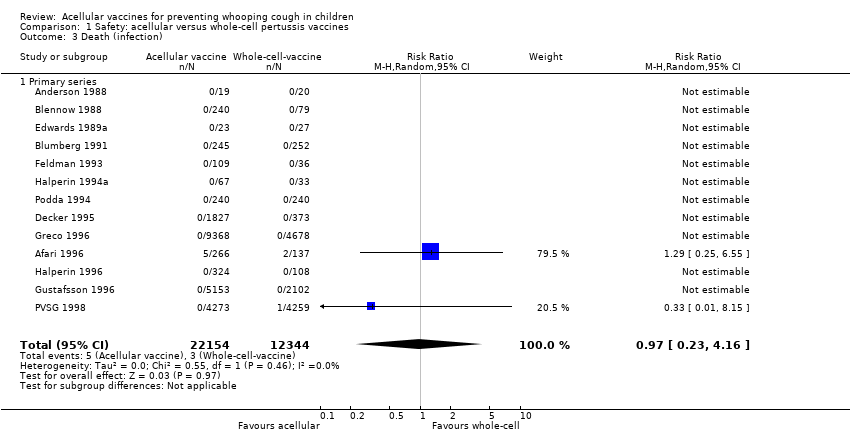
| 3 Death (infection) Show forest plot | 13 | 34498 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.23, 4.16] |
|
| 3.1 Primary series | 13 | 34498 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.23, 4.16] |
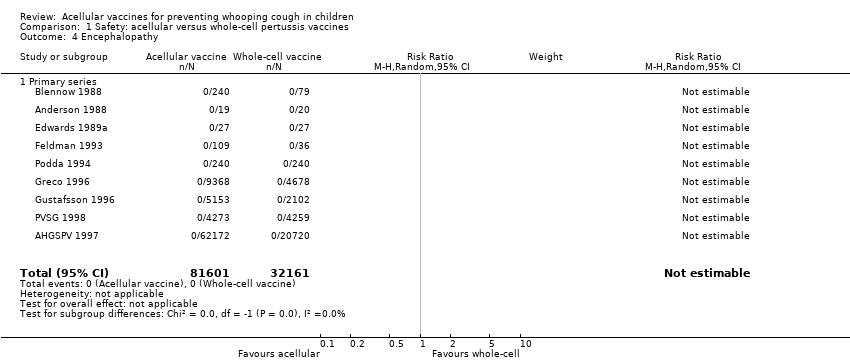
| 4 Encephalopathy Show forest plot | 9 | 113762 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
|
| 4.1 Primary series | 9 | 113762 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
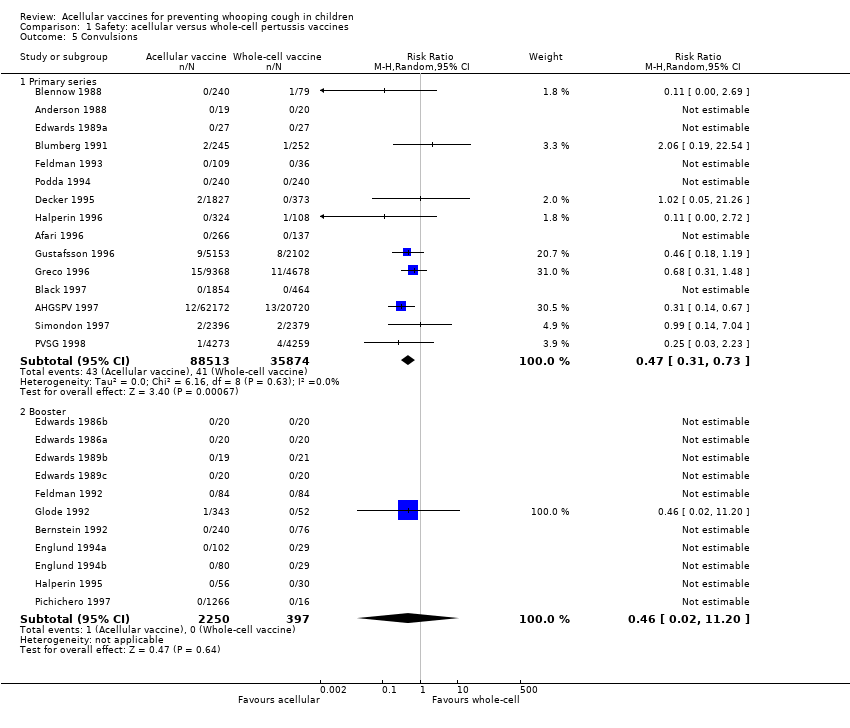
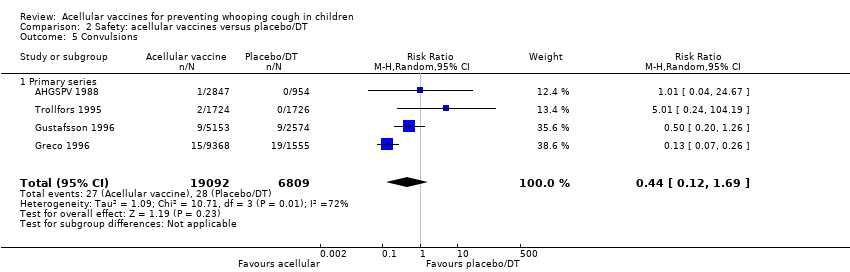
| 5 Convulsions Show forest plot | 26 | | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only |
|
| 5.1 Primary series | 15 | 124387 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.31, 0.73] |
| 5.2 Booster | 11 | 2647 | Risk Ratio (M‐H, Random, 95% CI) | 0.46 [0.02, 11.20] |
| 6 Hypotonic hyporesponsive episodes Show forest plot | 18 | | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only |
|
| 6.1 Primary series | 11 | 121573 | Risk Ratio (M‐H, Random, 95% CI) | 0.26 [0.08, 0.81] |
| 6.2 Booster | 7 | 2487 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Anorexia Show forest plot | 26 | | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only |
|
| 7.1 Primary series: Dose 1 | 11 | 19632 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.32, 0.57] |
| 7.2 Primary series: Dose 2 | 8 | 18501 | Risk Ratio (M‐H, Random, 95% CI) | 0.45 [0.33, 0.60] |
| 7.3 Primary series: Dose 3 | 9 | 18646 | Risk Ratio (M‐H, Random, 95% CI) | 0.50 [0.43, 0.60] |
| 7.4 aP booster (previous wP) versus wP booster (previous wP) | 14 | 1939 | Risk Ratio (M‐H, Random, 95% CI) | 0.40 [0.30, 0.54] |
| 7.5 aP booster (previous aP) versus wP booster (previous wP) | 4 | 8447 | Risk Ratio (M‐H, Random, 95% CI) | 0.42 [0.31, 0.58] |
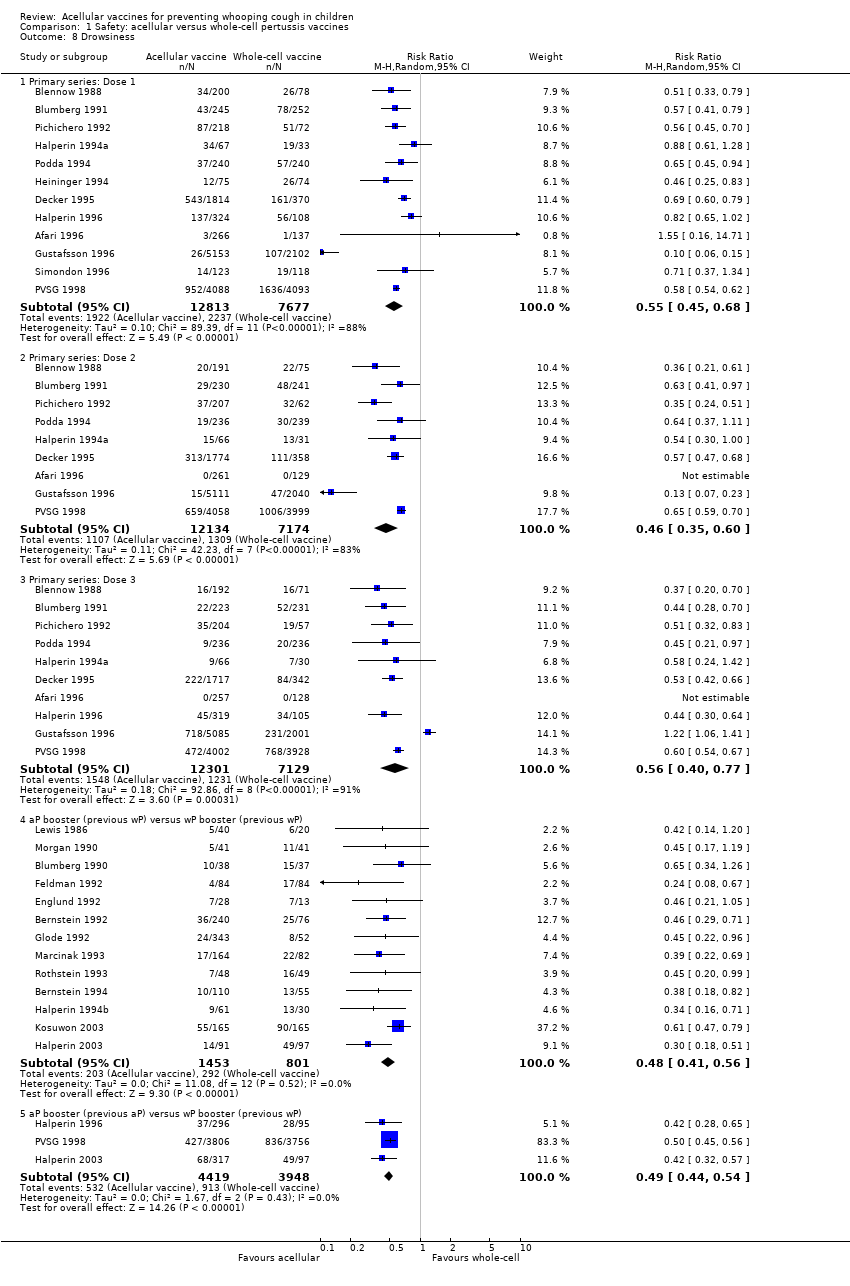
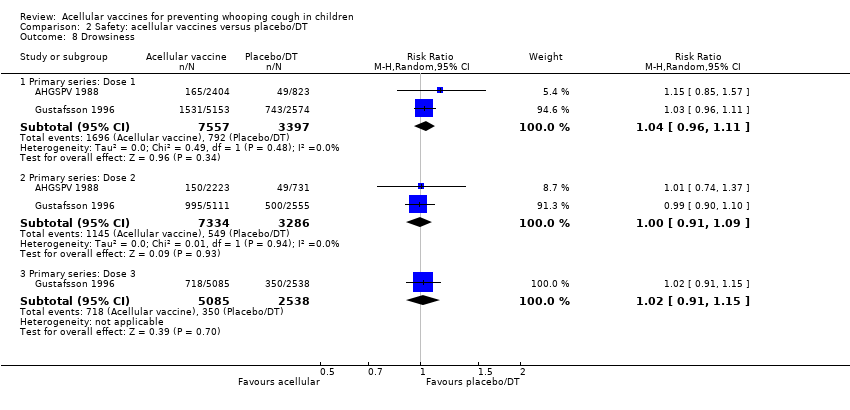
| 8 Drowsiness Show forest plot | 25 | | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only |
|
| 8.1 Primary series: Dose 1 | 12 | 20490 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.45, 0.68] |
| 8.2 Primary series: Dose 2 | 9 | 19308 | Risk Ratio (M‐H, Random, 95% CI) | 0.46 [0.35, 0.60] |
| 8.3 Primary series: Dose 3 | 10 | 19430 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.40, 0.77] |
| 8.4 aP booster (previous wP) versus wP booster (previous wP) | 13 | 2254 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.41, 0.56] |
| 8.5 aP booster (previous aP) versus wP booster (previous wP) | 3 | 8367 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.44, 0.54] |
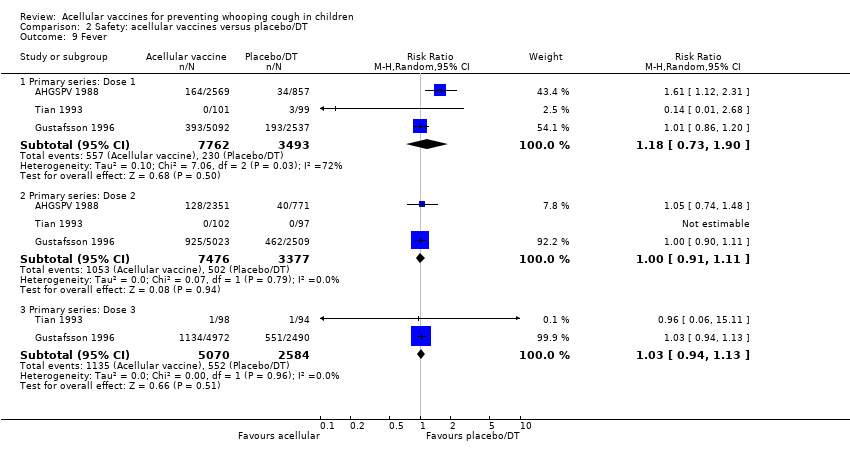
| 9 Fever Show forest plot | 46 | | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only |
|
| 9.1 Primary series: Dose 1 | 19 | 23267 | Risk Ratio (M‐H, Random, 95% CI) | 0.17 [0.13, 0.20] |
| 9.2 Primary series: Dose 2 | 17 | 22001 | Risk Ratio (M‐H, Random, 95% CI) | 0.31 [0.26, 0.37] |
| 9.3 Primary series: Dose 3 | 17 | 21731 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.30, 0.38] |
| 9.4 aP booster (previous wP) versus wP booster (previous wP) | 24 | 3381 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.26, 0.43] |
| 9.5 aP booster (previous aP) versus wP booster (previous wP) | 8 | 9879 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.22, 0.55] |
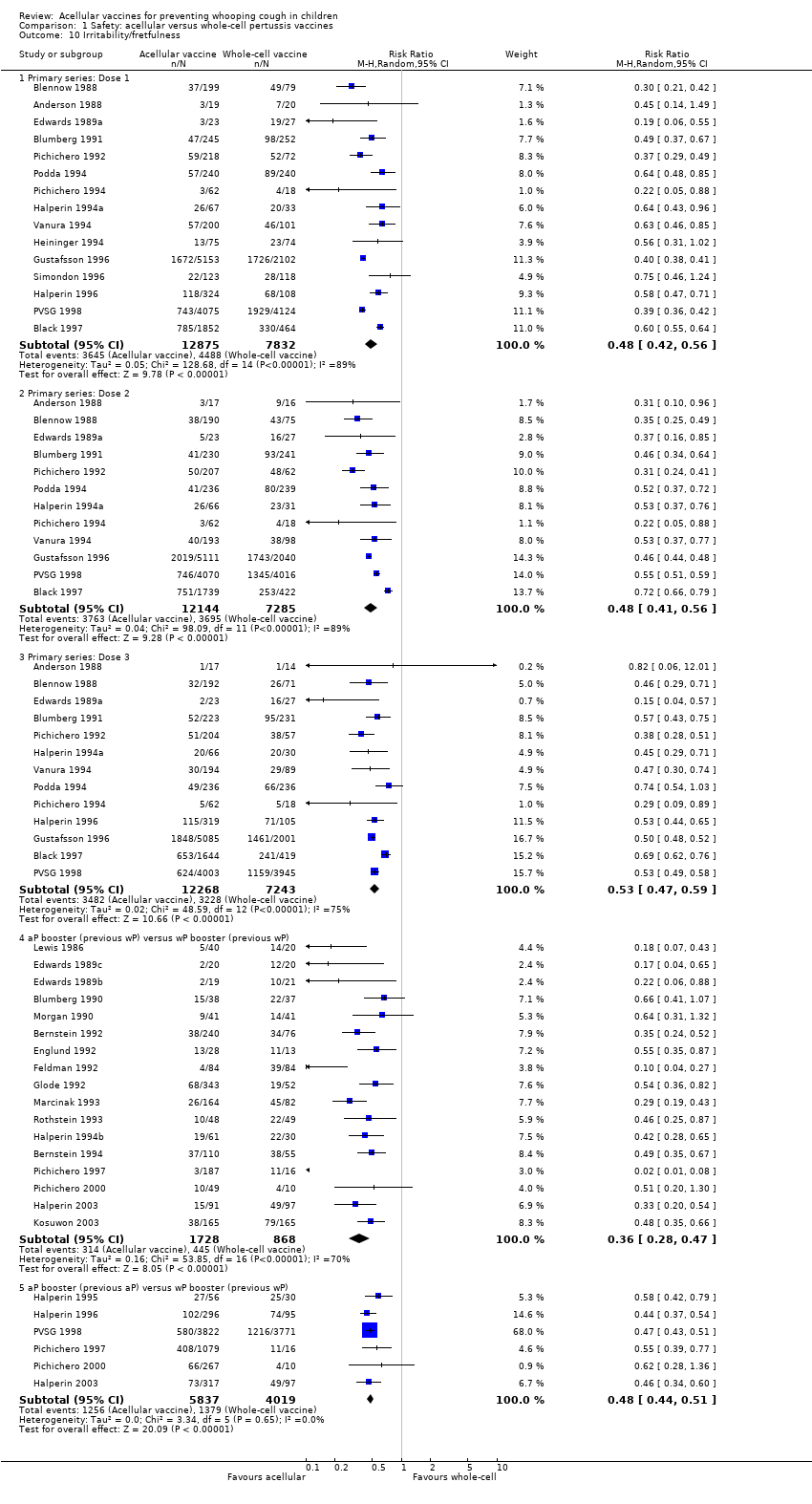
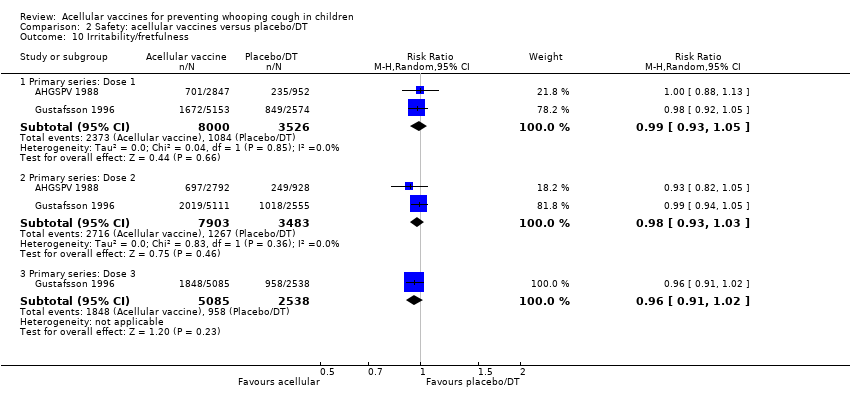
| 10 Irritability/fretfulness Show forest plot | 33 | | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only |
|
| 10.1 Primary series: Dose 1 | 15 | 20707 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.42, 0.56] |
| 10.2 Primary series: Dose 2 | 12 | 19429 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.41, 0.56] |
| 10.3 Primary series: Dose 3 | 13 | 19511 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.47, 0.59] |
| 10.4 aP booster (previous wP) versus wP booster (previous wP) | 17 | 2596 | Risk Ratio (M‐H, Random, 95% CI) | 0.36 [0.28, 0.47] |
| 10.5 aP booster (previous aP) versus wP booster (previous wP) | 6 | 9856 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.44, 0.51] |
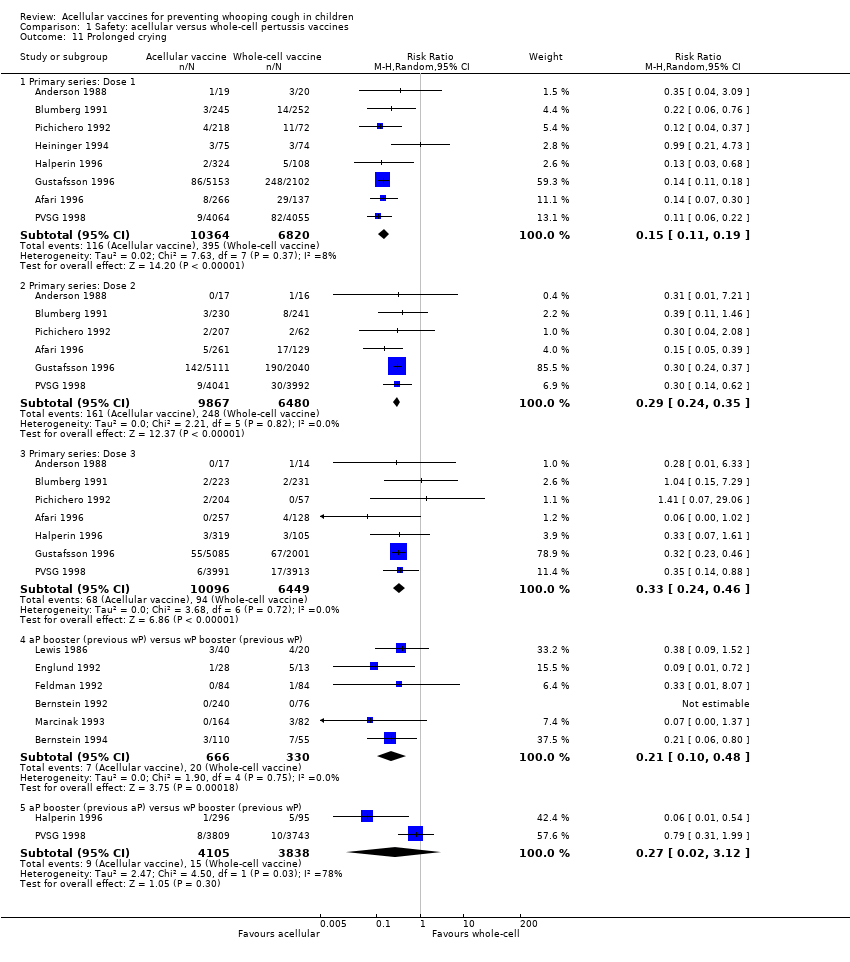
| 11 Prolonged crying Show forest plot | 14 | | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only |
|
| 11.1 Primary series: Dose 1 | 8 | 17184 | Risk Ratio (M‐H, Random, 95% CI) | 0.15 [0.11, 0.19] |
| 11.2 Primary series: Dose 2 | 6 | 16347 | Risk Ratio (M‐H, Random, 95% CI) | 0.29 [0.24, 0.35] |
| 11.3 Primary series: Dose 3 | 7 | 16545 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.24, 0.46] |
| 11.4 aP booster (previous wP) versus wP booster (previous wP) | 6 | 996 | Risk Ratio (M‐H, Random, 95% CI) | 0.21 [0.10, 0.48] |
| 11.5 aP booster (previous aP) versus wP booster (previous wP) | 2 | 7943 | Risk Ratio (M‐H, Random, 95% CI) | 0.27 [0.02, 3.12] |
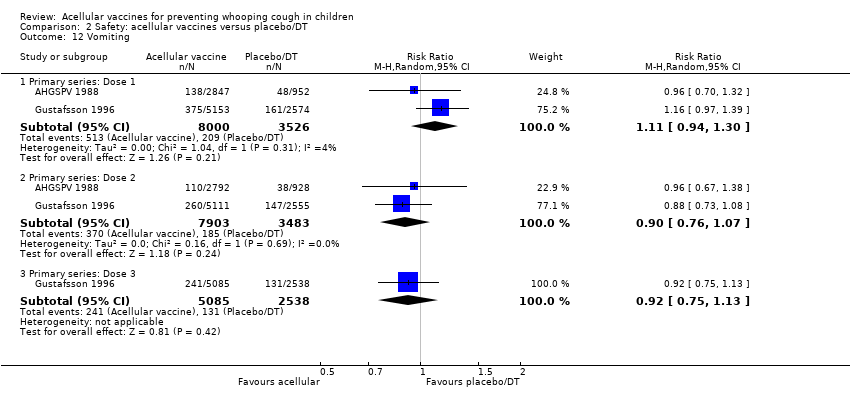
| 12 Vomiting Show forest plot | 15 | | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only |
|
| 12.1 Primary series: Dose 1 | 8 | 11450 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.66, 0.88] |
| 12.2 Primary series: Dose 2 | 7 | 10985 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.45, 0.86] |
| 12.3 Primary series: Dose 3 | 7 | 10813 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.46, 1.04] |
| 12.4 aP booster (previous wP) versus wP booster (previous wP) | 6 | 744 | Risk Ratio (M‐H, Random, 95% CI) | 0.50 [0.22, 1.11] |
| 12.5 aP booster (previous aP) versus wP booster (previous wP) | 1 | 86 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.10, 11.34] |
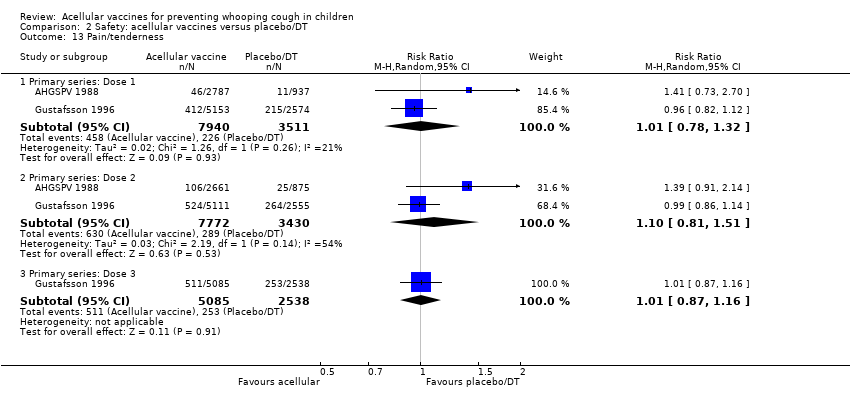
| 13 Pain/tenderness Show forest plot | 35 | | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only |
|
| 13.1 Primary series: Dose 1 | 13 | 14180 | Risk Ratio (M‐H, Random, 95% CI) | 0.20 [0.16, 0.25] |
| 13.2 Primary series: Dose 2 | 11 | 13186 | Risk Ratio (M‐H, Random, 95% CI) | 0.18 [0.15, 0.22] |
| 13.3 Primary series: Dose 3 | 12 | 13333 | Risk Ratio (M‐H, Random, 95% CI) | 0.20 [0.17, 0.24] |
| 13.4 aP booster (previous wP) versus wP booster (previous wP) | 21 | 3051 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.36, 0.53] |
| 13.5 aP booster (previous aP) versus wP booster (previous wP) | 5 | 2263 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.32, 0.58] |
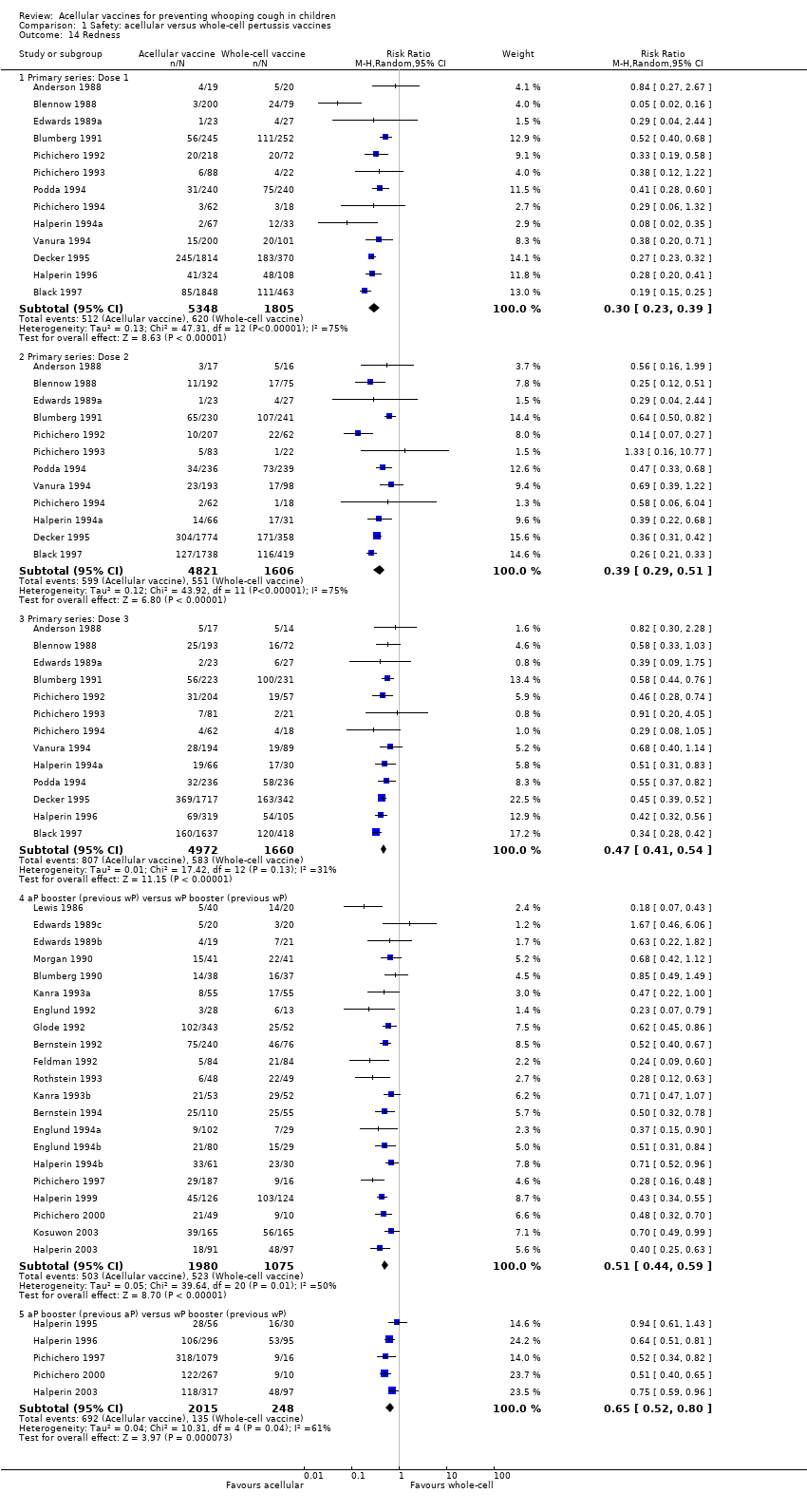
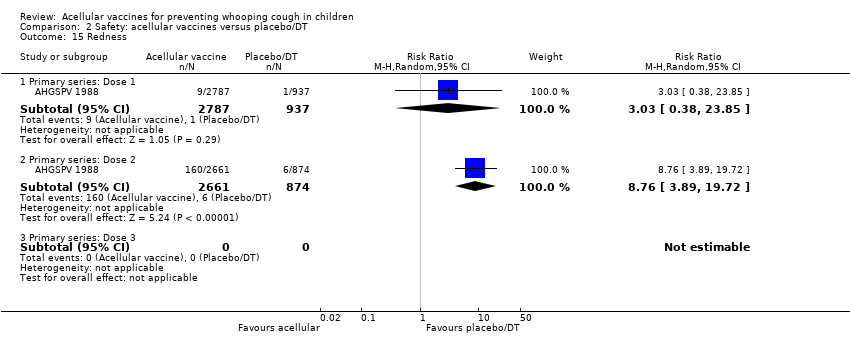
| 14 Redness Show forest plot | 35 | | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only |
|
| 14.1 Primary series: Dose 1 | 13 | 7153 | Risk Ratio (M‐H, Random, 95% CI) | 0.30 [0.23, 0.39] |
| 14.2 Primary series: Dose 2 | 12 | 6427 | Risk Ratio (M‐H, Random, 95% CI) | 0.39 [0.29, 0.51] |
| 14.3 Primary series: Dose 3 | 13 | 6632 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.41, 0.54] |
| 14.4 aP booster (previous wP) versus wP booster (previous wP) | 21 | 3055 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.44, 0.59] |
| 14.5 aP booster (previous aP) versus wP booster (previous wP) | 5 | 2263 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.52, 0.80] |
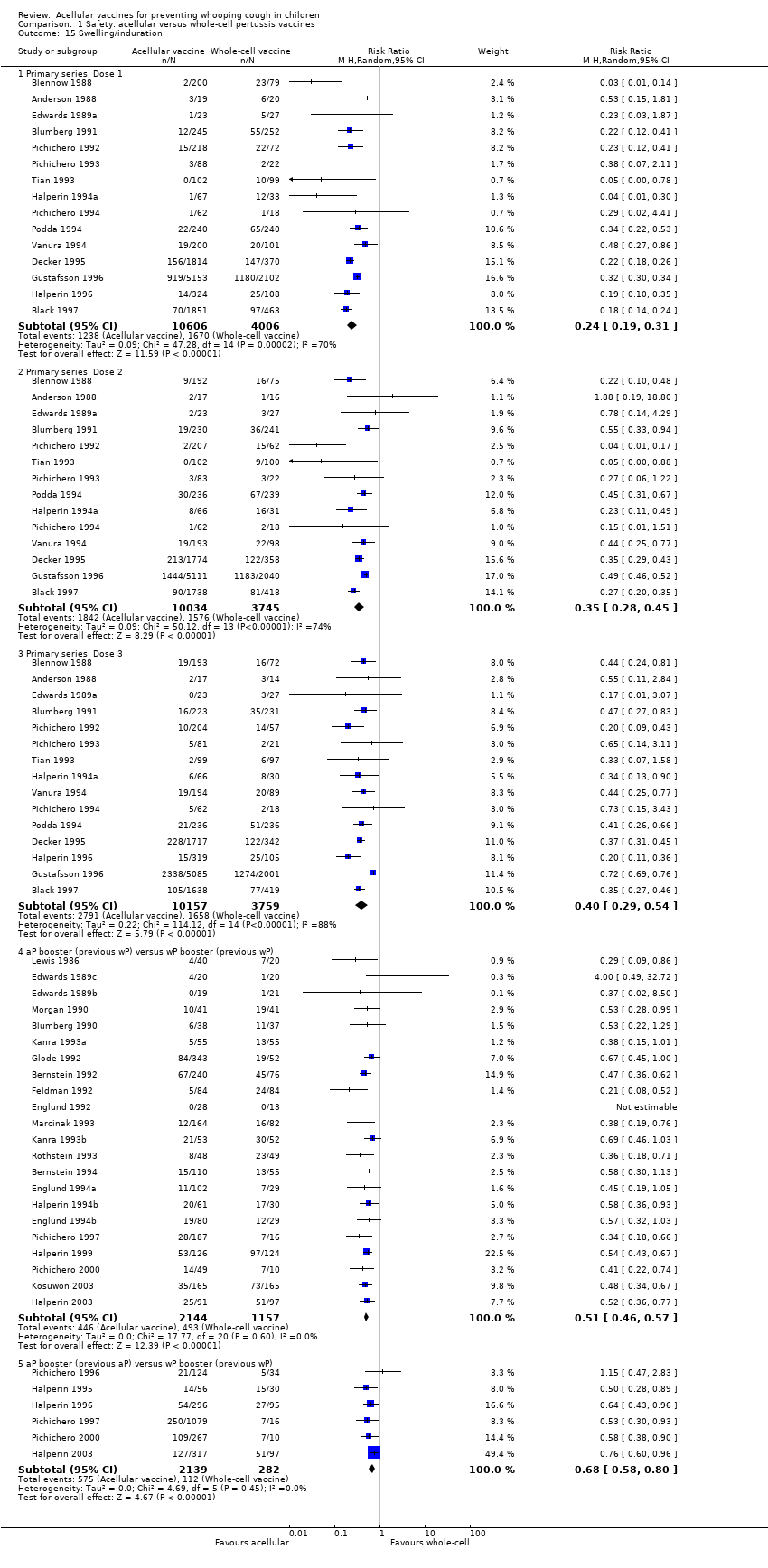
| 15 Swelling/induration Show forest plot | 39 | | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only |
|
| 15.1 Primary series: Dose 1 | 15 | 14612 | Risk Ratio (M‐H, Random, 95% CI) | 0.24 [0.19, 0.31] |
| 15.2 Primary series: Dose 2 | 14 | 13779 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.28, 0.45] |
| 15.3 Primary series: Dose 3 | 15 | 13916 | Risk Ratio (M‐H, Random, 95% CI) | 0.40 [0.29, 0.54] |
| 15.4 aP booster (previous wP) versus wP booster (previous wP) | 22 | 3301 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.46, 0.57] |
| 15.5 aP booster (previous aP) versus wP booster (previous wP) | 6 | 2421 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.58, 0.80] |