Medicamentos colinérgicos para la discinesia tardía inducida por neurolépticos
Referencias
References to studies included in this review
References to studies excluded from this review
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Allocation: randomised, no details. Setting: mostly inpatients, some outpatients, USA. | |
| Participants | Diagnosis: schizophrenia (21), affective disorder (3), OBS (7), neurosis (2). | |
| Interventions | 1. Lecithin: dose 60 g/day containing phosphatidylcholine 33 g/day. N = 25. | |
| Outcomes | TD symptoms: CGI. Unable to use ‐ | |
| Notes | ITT analysis not performed for continuous outcomes (CGI), results reported only for N = 31 who completed study (lecithin group 15, control group 16). Sponsorship source: Supported in part by a grant by the Veterans Administration. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Patients were randomly assigned", further details not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported. |
| Blinding of participants and personnel (performance bias) | Low risk | "double blind". "Only a member of the hospital pharmacy staff was aware of each patient's group assignment during the study. The investigator, patients, ward nurses, and physicians were all blind to patient status." "The control substance was a mixture of crushed graham cracker and corn oil which, when mixed with milk, resembled the lecithin mixture in taste, appearance, and viscosity. The mixtures were further disguised and made more palatable by the addition of artificial sweetener and vanilla extract." |
| Blinding of outcome assessment (detection bias) | Low risk | "double blind". "Only a member of the hospital pharmacy staff was aware of each patient's group assignment during the study. The investigator, patients, ward nurses, and physicians were all blind to patient status." "Treatment effect was assessed by blind evaluation of randomly sequenced videotapes wade during standard examinations before, during, and after treatment." "the sole rater was blind to patient treatment assignment". |
| Incomplete outcome data (attrition bias) | High risk | High drop‐out rate: 24%. 38/50 participants completed the trial (reasons reported per intervention group). Moreover, only 31/50 (62%) were included in the analysis (reasons reported). |
| Selective reporting (reporting bias) | Low risk | Dissertation. All outcomes seem to have been reported. |
| Other bias | Unclear risk | The groups seem to have had differences in their baseline dental status. |
| Methods | Allocation: randomised, no details. Setting: Patients treated in the Department of Veteran Affairs Medical Center, USA. | |
| Participants | Diagnosis: TD (research criteria), long‐duration schizophrenia (DSM‐IV criteria). History: Clinical diagnosis of TD lasting at least 3 months; treatment with antipsychotic drugs at least for 3 months. N = 38 Sex: all male. | |
| Interventions | 1. Galantamine: dose 4 mg twice daily for 4 weeks followed by 8 mg twice daily for 4 weeks, and 12 mg twice daily for an additional 4 weeks (followed by 4 weeks washout ad 12 weeks placebo). N = 19. Antipsychotics dose stable at least one month prior to the start of the study for oral medications and within 2 months for depot medications. Patients remained on a stable dose of antipsychotics throughout the study. Two patients were not receiving antipsychotics during the study. Any anticholinergic drugs or vitamin supplements were discontinued 2 weeks prior to randomisation. | |
| Outcomes | TD symptoms: total AIMS Leaving the study early Unable to use ‐ no report from first phase before crossing over separately: Simpson‐Angus Scale, BAS, BPRS, MMSE. | |
| Notes | Sponsorship source: Supported by a grant from Ortho‐McNeil Neurologics, Inc. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomized controlled trial," further details not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported. |
| Blinding of participants and personnel (performance bias) | Unclear risk | "double‐blind," details not reported. |
| Blinding of outcome assessment (detection bias) | Unclear risk | "double blind", details not reported. |
| Incomplete outcome data (attrition bias) | Unclear risk | "Overall, 10 (31.3%) of 32 patients receiving galantamine dropped out, and 6 (23.1%) of 26 patients receiving placebo dropped out. Twelve patients dropped out during phase 1 (galantamine, N=9; placebo, N=3), and 4 dropped out during phase 2 (galantamine, N = 1; placebo, N=3)." |
| Selective reporting (reporting bias) | High risk | Although the protocol specified that SAS (secondary outcome) and BAS should have been reported at the end of three months (phase I), data not reported per phase. Also data for BPRS not reported per phase. |
| Other bias | Unclear risk | Insufficient information to make a judgement. |
| Methods | Allocation: randomised, no details. Setting: from long‐term wards, Canada. | |
| Participants | Diagnosis: chronic schizophrenia. TD: significant (CGI Scale TD). | |
| Interventions | 1. Deanol: dose increased from 600 mg to 1500 mg/day during first week, constant thereafter. N = 10, for three weeks. | |
| Outcomes | Adverse effects. Death Unable to use ‐ | |
| Notes | Sponsorship source: Sponsorship source not reported Analysis of ESRS scores in publication did not detect significant treatment effect. Authors contacted ‐ no reply. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Patients were randomly assigned", further details not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported. |
| Blinding of participants and personnel (performance bias) | Unclear risk | "double‐blind" Details not reported. |
| Blinding of outcome assessment (detection bias) | Unclear risk | "The ESRS was completed independently by two psychiatrists during the same interview and a final rating was made by consensus." "double‐blind", further details of blinding not reported. |
| Incomplete outcome data (attrition bias) | Low risk | "All subjects completed the 3‐week trial" |
| Selective reporting (reporting bias) | High risk | TD symptoms (ESRS) and Mental State (BPRS) reported as means only. |
| Other bias | Low risk | The study seems to have been free of other sources of bias. |
| Methods | Allocation: randomised, procedure conducted independently by trial statistician, stratified by maintenance antipsychotic drug therapy. Setting: patients recruited from mental health centres and private physicians, USA. | |
| Participants | Diagnosis: schizophrenia (9), bipolar (6), major depression (3), generalised anxiety disorder (1), brief reactive psychosis (1), no psychiatric diagnosis (1). TD diagnosed by psychiatrist and neurologist using criteria. | |
| Interventions | 1. Lecithin: containing PC 20 g/day. N = 5 (completers).* Antipsychotics stable during trial. No anticholinergics permitted. Patients took the following concomitant psychoactive medications during the trial: antipsychotic agents alone (N = 7), antipsychotic drugs plus lithium (N = 3), antipsychotic drugs plus trazodone (N = 1), antipsychotic drugs plus an antianxiety agent (N = 1), antianxiety drugs alone (N = 1), antianxiety drugs plus lithium (N = 3), and lithium alone (N = 1). | |
| Outcomes | TD symptoms: AIMS. Unable to use ‐ | |
| Notes | * No information given on how many were originally allocated to each group. 14 of 21 completed the trial. Sponsorship source: Funded by National Institute of Mental Health grant, the Arbour Research Foundation, and the Center for Brain Sciences. ITT analysis not performed for AIMS scores (results reported only for completers). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "random‐order," "patients were stratified by whether they were on maintenance antipsychotic drug therapy." Details of sequence generation not reported. |
| Allocation concealment (selection bias) | Low risk | Allocation concealment not reported, but procedure confirmed as adequate from study authors. |
| Blinding of participants and personnel (performance bias) | Unclear risk | "double‐blind". "Several lecithin preparations were used during the study. We started with frappes prepared with chunks of 55% PC. That preparation was succeeded by chunks, chicken soup, and granola bars that contained 80% to 100% PC. Placebo included corn oil in frappes, ground corn flakes, and matching chicken noodle soup and granola bars." Unclear if the lecithin and placebo preparations were identical (color, taste, smell...)."The 14 completers were asked to fill out a questionnaire in which they specified (l) which of the two medications they thought was most helpful, (2) what effects (if any) they noted on their mood, and (3) whether they could guess which of the two medications was lecithin and which was placebo. Seven of the 14 patients felt that one treatment was definitely more helpful than the other; of those, 6 indicated that lecithin was the more helpful treatment." |
| Blinding of outcome assessment (detection bias) | Unclear risk | "We used two clinical raters, one blind rater who assessed TD and psychopathology and one open rater who rated side effects and distributed medication." "Both the blind rater and the patient completed Clinical Global Impressions and Improvement ratings at each visit and the blind rater assessed extrapyramidal effects with the Target Abnormal Kinetic Effects (TAKE) scale." |
| Incomplete outcome data (attrition bias) | High risk | "Fourteen patients‐7 men and 7 women‐completed at least 3 visits on the second leg of the trial. Data from these 14 completers were used in the efficacy analyses." Number completed the first period and number completed the trial not reported. 14/21 participants were entered to the analyses (approximately 33% drop out). |
| Selective reporting (reporting bias) | High risk | Clinical Global Impressions and Improvement, Target Abnormal Kinetic Effects (TAKE) scale, Mental State (BPRS and HAM‐D), adverse effects, and leaving the study early not fully reported. |
| Other bias | Low risk | The study seems to be free from other sources of bias. |
| Methods | Allocation: randomised, stratified by severity of TD. Setting: chronic psychiatric hospital residents, Australia. | |
| Participants | Diagnosis: chronic psychiatric hospital residents suffering from oral TD; having been treated with antipsychotics. | |
| Interventions | 1. Deanol: dose 2000 mg/day for four weeks. N = 11. Seven participants on antipsychotics during trial, CPE range 50 mg to 800 mg/day. Other concomitant medication not reported. | |
| Outcomes | TD symptoms. Unable to use ‐ | |
| Notes | Sponsorship source: The drug used in this trial was supplied by Riker Laboratories Pty. Ltd. who in addition, provided a grant for expenses involved in this project. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly assigned", further details not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported. |
| Blinding of participants and personnel (performance bias) | Unclear risk | "double‐blind", further details not reported. |
| Blinding of outcome assessment (detection bias) | Low risk | "The baseline rating of filming 1 and the ratings of filming 2, 3 and 4 were carried out by randomizing. All film segments and showing them unidentified to the raters on the 30th day of the study." |
| Incomplete outcome data (attrition bias) | Low risk | "all patients completed the trial." |
| Selective reporting (reporting bias) | High risk | TD symptoms reported only as means, Adverse events not fully reported. |
| Other bias | Unclear risk | "One subject in Group A showed 'substantial improvement', however, on preliminary and baseline rating that patient was one of the less severely afflicted. In Group B one patient also showed 'substantial improvement' and this patient was receiving thioridazine 200mg three times a day in addition to deanol." Possible confounding variables. |
| Methods | Allocation: randomised, no details. Setting: long‐term inpatients, USA. | |
| Participants | Diagnosis: schizophrenia + TD (Global AIMS rating of moderate to severe). | |
| Interventions | 1. Deanol: dose gradually increased to 1500 mg/day over 4 weeks. N = 4. | |
| Outcomes | TD symptoms: AIMS. Unable to use ‐ | |
| Notes | No participants developed clinical parkinsonism. Sponsorship source: Sponsorship source not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "subjects were randomly assigned", further details not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported. |
| Blinding of participants and personnel (performance bias) | Unclear risk | "double‐blind", details not reported. |
| Blinding of outcome assessment (detection bias) | Low risk | "A 20‐minute videotape of the patient sitting alone and then of an examination following the schema for the AIMS, by the same psychiatrist known to the patient. At the end of the study, the three videotapes for each patient were presented in random temporal sequence and rated "blind" by 4 psychiatrists using the AlMS." "Additional AIMS ratings were made by the same psychiatrist every 4 weeks on the ward without the disturbance of the videotape equipment. A weekly Global AIMS and Missouri In‐Patient Behavior Seale (MlBS) was per formed by a ward nurse" |
| Incomplete outcome data (attrition bias) | Unclear risk | Insufficient information to judge. |
| Selective reporting (reporting bias) | High risk | AIMS scores reported as means only. Data for Simpson Angus Scale not reported: "there was no significant change in the Simpson and Angus ratings". Data for MIBS not reported: "There was no significant or sustained change in the Missouri In‐Patient Behavior Scale ratings" |
| Other bias | High risk | "During the 32 weeks of the study, interrater variability, day‐to‐day changes in patient condition, and non‐drug related trends across time reduced the power of the single crossover design to the point where it would be unlikely to detect any but the most clearcut changes in a single patient." |
| Methods | Allocation: randomised, no details. Setting: long‐term inpatients, USA. | |
| Participants | Diagnosis: long‐term schizophrenia + TD (moderate or severe on AIMS global rating). | |
| Interventions | 1. Lecithin: dose 50 g/day containing PC 35 g/day. N = 3. | |
| Outcomes | TD symptoms: AIMS. Unable to use ‐ | |
| Notes | Sponsorship source: Sponsorship source not reported One person withdrawn early due to nausea and vomiting on a lecithin/water/orange flavour mix. Protocol changed to lecithin/ice cream/chocolate mix ‐ well tolerated! | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly assigned", further details not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported |
| Blinding of participants and personnel (performance bias) | Low risk | "double‐blind"; "Each dose of lecithin or placebo was prepared in a coded bottle independent of the patient clinical staff, and raters". "No attempt was made to systematically and objectively rate body odour, but no significant change, and particularly no "fishy odour," was noted by the subjects, ward staff, or raters." |
| Blinding of outcome assessment (detection bias) | Low risk | "Each dose of lecithin or placebo was prepared in a coded bottle independent of the patient clinical staff, and raters". "At the end of the study the 12 videotapes for each patient were presented in random temporal sequence and rated blind and independently by 2 psychiatrists using the AIMS...two raters' total AIMS scores, rated blind and independently from videotapes.." |
| Incomplete outcome data (attrition bias) | Unclear risk | Figure 1 reports data from all 6 participants. ITT is not mentioned. One participant was withdrawn from the study; reason reported. |
| Selective reporting (reporting bias) | High risk | BPRS and MIBS data not reported. |
| Other bias | Unclear risk | Insufficeint information to make a judgement. 1/6 participants was antipsychotic‐free throughout the study. |
| Methods | Allocation: "randomly assigned" no details reported. Setting: "Razi Psychiatric Center, Iran". | |
| Participants | Diagnosis: Patients with schizophrenia and TD based on DSM‐IV‐TR diagnosed by psychiatrist. N = 40. Age: range 18‐65 years Sex: not reported | |
| Interventions | 1. Rivastigmine: dose: 1.5 mg twice daily. N = 20 2. Placebo: no details reported. N = 20. | |
| Outcomes | TD symptoms: AIMS | |
| Notes | Sponsorship source: "no financial support". | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomly". No details reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) | Unclear risk | "Double blind". No details reported. |
| Blinding of outcome assessment (detection bias) | Unclear risk | "Double blind". No details reported. |
| Incomplete outcome data (attrition bias) | Unclear risk | Not reported. |
| Selective reporting (reporting bias) | Low risk | Outcomes have been reported based on the registered protocol IRCT2012092910964N1. |
| Other bias | Unclear risk | Insufficient information to make a judgement |
| Methods | Allocation: randomised, no details. Setting: long‐term inpatients. | |
| Participants | Diagnosis: schizophrenia (17), senile dementia (3) + TD (diagnosed by 2 physicians). | |
| Interventions | 1. Deanol: dose gradually increased to 1500 mg/day. N =1 0. | |
| Outcomes | TD symptoms. Unable to use ‐ | |
| Notes | Deanol well tolerated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomised", no details. |
| Allocation concealment (selection bias) | Unclear risk | No allocation concealment details. |
| Blinding of participants and personnel (performance bias) | Unclear risk | "double blind" not described. |
| Blinding of outcome assessment (detection bias) | Low risk | Two independent raters assessed outcomes. |
| Incomplete outcome data (attrition bias) | Low risk | No dropouts in study. |
| Selective reporting (reporting bias) | Unclear risk | No study protocol and the results are not proper reported. |
| Other bias | Unclear risk | No further information provided. |
| Methods | Allocation: matched pairs were randomised. Allocation procedure conducted independently by hospital pharmacist and not reported to trialists. Setting: long‐term inpatients. | |
| Participants | Diagnosis: schizophrenia (8), bipolar (1), cerebral sclerosis (1) + TD (diagnosed by 3 physicians using criteria). | |
| Interventions | 1. Deanol: dose gradually increased to 1500 mg/day. N = 5. | |
| Outcomes | TD symptoms. Unable to use ‐ | |
| Notes | Original study N = 20. Due to information about toxic effects of clozapine in July 1975, antipsychotic medication abruptly changed. In dissertation, detailed individual patient data supplied. Data extracted for 10 participants whose antipsychotic medication was stable during study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomised" No further details. |
| Allocation concealment (selection bias) | Low risk | "pharmacy‐controlled allocation, identical sequentially number drug containers". |
| Blinding of participants and personnel (performance bias) | Unclear risk | Identical capsules planned, but apparently differences in form and taste. |
| Blinding of outcome assessment (detection bias) | Low risk | "two independent raters under standardised conditions". |
| Incomplete outcome data (attrition bias) | Unclear risk | Dropouts with reasons reported, but unclear in which treatment phase of cross‐over study. |
| Selective reporting (reporting bias) | Unclear risk | No protocol available and the study outcomes are unclear if all were reported. |
| Other bias | Unclear risk | Unclear the cross‐over phases. |
| Methods | Allocation: "randomized" Blinding: "double blind" Design: cross‐over* Duration: Trial I*: 12 weeks (4 weeks, 4 weeks washout, then crossed over to another 4 weeks); Trial II: 16 weeks (6 weeks, 4 weeks washout, then crossed over to another 6 weeks) Setting: not reported. | |
| Participants | Diagnosis: TD diagnosis according to Schooler and Kane research diagnostic criteria History: average duration that participants experienced TD was 6.4 years (range, 2‐10 years). N (Trial I) = 7; N (Trial II) = 5* Age: mean 61.4 years Sex: 7 M, 3 F. | |
| Interventions | Trial I: 5 mg** donepezil daily (N = 4) vs placebo (N = 3). Trial II: 10 mg donepezil daily (N = 3) vs placebo (N = 2). Permitted to stay on current antipsychotic medication, but not allowed to take anticholinergic medication during the study. | |
| Outcomes | TD symptoms: improved/deteriorated, AIMS scale scores Adverse event Leaving the study early Unable to use ‐ SAS, BPRS, MMSE (data not fully reported) | |
| Notes | *Two individuals participated in both studies, in which case, their data from the earlier 5 mg study were used. **Because there was no significant effect of donepezil 5 mg daily on dyskinetic movements the same trial design was continued but with increased daily dose to 10 mg. We have analysed the two doses together. Study author kindly replied to our request for outcome‐ and 'Risk of bias' data. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Random digit generation: even‐‐donepezil; odd—placebo" (personal communication). |
| Allocation concealment (selection bias) | Unclear risk | No details reported. |
| Blinding of participants and personnel (performance bias) | Unclear risk | "double‐blind", no further details reported. |
| Blinding of outcome assessment (detection bias) | Low risk | One to two raters blindly scored subjects for each outcome scale. |
| Incomplete outcome data (attrition bias) | Unclear risk | 2/10 participants discontinued, not reported reasons or from which group. |
| Selective reporting (reporting bias) | High risk | MMSE and BPRS not reported. |
| Other bias | High risk | |
| Methods | Allocation: randomised, no details. Setting: inpatients, USA. | |
| Participants | Diagnosis: schizophrenia (69%), OBS (29%), bipolar (2%) + TD (diagnosed by criteria), thorough evaluation to rule out differential diagnostic categories. | |
| Interventions | 1. Lecithin: dose 60 g/day containing PC dose of 33 g/day. N = 15. | |
| Outcomes | TD symptoms. Unable to use ‐ | |
| Notes | Sponsorship source: Sponsorship source not reported. Review uses data only from lecithin and placebo groups for whom blinding adequate and reporting consistent. (N = 15 + 15 = 30). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly selected". Details not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported. |
| Blinding of participants and personnel (performance bias) | Low risk | "double‐blind". "Although the subjects assigned to one of the treatment groups were informed that they would receive either the lecithin treatment or a placebo control treatment, neither the patients nor the researcher knew to which group any individual had been assigned." "The placebo substance resembled the lecithin mixture in taste, .appearance, and thickness" The no treatment group's participants could not have been blinded |
| Blinding of outcome assessment (detection bias) | Low risk | Rater and self‐report were used as outcome measures. Research personnel and participants seem to have been blinded to the assignment. Self‐report ratings were not recorded for the "no treatment" group. |
| Incomplete outcome data (attrition bias) | Low risk | All participants completed the trial. |
| Selective reporting (reporting bias) | High risk | Dissertation. All outcomes seem to have been reported. However, adverse effects expected to be reported in such trials have not been reported. Data for TD scores are not extractable. |
| Other bias | Low risk | "A one‐way analysis of variance was performed on subject variables to determine if there were initial differences among the groups. These analyses show no significant differences for age... , duration of antipsychotic treatment..., or initial symptom severity. A chi‐square analysis of diagnostic categories demonstrates no significant difference among the groups...". The study seems to have been free of other sources of bias. |
| Methods | Allocation: randomised, by table of random numbers, concealment unclear. Setting: outpatients and inpatients, USA. | |
| Participants | Diagnosis: psychiatric disorder. | |
| Interventions | 1. Deanol: dose 1000 mg/day for 4 weeks, then 2000 mg/day for next 4 weeks. N = 4. Concomitant medication not reported. | |
| Outcomes | TD symptoms: modified Simpson TDRS. | |
| Notes | Sponsorship source: Supported by Veterans Administration research funds. Osvaldo N. Re, MD, and Riker Laboratories provided assistance. No parkinsonian adverse effects or mood changes observed. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Order of treatment was determined by a table of random numbers". |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported. |
| Blinding of participants and personnel (performance bias) | Unclear risk | "double blind" no details reported. |
| Blinding of outcome assessment (detection bias) | Unclear risk | "double blind" no details reported. |
| Incomplete outcome data (attrition bias) | Low risk | All participants completed the study. |
| Selective reporting (reporting bias) | Unclear risk | Outcomes have not been clearly defined to make a judgement. |
| Other bias | Unclear risk | Insufficient information to make a judgement. |
| Methods | Allocation: randomised, blocks of 4. Setting: 97% long‐term inpatients, Japan. | |
| Participants | Diagnosis: schizophrenia (90%), other (10%). | |
| Interventions | 1. Meclofenoxate hydrochloride (MF): dose 900 mg/day. N = 31. | |
| Outcomes | TD symptoms: AIMS, FGIR. Unable to use ‐ | |
| Notes | For blood test no differences between MF and placebo groups. According to Overall Safety Rating MF caused no severe adverse effects, as did not placebo. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomized" No further information |
| Allocation concealment (selection bias) | Unclear risk | randomised in blocks of 4 |
| Blinding of participants and personnel (performance bias) | Unclear risk | "double blind" not described |
| Blinding of outcome assessment (detection bias) | Unclear risk | "double blind", details on blinding not reported for primary outcome |
| Incomplete outcome data (attrition bias) | Low risk | All subjects completed the trial |
| Selective reporting (reporting bias) | Unclear risk | Outcomes are reported but not with all the necessary information |
| Other bias | Unclear risk | Insufficient information to make a judgement. |
Scales:
AIMS = Abnormal Involuntary Movement Scale
BAS = Barnes Akathisia Scale
BPRS = Brief Psychiatric Rating Scale
CGI = Clinical Global Impressions
DSM IV = Diagnostic and Statistical Manual, 4th edition
ESRS = Extrapyramidal Symptom Rating Scale
HAM‐D = Hamilton Rating Scale for Depression
MIBS = Missouri In‐Patient Behavior Scale
MMSE = Mini‐Mental State Examination
SAS = Simpson Angus Scale
SRTDRS = Self‐Report Tardive Dyskinesia Rating Scale
STDRS = Simpson (Rockland) Tardive Dyskinesia Rating Scale
TAKE = Target Abnormal Kinetic Effects
Other abbreviations:
ANCOVA = Analysis of covariance
CPE = Chlorpromazine equivalent
ECG = Electrocardiogram
GI = gastrointestinal
ITT = intention‐to‐treat
OBS = Organic Brain Syndrome
PC = Phosphatidylcholine
SD = standard deviation
TD = Tardive dyskinesia
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Allocation: publication does not specify if trial was randomised; authors contacted to confirm lack of additional data. | |
| Allocation: not randomised. | |
| Allocation: no mention of randomisation; authors contacted twice, no reply. | |
| Allocation: not randomised. | |
| Allocation: not randomised, case study. | |
| Allocation: not randomised, ABAB design. | |
| Allocation: not randomised, clinical trial. | |
| Allocation: randomised. | |
| Allocation: not randomised, case series. | |
| Allocation: not randomised, case study. | |
| Allocation: not randomised, case study. | |
| Allocation: not randomised, cohort study, AB(A). | |
| Allocation: not randomised, AB design. | |
| Allocation: not randomised, cohort study, AB. | |
| Allocation: not randomised, case reports. | |
| Allocation: randomised, cross‐over. | |
| Allocation: not randomised, case studies. | |
| Allocation: not randomised, clinical trial. | |
| Allocation: not randomised, cohort study. | |
| Allocation: not randomised, case series. | |
| Allocation: not randomised, cohort study. | |
| Allocation: randomised. | |
| Allocation: not randomised, case series. | |
| Allocation: not randomised. | |
| Allocation: not randomised, open clinical study. | |
| Allocation: not randomised, open clinical study. | |
| Allocation: not randomised, open‐study. | |
| Allocation: randomised. Participants: people with chronic schizophrenia diagnosed by DSM III who had taken antipsychotic drugs for at least 3 months, abnormal involuntary body movement in at least one part of body (face, lip and perioral, jaw, tongue, upper extremity, lower extremity, trunk (neck, shoulder, hips)) rated at least 2 point, who has no other kind of neurological disease which may cause the abnormal involuntary movement. Interventions: Lecithin versus placebo. Outcomes: no outcome data provided for first period before cross‐over; author contacted ‐ no additional information received. | |
| Allocation: randomised, cross‐over. | |
| Allocation: not randomised, case series. | |
| Allocation: not randomised, case study. | |
| Allocation: not randomised, case study. | |
| Allocation: randomised. Participants: people with TD. Interventions: physostigmine vs bromocriptine vs benztropine vs haloperidol. Outcomes: no outcome data provided for first period before cross‐over; study author contacted ‐ no additional information received. | |
| Allocation: not randomised, controlled clinical trial. | |
| Allocation: not randomised, open‐trial. | |
| Allocation: randomised. Participants: people with TD (17 schizophrenia, 5 schizoaffective disorder and 1 atypical psychosis). Interventions: 7‐methoxytacrine (7‐MEOTA) vs placebo. Outcomes: therapeutic efficacy and adverse events ‐ no usable data from this brief report; unable to identify up‐to‐date contact details of authors. | |
| Allocation: not randomised, case reports. | |
| Allocation: randomised. Participants: people with TD. | |
| Allocation: not randomised, cohort study, ABA. | |
| Allocation: randomised. Participants: schizophrenia, paranoid disorder, and schizoaffective disorder + persistent TD Interventions: AMPT vs L‐DOPA vs choline chloride vs valproic acid vs hydroxytryptophan. Outcomes: no outcome data provided for first period before cross‐over; author contacted ‐ no additional information received. | |
| Allocation: not randomised, controlled single‐dose trial. | |
| Allocation: randomised, cross‐over. | |
| Allocation: randomised, cross‐over. Participants: chronic psychiatric disorders; severe persistent TD of more than six months. Interventions: lecithin versus placebo. Outcomes: no outcome data reported for first treatment phase before cross‐over; authors contacted but no new information received. | |
| Allocation: not randomised, case series. | |
| Allocation: not randomised. | |
| Allocation: randomised. | |
| Allocation: not randomised, ABA design. | |
| Allocation: not randomised. | |
| Allocation: not randomised, open‐study. |
Abbreviations:
AIMS = Abnormal Involuntary Movement Scale
BPRS = Brief Psychiatric Rating Scale
CGI = Clinical Global Impressions
DSM IV = Diagnostic and Statistical Manual, 4th edition
ESS = Emergent Symptom Scale (adverse effects)
i.m. = intramuscular
i.v. = intravenous
NOSIE = Nurses´ Observation Scale for Inpatient Evaluation
RBC = Red blood cell
TD = Tardive dyskinesia
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size | ||||||||||||||||||||||||||||||
| 1 Tardive dyskinesia: 1. No clinically important improvement (50% or more change on any validated TD scale) Show forest plot | 4 | 27 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.65, 1.23] | ||||||||||||||||||||||||||||||
| Analysis 1.1 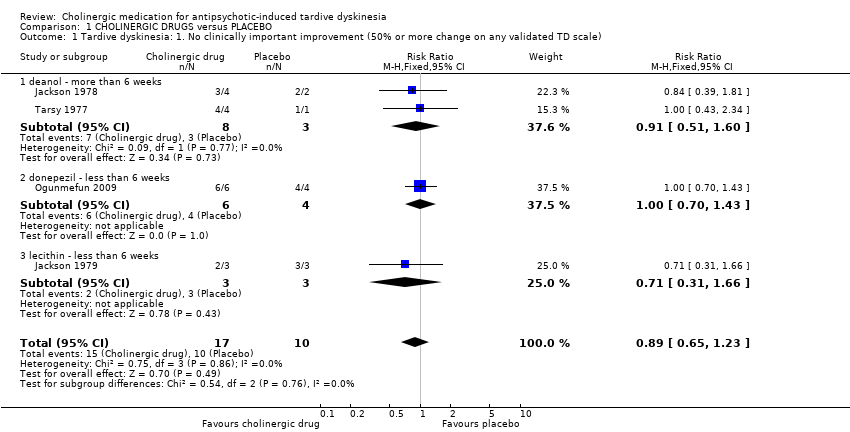 Comparison 1 CHOLINERGIC DRUGS versus PLACEBO, Outcome 1 Tardive dyskinesia: 1. No clinically important improvement (50% or more change on any validated TD scale). | ||||||||||||||||||||||||||||||||||
| 1.1 deanol ‐ more than 6 weeks | 2 | 11 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.51, 1.60] | ||||||||||||||||||||||||||||||
| 1.2 donepezil ‐ less than 6 weeks | 1 | 10 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.70, 1.43] | ||||||||||||||||||||||||||||||
| 1.3 lecithin ‐ less than 6 weeks | 1 | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.31, 1.66] | ||||||||||||||||||||||||||||||
| 2 Tardive dyskinesia: 2a. Not any improvement (as assessed by rater) Show forest plot | 9 | 180 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.71, 1.07] | ||||||||||||||||||||||||||||||
| Analysis 1.2 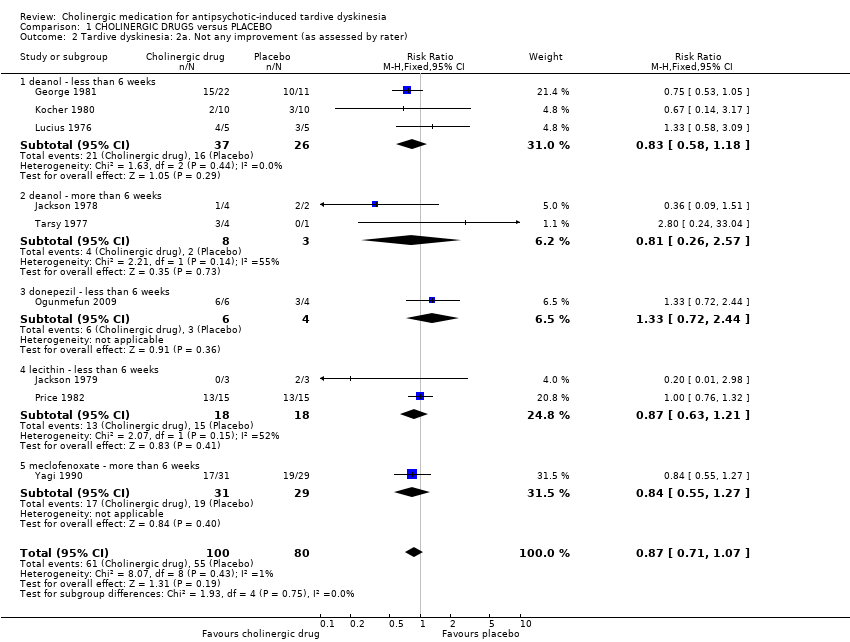 Comparison 1 CHOLINERGIC DRUGS versus PLACEBO, Outcome 2 Tardive dyskinesia: 2a. Not any improvement (as assessed by rater). | ||||||||||||||||||||||||||||||||||
| 2.1 deanol ‐ less than 6 weeks | 3 | 63 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.58, 1.18] | ||||||||||||||||||||||||||||||
| 2.2 deanol ‐ more than 6 weeks | 2 | 11 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.26, 2.57] | ||||||||||||||||||||||||||||||
| 2.3 donepezil ‐ less than 6 weeks | 1 | 10 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.72, 2.44] | ||||||||||||||||||||||||||||||
| 2.4 lecithin ‐ less than 6 weeks | 2 | 36 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.63, 1.21] | ||||||||||||||||||||||||||||||
| 2.5 meclofenoxate ‐ more than 6 weeks | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.55, 1.27] | ||||||||||||||||||||||||||||||
| 3 Tardive dyskinesia: 2b. Not any improvement (as assessed by self report) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |||||||||||||||||||||||||||||||
| Analysis 1.3  Comparison 1 CHOLINERGIC DRUGS versus PLACEBO, Outcome 3 Tardive dyskinesia: 2b. Not any improvement (as assessed by self report). | ||||||||||||||||||||||||||||||||||
| 3.1 lecithin ‐ less than 6 weeks | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.62, 1.36] | ||||||||||||||||||||||||||||||
| 4 Tardive dyskinesia: 3a. Average endpoint score on AIMS (low score = better) Show forest plot | 7 | 171 | Mean Difference (IV, Fixed, 95% CI) | ‐0.12 [‐0.44, 0.21] | ||||||||||||||||||||||||||||||
| Analysis 1.4 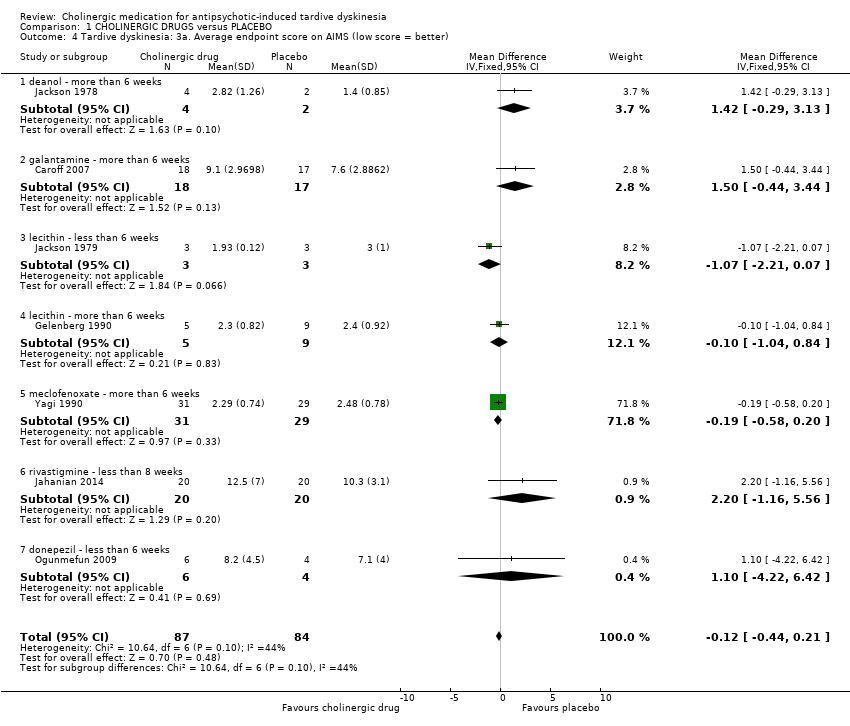 Comparison 1 CHOLINERGIC DRUGS versus PLACEBO, Outcome 4 Tardive dyskinesia: 3a. Average endpoint score on AIMS (low score = better). | ||||||||||||||||||||||||||||||||||
| 4.1 deanol ‐ more than 6 weeks | 1 | 6 | Mean Difference (IV, Fixed, 95% CI) | 1.42 [‐0.29, 3.13] | ||||||||||||||||||||||||||||||
| 4.2 galantamine ‐ more than 6 weeks | 1 | 35 | Mean Difference (IV, Fixed, 95% CI) | 1.5 [‐0.44, 3.44] | ||||||||||||||||||||||||||||||
| 4.3 lecithin ‐ less than 6 weeks | 1 | 6 | Mean Difference (IV, Fixed, 95% CI) | ‐1.07 [‐2.21, 0.07] | ||||||||||||||||||||||||||||||
| 4.4 lecithin ‐ more than 6 weeks | 1 | 14 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐1.04, 0.84] | ||||||||||||||||||||||||||||||
| 4.5 meclofenoxate ‐ more than 6 weeks | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐0.19 [‐0.58, 0.20] | ||||||||||||||||||||||||||||||
| 4.6 rivastigmine ‐ less than 8 weeks | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | 2.20 [‐1.16, 5.56] | ||||||||||||||||||||||||||||||
| 4.7 donepezil ‐ less than 6 weeks | 1 | 10 | Mean Difference (IV, Fixed, 95% CI) | 1.10 [‐4.22, 6.42] | ||||||||||||||||||||||||||||||
| 5 Tardive dyskinesia: 3b. Average endpoint score on modified Simpson TDRS (low score = better) Show forest plot | Other data | No numeric data | ||||||||||||||||||||||||||||||||
| Analysis 1.5
Comparison 1 CHOLINERGIC DRUGS versus PLACEBO, Outcome 5 Tardive dyskinesia: 3b. Average endpoint score on modified Simpson TDRS (low score = better). | ||||||||||||||||||||||||||||||||||
| 5.1 deanol ‐ more than 6 weeks | Other data | No numeric data | ||||||||||||||||||||||||||||||||
| 6 Tardive dyskinesia: 4a. Deterioration (as assessed by rater) Show forest plot | 8 | 147 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.55, 2.24] | ||||||||||||||||||||||||||||||
| Analysis 1.6 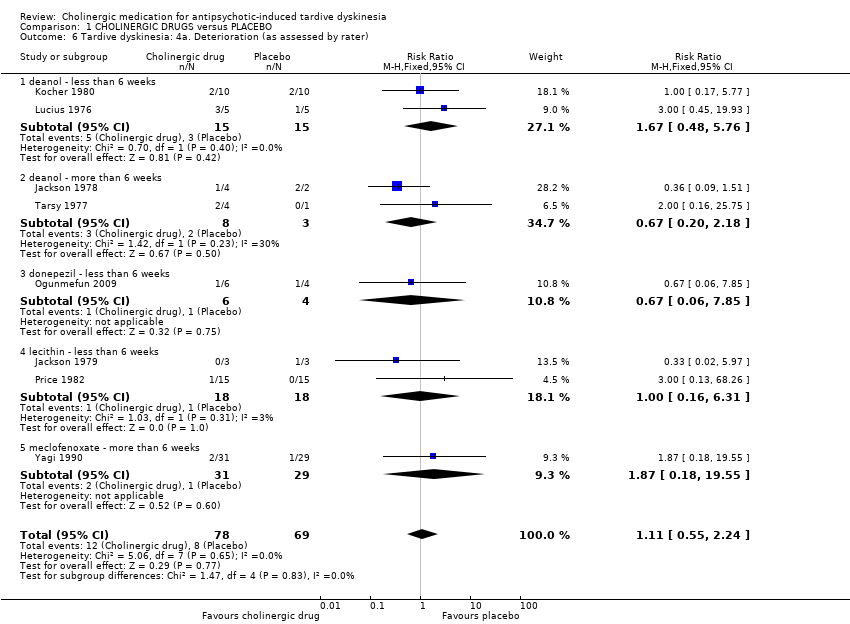 Comparison 1 CHOLINERGIC DRUGS versus PLACEBO, Outcome 6 Tardive dyskinesia: 4a. Deterioration (as assessed by rater). | ||||||||||||||||||||||||||||||||||
| 6.1 deanol ‐ less than 6 weeks | 2 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.67 [0.48, 5.76] | ||||||||||||||||||||||||||||||
| 6.2 deanol ‐ more than 6 weeks | 2 | 11 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.20, 2.18] | ||||||||||||||||||||||||||||||
| 6.3 donepezil ‐ less than 6 weeks | 1 | 10 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.06, 7.85] | ||||||||||||||||||||||||||||||
| 6.4 lecithin ‐ less than 6 weeks | 2 | 36 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.16, 6.31] | ||||||||||||||||||||||||||||||
| 6.5 meclofenoxate ‐ more than 6 weeks | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.87 [0.18, 19.55] | ||||||||||||||||||||||||||||||
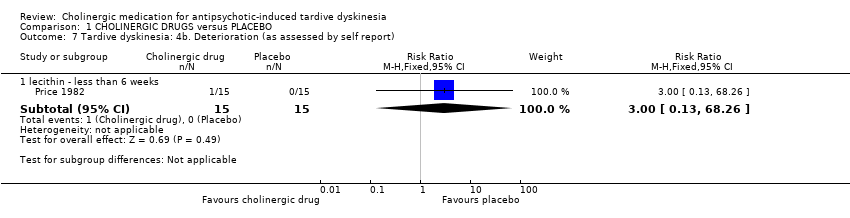
| 7 Tardive dyskinesia: 4b. Deterioration (as assessed by self report) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |||||||||||||||||||||||||||||||
| Analysis 1.7 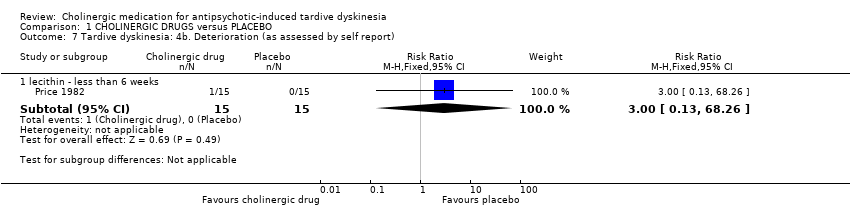 Comparison 1 CHOLINERGIC DRUGS versus PLACEBO, Outcome 7 Tardive dyskinesia: 4b. Deterioration (as assessed by self report). | ||||||||||||||||||||||||||||||||||
| 7.1 lecithin ‐ less than 6 weeks | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 68.26] | ||||||||||||||||||||||||||||||
| 8 Global outcome: Death for any reason Show forest plot | 11 | 278 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | ||||||||||||||||||||||||||||||
| Analysis 1.8  Comparison 1 CHOLINERGIC DRUGS versus PLACEBO, Outcome 8 Global outcome: Death for any reason. | ||||||||||||||||||||||||||||||||||
| 8.1 deanol ‐ less than 6 weeks | 4 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | ||||||||||||||||||||||||||||||
| 8.2 deanol ‐ more than 6 weeks | 2 | 11 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | ||||||||||||||||||||||||||||||
| 8.3 galantamine ‐ more than 6 weeks | 1 | 38 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | ||||||||||||||||||||||||||||||
| 8.4 lecithin ‐ less than 6 weeks | 3 | 86 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | ||||||||||||||||||||||||||||||
| 8.5 meclofenoxate ‐ more than 6 weeks | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | ||||||||||||||||||||||||||||||
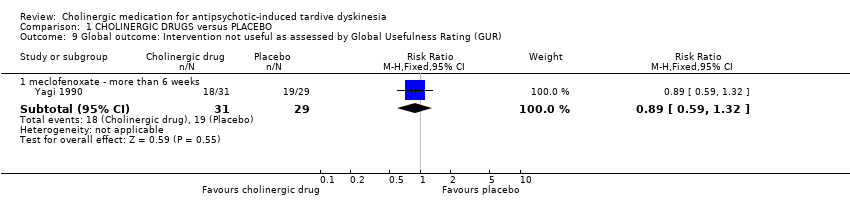
| 9 Global outcome: Intervention not useful as assessed by Global Usefulness Rating (GUR) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |||||||||||||||||||||||||||||||
| Analysis 1.9  Comparison 1 CHOLINERGIC DRUGS versus PLACEBO, Outcome 9 Global outcome: Intervention not useful as assessed by Global Usefulness Rating (GUR). | ||||||||||||||||||||||||||||||||||
| 9.1 meclofenoxate ‐ more than 6 weeks | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.59, 1.32] | ||||||||||||||||||||||||||||||
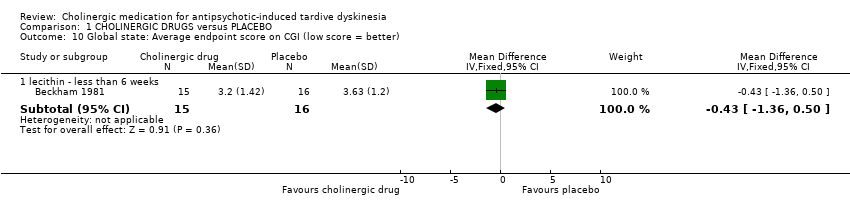
| 10 Global state: Average endpoint score on CGI (low score = better) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |||||||||||||||||||||||||||||||
| Analysis 1.10  Comparison 1 CHOLINERGIC DRUGS versus PLACEBO, Outcome 10 Global state: Average endpoint score on CGI (low score = better). | ||||||||||||||||||||||||||||||||||
| 10.1 lecithin ‐ less than 6 weeks | 1 | 31 | Mean Difference (IV, Fixed, 95% CI) | ‐0.43 [‐1.36, 0.50] | ||||||||||||||||||||||||||||||
| 11 Mental state: Deterioration Show forest plot | 5 | 77 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.10, 2.61] | ||||||||||||||||||||||||||||||
| Analysis 1.11 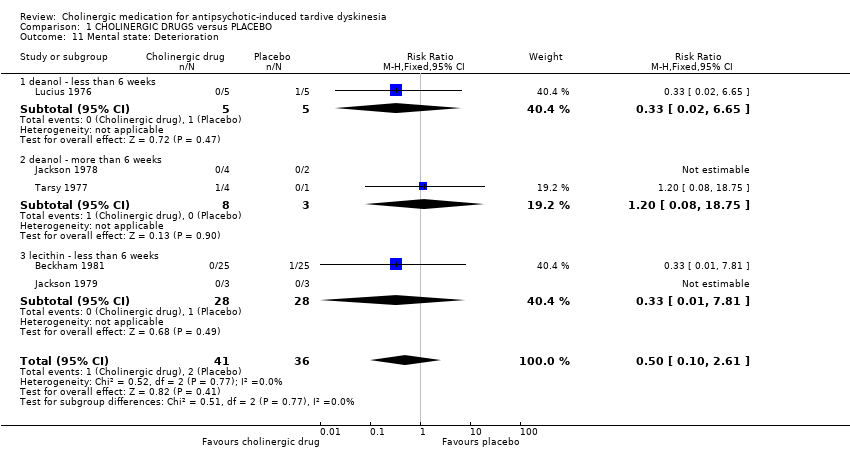 Comparison 1 CHOLINERGIC DRUGS versus PLACEBO, Outcome 11 Mental state: Deterioration. | ||||||||||||||||||||||||||||||||||
| 11.1 deanol ‐ less than 6 weeks | 1 | 10 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.02, 6.65] | ||||||||||||||||||||||||||||||
| 11.2 deanol ‐ more than 6 weeks | 2 | 11 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.2 [0.08, 18.75] | ||||||||||||||||||||||||||||||
| 11.3 lecithin ‐ less than 6 weeks | 2 | 56 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.81] | ||||||||||||||||||||||||||||||
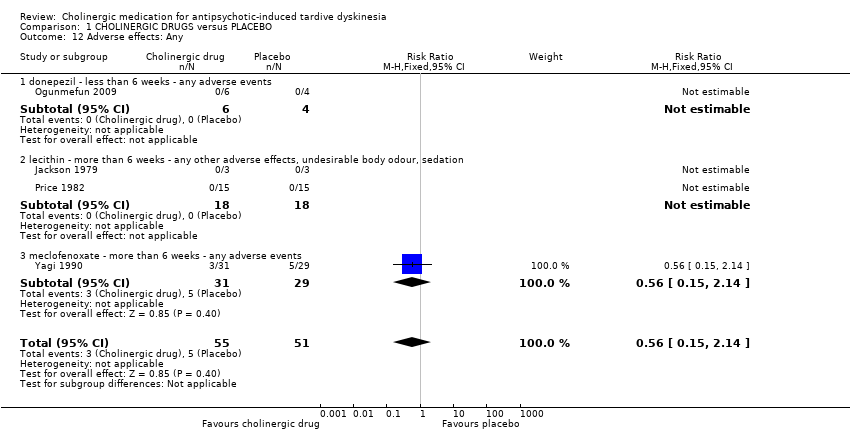
| 12 Adverse effects: Any Show forest plot | 4 | 106 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.15, 2.14] | ||||||||||||||||||||||||||||||
| Analysis 1.12  Comparison 1 CHOLINERGIC DRUGS versus PLACEBO, Outcome 12 Adverse effects: Any. | ||||||||||||||||||||||||||||||||||
| 12.1 donepezil ‐ less than 6 weeks ‐ any adverse events | 1 | 10 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | ||||||||||||||||||||||||||||||
| 12.2 lecithin ‐ more than 6 weeks ‐ any other adverse effects, undesirable body odour, sedation | 2 | 36 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | ||||||||||||||||||||||||||||||
| 12.3 meclofenoxate ‐ more than 6 weeks ‐ any adverse events | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.15, 2.14] | ||||||||||||||||||||||||||||||
| 13 Adverse effects: Various specific Show forest plot | 8 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |||||||||||||||||||||||||||||||
| Analysis 1.13 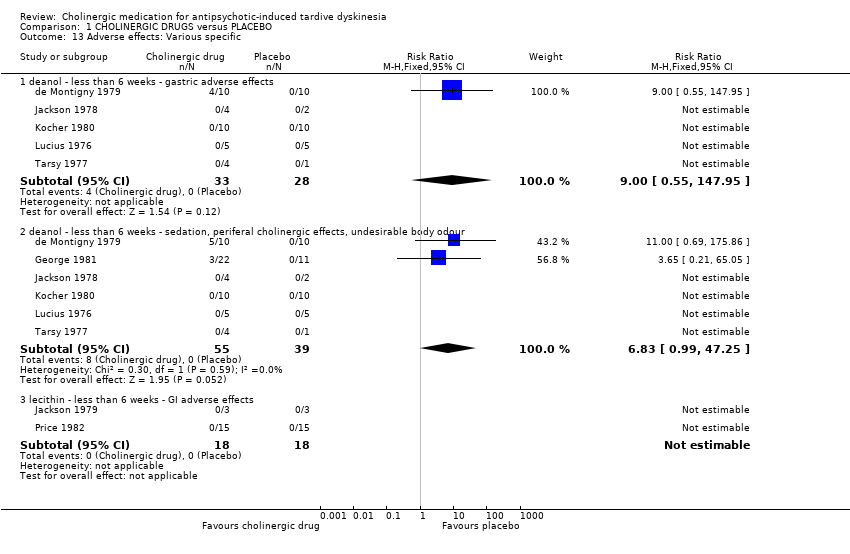 Comparison 1 CHOLINERGIC DRUGS versus PLACEBO, Outcome 13 Adverse effects: Various specific. | ||||||||||||||||||||||||||||||||||
| 13.1 deanol ‐ less than 6 weeks ‐ gastric adverse effects | 5 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 9.0 [0.55, 147.95] | ||||||||||||||||||||||||||||||
| 13.2 deanol ‐ less than 6 weeks ‐ sedation, periferal cholinergic effects, undesirable body odour | 6 | 94 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.83 [0.99, 47.25] | ||||||||||||||||||||||||||||||
| 13.3 lecithin ‐ less than 6 weeks ‐ GI adverse effects | 2 | 36 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | ||||||||||||||||||||||||||||||
| 14 Leaving the study early Show forest plot | 12 | 288 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.56, 2.10] | ||||||||||||||||||||||||||||||
| Analysis 1.14 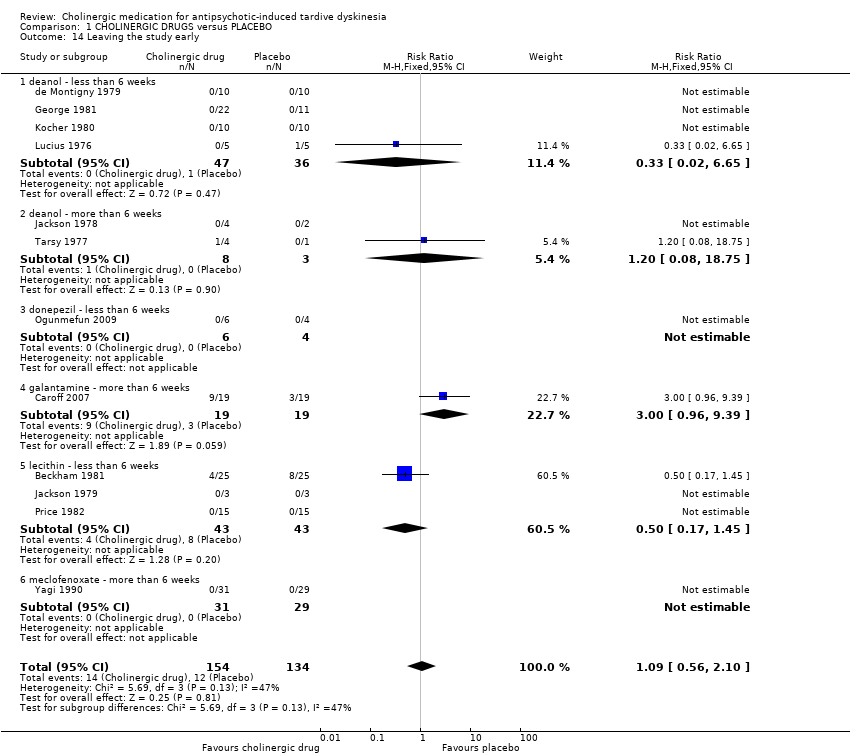 Comparison 1 CHOLINERGIC DRUGS versus PLACEBO, Outcome 14 Leaving the study early. | ||||||||||||||||||||||||||||||||||
| 14.1 deanol ‐ less than 6 weeks | 4 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.02, 6.65] | ||||||||||||||||||||||||||||||
| 14.2 deanol ‐ more than 6 weeks | 2 | 11 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.2 [0.08, 18.75] | ||||||||||||||||||||||||||||||
| 14.3 donepezil ‐ less than 6 weeks | 1 | 10 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | ||||||||||||||||||||||||||||||
| 14.4 galantamine ‐ more than 6 weeks | 1 | 38 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.96, 9.39] | ||||||||||||||||||||||||||||||
| 14.5 lecithin ‐ less than 6 weeks | 3 | 86 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.17, 1.45] | ||||||||||||||||||||||||||||||
| 14.6 meclofenoxate ‐ more than 6 weeks | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | ||||||||||||||||||||||||||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Tardive dyskinesia: 2a. Not any improvement (as assessed by rater) ‐ less than 6 weeks Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.1  Comparison 2 CHOLINERGIC DRUGS versus OTHER CHOLINERGIC DRUGS, Outcome 1 Tardive dyskinesia: 2a. Not any improvement (as assessed by rater) ‐ less than 6 weeks. | ||||
| 1.1 deanol 2g vs deanol 1g | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Global outcome: Death for any reason ‐ less than 6 weeks Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.2  Comparison 2 CHOLINERGIC DRUGS versus OTHER CHOLINERGIC DRUGS, Outcome 2 Global outcome: Death for any reason ‐ less than 6 weeks. | ||||
| 2.1 deanol 2g vs deanol 1g | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Leaving the study early ‐ less than 6 weeks Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.3  Comparison 2 CHOLINERGIC DRUGS versus OTHER CHOLINERGIC DRUGS, Outcome 3 Leaving the study early ‐ less than 6 weeks. | ||||
| 3.1 deanol 2g vs deanol 1g | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |

Message from one of the participants of the Public and patient involvement consultation of service user perspectives on tardive dyskinesia research.
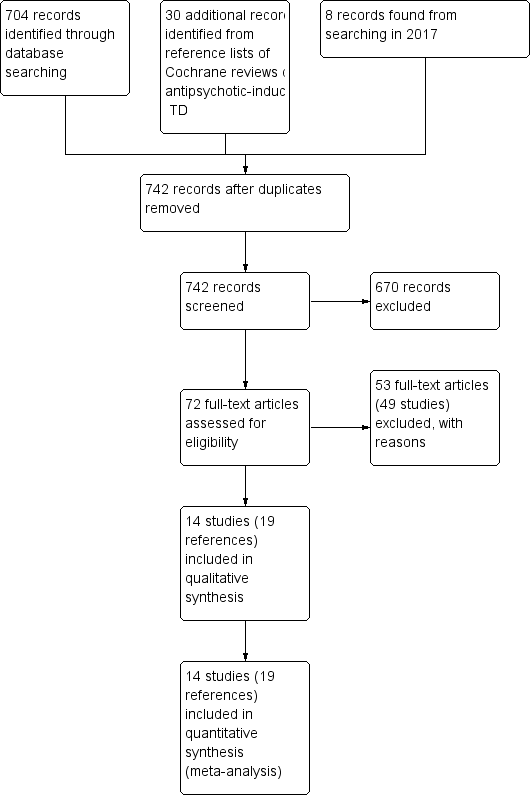
Study flow diagram for 2015 and 2017 searches.

'Risk of bias, summary: review authors' judgements about each risk of bias item for each included study.
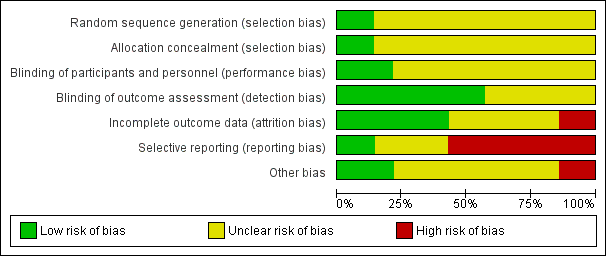
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
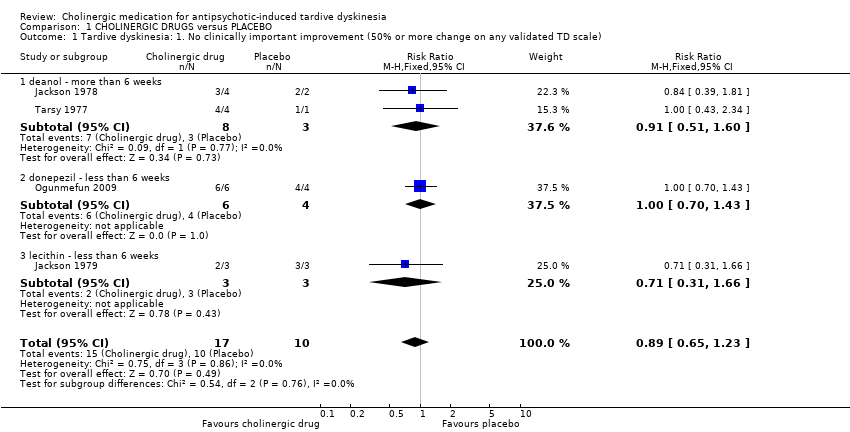
Comparison 1 CHOLINERGIC DRUGS versus PLACEBO, Outcome 1 Tardive dyskinesia: 1. No clinically important improvement (50% or more change on any validated TD scale).

Comparison 1 CHOLINERGIC DRUGS versus PLACEBO, Outcome 2 Tardive dyskinesia: 2a. Not any improvement (as assessed by rater).
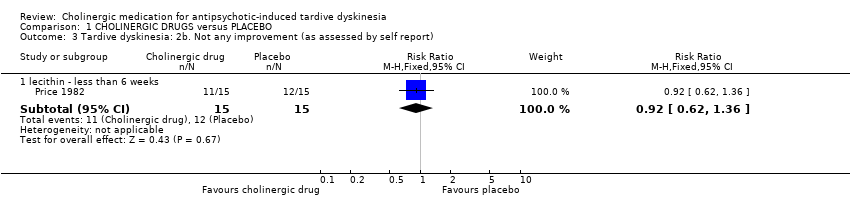
Comparison 1 CHOLINERGIC DRUGS versus PLACEBO, Outcome 3 Tardive dyskinesia: 2b. Not any improvement (as assessed by self report).

Comparison 1 CHOLINERGIC DRUGS versus PLACEBO, Outcome 4 Tardive dyskinesia: 3a. Average endpoint score on AIMS (low score = better).
| Study | Intervention | Mean | SD | N | Comments |
| deanol ‐ more than 6 weeks | |||||
| Tarsy 1977 | Deanol | 10 | 5.48 | 4 | |
| Tarsy 1977 | Placebo | 10 | 0 | 1 | The confidence interval of mean difference was not estimable because the placebo group only had one participant. |
Comparison 1 CHOLINERGIC DRUGS versus PLACEBO, Outcome 5 Tardive dyskinesia: 3b. Average endpoint score on modified Simpson TDRS (low score = better).

Comparison 1 CHOLINERGIC DRUGS versus PLACEBO, Outcome 6 Tardive dyskinesia: 4a. Deterioration (as assessed by rater).
Comparison 1 CHOLINERGIC DRUGS versus PLACEBO, Outcome 7 Tardive dyskinesia: 4b. Deterioration (as assessed by self report).

Comparison 1 CHOLINERGIC DRUGS versus PLACEBO, Outcome 8 Global outcome: Death for any reason.
Comparison 1 CHOLINERGIC DRUGS versus PLACEBO, Outcome 9 Global outcome: Intervention not useful as assessed by Global Usefulness Rating (GUR).
Comparison 1 CHOLINERGIC DRUGS versus PLACEBO, Outcome 10 Global state: Average endpoint score on CGI (low score = better).

Comparison 1 CHOLINERGIC DRUGS versus PLACEBO, Outcome 11 Mental state: Deterioration.
Comparison 1 CHOLINERGIC DRUGS versus PLACEBO, Outcome 12 Adverse effects: Any.

Comparison 1 CHOLINERGIC DRUGS versus PLACEBO, Outcome 13 Adverse effects: Various specific.
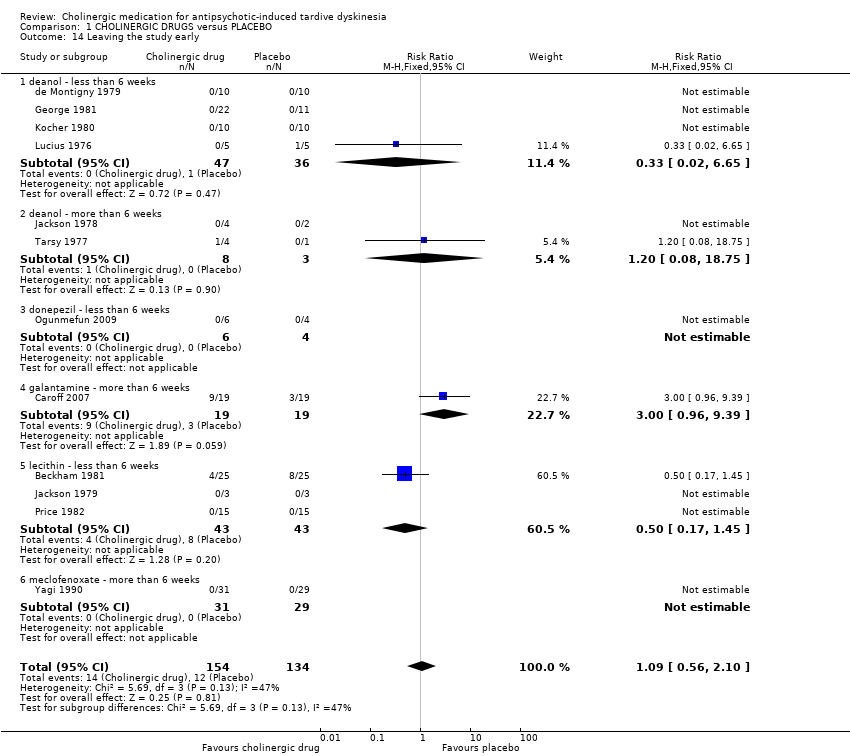
Comparison 1 CHOLINERGIC DRUGS versus PLACEBO, Outcome 14 Leaving the study early.

Comparison 2 CHOLINERGIC DRUGS versus OTHER CHOLINERGIC DRUGS, Outcome 1 Tardive dyskinesia: 2a. Not any improvement (as assessed by rater) ‐ less than 6 weeks.

Comparison 2 CHOLINERGIC DRUGS versus OTHER CHOLINERGIC DRUGS, Outcome 2 Global outcome: Death for any reason ‐ less than 6 weeks.

Comparison 2 CHOLINERGIC DRUGS versus OTHER CHOLINERGIC DRUGS, Outcome 3 Leaving the study early ‐ less than 6 weeks.
| Excluded study | Comparison | Treatment category | Relevant review | ||||
| #1 | #2 | ||||||
| Alpha‐methyl‐p‐tyrosine (AMPT) | versus choline chloride | Amino acid | Organic salt | ‐ | |||
| versus hydroxytryptophan | Amino acid (serotonin precursor) | ‐ | |||||
| versus valproic acid | Mood stabilisers | ‐ | |||||
| versus L‐DOPA | Amino acid | ‐ | |||||
| Benztropine versus bromocriptine | Anticholinergic | Dopamine agonist | ‐ | ||||
| Bromocriptine versus haloperidol | Dopamine agonist | Antipsychotic | ‐ | ||||
| Choline chloride | versus L‐DOPA | Organic salt | Amino acid | ‐ | |||
| versus hydroxytryptophan | ‐ | ||||||
| versus valproic | Anticonvulsant | ‐ | |||||
| Deanol | versus lithium carbonate | Antidepressant | Organic salt | ‐ | |||
| versus placebo | Placebo | ‐ | |||||
| versus sodium valproate | Anticonvulsant | ‐ | |||||
| versus oxpertine | Antipsychotic | ‐ | |||||
| Hydroxytryptophan versus L‐DOPA | Amino acid | Amino acid | ‐ | ||||
| L‐DOPA versus valproic acid | Anticonvulsant | ‐ | |||||
| Lithium carbonate versus placebo | Mood stabiliser | Placebo | ‐ | ||||
| Oxypertine versus sodium valproate | Antipsychotic | Anticonvulsant | ‐ | ||||
| Methods | Allocation: randomised, with sequence generation and concealment of allocation clearly described. |
| Participants | People with antipsychotic‐induced tardive dyskinesia.* |
| Interventions | Specific cholinergic drug (N = 150) versus placebo (N = 150) |
| Outcomes | Tardive dyskinesia: any clinically important improvement in TD, any improvement, deterioration.*** |
| Notes | * This could be diagnosed by clinical decision. If funds were permitting all participants could be screened using operational criteria, otherwise a random sample should suffice. ** Size of study with sufficient power to highlight about a 10% difference between groups for primary outcome. |
| CHOLINERGIC DRUGS versus PLACEBO for antipsychotic‐induced tardive dyskinesia | ||||||
| Patient or population: people with various psychiatric disorders (mainly schizophrenia) and antipsychotic‐induced tardive dyskinesia | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| PLACEBO | CHOLINERGIC DRUGS | |||||
| Tardive dyskinesia: No clinically important improvement follow‐up: 2 to 12 weeks | 1000 per 1000 | 890 per 1000 | RR 0.89 | 27 | ⊕⊝⊝⊝ | None of the subgroups that reported on this outcome (deanol, donepezil, lecithin) found a significant difference between cholinergic drug and placebo. |
| Tardive dyskinesia: Deterioration follow‐up: 9 days to 12 weeks | 116 per 1000 | 129 per 1000 | RR 1.11 | 147 | ⊕⊕⊝⊝ | None of the subgroups that reported on this outcome (deanol, donepezil, lecithin, meclofenoxate) found a significant difference between cholinergic drug and placebo. |
| Mental state: Deterioration follow‐up: 11 days to 12 weeks | 56 per 1000 | 28 per 1000 | RR 0.50 | 77 | ⊕⊝⊝⊝ | None of the subgroups that reported on this outcome (deanol, lecithin) found a significant difference between cholinergic drug and placebo. |
| Adverse effects: Any adverse events follow‐up: 9 days to 8 weeks | 98 per 1000 | 55 per 1000 | RR 0.56 | 106 | ⊕⊝⊝⊝ | None of the subgroups that reported on this outcome (donepezil, lecithin, meclofenoxate) found a significant difference between cholinergic drug and placebo. |
| Acceptability of treatment: Leaving the study early follow‐up: 9 days to 12 weeks | 90 per 1000 | 98 per 1000 | RR 1.09 | 288 | ⊕⊝⊝⊝ | None of the subgroups that reported on this outcome (deanol, donepezil, galantamine, meclofenoxate, lecithin) found a significant difference between cholinergic drug and placebo. |
| Social confidence, social inclusion, social networks, or personalised quality of life ‐ not reported | None of the included studies reported on this outcome. | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded one step for risk of bias: for many studies it was unclear whether randomisation procedure and allocation concealment were carried out adequately. | ||||||
| Interventions | Reference |
| Anticholinergic medication | |
| Benzodiazepines | |
| Calcium channel blockers | |
| Cholinergic medication | This review |
| Gamma‐aminobutyric acid agonists | |
| Miscellaneous treatments | |
| Neuroleptic reduction and/or cessation and neuroleptics | |
| Non‐neuroleptic catecholaminergic drugs | |
| Vitamin E |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Tardive dyskinesia: 1. No clinically important improvement (50% or more change on any validated TD scale) Show forest plot | 4 | 27 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.65, 1.23] |
| 1.1 deanol ‐ more than 6 weeks | 2 | 11 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.51, 1.60] |
| 1.2 donepezil ‐ less than 6 weeks | 1 | 10 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.70, 1.43] |
| 1.3 lecithin ‐ less than 6 weeks | 1 | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.31, 1.66] |
| 2 Tardive dyskinesia: 2a. Not any improvement (as assessed by rater) Show forest plot | 9 | 180 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.71, 1.07] |
| 2.1 deanol ‐ less than 6 weeks | 3 | 63 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.58, 1.18] |
| 2.2 deanol ‐ more than 6 weeks | 2 | 11 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.26, 2.57] |
| 2.3 donepezil ‐ less than 6 weeks | 1 | 10 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.72, 2.44] |
| 2.4 lecithin ‐ less than 6 weeks | 2 | 36 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.63, 1.21] |
| 2.5 meclofenoxate ‐ more than 6 weeks | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.55, 1.27] |
| 3 Tardive dyskinesia: 2b. Not any improvement (as assessed by self report) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 lecithin ‐ less than 6 weeks | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.62, 1.36] |
| 4 Tardive dyskinesia: 3a. Average endpoint score on AIMS (low score = better) Show forest plot | 7 | 171 | Mean Difference (IV, Fixed, 95% CI) | ‐0.12 [‐0.44, 0.21] |
| 4.1 deanol ‐ more than 6 weeks | 1 | 6 | Mean Difference (IV, Fixed, 95% CI) | 1.42 [‐0.29, 3.13] |
| 4.2 galantamine ‐ more than 6 weeks | 1 | 35 | Mean Difference (IV, Fixed, 95% CI) | 1.5 [‐0.44, 3.44] |
| 4.3 lecithin ‐ less than 6 weeks | 1 | 6 | Mean Difference (IV, Fixed, 95% CI) | ‐1.07 [‐2.21, 0.07] |
| 4.4 lecithin ‐ more than 6 weeks | 1 | 14 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐1.04, 0.84] |
| 4.5 meclofenoxate ‐ more than 6 weeks | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐0.19 [‐0.58, 0.20] |
| 4.6 rivastigmine ‐ less than 8 weeks | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | 2.20 [‐1.16, 5.56] |
| 4.7 donepezil ‐ less than 6 weeks | 1 | 10 | Mean Difference (IV, Fixed, 95% CI) | 1.10 [‐4.22, 6.42] |
| 5 Tardive dyskinesia: 3b. Average endpoint score on modified Simpson TDRS (low score = better) Show forest plot | Other data | No numeric data | ||
| 5.1 deanol ‐ more than 6 weeks | Other data | No numeric data | ||
| 6 Tardive dyskinesia: 4a. Deterioration (as assessed by rater) Show forest plot | 8 | 147 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.55, 2.24] |
| 6.1 deanol ‐ less than 6 weeks | 2 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.67 [0.48, 5.76] |
| 6.2 deanol ‐ more than 6 weeks | 2 | 11 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.20, 2.18] |
| 6.3 donepezil ‐ less than 6 weeks | 1 | 10 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.06, 7.85] |
| 6.4 lecithin ‐ less than 6 weeks | 2 | 36 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.16, 6.31] |
| 6.5 meclofenoxate ‐ more than 6 weeks | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.87 [0.18, 19.55] |
| 7 Tardive dyskinesia: 4b. Deterioration (as assessed by self report) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 lecithin ‐ less than 6 weeks | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 68.26] |
| 8 Global outcome: Death for any reason Show forest plot | 11 | 278 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.1 deanol ‐ less than 6 weeks | 4 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.2 deanol ‐ more than 6 weeks | 2 | 11 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.3 galantamine ‐ more than 6 weeks | 1 | 38 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.4 lecithin ‐ less than 6 weeks | 3 | 86 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.5 meclofenoxate ‐ more than 6 weeks | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Global outcome: Intervention not useful as assessed by Global Usefulness Rating (GUR) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9.1 meclofenoxate ‐ more than 6 weeks | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.59, 1.32] |
| 10 Global state: Average endpoint score on CGI (low score = better) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 10.1 lecithin ‐ less than 6 weeks | 1 | 31 | Mean Difference (IV, Fixed, 95% CI) | ‐0.43 [‐1.36, 0.50] |
| 11 Mental state: Deterioration Show forest plot | 5 | 77 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.10, 2.61] |
| 11.1 deanol ‐ less than 6 weeks | 1 | 10 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.02, 6.65] |
| 11.2 deanol ‐ more than 6 weeks | 2 | 11 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.2 [0.08, 18.75] |
| 11.3 lecithin ‐ less than 6 weeks | 2 | 56 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.81] |
| 12 Adverse effects: Any Show forest plot | 4 | 106 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.15, 2.14] |
| 12.1 donepezil ‐ less than 6 weeks ‐ any adverse events | 1 | 10 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12.2 lecithin ‐ more than 6 weeks ‐ any other adverse effects, undesirable body odour, sedation | 2 | 36 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12.3 meclofenoxate ‐ more than 6 weeks ‐ any adverse events | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.15, 2.14] |
| 13 Adverse effects: Various specific Show forest plot | 8 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 13.1 deanol ‐ less than 6 weeks ‐ gastric adverse effects | 5 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 9.0 [0.55, 147.95] |
| 13.2 deanol ‐ less than 6 weeks ‐ sedation, periferal cholinergic effects, undesirable body odour | 6 | 94 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.83 [0.99, 47.25] |
| 13.3 lecithin ‐ less than 6 weeks ‐ GI adverse effects | 2 | 36 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Leaving the study early Show forest plot | 12 | 288 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.56, 2.10] |
| 14.1 deanol ‐ less than 6 weeks | 4 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.02, 6.65] |
| 14.2 deanol ‐ more than 6 weeks | 2 | 11 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.2 [0.08, 18.75] |
| 14.3 donepezil ‐ less than 6 weeks | 1 | 10 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14.4 galantamine ‐ more than 6 weeks | 1 | 38 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.96, 9.39] |
| 14.5 lecithin ‐ less than 6 weeks | 3 | 86 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.17, 1.45] |
| 14.6 meclofenoxate ‐ more than 6 weeks | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Tardive dyskinesia: 2a. Not any improvement (as assessed by rater) ‐ less than 6 weeks Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 deanol 2g vs deanol 1g | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Global outcome: Death for any reason ‐ less than 6 weeks Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 deanol 2g vs deanol 1g | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Leaving the study early ‐ less than 6 weeks Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 deanol 2g vs deanol 1g | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |

