Medicamentos colinérgicos para la discinesia tardía inducida por neurolépticos
Información
- DOI:
- https://doi.org/10.1002/14651858.CD000207.pub2Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 19 marzo 2018see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Esquizofrenia
- Copyright:
-
- Copyright © 2018 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
Irina Tammenmaa‐ Aho ‐ searching for trials, evaluating trials, data extraction, analysis, writing of final report (2002).
Rosie Asher ‐ study screening, study selection, data extraction (2017).
Karla Soares‐Weiser ‐ protocol development, author of first version of review (2002 ‐ 2017).
Hanna Bergman ‐ study selection, data extraction and assimilation, summary of findings, report writing (2017 update).
Sources of support
Internal sources
-
Queensland Health, Australia.
-
CAPES ‐ Ministry of Education, Brazil.
-
Clinic of Psychiatry, Helsinki University Central Hospital (HUCS), Finland.
At the time of writing the review, employed Irina Tammenmaa as a resident physician.
-
Enhance Reviews Ltd., UK.
Logistics support for Hanna Bergman.
External sources
-
Universidade Federal de Sao Paulo, Brazil.
-
FinOHTA, STAKES, Finland.
-
NIHR HTA Project Grant, reference number: 14/27/02, UK.
Salary support for Hanna Bergman.
Support for patient involvement.
Support for traceable data database.
Declarations of interest
Irina Tammenmaa‐Aho ‐ None.
Karla Soares‐Weiser ‐ is the Deputy Editor‐in‐Chief for Cochrane and Cochrane Innovations. When the NHIR HTA programme grant was awarded that included to update this review, Karla was the Managing Director of Enhance Reviews Ltd.
Hanna Bergman ‐ worked for Enhance Reviews Ltd. during preparation of this review and was paid for her contribution to this review. Enhance Reviews Ltd. is a private company that performs systematic reviews of literature. HB works for Cochrane Response, an evidence consultancy that takes commissions from healthcare guideline developers and policy makers.
Rosie Asher ‐ worked for Enhance Reviews Ltd. during preparation of this review and was paid for her contribution to this review.
Acknowledgements
The review authors wish to thank Prof Kristian Wahlbeck, who acted as an advisor throughout the first version of the review process, for his constant help. The review authors also wish to acknowledge Prof Toshiaki Furukawa for great help with translating and extracting data from the Japanese articles. We wish to thank the authors and conductors of trials, S Bockenheimer, Joanne Doller‐Wojcik, Edward F Domino, Alan J Gelenberg, John H Growdon, Gabriele Lucius‐Hoene, David B Newgreen, Patricia E Penovich, Jorge Perez Cruet, Lynn A Price, Andre' Villeneuve and Jan Volavka for their kind replies to our inquiries about their studies and Stanley N Caroff who also acted as peer reviewer.
For the 2017 update, we wish to thank Antonio Grande for screening literature and helping with data extraction, Farhad Sokraneh for carrying out the trial search, helping to find full‐text papers, and assessing and extracting data from a study in Persian, Ben Gray for writing the Plain language summary, and Nicholas Henschke, Linda Levi, and Loukia Spineli for assistance with preparing the report. We are also grateful to Dawn‐Marie Walker, Ruth Sayers, Megan Lees, and Vanessa Pinfold from McPin Foundation for organising and holding the public and patient involvement consultation with TD service users that contributed to selecting outcomes for the 'Summary of findings' table and to guide future research. We wish to thank the author and trial conductor William T Regenold for his kind reply to our queries about his study. We would also like to thank John McGrath for his advice and support writing the first published version of this review and Dr Eila Sailas for contributing to earlier versions of this review.
Version history
| Published | Title | Stage | Authors | Version |
| 2018 Mar 19 | Cholinergic medication for antipsychotic‐induced tardive dyskinesia | Review | Irina Tammenmaa‐Aho, Rosie Asher, Karla Soares‐Weiser, Hanna Bergman | |
| 2002 Jul 22 | Cholinergic medication for neuroleptic‐induced tardive dyskinesia | Review | Irina Tammenmaa, John McGrath, Eila ES Sailas, Karla Soares‐Weiser | |
| Interventions | Reference |
| Anticholinergic medication | |
| Benzodiazepines | |
| Calcium channel blockers | |
| Cholinergic medication | This review |
| Gamma‐aminobutyric acid agonists | |
| Miscellaneous treatments | |
| Neuroleptic reduction and/or cessation and neuroleptics | |
| Non‐neuroleptic catecholaminergic drugs | |
| Vitamin E |
Differences between protocol and review
The protocol as published with this review has evolved over time. The revisions of protocol are in line with the development of RevMan and in keeping with Cochrane guidance. We think the revisions have greatly improved and enhanced this review. We do not think, however, that it has materially affected our conduct of the review or interpretation of the results.
There was a substantial update to the protocol in the 2017 review update. The biggest changes to affect the review were to:
-
broaden the inclusion criteria, and adding the comparison 'Cholinergic medication versus any other intervention for the treatment of tardive dyskinesia';
-
change the title from 'Cholinergic medication for neuroleptic‐induced tardive dyskinesia' to 'Cholinergic medication for antipsychotic‐induced tardive dyskinesia';
-
update the list of outcomes following consultation with consumers; and
-
add a 'Summary of findings' table.
Previous methods are reproduced in Appendix 1.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
Medical Subject Headings Check Words
Humans;
PICO

Message from one of the participants of the Public and patient involvement consultation of service user perspectives on tardive dyskinesia research.
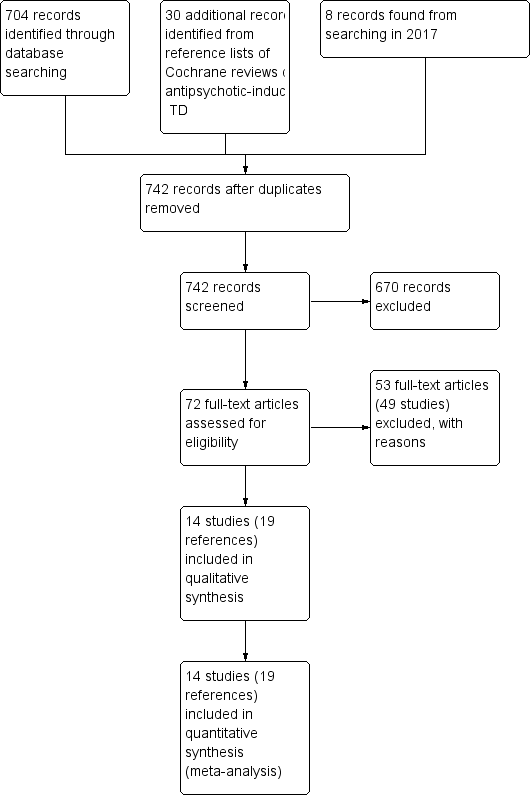
Study flow diagram for 2015 and 2017 searches.

'Risk of bias, summary: review authors' judgements about each risk of bias item for each included study.
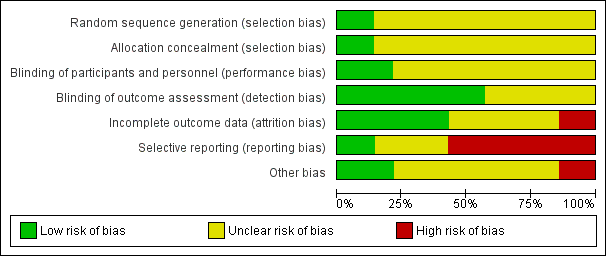
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
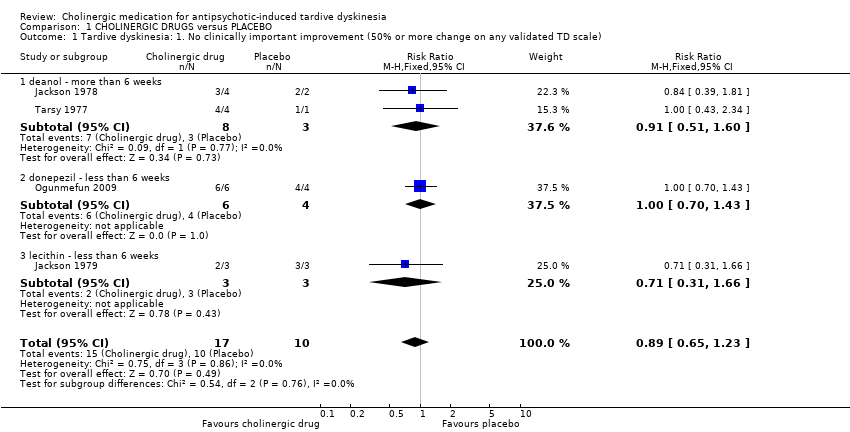
Comparison 1 CHOLINERGIC DRUGS versus PLACEBO, Outcome 1 Tardive dyskinesia: 1. No clinically important improvement (50% or more change on any validated TD scale).

Comparison 1 CHOLINERGIC DRUGS versus PLACEBO, Outcome 2 Tardive dyskinesia: 2a. Not any improvement (as assessed by rater).
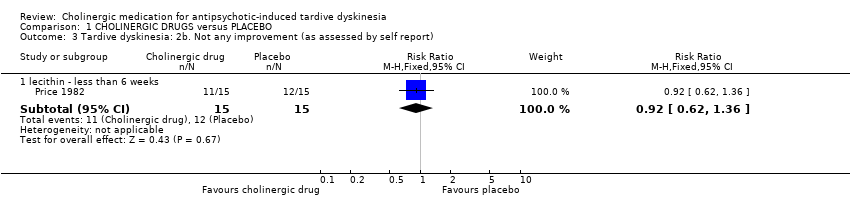
Comparison 1 CHOLINERGIC DRUGS versus PLACEBO, Outcome 3 Tardive dyskinesia: 2b. Not any improvement (as assessed by self report).

Comparison 1 CHOLINERGIC DRUGS versus PLACEBO, Outcome 4 Tardive dyskinesia: 3a. Average endpoint score on AIMS (low score = better).
| Study | Intervention | Mean | SD | N | Comments |
| deanol ‐ more than 6 weeks | |||||
| Tarsy 1977 | Deanol | 10 | 5.48 | 4 | |
| Tarsy 1977 | Placebo | 10 | 0 | 1 | The confidence interval of mean difference was not estimable because the placebo group only had one participant. |
Comparison 1 CHOLINERGIC DRUGS versus PLACEBO, Outcome 5 Tardive dyskinesia: 3b. Average endpoint score on modified Simpson TDRS (low score = better).

Comparison 1 CHOLINERGIC DRUGS versus PLACEBO, Outcome 6 Tardive dyskinesia: 4a. Deterioration (as assessed by rater).
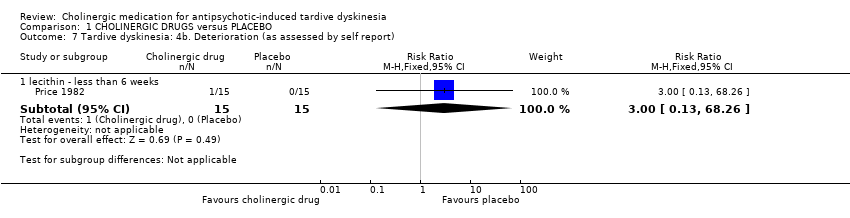
Comparison 1 CHOLINERGIC DRUGS versus PLACEBO, Outcome 7 Tardive dyskinesia: 4b. Deterioration (as assessed by self report).

Comparison 1 CHOLINERGIC DRUGS versus PLACEBO, Outcome 8 Global outcome: Death for any reason.
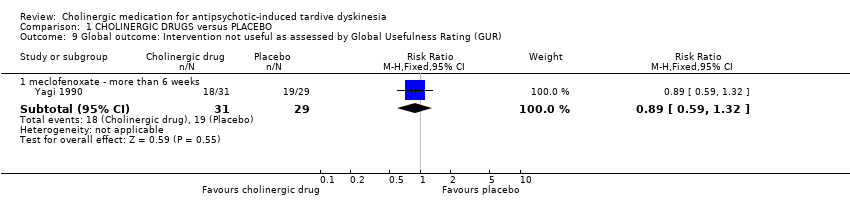
Comparison 1 CHOLINERGIC DRUGS versus PLACEBO, Outcome 9 Global outcome: Intervention not useful as assessed by Global Usefulness Rating (GUR).
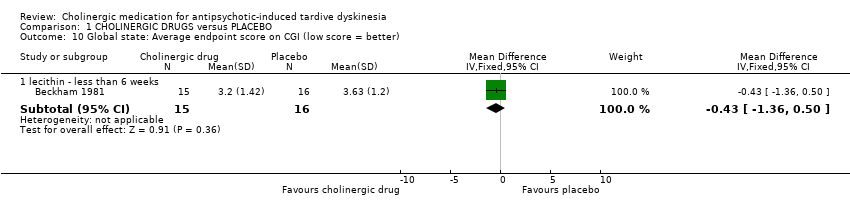
Comparison 1 CHOLINERGIC DRUGS versus PLACEBO, Outcome 10 Global state: Average endpoint score on CGI (low score = better).

Comparison 1 CHOLINERGIC DRUGS versus PLACEBO, Outcome 11 Mental state: Deterioration.
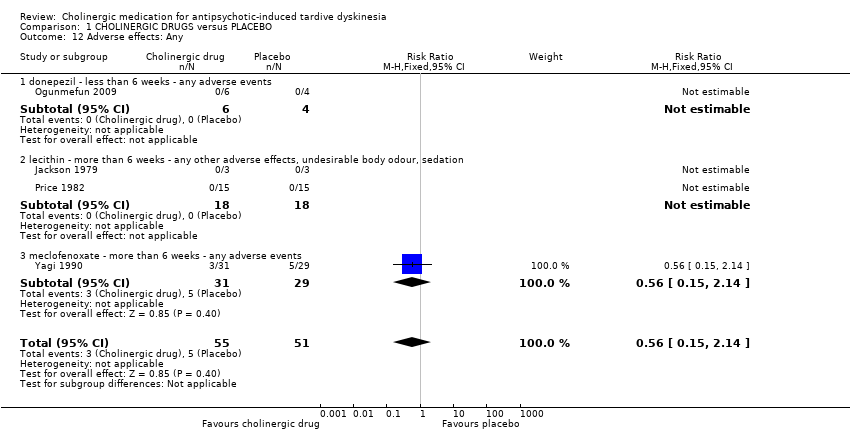
Comparison 1 CHOLINERGIC DRUGS versus PLACEBO, Outcome 12 Adverse effects: Any.

Comparison 1 CHOLINERGIC DRUGS versus PLACEBO, Outcome 13 Adverse effects: Various specific.
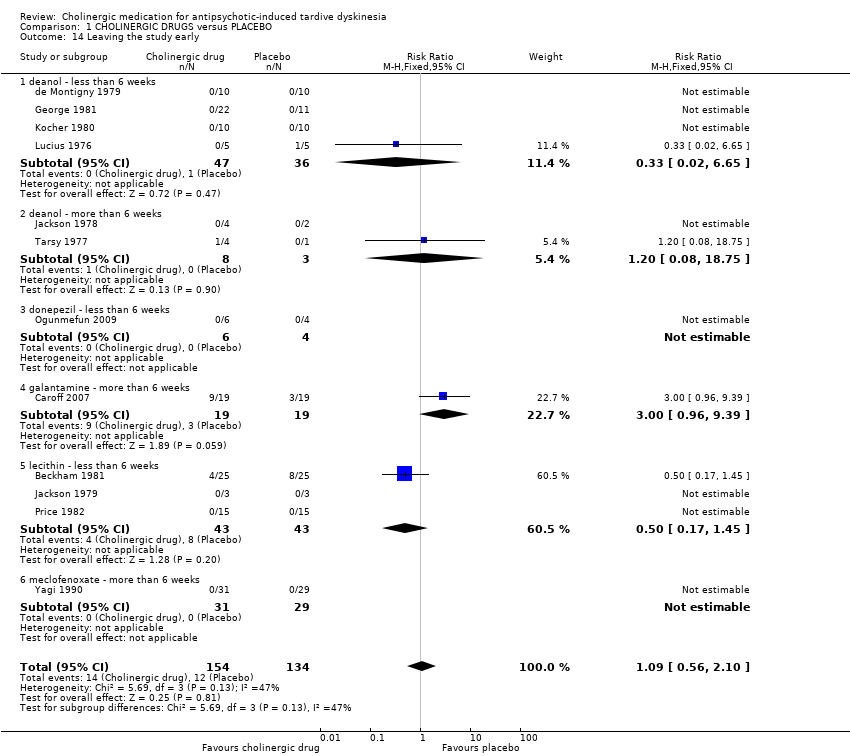
Comparison 1 CHOLINERGIC DRUGS versus PLACEBO, Outcome 14 Leaving the study early.

Comparison 2 CHOLINERGIC DRUGS versus OTHER CHOLINERGIC DRUGS, Outcome 1 Tardive dyskinesia: 2a. Not any improvement (as assessed by rater) ‐ less than 6 weeks.

Comparison 2 CHOLINERGIC DRUGS versus OTHER CHOLINERGIC DRUGS, Outcome 2 Global outcome: Death for any reason ‐ less than 6 weeks.

Comparison 2 CHOLINERGIC DRUGS versus OTHER CHOLINERGIC DRUGS, Outcome 3 Leaving the study early ‐ less than 6 weeks.
| Excluded study | Comparison | Treatment category | Relevant review | ||||
| #1 | #2 | ||||||
| Alpha‐methyl‐p‐tyrosine (AMPT) | versus choline chloride | Amino acid | Organic salt | ‐ | |||
| versus hydroxytryptophan | Amino acid (serotonin precursor) | ‐ | |||||
| versus valproic acid | Mood stabilisers | ‐ | |||||
| versus L‐DOPA | Amino acid | ‐ | |||||
| Benztropine versus bromocriptine | Anticholinergic | Dopamine agonist | ‐ | ||||
| Bromocriptine versus haloperidol | Dopamine agonist | Antipsychotic | ‐ | ||||
| Choline chloride | versus L‐DOPA | Organic salt | Amino acid | ‐ | |||
| versus hydroxytryptophan | ‐ | ||||||
| versus valproic | Anticonvulsant | ‐ | |||||
| Deanol | versus lithium carbonate | Antidepressant | Organic salt | ‐ | |||
| versus placebo | Placebo | ‐ | |||||
| versus sodium valproate | Anticonvulsant | ‐ | |||||
| versus oxpertine | Antipsychotic | ‐ | |||||
| Hydroxytryptophan versus L‐DOPA | Amino acid | Amino acid | ‐ | ||||
| L‐DOPA versus valproic acid | Anticonvulsant | ‐ | |||||
| Lithium carbonate versus placebo | Mood stabiliser | Placebo | ‐ | ||||
| Oxypertine versus sodium valproate | Antipsychotic | Anticonvulsant | ‐ | ||||
| Methods | Allocation: randomised, with sequence generation and concealment of allocation clearly described. |
| Participants | People with antipsychotic‐induced tardive dyskinesia.* |
| Interventions | Specific cholinergic drug (N = 150) versus placebo (N = 150) |
| Outcomes | Tardive dyskinesia: any clinically important improvement in TD, any improvement, deterioration.*** |
| Notes | * This could be diagnosed by clinical decision. If funds were permitting all participants could be screened using operational criteria, otherwise a random sample should suffice. ** Size of study with sufficient power to highlight about a 10% difference between groups for primary outcome. |
| CHOLINERGIC DRUGS versus PLACEBO for antipsychotic‐induced tardive dyskinesia | ||||||
| Patient or population: people with various psychiatric disorders (mainly schizophrenia) and antipsychotic‐induced tardive dyskinesia | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| PLACEBO | CHOLINERGIC DRUGS | |||||
| Tardive dyskinesia: No clinically important improvement follow‐up: 2 to 12 weeks | 1000 per 1000 | 890 per 1000 | RR 0.89 | 27 | ⊕⊝⊝⊝ | None of the subgroups that reported on this outcome (deanol, donepezil, lecithin) found a significant difference between cholinergic drug and placebo. |
| Tardive dyskinesia: Deterioration follow‐up: 9 days to 12 weeks | 116 per 1000 | 129 per 1000 | RR 1.11 | 147 | ⊕⊕⊝⊝ | None of the subgroups that reported on this outcome (deanol, donepezil, lecithin, meclofenoxate) found a significant difference between cholinergic drug and placebo. |
| Mental state: Deterioration follow‐up: 11 days to 12 weeks | 56 per 1000 | 28 per 1000 | RR 0.50 | 77 | ⊕⊝⊝⊝ | None of the subgroups that reported on this outcome (deanol, lecithin) found a significant difference between cholinergic drug and placebo. |
| Adverse effects: Any adverse events follow‐up: 9 days to 8 weeks | 98 per 1000 | 55 per 1000 | RR 0.56 | 106 | ⊕⊝⊝⊝ | None of the subgroups that reported on this outcome (donepezil, lecithin, meclofenoxate) found a significant difference between cholinergic drug and placebo. |
| Acceptability of treatment: Leaving the study early follow‐up: 9 days to 12 weeks | 90 per 1000 | 98 per 1000 | RR 1.09 | 288 | ⊕⊝⊝⊝ | None of the subgroups that reported on this outcome (deanol, donepezil, galantamine, meclofenoxate, lecithin) found a significant difference between cholinergic drug and placebo. |
| Social confidence, social inclusion, social networks, or personalised quality of life ‐ not reported | None of the included studies reported on this outcome. | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded one step for risk of bias: for many studies it was unclear whether randomisation procedure and allocation concealment were carried out adequately. | ||||||
| Interventions | Reference |
| Anticholinergic medication | |
| Benzodiazepines | |
| Calcium channel blockers | |
| Cholinergic medication | This review |
| Gamma‐aminobutyric acid agonists | |
| Miscellaneous treatments | |
| Neuroleptic reduction and/or cessation and neuroleptics | |
| Non‐neuroleptic catecholaminergic drugs | |
| Vitamin E |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Tardive dyskinesia: 1. No clinically important improvement (50% or more change on any validated TD scale) Show forest plot | 4 | 27 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.65, 1.23] |
| 1.1 deanol ‐ more than 6 weeks | 2 | 11 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.51, 1.60] |
| 1.2 donepezil ‐ less than 6 weeks | 1 | 10 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.70, 1.43] |
| 1.3 lecithin ‐ less than 6 weeks | 1 | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.31, 1.66] |
| 2 Tardive dyskinesia: 2a. Not any improvement (as assessed by rater) Show forest plot | 9 | 180 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.71, 1.07] |
| 2.1 deanol ‐ less than 6 weeks | 3 | 63 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.58, 1.18] |
| 2.2 deanol ‐ more than 6 weeks | 2 | 11 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.26, 2.57] |
| 2.3 donepezil ‐ less than 6 weeks | 1 | 10 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.72, 2.44] |
| 2.4 lecithin ‐ less than 6 weeks | 2 | 36 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.63, 1.21] |
| 2.5 meclofenoxate ‐ more than 6 weeks | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.55, 1.27] |
| 3 Tardive dyskinesia: 2b. Not any improvement (as assessed by self report) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 lecithin ‐ less than 6 weeks | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.62, 1.36] |
| 4 Tardive dyskinesia: 3a. Average endpoint score on AIMS (low score = better) Show forest plot | 7 | 171 | Mean Difference (IV, Fixed, 95% CI) | ‐0.12 [‐0.44, 0.21] |
| 4.1 deanol ‐ more than 6 weeks | 1 | 6 | Mean Difference (IV, Fixed, 95% CI) | 1.42 [‐0.29, 3.13] |
| 4.2 galantamine ‐ more than 6 weeks | 1 | 35 | Mean Difference (IV, Fixed, 95% CI) | 1.5 [‐0.44, 3.44] |
| 4.3 lecithin ‐ less than 6 weeks | 1 | 6 | Mean Difference (IV, Fixed, 95% CI) | ‐1.07 [‐2.21, 0.07] |
| 4.4 lecithin ‐ more than 6 weeks | 1 | 14 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐1.04, 0.84] |
| 4.5 meclofenoxate ‐ more than 6 weeks | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐0.19 [‐0.58, 0.20] |
| 4.6 rivastigmine ‐ less than 8 weeks | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | 2.20 [‐1.16, 5.56] |
| 4.7 donepezil ‐ less than 6 weeks | 1 | 10 | Mean Difference (IV, Fixed, 95% CI) | 1.10 [‐4.22, 6.42] |
| 5 Tardive dyskinesia: 3b. Average endpoint score on modified Simpson TDRS (low score = better) Show forest plot | Other data | No numeric data | ||
| 5.1 deanol ‐ more than 6 weeks | Other data | No numeric data | ||
| 6 Tardive dyskinesia: 4a. Deterioration (as assessed by rater) Show forest plot | 8 | 147 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.55, 2.24] |
| 6.1 deanol ‐ less than 6 weeks | 2 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.67 [0.48, 5.76] |
| 6.2 deanol ‐ more than 6 weeks | 2 | 11 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.20, 2.18] |
| 6.3 donepezil ‐ less than 6 weeks | 1 | 10 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.06, 7.85] |
| 6.4 lecithin ‐ less than 6 weeks | 2 | 36 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.16, 6.31] |
| 6.5 meclofenoxate ‐ more than 6 weeks | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.87 [0.18, 19.55] |
| 7 Tardive dyskinesia: 4b. Deterioration (as assessed by self report) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 lecithin ‐ less than 6 weeks | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 68.26] |
| 8 Global outcome: Death for any reason Show forest plot | 11 | 278 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.1 deanol ‐ less than 6 weeks | 4 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.2 deanol ‐ more than 6 weeks | 2 | 11 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.3 galantamine ‐ more than 6 weeks | 1 | 38 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.4 lecithin ‐ less than 6 weeks | 3 | 86 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.5 meclofenoxate ‐ more than 6 weeks | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Global outcome: Intervention not useful as assessed by Global Usefulness Rating (GUR) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9.1 meclofenoxate ‐ more than 6 weeks | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.59, 1.32] |
| 10 Global state: Average endpoint score on CGI (low score = better) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 10.1 lecithin ‐ less than 6 weeks | 1 | 31 | Mean Difference (IV, Fixed, 95% CI) | ‐0.43 [‐1.36, 0.50] |
| 11 Mental state: Deterioration Show forest plot | 5 | 77 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.10, 2.61] |
| 11.1 deanol ‐ less than 6 weeks | 1 | 10 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.02, 6.65] |
| 11.2 deanol ‐ more than 6 weeks | 2 | 11 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.2 [0.08, 18.75] |
| 11.3 lecithin ‐ less than 6 weeks | 2 | 56 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.81] |
| 12 Adverse effects: Any Show forest plot | 4 | 106 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.15, 2.14] |
| 12.1 donepezil ‐ less than 6 weeks ‐ any adverse events | 1 | 10 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12.2 lecithin ‐ more than 6 weeks ‐ any other adverse effects, undesirable body odour, sedation | 2 | 36 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12.3 meclofenoxate ‐ more than 6 weeks ‐ any adverse events | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.15, 2.14] |
| 13 Adverse effects: Various specific Show forest plot | 8 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 13.1 deanol ‐ less than 6 weeks ‐ gastric adverse effects | 5 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 9.0 [0.55, 147.95] |
| 13.2 deanol ‐ less than 6 weeks ‐ sedation, periferal cholinergic effects, undesirable body odour | 6 | 94 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.83 [0.99, 47.25] |
| 13.3 lecithin ‐ less than 6 weeks ‐ GI adverse effects | 2 | 36 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Leaving the study early Show forest plot | 12 | 288 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.56, 2.10] |
| 14.1 deanol ‐ less than 6 weeks | 4 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.02, 6.65] |
| 14.2 deanol ‐ more than 6 weeks | 2 | 11 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.2 [0.08, 18.75] |
| 14.3 donepezil ‐ less than 6 weeks | 1 | 10 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14.4 galantamine ‐ more than 6 weeks | 1 | 38 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.96, 9.39] |
| 14.5 lecithin ‐ less than 6 weeks | 3 | 86 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.17, 1.45] |
| 14.6 meclofenoxate ‐ more than 6 weeks | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Tardive dyskinesia: 2a. Not any improvement (as assessed by rater) ‐ less than 6 weeks Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 deanol 2g vs deanol 1g | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Global outcome: Death for any reason ‐ less than 6 weeks Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 deanol 2g vs deanol 1g | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Leaving the study early ‐ less than 6 weeks Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 deanol 2g vs deanol 1g | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |

