Peroxisome proliferator‐activated receptor gamma agonists for preventing recurrent stroke and other vascular events in people with stroke or transient ischaemic attack
Abstract
Background
Peroxisome proliferator‐activated receptor gamma (PPAR‐γ) agonists are insulin‐sensitising drugs used for the treatment of insulin resistance. In addition to lowering glucose in diabetes, these drugs may also protect against hyperlipidaemia and arteriosclerosis, which are risk factors for stroke. This is an update of a review first published in January 2014 and subsequently updated in October 2015.
Objectives
To assess the efficacy and safety of PPAR‐γ agonists in the secondary prevention of stroke and related vascular events for people with stroke or transient ischaemic attack (TIA).
Search methods
We searched the Cochrane Stroke Group Trials Register (16 May 2017), the Cochrane Central Register of Controlled Trials (CENTRAL; 2017, Issue 5), MEDLINE (1949 to 16 May 2017), Embase (1980 to 16 May 2017), CINAHL (1982 to 16 May 2017), AMED (1985 to 16 May 2017), and 11 Chinese databases (16 May 2017). In an effort to identify further published, unpublished, and ongoing trials, we searched ongoing trials registers, reference lists, and relevant conference proceedings, and contacted authors and pharmaceutical companies. We did not impose any language restrictions.
Selection criteria
We included randomised controlled trials (RCTs) evaluating PPAR‐γ agonists versus placebo for the secondary prevention of stroke and related vascular events in people with stroke or TIA, with the outcomes of recurrent stroke, vascular events, and adverse events.
Data collection and analysis
Two review authors independently screened the titles and abstracts of identified records, selected studies for inclusion, extracted eligible data, cross‐checked the data for accuracy, and assessed methodological quality and risk of bias. We evaluated the quality of evidence for each outcome using the GRADE approach.
Main results
We identified five RCTs with 5039 participants; two studies had a low risk of bias for all domains. Four studies evaluated the drug pioglitazone, and one study evaluated rosiglitazone. The participants in different studies were heterogeneous.
Recurrent stroke
Three studies evaluated the number of participants with recurrent stroke (4979 participants, a single study contributing 3876 of these). Peroxisome proliferator‐activated receptor gamma agonists probably reduce the recurrence of stroke compared with placebo (risk ratio (RR) 0.66, 95% confidence interval (CI) 0.44 to 0.99; moderate‐quality evidence).
Adverse events
Evidence that adverse events occurred more frequently in participants treated with PPAR‐γ agonists when compared with placebo was uncertain due to wide confidence interval and high levels of statistical heterogeneity: risk difference 10%, 95% CI ‐8% to 28%; low‐quality evidence).
Data were available on additional composite outcomes reflecting serious vascular events (all‐cause death and other major vascular events; all‐cause mortality, non‐fatal myocardial infarction or non‐fatal stroke) from one study in 984 people. This study provided low‐quality evidence that PPAR‐γ agonists led to fewer events (data not meta‐analysed).
Vascular events
Peroxisome proliferator‐activated receptor gamma agonists given over a mean duration of 34.5 months in a single trial of 984 participants may reduce serious vascular events expressed as a composite outcome of total events of cardiovascular death, non‐fatal myocardial infarction or non‐fatal stroke (RR 0.73, 95% CI 0.54 to 0.99; low‐quality evidence).
Other outcomes
One study in 20 people measured insulin sensitivity, and one study in 40 people measured the ubiquitin‐proteasome activity in carotid plaques. Our confidence in the improvements observed with PPAR‐γ agonists were limited by small sample sizes and risk of bias. None of the studies reported the number of participants with disability due to vascular events or improvement in quality of life.
Authors' conclusions
Peroxisome proliferator‐activated receptor gamma agonists probably reduce recurrent stroke and total events of cardiovascular death, non‐fatal myocardial infarction or non‐fatal stroke, and may improve insulin sensitivity and the stabilisation of carotid plaques. Their effects on adverse events are uncertain. Our conclusions should be interpreted with caution considering the small number and the quality of the included studies. Further well‐designed, double‐blind RCTs with large samples are required to assess the efficacy and safety of PPAR‐γ agonists in the secondary prevention of stroke and related vascular events in people with stroke or TIA.
PICOs
Plain language summary
Diabetes drugs for preventing stroke and other blood vessel disease in people who have had a previous stroke or transient ischaemic attack
Question
We wanted to evaluate the effectiveness and safety of new diabetes drugs (peroxisome proliferator‐activated receptor gamma (PPAR‐γ) agonists) in the prevention of stroke and related blood vessel disease in people who have already had a stroke or transient ischaemic attack.
Background
Peroxisome proliferator‐activated receptor gamma agonists are drugs that improve the way insulin works in the human body. They are widely used in the treatment of adult type diabetes (type 2 diabetes). Moreover, they may also protect against the presence of excess fats in the blood and disease of the artery walls, which are both risk factors for stroke.
Study characteristics
We identified five studies to 16 May 2017 including a total of 5039 participants. Four studies evaluated the drug pioglitazone, and one study evaluated rosiglitazone. Four studies included participants who had no history of diabetes, and one study included only participants with diabetes.
Key results
Compared with placebo tablets, PPAR‐γ agonists reduced recurrent strokes and other blood vessel disease, improved the body's response to insulin, and stabilised fatty deposits in artery walls. The drugs also appeared to be well tolerated, but the evidence for this was inconclusive.
Quality of the evidence
Our conclusions should be interpreted with caution considering the small number of included studies and the limited quality of some of the studies. Further well‐designed randomised controlled trials with large sample sizes are required.
Authors' conclusions
Summary of findings
| Peroxisome proliferator‐activated receptor gamma (PPAR‐γ) agonists for preventing recurrent stroke and other vascular events in people with stroke or transient ischaemic attack | ||||||
| Patient or population: people with stroke or transient ischaemic attack Settings: inpatients Intervention: PPAR‐γ agonists Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | PPAR‐γ agonists | |||||
| Recurrence of stroke Follow‐up: 25 to 57.6 months | 85 per 1000 | 56 per 1000 (37 to 84) | RR 0.66 (0.44 to 0.99) | 4979 | ⊕⊕⊕⊝ | |
| Reported adverse events Follow‐up: 3 to 34.5 months | 492 per 1000 | 502 per 1000 (412 to 530) | RD 0.10 (‐0.08 to 0.28) | 1044 | ⊕⊕⊝⊝ | |
| Serious vascular events | For the total events of all‐cause death, non‐fatal myocardial infarction, acute coronary syndrome and cardiac intervention, stroke, major leg amputation, or bypass surgery or leg revascularisation, there were 126 of 498 (25%). For the total events of all‐cause death, non‐fatal myocardial infarction or non‐fatal stroke, there were 98 of 498 (20%). | For the total events of all‐cause death, non‐fatal myocardial infarction, acute coronary syndrome and cardiac intervention, stroke, major leg amputation, or bypass surgery or leg revascularisation, there were 98 of 486 (20%). For the total events of all‐cause death, non‐fatal myocardial infarction or non‐fatal stroke, there were 76 of 486 (16%). | For the total events of all‐cause death, non‐fatal myocardial infarction, acute coronary syndrome and cardiac intervention, stroke, major leg amputation, or bypass surgery or leg revascularisation (RR 0.80, 95% CI 0.63 to 1.01). For the total events of all‐cause death, non‐fatal myocardial infarction or non‐fatal stroke (RR 0.79, 95% CI 0.61 to 1.04). Pioglitazone reduced fatal or non‐fatal stroke (RR 0.54, 95% CI 0.35 to 0.85) and total events of cardiovascular death, non‐fatal myocardial infarction or non‐fatal stroke (RR 0.73, 95% CI 0.54 to 0.99). | 984 | ⊕⊕⊝⊝ | Pioglitazone reduced fatal or non‐fatal stroke and total events of cardiovascular death, non‐fatal myocardial infarction or non‐fatal stroke. |
| Deaths due to vascular events | Not reported | Not reported | — | — | — | |
| Disability due to vascular events | Not reported | Not reported | — | — | — | |
| Improvement in quality of life | Not reported | Not reported | — | — | — | |
| Insulin sensitivity Assessed with composite insulin sensitivity index | The change in the composite index was ‐0.1 ± 0.6. The C‐reactive protein concentration increased from 0.41 to 0.45 mg/L. | The change in the composite index was 1.2 ± 0.6. The C‐reactive protein concentration declined from 0.30 to 0.20 mg/L. | The change in the composite index was significantly increased in the pioglitazone group in comparison with the placebo group (P = 0.0003). | 20 | ⊕⊕⊝⊝ | |
| Ubiquitin‐proteasome activity in carotid plaques | Ubiquitin 468.7 ± 89 ng/mg; proteasome 20S 79.8 ± 25 pmol/mg; nitrotyrosine 3.5 ± 0.42 nmol/pg; superoxide anion production 6.26 ± 1.4 pmol/L | Ubiquitin 322 ± 79 ng/mg; proteasome 20S 46.8 ± 10 pmol/mg; nitrotyrosine 2.2 ± 0.21 nmol/pg; superoxide anion production 3.57 ± 1.1 pmol/L | Compared with the placebo group, symptomatic carotid plaques in the rosiglitazone group showed fewer inflammatory cells (P < 0.01) with less ubiquitin (P < 0.01), proteasome 20S (P < 0.01), nuclear factor kappa B (P < 0.01), nitrotyrosine (P < 0.01), superoxide anion production (P < 0.01), and more collagen content (P < 0.01). | 40 | ⊕⊕⊝⊝ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded one level due to risk of bias. | ||||||
Background
Description of the condition
Stroke is one of the most common neurological diseases and one of the most common causes of mortality worldwide (WHO 2010). On average 80% of strokes are caused by ischaemia, and recurrent strokes account for around 30% of all events (Goldstein 2006; Simon 2009). Significantly higher mortality was found in recurrent stroke compared with first‐ever stroke (Jørgensen 1997). Secondary prevention therefore plays an important role in reducing stroke recurrence and other related vascular events. Diabetes is an important risk factor for ischaemic stroke. It has been estimated that around one in eight or nine strokes in people with a history of stroke or transient ischaemic attack (TIA) could be attributed to diabetes (Emerging Risk Factors Collaboration 2010). Management of blood glucose can therefore be regarded as one possible target for stroke prevention.
Description of the intervention
Peroxisome proliferator‐activated receptor gamma (PPAR‐γ) agonists are insulin‐sensitising drugs used for the treatment of hyperglycaemia with insulin resistance. To date, PPAR‐γ agonists such as rosiglitazone and pioglitazone have been widely recommended in the treatment of people with type 2 diabetes (Mooradian 2002). In certain cases PPAR‐γ agonists can be used in combination with insulin or other hypoglycaemic agents. In view of their effect on lowering glucose, PPAR‐γ agonists are believed to be beneficial for stroke prevention. Common adverse events associated with PPAR‐γ agonists include oedema, anaemia, liver dysfunction, and cardiac failure (Fogg 2009). Moreover, an increased risk of mortality and vascular events was found with rosiglitazone compared with pioglitazone in people with diabetes older than 65 years of age (Graham 2010).
How the intervention might work
The mechanisms of hyperglycaemia and stroke have been widely discussed. Blood flow and vascular reactivity can be affected by hyperglycaemia due to the abnormal metabolism of endothelium‐derived nitric oxide (Melikian 2009). Hyperglycaemia is also associated with reduced penumbral salvage in the large‐vessel thromboembolic stroke (Els 2002). Peroxisome proliferator‐activated receptor gamma agonists can therefore prevent these pathological processes by controlling blood glucose. In addition to lowering glucose, PPAR‐γ agonists may also protect against hyperlipidaemia and arteriosclerosis, which are complications of diabetes and risk factors for stroke (Collino 2010; Dasu 2009). Moreover, PPAR‐γ agonists have been shown to reduce inflammation, which may prevent vascular events to some extent (Nakamura 2007).
Why it is important to do this review
Evidence from trials indicates the benefits of aspirin, clopidogrel, ticlopidine, triflusal, and the combination of aspirin and dipyridamole in the secondary prevention of stroke and other vascular events (Costa 2005; De Schryver 2007; Sudlow 2009). Anticoagulants appear to be less effective, at least where the cause is not cardioembolic (Sandercock 2009). Vitamin K antagonists are not more efficacious than antiplatelet therapy (De Schryver 2012). Peroxisome proliferator‐activated receptor gamma agonists have been tested in clinical trials focusing on the prevention of stroke and other vascular events, particularly for people with diabetes. We thus aimed to evaluate the efficacy and safety of PPAR‐γ agonists for preventing recurrent stroke and other vascular events in people with stroke or TIA. To our knowledge, no other systematic review or meta‐analysis on this topic exists in the literature.
Objectives
To assess the efficacy and safety of PPAR‐γ agonists in the secondary prevention of stroke and related vascular events for people with stroke or TIA.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials and excluded quasi‐randomised or confounded studies.
Types of participants
We included studies of people over the age of 18 years with a history of stroke or TIA. We used the definition of TIA as provided in the original publications. We excluded studies of people with diabetes who lacked a clear history of stroke or TIA.
Types of interventions
We included trials comparing PPAR‐γ agonists (including pioglitazone, rosiglitazone, glitazone, troglitazone, netoglitazone, rivoglitazone, ciglitazone, balaglitazone, darglitazone, edaglitazone, englitazone, and lobeglitazone) with placebo, regardless of the length of treatment period and dosage of treatment. We included other concomitant therapies providing they were administered to both the intervention and control groups.
Types of outcome measures
We measured all outcomes at the end of follow‐up.
Primary outcomes
-
The number of participants with recurrent stroke, as defined in the original publications.
-
The number of participants who experienced any adverse events, such as oedema, anaemia, or cardiac failure.
Secondary outcomes
-
The number of participants with serious vascular events, such as myocardial infarction, stroke, or vascular death.
-
The number of deaths due to vascular events.
-
The number of participants with disability due to vascular events.
-
Improvement in quality of life.
-
Insulin sensitivity.
-
Ubiquitin‐proteasome activity in carotid plaques.
Search methods for identification of studies
See the 'Specialized register' section in the Cochrane Stroke Group module. We searched for trials in all languages and arranged for the translation of relevant articles where necessary.
Electronic searches
We searched the Cochrane Stroke Group Trials Register (16 May 2017) and the following electronic bibliographic databases:
-
Cochrane Central Register of Controlled Trials (CENTRAL; 2017, Issue 5) in the Cochrane Library (searched 16 May 2017) (Appendix 1);
-
MEDLINE (Ovid) (1949 to 16 May 2017) (Appendix 2);
-
Embase (Ovid) (1980 to 16 May 2017) (Appendix 3);
-
CINAHL (EBSCO) (Cumulative Index to Nursing and Allied Health Literature; 1982 to 16 May 2017) (Appendix 4);
-
AMED (Ovid) (Allied and Complementary Medicine Database; 1985 to 16 May 2017) (Appendix 5).
We developed all the search strategies with the help of the Cochrane Stroke Group Information Specialist.
We also searched the following ongoing trials registers on 16 May 2017 with keywords including PPAR‐γ agonist, thiazolidinedione, pioglitazone, rosiglitazone, glitazone, troglitazone, netoglitazone, rivoglitazone, ciglitazone, balaglitazone, darglitazone, edaglitazone, englitazone, lobeglitazone, stroke, and TIA:
-
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov/);
-
World Health Organization International Clinical Trials Registry Platform (apps.who.int/trialsearch);
-
ISRCTN registry (www.isrctn.com/);
-
EU Clinical Trials Register (www.clinicaltrialsregister.eu);
-
The Internet Stroke Center Stroke Trials Registry (www.strokecenter.org/trials).
Searching other resources
We searched the following resources with the Chinese equivalents of keywords such as PPAR‐γ agonist, thiazolidinedione, pioglitazone, rosiglitazone, glitazone, troglitazone, netoglitazone, rivoglitazone, ciglitazone, balaglitazone, darglitazone, edaglitazone, englitazone, lobeglitazone, stroke, and TIA:
-
Chinese Clinical Trials Registry (searched on 16 May 2017);
-
CBM‐disc (China Biological Medicine Databases) (1979 to 16 May 2017);
-
CNKI (China National Knowledge Infrastructure) (1979 to 16 May 2017);
-
Chinese MD and DD Dissertations in CNKI (searched on 16 May 2017);
-
CACP (Chinese Academic Conference Papers Database) (1998 to 16 May 2017);
-
CDDB (Chinese Dissertations Database) (1977 to 16 May 2017);
-
Chinese Evidence‐Based Medicine Database (searched on 16 May 2017);
-
CMAC (China Medical Academic Conferences) (1994 to 16 May 2017);
-
CMCC (Chinese Medical Current Contents) (1994 to 16 May 2017);
-
Chinese Science and Technique Journals Database (VIP) (1989 to 16 May 2017);
-
Wanfang Data (www.wanfangdata.com/) (1984 to 16 May 2017).
We also:
-
used Science Citation Index Cited Reference Search for forward tracking of important articles;
-
searched reference lists of relevant reviews and retrieved articles;
-
searched relevant conference proceedings, including the 6th to 25th European Stroke Conference (from 1997 to 2016) and the 4th to 10th World Stroke Congress (from 2000 to 2016);
-
contacted authors where necessary for missing information;
-
contacted the manufacturers (Takeda Pharmaceutical Company and GlaxoSmithKline Pharmaceuticals) for updated information on 16 May 2017.
Data collection and analysis
Selection of studies
Two review authors (JL, LW) independently screened titles and abstracts of the references identified by the search and excluded obviously irrelevant reports. We retrieved the full‐text articles for the remaining references, and the same two review authors independently screened the full‐text articles and identified studies for inclusion, and also identified and recorded reasons for exclusion of the ineligible studies. We resolved any disagreements through discussion or, if required, we consulted an external third party (Professor Si‐yan Zhan (SZ)). We collated multiple reports of the same study so that each study, not each reference, was the unit of interest in the review. We recorded the selection process and completed a PRISMA flow diagram (Figure 1).
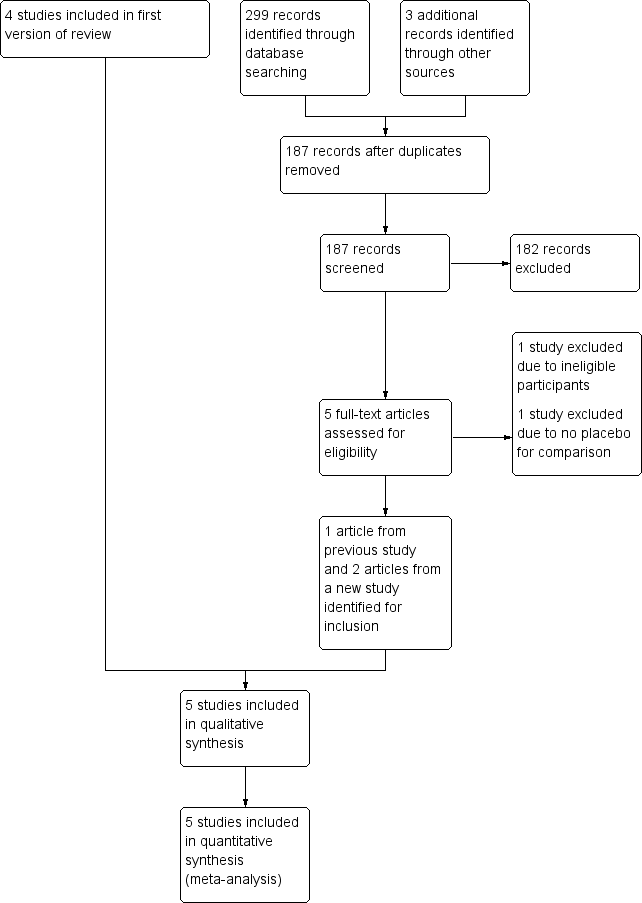
Study flow diagram.
Data extraction and management
Two review authors (JL, LW) independently extracted data from the published reports onto standardised forms, and cross‐checked the data for accuracy. We used checklists to independently record details including methods of generating the randomisation schedule, method of concealment of allocation, blinding of assessors, intention‐to‐treat analysis, adverse events and dropouts for all reasons, important imbalance in prognostic factors, participants (socio‐demographic and related clinical information), interventions (medications and non‐pharmacological interventions), and outcomes. We resolved disagreements with an external third party (SZ).
Assessment of risk of bias in included studies
Two review authors (JL, LW) independently assessed the risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreements by discussion or by involving an external third party (SZ). We assessed the risk of bias according to the following domains.
-
Random sequence generation.
-
Allocation concealment.
-
Blinding of participants and personnel.
-
Blinding of outcome assessment.
-
Incomplete outcome data.
-
Selective outcome reporting.
-
Other bias.
We graded the risk of bias for each domain as high, low, or unclear and provided information from the study report together with a justification for our judgement in the 'Risk of bias' tables.
Measures of treatment effect
We expected the randomised controlled trials to measure dichotomous data. We expressed dichotomised data as risk ratios (RRs) with their 95% confidence intervals (CI). If a trial (or group within a trial) reported no adverse events or dropouts, we calculated risk differences (RDs) instead of RRs with 95% CI. We entered and analysed data using Review Manager 5 (RevMan 2014).
Unit of analysis issues
We dealt with any unit of analysis issues according to the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Dealing with missing data
We planned to contact the authors of the studies for further details if any data were missing or to establish the characteristics of unpublished trials. According to the intention‐to‐treat principle, all randomised participants should be included. We considered different scenarios (best‐case and worst‐case) to account for missing data.
Assessment of heterogeneity
We used the I² statistic to measure heterogeneity among the trials in each analysis, considering I² values over 50% as suggestive of substantial heterogeneity.
Assessment of reporting biases
We planned to use funnel plots to examine potential publication bias if there was a sufficient number of trials (Egger 1997).
Data synthesis
Where we considered studies to be sufficiently similar, we conducted a meta‐analysis by pooling the appropriate data using Review Manager 5 (RevMan 2014). We expressed dichotomised data as RRs with their 95% CI. If a trial (or group within a trial) reported no adverse events or dropouts, we calculated RDs instead of RRs with 95% CI. We calculated the overall effects using a random‐effects model regardless of the level of heterogeneity. We provided a descriptive summary of the results when substantial heterogeneity between the studies prevented us from combining outcome data.
'Summary of findings' and GRADE
We included each of the main analyses in a 'Summary of findings' table (summary of findings Table for the main comparison). We determined the quality of the evidence using the GRADE approach and downgraded evidence due to the presence of high risk of bias in at least one study, indirectness of the evidence, unexplained heterogeneity or inconsistency, imprecision of results, high probability of publication bias. We downgraded evidence by one level if we considered the limitation to be serious and by two levels if very serious.
Subgroup analysis and investigation of heterogeneity
We intended to undertake subgroup analyses according to the age and ethnicity of participants, TIA definition, different PPAR‐γ agonists, and dosage and duration of treatment. We intended to use the Chi² test to examine the significance of differences between subgroups.
Sensitivity analysis
We analysed sensitivity by assessing the robustness of results in fixed‐effect versus random‐effects models, and studies at high risk versus low risk of bias. We also examined potential sources of methodological heterogeneity.
Results
Description of studies
Results of the search
Our original review included four studies. On re‐running the searches in 16 May 2017, we identified 187 papers after de‐duplicating the results (Figure 1). We acquired and screened the full texts of five articles. Three articles (one article from a previous study and two articles from a new study) met the inclusion criteria. We therefore included a total of five studies with 16 articles. Agreement between the review authors on exclusion was 100%.
Included studies
In accordance with the inclusion criteria, we included five studies with 5039 participants. IRIS investigated the efficacy of pioglitazone in participants with insulin resistance and ischaemic stroke or TIA no less than 14 days and no more than six months before randomisation. J‐SPIRIT tested the effect of pioglitazone on the reduction of recurrent stroke in participants with abnormal glucose metabolisms and insulin resistance after ischaemic stroke. Kernan 2003 evaluated the effect of pioglitazone in improving insulin sensitivity among non‐diabetic patients with a recent TIA or non‐disabling ischaemic stroke. Marfella 2006 investigated the effect of rosiglitazone in participants with symptomatic carotid stenosis by testing ubiquitin‐proteasome activity in carotid plaques. PROactive focused on the efficacy and safety of pioglitazone on the reduction of stroke recurrence and related vascular events in participants with type 2 diabetes. The details of the included studies are provided in the Characteristics of included studies table.
Excluded studies
We excluded 14 studies after full‐text evaluation (CIMT Trial; ISRCTN54951661; Erdmann 2015; Forst 2008; Hedblad 2007; Koshiyama 2001; Lincoff 2014; Meisner 2006; Sidhu 2004; Tanaka 2015; TART; NCT00879970; TRIPOD; Varghese 2009). The reasons for exclusion are provided in the Characteristics of excluded studies table.
Risk of bias in included studies
Information regarding risk of bias is provided in Figure 2 and Figure 3. The data for J‐SPIRIT were only released in abstracts, therefore insufficient information was available for us to judge the risk of bias.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Two studies clearly stated the methods of randomisation and allocation concealment (IRIS; Kernan 2003). Information for the remaining studies was insufficient to make a judgement of high or low risk of bias, therefore we judged selection bias as at unclear risk of bias.
Blinding
Information regarding blinding was insufficient in two studies (J‐SPIRIT; PROactive). In Kernan 2003 and IRIS, information about performance bias and detection bias was provided, while only information about detection bias was reported in Marfella 2006. We therefore assessed this as at unclear risk of bias.
Incomplete outcome data
J‐SPIRIT did not report on completeness of outcome data. No dropouts were reported in Kernan 2003 or Marfella 2006. In IRIS, a total of 227 participants (5.9%) withdrew consent and 99 (2.6%) were lost to follow‐up; we assessed this study as at low risk of bias. In PROactive, 882 of 984 participants (90%) completed the final visit: 439/486 (90%) in the pioglitazone group and 443/498 (89%) in the placebo group. We assessed this as at high risk of bias.
Selective reporting
With the exception of J‐SPIRIT, all prespecified outcomes were reported in the included trials. We therefore assessed this as at low risk of bias.
Other potential sources of bias
The role of financial support was clearly stated in Kernan 2003, therefore we judged this study as at low risk of bias. We judged the remaining four studies as at unclear risk of bias (IRIS; J‐SPIRIT; Marfella 2006; PROactive).
Effects of interventions
Primary outcome measures
Number of participants with recurrent stroke
Three studies with 4979 participants reported the number of recurrent strokes at the end of the study: 156/2483 (6%) participants in the PPAR‐γ agonist group and 212/2496 (8%) participants in the placebo group (risk ratio (RR) 0.66, 95% confidence interval (CI) 0.44 to 0.99; moderate‐quality evidence) (Analysis 1.1) (IRIS; J‐SPIRIT; PROactive). When we conducted a sensitivity analysis with a fixed‐effect model, the results did not vary substantially (RR 0.74, 95% CI 0.61 to 0.90).
Number of participants who experienced any adverse events
Three studies reported the number of participants with any adverse events (Kernan 2003; Marfella 2006; PROactive). Adverse events were experienced by 247/516 (48%) and 260/528 (49%) participants in the PPAR‐γ agonists and placebo groups, respectively (risk difference (RD) 10%, 95% CI ‐8% to 28%; low‐quality evidence), with strong heterogeneity (test for heterogeneity I² = 86%) (Analysis 1.2). When we conducted a sensitivity analysis with a fixed‐effect model, the result was: RD ‐1%, 95% CI ‐7% to 5%. The RDs of the random‐effects and fixed‐effect models were essentially the same. In Kernan 2003, certain adverse events such as nausea, oedema, muscle aches, sore throat, and dizziness were more common in the pioglitazone group. In Marfella 2006, no clinical events were reported in either group during the study. The proportion of participants who experienced adverse events was the highest in PROactive, with definitions of adverse events including: resulting in death, life‐threatening, needing or prolonging in‐patient admission, resulting in persistent or significant disability, or needing intervention to prevent any of the above. As a result, heart failure requiring hospitalisation was reported in 31 (6.4%) participants in the pioglitazone group versus 20 (4.0%) in the placebo group (P = 0.09). Fatal heart failure was reported in 6 (1.2%) participants in the pioglitazone group versus 4 (0.8%) participants in the placebo group (P = 0.50). IRIS only reported the number of each adverse event. In general, participants in the pioglitazone group had more weight gain, oedema, shortness of breath, and bone fractures than did participants in the placebo group.
Secondary outcome measures
Number of participants with serious vascular events
Only one study reported the number of participants with serious vascular events (PROactive). For the total events of all‐cause death, non‐fatal myocardial infarction, acute coronary syndrome and cardiac intervention, stroke, major leg amputation, or bypass surgery or leg revascularisation, there were 98/486 (20%) and 126/498 (25%) participants in the PPAR‐γ agonists and placebo groups, respectively (RR 0.80, 95% CI 0.63 to 1.01; low‐quality evidence). For the total events of all‐cause death, non‐fatal myocardial infarction or non‐fatal stroke, there were 76/486 (16%) and 98/498 (20%) participants in the PPAR‐γ agonists and placebo groups, respectively (RR 0.79, 95% CI 0.61 to 1.04; low‐quality evidence). Pioglitazone reduced fatal or non‐fatal stroke (RR 0.54, 95% CI 0.35 to 0.85; low‐quality evidence) and total events of cardiovascular death, non‐fatal myocardial infarction or non‐fatal stroke (RR 0.73, 95% CI 0.54 to 0.99; low‐quality evidence).
Number of deaths due to vascular events
None of the studies reported the number of deaths due to vascular events. However, PROactive reported all‐cause mortality: 46/486 (9%) and 49/498 (10%) deaths in the PPAR‐γ agonists and placebo groups, respectively (RR 0.96, 95% CI 0.66 to 1.41).
Number of participants with disability due to vascular events
None of the studies reported the number of participants with disability due to vascular events.
Improvement in quality of life
None of the studies reported improvement in quality of life.
Insulin sensitivity
In Kernan 2003, insulin sensitivity was measured with the composite insulin sensitivity index. The change in the composite index was 1.2 ± 0.6 (mean ± standard deviation) in the pioglitazone group and ‐0.1 ± 0.6 in the placebo group (P = 0.0003). The C‐reactive protein concentration declined from 0.30 to 0.20 mg/L in the pioglitazone group, but increased from 0.41 to 0.45 mg/L in the placebo group (P = 0.06).
Ubiquitin‐proteasome activity in carotid plaques
Marfella 2006 examined the ubiquitin‐proteasome activity in carotid plaques. Compared with the placebo group, symptomatic carotid plaques in the rosiglitazone group showed fewer inflammatory cells (P < 0.01); less ubiquitin (322 ± 79 ng/mg in the rosiglitazone group and 468.7 ± 89 ng/mg in the placebo group, P < 0.01), proteasome 20S (46.8 ± 10 pmol/mg in the rosiglitazone group and 79.8 ± 25 pmol/mg in the placebo group, P < 0.01), and nuclear factor kappa B (NFkB) (P < 0.01); less nitrotyrosine (2.2 ± 0.21 nmol/pg in the rosiglitazone group and 3.5 ± 0.42 nmol/pg in the placebo group, P < 0.01) and superoxide anion production (3.57 ± 1.1 pmol/L in the rosiglitazone group and 6.26 ± 1.4 pmol/L in the placebo group, P < 0.01); and more collagen content (P < 0.01), suggesting greater plaque stabilisation.
Discussion
Summary of main results
Five studies with 5039 participants met the inclusion criteria. Four of these studies evaluated the effect of pioglitazone versus placebo (IRIS; J‐SPIRIT; Kernan 2003; PROactive); the fifth focused on rosiglitazone versus placebo (Marfella 2006). Three studies evaluated the number of participants with recurrent stroke (IRIS; J‐SPIRIT; PROactive), where PPAR‐γ agonists reduced the recurrence of stroke compared with placebo (RR 0.66, 95% CI 0.44 to 0.99; moderate‐quality evidence). Moreover, PPAR‐γ agonists reduced total events of cardiovascular death, non‐fatal myocardial infarction or non‐fatal stroke and improved insulin sensitivity and stabilisation of carotid plaques (Kernan 2003; Marfella 2006; PROactive). Regarding safety, evidence was inconclusive of any difference in reported adverse events in the PPAR‐γ agonists group versus the placebo group (RD 10%, 95% CI ‐8% to 28%; low‐quality evidence) in three studies (Kernan 2003; Marfella 2006; PROactive).
Overall completeness and applicability of evidence
All participants had a past history of stroke or TIA; however, none of the randomised controlled trials precisely described stroke type or diagnosis criteria. Kernan 2003 evaluated non‐diabetic participants, IRIS and J‐SPIRIT participants with an abnormal glucose metabolism and insulin resistance, PROactive participants with type 2 diabetes, and Marfella 2006 participants with carotid plaques. The clinical heterogeneity of the participants in the included studies could potentially induce heterogeneity in the results of the meta‐analysis. Four studies evaluated pioglitazone versus placebo (IRIS; J‐SPIRIT; Kernan 2003; PROactive), and one study evaluated rosiglitazone versus placebo (Marfella 2006). Regarding outcomes, three studies recorded recurrent stroke (IRIS; J‐SPIRIT; PROactive). The other two studies investigated insulin sensitivity and ubiquitin‐proteasome activity in carotid plaques, respectively (Kernan 2003; Marfella 2006). Due to insufficient data, we did not undertake any subgroup analyses.
Quality of the evidence
We judged IRIS and Kernan 2003 as of high quality with a low risk of bias for all domains. J‐SPIRIT was only published as abstracts, therefore information was insufficient to judge. We assessed Marfella 2006 and PROactive as at unclear risk of bias forthe method of randomisation, allocation concealment, and blinding., and PROactive as at high risk of bias for incomplete outcome data. In summary, the methodological limitations of the included studies should be considered when interpreting the results. In addition, the sample sizes in Kernan 2003 and Marfella 2006 were relatively small.
Potential biases in the review process
We performed the search strategy as per protocol and identified five completed studies. However, we cannot be certain that we have not missed other unpublished studies. Our contact with relevant authors and manufacturers yielded no additional information. In preparing this review, we independently screened trials for inclusion, extracted data, and assessed the quality of included trials to minimise potential biases. We used the RD as a way of addressing studies with zero events. The event rates of the number of participants with adverse events reported, based on the aggregate event rates for the two groups, were 48% versus 49%, a difference of 1%. With the random‐effects model, the outlying estimate from Kernan 2003 effectively pushed the RD out towards 10%. This might well explain why the difference in the aggregate data of 1% did not translate to a RD of 10% (the fixed‐effect result was much closer to the aggregate events). We found no other potential biases.
Agreements and disagreements with other studies or reviews
To our knowledge, this is the first systematic review of this intervention in the field of secondary prevention of stroke and related vascular events for people with stroke or TIA.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
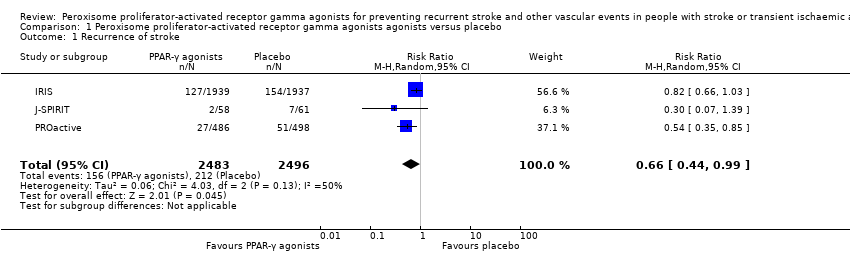
Comparison 1 Peroxisome proliferator‐activated receptor gamma agonists agonists versus placebo, Outcome 1 Recurrence of stroke.

Comparison 1 Peroxisome proliferator‐activated receptor gamma agonists agonists versus placebo, Outcome 2 Reported adverse events.
| Peroxisome proliferator‐activated receptor gamma (PPAR‐γ) agonists for preventing recurrent stroke and other vascular events in people with stroke or transient ischaemic attack | ||||||
| Patient or population: people with stroke or transient ischaemic attack Settings: inpatients Intervention: PPAR‐γ agonists Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | PPAR‐γ agonists | |||||
| Recurrence of stroke Follow‐up: 25 to 57.6 months | 85 per 1000 | 56 per 1000 (37 to 84) | RR 0.66 (0.44 to 0.99) | 4979 | ⊕⊕⊕⊝ | |
| Reported adverse events Follow‐up: 3 to 34.5 months | 492 per 1000 | 502 per 1000 (412 to 530) | RD 0.10 (‐0.08 to 0.28) | 1044 | ⊕⊕⊝⊝ | |
| Serious vascular events | For the total events of all‐cause death, non‐fatal myocardial infarction, acute coronary syndrome and cardiac intervention, stroke, major leg amputation, or bypass surgery or leg revascularisation, there were 126 of 498 (25%). For the total events of all‐cause death, non‐fatal myocardial infarction or non‐fatal stroke, there were 98 of 498 (20%). | For the total events of all‐cause death, non‐fatal myocardial infarction, acute coronary syndrome and cardiac intervention, stroke, major leg amputation, or bypass surgery or leg revascularisation, there were 98 of 486 (20%). For the total events of all‐cause death, non‐fatal myocardial infarction or non‐fatal stroke, there were 76 of 486 (16%). | For the total events of all‐cause death, non‐fatal myocardial infarction, acute coronary syndrome and cardiac intervention, stroke, major leg amputation, or bypass surgery or leg revascularisation (RR 0.80, 95% CI 0.63 to 1.01). For the total events of all‐cause death, non‐fatal myocardial infarction or non‐fatal stroke (RR 0.79, 95% CI 0.61 to 1.04). Pioglitazone reduced fatal or non‐fatal stroke (RR 0.54, 95% CI 0.35 to 0.85) and total events of cardiovascular death, non‐fatal myocardial infarction or non‐fatal stroke (RR 0.73, 95% CI 0.54 to 0.99). | 984 | ⊕⊕⊝⊝ | Pioglitazone reduced fatal or non‐fatal stroke and total events of cardiovascular death, non‐fatal myocardial infarction or non‐fatal stroke. |
| Deaths due to vascular events | Not reported | Not reported | — | — | — | |
| Disability due to vascular events | Not reported | Not reported | — | — | — | |
| Improvement in quality of life | Not reported | Not reported | — | — | — | |
| Insulin sensitivity Assessed with composite insulin sensitivity index | The change in the composite index was ‐0.1 ± 0.6. The C‐reactive protein concentration increased from 0.41 to 0.45 mg/L. | The change in the composite index was 1.2 ± 0.6. The C‐reactive protein concentration declined from 0.30 to 0.20 mg/L. | The change in the composite index was significantly increased in the pioglitazone group in comparison with the placebo group (P = 0.0003). | 20 | ⊕⊕⊝⊝ | |
| Ubiquitin‐proteasome activity in carotid plaques | Ubiquitin 468.7 ± 89 ng/mg; proteasome 20S 79.8 ± 25 pmol/mg; nitrotyrosine 3.5 ± 0.42 nmol/pg; superoxide anion production 6.26 ± 1.4 pmol/L | Ubiquitin 322 ± 79 ng/mg; proteasome 20S 46.8 ± 10 pmol/mg; nitrotyrosine 2.2 ± 0.21 nmol/pg; superoxide anion production 3.57 ± 1.1 pmol/L | Compared with the placebo group, symptomatic carotid plaques in the rosiglitazone group showed fewer inflammatory cells (P < 0.01) with less ubiquitin (P < 0.01), proteasome 20S (P < 0.01), nuclear factor kappa B (P < 0.01), nitrotyrosine (P < 0.01), superoxide anion production (P < 0.01), and more collagen content (P < 0.01). | 40 | ⊕⊕⊝⊝ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded one level due to risk of bias. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Recurrence of stroke Show forest plot | 3 | 4979 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.44, 0.99] |
| 2 Reported adverse events Show forest plot | 3 | 1044 | Risk Difference (M‐H, Random, 95% CI) | 0.10 [‐0.08, 0.28] |

