Peroxisome proliferator‐activated receptor gamma agonists for preventing recurrent stroke and other vascular events in people with stroke or transient ischaemic attack
Information
- DOI:
- https://doi.org/10.1002/14651858.CD010693.pub4Copy DOI
- Database:
-
- Cochrane Database of Systematic Reviews
- Version published:
-
- 02 December 2017see what's new
- Type:
-
- Intervention
- Stage:
-
- Review
- Cochrane Editorial Group:
-
Cochrane Stroke Group
- Copyright:
-
- Copyright © 2017 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Article metrics
Altmetric:
Cited by:
Authors
Contributions of authors
Liu J and Wang L conceived and developed the review. Liu J was in charge of searching, identifying and assessing studies, data extraction and analyses, and writing the draft of the review. Wang L gave general advice on the review, identified and assessed studies, extracted data, and revised the draft. Liu J supervised the quality of the methodology and statistics. Liu J will be responsible for future review updates.
Declarations of interest
Jia Liu: none known.
Lu‐Ning Wang: none known.
Acknowledgements
The authors acknowledge the help provided by the Cochrane Stroke Group.
Version history
| Published | Title | Stage | Authors | Version |
| 2023 Jan 10 | Peroxisome proliferator‐activated receptor gamma agonists for preventing recurrent stroke and other vascular events in people with stroke or transient ischaemic attack | Review | Jia Liu, Lu-Ning Wang | |
| 2019 Oct 09 | Peroxisome proliferator‐activated receptor gamma agonists for preventing recurrent stroke and other vascular events in people with stroke or transient ischaemic attack | Review | Jia Liu, Lu‐Ning Wang | |
| 2017 Dec 02 | Peroxisome proliferator‐activated receptor gamma agonists for preventing recurrent stroke and other vascular events in people with stroke or transient ischaemic attack | Review | Jia Liu, Lu‐Ning Wang | |
| 2015 Oct 29 | Peroxisome proliferator‐activated receptor gamma agonists for preventing recurrent stroke and other vascular events in patients with stroke or transient ischaemic attack | Review | Jia Liu, Lu‐Ning Wang | |
| 2014 Jan 08 | Peroxisome proliferator‐activated receptor gamma agonists for preventing recurrent stroke and other vascular events in patients with stroke or transient ischaemic attack | Review | Jia Liu, Lu‐Ning Wang | |
| 2013 Aug 02 | Peroxisome proliferator‐activated receptor gamma agonists for preventing recurrent stroke and other vascular events in patients with stroke or transient ischaemic attack | Protocol | Jia Liu, Lu‐Ning Wang | |
Differences between protocol and review
Some of the subgroup and sensitivity analyses specified were not conducted due to insufficient data.
We added insulin sensitivity and ubiquitin‐proteasome activity in carotid plaques as secondary outcomes, which were not included in the protocol.
We calculated the overall effects using a random‐effects model regardless of the level of heterogeneity. If heterogeneity was 0%, then the results using a random‐effects model would be the same as the results from a fixed‐effect model.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
- Cardiovascular Diseases [mortality, prevention & control];
- Carotid Artery Diseases [enzymology];
- Hypoglycemic Agents [adverse effects, therapeutic use];
- Insulin Resistance;
- Ischemic Attack, Transient [*prevention & control];
- Myocardial Infarction [mortality, prevention & control];
- PPAR gamma [*agonists];
- Pioglitazone;
- Proteasome Endopeptidase Complex [metabolism];
- Randomized Controlled Trials as Topic;
- Recurrence;
- Rosiglitazone;
- Secondary Prevention [*methods];
- Stroke [mortality, *prevention & control];
- Thiazolidinediones [adverse effects, *therapeutic use];
- Ubiquitin [metabolism];
Medical Subject Headings Check Words
Humans;
PICOs

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
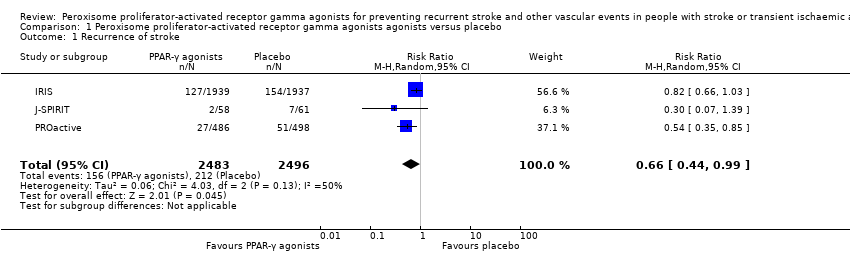
Comparison 1 Peroxisome proliferator‐activated receptor gamma agonists agonists versus placebo, Outcome 1 Recurrence of stroke.

Comparison 1 Peroxisome proliferator‐activated receptor gamma agonists agonists versus placebo, Outcome 2 Reported adverse events.
| Peroxisome proliferator‐activated receptor gamma (PPAR‐γ) agonists for preventing recurrent stroke and other vascular events in people with stroke or transient ischaemic attack | ||||||
| Patient or population: people with stroke or transient ischaemic attack Settings: inpatients Intervention: PPAR‐γ agonists Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | PPAR‐γ agonists | |||||
| Recurrence of stroke Follow‐up: 25 to 57.6 months | 85 per 1000 | 56 per 1000 (37 to 84) | RR 0.66 (0.44 to 0.99) | 4979 | ⊕⊕⊕⊝ | |
| Reported adverse events Follow‐up: 3 to 34.5 months | 492 per 1000 | 502 per 1000 (412 to 530) | RD 0.10 (‐0.08 to 0.28) | 1044 | ⊕⊕⊝⊝ | |
| Serious vascular events | For the total events of all‐cause death, non‐fatal myocardial infarction, acute coronary syndrome and cardiac intervention, stroke, major leg amputation, or bypass surgery or leg revascularisation, there were 126 of 498 (25%). For the total events of all‐cause death, non‐fatal myocardial infarction or non‐fatal stroke, there were 98 of 498 (20%). | For the total events of all‐cause death, non‐fatal myocardial infarction, acute coronary syndrome and cardiac intervention, stroke, major leg amputation, or bypass surgery or leg revascularisation, there were 98 of 486 (20%). For the total events of all‐cause death, non‐fatal myocardial infarction or non‐fatal stroke, there were 76 of 486 (16%). | For the total events of all‐cause death, non‐fatal myocardial infarction, acute coronary syndrome and cardiac intervention, stroke, major leg amputation, or bypass surgery or leg revascularisation (RR 0.80, 95% CI 0.63 to 1.01). For the total events of all‐cause death, non‐fatal myocardial infarction or non‐fatal stroke (RR 0.79, 95% CI 0.61 to 1.04). Pioglitazone reduced fatal or non‐fatal stroke (RR 0.54, 95% CI 0.35 to 0.85) and total events of cardiovascular death, non‐fatal myocardial infarction or non‐fatal stroke (RR 0.73, 95% CI 0.54 to 0.99). | 984 | ⊕⊕⊝⊝ | Pioglitazone reduced fatal or non‐fatal stroke and total events of cardiovascular death, non‐fatal myocardial infarction or non‐fatal stroke. |
| Deaths due to vascular events | Not reported | Not reported | — | — | — | |
| Disability due to vascular events | Not reported | Not reported | — | — | — | |
| Improvement in quality of life | Not reported | Not reported | — | — | — | |
| Insulin sensitivity Assessed with composite insulin sensitivity index | The change in the composite index was ‐0.1 ± 0.6. The C‐reactive protein concentration increased from 0.41 to 0.45 mg/L. | The change in the composite index was 1.2 ± 0.6. The C‐reactive protein concentration declined from 0.30 to 0.20 mg/L. | The change in the composite index was significantly increased in the pioglitazone group in comparison with the placebo group (P = 0.0003). | 20 | ⊕⊕⊝⊝ | |
| Ubiquitin‐proteasome activity in carotid plaques | Ubiquitin 468.7 ± 89 ng/mg; proteasome 20S 79.8 ± 25 pmol/mg; nitrotyrosine 3.5 ± 0.42 nmol/pg; superoxide anion production 6.26 ± 1.4 pmol/L | Ubiquitin 322 ± 79 ng/mg; proteasome 20S 46.8 ± 10 pmol/mg; nitrotyrosine 2.2 ± 0.21 nmol/pg; superoxide anion production 3.57 ± 1.1 pmol/L | Compared with the placebo group, symptomatic carotid plaques in the rosiglitazone group showed fewer inflammatory cells (P < 0.01) with less ubiquitin (P < 0.01), proteasome 20S (P < 0.01), nuclear factor kappa B (P < 0.01), nitrotyrosine (P < 0.01), superoxide anion production (P < 0.01), and more collagen content (P < 0.01). | 40 | ⊕⊕⊝⊝ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded one level due to risk of bias. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Recurrence of stroke Show forest plot | 3 | 4979 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.44, 0.99] |
| 2 Reported adverse events Show forest plot | 3 | 1044 | Risk Difference (M‐H, Random, 95% CI) | 0.10 [‐0.08, 0.28] |