Peroxisome proliferator‐activated receptor gamma agonists for preventing recurrent stroke and other vascular events in people with stroke or transient ischaemic attack
References
References to studies included in this review
References to studies excluded from this review
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Jump to:
| Methods | A multicentre, double‐blind, placebo‐controlled clinical trial to test the effectiveness of pioglitazone for insulin resistant, non‐diabetic patients with a recent ischaemic stroke or TIA | |
| Participants | People aged at least 40 years with qualifying ischaemic stroke or TIA during the 6 months before randomisation, as well as insulin resistance, defined as a value of more than 3.0 on the homeostasis model assessment of insulin resistance index. 3876 participants were randomised. | |
| Interventions | Pioglitazone or matching placebo. The initial dose was 15 mg of pioglitazone daily or placebo. The dose was increased to 2 pills daily (30 mg of pioglitazone or placebo) at 4 weeks and to 3 pills daily (45 mg of pioglitazone or placebo) at 8 weeks. At 12 weeks, participants were started on 1 x 45 mg pioglitazone tablet or placebo tablet daily. | |
| Outcomes | Fatal or non‐fatal stroke; fatal or non‐fatal myocardial infarction; heart failure resulting in hospitalisation or death; death from any cause; diabetes; and cognitive decline | |
| Notes | Participants were contacted every 4 months with a median follow‐up of 4.8 years. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed using a random permuted block design with variable block sizes stratified by site. |
| Allocation concealment (selection bias) | Low risk | To conceal the allocation sequence, randomisation lists were kept only at the central pharmacy and the statistical centre. |
| Blinding of participants and personnel (performance bias) | Low risk | The Investigational Drug Service prepared medication bottles, including starter supplies, which were stored at the research sites. At the baseline visit, a structured interview was administered and the starter bottle with the participant's assigned randomisation number was dispensed. |
| Blinding of outcome assessment (detection bias) | Low risk | Reviewers were blinded to treatment allocation. |
| Incomplete outcome data (attrition bias) | Low risk | During a median follow‐up of 4.8 years, a total of 227 participants (5.9%) withdrew consent and 99 participants (2.6%) were lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported. |
| Other bias | Unclear risk | This study was supported by the National Institute of Neurological Disorders and Stroke (NINDS) and monitored by an independent data and safety monitoring board appointed by NINDS. Pioglitazone and placebo were provided by Takeda Pharmaceuticals International. |
| Methods | A multicentre, randomised, double‐blind, placebo‐controlled trial to test the effect of pioglitazone on the reduction of recurrent stroke in people with abnormal glucose metabolism and insulin resistance after ischaemic stroke | |
| Participants | People aged 35 to 85 years with symptomatic ischaemic stroke and no history of diabetes and no evidence of diabetes by initial blood test were included. 119 eligible people from 3 hospitals in Tokyo or neighbouring cities in Japan were randomised. | |
| Interventions | Pioglitazone or matching placebo | |
| Outcomes | Recurrence of stroke; recurrence of ischaemic stroke | |
| Notes | Mean observation periods were 25 ± 19.9 months in the pioglitazone group and 30 ± 16 months in the control group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of random sequence generation was not described. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information to judge |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information to judge |
| Incomplete outcome data (attrition bias) | Unclear risk | Insufficient information to judge |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to judge |
| Other bias | Unclear risk | Information about financial support was not provided. |
| Methods | A randomised, double‐blind, placebo‐controlled trial to test the effect of pioglitazone compared with placebo for improving insulin sensitivity among non‐diabetic patients with a recent TIA or non‐disabling ischaemic stroke | |
| Participants | Non‐diabetic men and women aged > 45 years with TIA or non‐disabling ischaemic stroke were included. 20 eligible patients from 3 hospitals were randomised as 1:1 into the trial. | |
| Interventions | Pioglitazone 45 mg per day or placebo was given for 3 months. | |
| Outcomes | Mean proportional changes in insulin sensitivity; mean C‐reactive protein concentration; adverse events | |
| Notes | A repeated oral glucose tolerance test was done at the endpoint of the 3 months of therapy. The mean age was 66 years among participants assigned to pioglitazone and 67 years among participants assigned to placebo. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A master schedule of computer‐generated random treatment assignments (placebo or pioglitazone) was stored at the investigational pharmacy at Yale‐New Haven hospital. |
| Allocation concealment (selection bias) | Low risk | After screening for eligibility, a research associate contacted the investigational pharmacist, who assigned the participant to the next available treatment as specified by the master schedule. |
| Blinding of participants and personnel (performance bias) | Low risk | Participants were blinded to treatment assignment throughout the study. |
| Blinding of outcome assessment (detection bias) | Low risk | Research staff and investigators were blinded to treatment assignment throughout the study. |
| Incomplete outcome data (attrition bias) | Low risk | No participant permanently discontinued the treatment. |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported. |
| Other bias | Low risk | The study was funded by an investigator‐initiated grant from Takeda Pharmaceuticals North America. Takeda had no involvement in data collection, data analysis, or report composition. By contract, Takeda could not suppress publication of this report. |
| Methods | A randomised, placebo‐controlled trial | |
| Participants | 40 people who presented with symptoms of cerebral ischaemic attack were included and randomised as 1:1 into the trial. | |
| Interventions | Rosiglitazone 8 mg per day or placebo was given for 4 months. | |
| Outcomes | Ubiquitin‐proteasome activity | |
| Notes | We did not include 38 people with asymptomatic carotid stenosis in this review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of random sequence generation was not described. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information to judge |
| Blinding of outcome assessment (detection bias) | Low risk | The specimens were analysed by an expert pathologist blinded to the participant's diagnosis. |
| Incomplete outcome data (attrition bias) | Low risk | No participant in either group discontinued the treatment. |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported. |
| Other bias | Unclear risk | Information about financial support was not provided. |
| Methods | A multicentre, randomised, double‐blind, placebo‐controlled trial | |
| Participants | People aged 35 to 75 years with type 2 diabetes and a previous stroke (6 months before randomisation) were included. | |
| Interventions | Pioglitazone titration from 15 mg to 45 mg per day depending on tolerability or placebo was given until the first occurrence of any of the events described in the primary outcomes. The mean duration was 34.5 months. | |
| Outcomes | Primary outcomes: all‐cause mortality, non‐fatal myocardial infarction (including silent myocardial infarction), stroke, acute coronary syndrome, cardiac intervention (including coronary artery bypass graft surgery or percutaneous coronary intervention), leg revascularisation, and amputation above the ankle Secondary outcomes: all‐cause death, non‐fatal myocardial infarction or non‐fatal stroke; adverse events | |
| Notes | We did not include data for participants without previous stroke. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of random sequence generation was not described. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information to judge |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information to judge |
| Incomplete outcome data (attrition bias) | High risk | 882/984 (90%) participants completed the final visit: 439/486 (90%) in the pioglitazone group and 443/498 (89%) in the placebo group. Intention‐to‐treat analysis was used. |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported. |
| Other bias | Unclear risk | This study was funded by Takeda Pharmaceuticals and Eli Lilly, and designed by the international steering committee, who also approved the protocol and amendments. The sponsors had 2 representatives on the international steering committee; the same 2 were also members of the executive committee. Data analysis, data interpretation, and writing of the report was done by the executive committee, with contributions from the international steering committee, the data and safety monitoring committee, and the endpoint adjudication committee. All the authors had full access to all the data in the study and had final responsibility for the decision to submit for publication. |
TIA: transient ischaemic attack
Characteristics of excluded studies [ordered by study ID]
Jump to:
| Study | Reason for exclusion |
| The intervention was not PPAR‐γ agonist. | |
| The participants were not eligible. | |
| There was no subgroup of stroke patients for whom separate results were available. | |
| The participants were not eligible. | |
| The participants were not eligible. | |
| The participants were not eligible. | |
| The participants were not eligible. | |
| The participants were not eligible. | |
| There was no subgroup of stroke patients for whom separate results were available. | |
| The participants were not eligible. | |
| There was no placebo for comparison. | |
| The participants were not eligible. | |
| The participants were not eligible. | |
| The participants were not eligible. |
PPAR‐γ: peroxisome proliferator‐activated receptor gamma
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Recurrence of stroke Show forest plot | 3 | 4979 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.44, 0.99] |
| Analysis 1.1 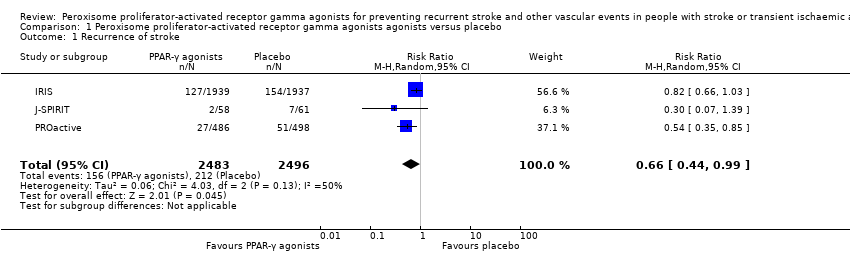 Comparison 1 Peroxisome proliferator‐activated receptor gamma agonists agonists versus placebo, Outcome 1 Recurrence of stroke. | ||||
| 2 Reported adverse events Show forest plot | 3 | 1044 | Risk Difference (M‐H, Random, 95% CI) | 0.10 [‐0.08, 0.28] |
| Analysis 1.2  Comparison 1 Peroxisome proliferator‐activated receptor gamma agonists agonists versus placebo, Outcome 2 Reported adverse events. | ||||

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Comparison 1 Peroxisome proliferator‐activated receptor gamma agonists agonists versus placebo, Outcome 1 Recurrence of stroke.

Comparison 1 Peroxisome proliferator‐activated receptor gamma agonists agonists versus placebo, Outcome 2 Reported adverse events.
| Peroxisome proliferator‐activated receptor gamma (PPAR‐γ) agonists for preventing recurrent stroke and other vascular events in people with stroke or transient ischaemic attack | ||||||
| Patient or population: people with stroke or transient ischaemic attack Settings: inpatients Intervention: PPAR‐γ agonists Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | PPAR‐γ agonists | |||||
| Recurrence of stroke Follow‐up: 25 to 57.6 months | 85 per 1000 | 56 per 1000 (37 to 84) | RR 0.66 (0.44 to 0.99) | 4979 | ⊕⊕⊕⊝ | |
| Reported adverse events Follow‐up: 3 to 34.5 months | 492 per 1000 | 502 per 1000 (412 to 530) | RD 0.10 (‐0.08 to 0.28) | 1044 | ⊕⊕⊝⊝ | |
| Serious vascular events | For the total events of all‐cause death, non‐fatal myocardial infarction, acute coronary syndrome and cardiac intervention, stroke, major leg amputation, or bypass surgery or leg revascularisation, there were 126 of 498 (25%). For the total events of all‐cause death, non‐fatal myocardial infarction or non‐fatal stroke, there were 98 of 498 (20%). | For the total events of all‐cause death, non‐fatal myocardial infarction, acute coronary syndrome and cardiac intervention, stroke, major leg amputation, or bypass surgery or leg revascularisation, there were 98 of 486 (20%). For the total events of all‐cause death, non‐fatal myocardial infarction or non‐fatal stroke, there were 76 of 486 (16%). | For the total events of all‐cause death, non‐fatal myocardial infarction, acute coronary syndrome and cardiac intervention, stroke, major leg amputation, or bypass surgery or leg revascularisation (RR 0.80, 95% CI 0.63 to 1.01). For the total events of all‐cause death, non‐fatal myocardial infarction or non‐fatal stroke (RR 0.79, 95% CI 0.61 to 1.04). Pioglitazone reduced fatal or non‐fatal stroke (RR 0.54, 95% CI 0.35 to 0.85) and total events of cardiovascular death, non‐fatal myocardial infarction or non‐fatal stroke (RR 0.73, 95% CI 0.54 to 0.99). | 984 | ⊕⊕⊝⊝ | Pioglitazone reduced fatal or non‐fatal stroke and total events of cardiovascular death, non‐fatal myocardial infarction or non‐fatal stroke. |
| Deaths due to vascular events | Not reported | Not reported | — | — | — | |
| Disability due to vascular events | Not reported | Not reported | — | — | — | |
| Improvement in quality of life | Not reported | Not reported | — | — | — | |
| Insulin sensitivity Assessed with composite insulin sensitivity index | The change in the composite index was ‐0.1 ± 0.6. The C‐reactive protein concentration increased from 0.41 to 0.45 mg/L. | The change in the composite index was 1.2 ± 0.6. The C‐reactive protein concentration declined from 0.30 to 0.20 mg/L. | The change in the composite index was significantly increased in the pioglitazone group in comparison with the placebo group (P = 0.0003). | 20 | ⊕⊕⊝⊝ | |
| Ubiquitin‐proteasome activity in carotid plaques | Ubiquitin 468.7 ± 89 ng/mg; proteasome 20S 79.8 ± 25 pmol/mg; nitrotyrosine 3.5 ± 0.42 nmol/pg; superoxide anion production 6.26 ± 1.4 pmol/L | Ubiquitin 322 ± 79 ng/mg; proteasome 20S 46.8 ± 10 pmol/mg; nitrotyrosine 2.2 ± 0.21 nmol/pg; superoxide anion production 3.57 ± 1.1 pmol/L | Compared with the placebo group, symptomatic carotid plaques in the rosiglitazone group showed fewer inflammatory cells (P < 0.01) with less ubiquitin (P < 0.01), proteasome 20S (P < 0.01), nuclear factor kappa B (P < 0.01), nitrotyrosine (P < 0.01), superoxide anion production (P < 0.01), and more collagen content (P < 0.01). | 40 | ⊕⊕⊝⊝ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded one level due to risk of bias. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Recurrence of stroke Show forest plot | 3 | 4979 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.44, 0.99] |
| 2 Reported adverse events Show forest plot | 3 | 1044 | Risk Difference (M‐H, Random, 95% CI) | 0.10 [‐0.08, 0.28] |