Antibióticos tópicos para la otitis media supurativa crónica
Resumen
Antecedentes
La otitis media supurativa crónica (OMSC), a veces denominada otitis media crónica (OMC), es una inflamación crónica y a menudo una infección polimicrobiana (que involucra más de un microorganismo) del oído medio y la cavidad mastoidea, caracterizada por la secreción del oído (otorrea) a través de una membrana timpánica perforada. Los síntomas predominantes de la OMSC son la secreción del oído y la pérdida de audición. Los antibióticos tópicos, el tratamiento más común para la OMSC, actúan para eliminar o inhibir el crecimiento de los microorganismos que pueden ser responsables de la infección. Los antibióticos se pueden utilizar solos o además de otros tratamientos para la OMSC, como los antisépticos o la limpieza de los oídos (lavado ótico).
Objetivos
Evaluar los efectos de los antibióticos tópicos (sin esteroides) para los pacientes con OMSC.
Métodos de búsqueda
El Especialista en Información del Grupo Cochrane de Enfermedades de Oído, Nariz y Garganta (Cochrane ENT Group Information Specialist) realizó búsquedas en el Registro de Ensayos del Grupo Cochrane de Enfermedades de Oído, Nariz y Garganta; el Registro Cochrane de Ensayos Controlados (a través del Registro de Estudios Cochrane [Cochrane Register of Studies]); Ovid MEDLINE; Ovid Embase; CINAHL; Web of Science; ClinicalTrials.gov; ICTRP y fuentes adicionales para obtener ensayos publicados y no publicados. La fecha de la búsqueda fue el 1 de abril 2019.
Criterios de selección
Se incluyeron ensayos controlados aleatorizados (ECA) con al menos una semana de seguimiento que incluían a participantes (adultos y niños) con secreción ótica crónica de causa desconocida u OMSC, en los que la secreción ótica había continuado durante más de dos semanas.
Las intervenciones fueron cualquier agente antibiótico tópico único o una combinación de agentes de cualquier clase, aplicados directamente en el conducto auditivo externo en forma de gotas, polvos o irrigaciones, o como parte de un procedimiento de lavado ótico.
Las dos comparaciones principales fueron un antibiótico tópico comparado con a) placebo o ninguna intervención y b) otro antibiótico tópico (p.ej. antibiótico tópico A versus antibiótico tópico B).
Dentro de cada comparación se separaron los estudios en los que ambos grupos de participantes habían recibido un antibiótico tópico a) solo o con lavado ótico y b) además del tratamiento de base (como los antibióticos sistémicos).
Obtención y análisis de los datos
Se utilizaron los procedimientos metodológicos Cochrane estándar. Se utilizaron los criterios GRADE para evaluar la certeza de la evidencia de cada resultado.
Los resultados primarios fueron: resolución de la secreción del oído u "oído seco" (confirmada o no otoscópicamente), medida entre una y hasta dos semanas, dos y hasta cuatro semanas y después de cuatro semanas; calidad de vida relacionada con la salud mediante un instrumento validado; dolor de oído (otalgia) o malestar o irritación local. Los resultados secundarios incluyeron la audición, las complicaciones graves y la ototoxicidad medida de varias maneras.
Resultados principales
Se incluyeron 17 estudios con un total de 2198 participantes. Doce estudios informaron el tamaño de la muestra en términos de los participantes (no de los oídos); los mismos tuvieron un total de 1797 participantes. Los cinco estudios restantes informaron tanto el número de participantes como de oídos, lo cual representa a 401 participantes o 510 oídos.
A: Antibióticos tópicos versus placebo o ningún tratamiento (con lavado ótico en ambos brazos y ningún otro tratamiento de base)
Un estudio pequeño comparó un antibiótico tópico (ciprofloxacino) con placebo (solución salina). Todos los participantes recibieron un lavado ótico. Aunque la ciprofloxacina fue mejor que la solución salina en cuanto a la resolución de la secreción a la semana o dos semanas: 84% versus 12% (riesgo relativo [RR] 6,74; intervalo de confianza [IC] del 95%: 1,82 a 24,99; 35 participantes, evidencia de certeza muy baja), la certeza muy baja de la evidencia significa que no se conoce si una intervención es mejor o peor que la otra. Los autores del estudio informaron que "no se detectaron efectos secundarios médicos ni el empeoramiento de las mediciones audiológicas relacionadas con este medicamento tópico" (evidencia de certeza muy baja).
B: Antibióticos tópicos versus placebo o ningún tratamiento (con uso de antibióticos orales en ambos brazos)
Cuatro estudios compararon ciprofloxacina tópica con ningún tratamiento (tres estudios; 190 participantes) o ceftizoxima tópica con ningún tratamiento (un estudio; 248 participantes). En cada estudio todos los participantes recibieron el mismo antibiótico de forma sistémica (ciprofloxacino oral, ceftizoxima inyectada). En al menos un estudio todos los participantes recibieron un lavado ótico. Solo hubo datos disponibles utilizables en los tres primeros estudios; la ciprofloxacina fue mejor que ningún tratamiento, la resolución de la secreción se produjo en el 88,2% versus el 60% a la semana o dos semanas (RR 1,47; IC del 95%: 1,20 a 1,80; dos estudios, 150 participantes; evidencia de certeza baja). Ninguno de los estudios informó de dolor de oído o malestar/ irritación local.
C: Comparaciones de diferentes antibióticos tópicos
La certeza de la evidencia para todos los resultados en estas comparaciones es muy baja.
Quinolonas versus aminoglucósidos
Siete estudios compararon un aminoglucósido (gentamicina, neomicina o tobramicina) con ciprofloxacina (734 participantes) u ofloxacina (214 participantes). Aunque la resolución de la secreción a la semana o dos semanas fue mayor en el grupo de quinolonas, la certeza muy baja de la evidencia significa que no se conoce si una intervención es mejor o peor que la otra (RR 1,95; IC del 95%: 0,88 a 4,29; seis estudios, 694 participantes). Un estudio midió el dolor de oído y no informó de ninguna diferencia entre los grupos.
Quinolonas versus combinación de aminoglucósidos/polimixina B ± gramicidina
Se identificaron tres estudios, aunque solo hubo datos disponibles sobre el resultado primario en un estudio. Al comparar la ciprofloxacina con una combinación de neomicina/polimixina B/gramicidina, para un tratamiento de duración desconocida (probablemente cuatro semanas), la ciprofloxacina fue mejor (RR 1,12; IC del 95%: 1,03 a 1,22; 186 participantes). Unos "pocos" pacientes experimentaron irritación local en la primera instilación del tratamiento tópico (números/grupos no declarados).
Otros
Otros estudios examinaron la gentamicina tópica versus una combinación de trimetoprima/sulfacetamida/polimixina B (91 participantes) y rifampicina versus cloranfenicol (160 participantes). Hubo datos limitados disponibles y los hallazgos fueron muy inciertos.
Conclusiones de los autores
No se conoce con certeza la efectividad de los antibióticos tópicos para mejorar la resolución de la secreción del oído en pacientes con OMSC debido a la cantidad limitada de evidencia de calidad baja disponible. Sin embargo, entre estas dudas hay alguna evidencia que sugiere que el uso de antibióticos tópicos puede ser efectivo en comparación con placebo, o cuando se utilizan además de un antibiótico sistémico. También existe incertidumbre acerca de la efectividad relativa de los diferentes tipos de antibióticos; no es posible determinar con ninguna certeza si las quinolonas son mejores o peores que los aminoglucósidos. Estos dos grupos de compuestos tienen diferentes perfiles de efectos adversos, aunque no hay evidencia suficiente a partir de los estudios incluidos para realizar algún comentario sobre los mismos. En general, los efectos adversos se informaron de manera deficiente.
PICO
Resumen en términos sencillos
Antibióticos tópicos para pacientes con otitis media supurativa crónica
¿Cuál era el objetivo de esta revisión?
El objetivo de esta revisión Cochrane fue averiguar si los antibióticos tópicos son efectivos en el tratamiento de la otitis media supurativa crónica y si un tipo de tratamiento con antibióticos tópicos es más efectivo que cualquier otro. Se recopilaron y analizaron todos los estudios relevantes para responder esta pregunta.
Mensajes clave
Existe mucha incertidumbre acerca de si los antibióticos tópicos mejoran o no la resolución de la secreción del oído en los pacientes con otitis media supurativa crónica (OMSC). Sin embargo, entre estas dudas hay alguna evidencia que sugiere que el uso de antibióticos tópicos puede ser efectivo cuando se compara con placebo, o cuando se usan además de un antibiótico sistémico (oral o inyectado). También hay mucha incertidumbre sobre qué tipo de antibiótico tópico es el más efectivo. En general, la certeza de la evidencia fue muy baja.
¿Qué se estudió en la revisión?
La otitis media supurativa crónica, a veces denominada otitis media crónica (OMC), es una inflamación e infección a largo plazo (crónica) del oído medio, con secreción del oído (otorrea) a través de una membrana timpánica perforada (tímpano). Los síntomas principales de OMSC son la secreción del oído y la pérdida de audición. Los antibióticos tópicos (administrados en el canal auditivo como gotas, ungüentos, aerosoles o cremas) son el tratamiento utilizado con mayor frecuencia para la OMSC. Los antibióticos tópicos eliminan o detienen el crecimiento de los microorganismos que pueden ser responsables de la infección. Los antibióticos tópicos se pueden usar solos o se pueden agregar a otros tratamientos para la OMSC, como los antisépticos o la limpieza de los oídos (lavado ótico) o a los antibióticos sistémicos (antibióticos administrados ya sea por la boca o mediante una inyección en un músculo o una vena). En esta revisión fue importante examinar si hubo algún efecto adverso causado por el uso de los antibióticos tópicos, ya que pueden causar irritación de la piel dentro del oído externo, lo cual puede dar lugar a malestar, dolor o picazón. Esta revisión también examinó si diferentes tipos de antibióticos eran más efectivos que otros para tratar la OMSC, ya que algunos antibióticos (como los aminoglucósidos) pueden tener el potencial de ser tóxicos para el oído interno (ototoxicidad), con la posibilidad de causar pérdida de la audición irreparable (neurosensorial), mareos o zumbido en el oído (tinnitus).
¿Cuáles son los principales resultados de la revisión?
Se encontraron 17 estudios que examinaban a al menos 2126 participantes, aunque fue difícil determinar con precisión cuántos participantes se incluyeron ya que varios estudios no informaron el número de manera clara. Se utilizaron varios tipos y combinaciones diferentes de antibióticos.
Comparación de los antibióticos tópicos con placebo o ningún tratamiento
Un estudio comparó los antibióticos tópicos con el lavado del oído con solución salina (agua salada). Los antibióticos tópicos parecieron ser más efectivos que el lavado del oído con solución salina cuando se evaluaron de una a dos semanas después del tratamiento, aunque este estudio fue demasiado pequeño para proporcionar resultados de cualquier certeza (evidencia de certeza muy baja).
Comparación de antibióticos tópicos además de antibióticos sistémicos (orales o inyectados)
Cuatro estudios compararon el tratamiento con un antibiótico tópico en forma de gotas (ciprofloxacina) además de un antibiótico sistémico (oral o inyectado). El tratamiento favoreció de forma marginal a los antibióticos tópicos y orales combinados en comparación con los antibióticos orales solamente para la resolución de la secreción a la semana o dos semanas y a las dos o cuatro semanas. Estos estudios fueron demasiado pequeños para proporcionar cualquier certeza de los resultados (evidencia de certeza baja).
Comparaciones de diferentes antibióticos tópicos
Hubo 12 estudios que examinaron la efectividad de diferentes tipos de antibióticos. La certeza de la evidencia para todos los resultados en estas comparaciones es muy baja. Dos estudios no informaron el número de participantes incluidos, o informaron solo el número de oídos tratados, por lo que no se pudo calcular el número total de participantes. Debido a la certeza baja de la evidencia, no se sabe qué tipo de antibiótico tópico es el más efectivo.
¿Cómo de actualizada está esta revisión?
La evidencia está actualizada hasta abril 2019.
Authors' conclusions
Summary of findings
| Topical antibiotics (ciprofloxacin) versus placebo/no treatment for chronic suppurative otitis media | |||||||
| Patient or population: patients with mucopurulent otorrhoea Settings: specialist hospital in Thailand Intervention: ciprofloxacin ear drops Comparison: saline | |||||||
| Outcomes | Relative effect | Number of participants (studies) | Anticipated absolute effects* (95% CI) | Certainty of the evidence | Comments | ||
| Without topical antibiotic | With topical antibiotic | Difference | |||||
| Resolution of ear discharge ‐ measured at 1 to 2 weeks Follow‐up: 7 days | RR 6.74 (1.82 to 24.99) | 35 (1 RCT) | Study population | ⊕⊝⊝⊝ | Topical antibiotics may increase the number of patients with resolution of ear discharge at 7 days compared with placebo, but we are very uncertain about the results. | ||
| 12.5% | 84.2% (22.8 to 100.0) | 71.8% more (10.3 to 299.9) | |||||
| Resolution of ear discharge ‐ measured after 4 weeks | No study measured this outcome. | ||||||
| Health‐related quality of life | No study measured this outcome. | ||||||
| Ear pain (otalgia) or discomfort or local irritation Follow‐up: 7 days | — | 35 (1 RCT) | Authors reported "no medical side‐effects and worsening of audiological measurements related to this topical medication were detected". | ⊕⊝⊝⊝ | — | ||
| Hearing Follow‐up: 7 days | — | 35 (1 RCT) | Authors reported "no ... worsening of audiological measurements related to this topical medication were detected." | ⊕⊝⊝⊝ | — | ||
| Serious complications | No studies reported that any participant died or had any intracranial or extracranial complications. | ||||||
| Suspected ototoxicity Follow‐up: 7 days | — | 35 (1 RCT) | Authors report "no suspected ototoxicity" but it is unclear how this was measured. | ⊕⊝⊝⊝ | — | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group | |||||||
| GRADE Working Group grades of evidence | |||||||
| 1Downgraded to very low certainty: downgraded by one level due to study limitations (risk of bias) because there were concerns about incomplete data (50 people entered the study but results are only reported for 35). Downgraded by two levels due to imprecision as there was one very small study (35 participants) with wide confidence intervals. 2Downgraded to very low certainty: downgraded by two levels due to study limitations (risk of bias) because there were concerns about incomplete data (50 people entered the study but results are only reported for 35) and it is unclear how this outcome was measured as the paper just reports "no medical side effects". Downgraded by one level due to imprecision as numeric results were not provided and it was only one very small study (35 participants). 3Downgraded to very low certainty: downgraded by two levels due to study limitations (risk of bias) because of concerns about incomplete data (50 people entered the study but results are only reported for 35) and the methods used for measuring hearing were not provided in the paper. Downgraded by one level due to imprecision as numeric results were not reported and it was only one very small study (35 participants). | |||||||
| Topical antibiotics (ciprofloxacin) on top of systemic antibiotics (ciprofloxacin) for chronic suppurative otitis media | |||||||
| Patient or population: CSOM, recurrence of CSOM or suppuration following mastoidectomy or tympanoplasty Settings: secondary care clinics in Spain and Italy Intervention: ciprofloxacin (topical) plus ciprofloxacin (systemic) Comparison: ciprofloxacin (systemic) | |||||||
| Outcomes | Relative effect | Number of participants (studies) | Anticipated absolute effects* (95% CI) | Certainty of the evidence | Comments | ||
| Without topical antibiotics | With topical antibiotics | Difference | |||||
| Resolution of ear discharge‐ measured at 1 to 2 weeks Follow‐up: 7 to 10 days | RR 1.47 (1.20 to 1.80) | 150 (2 RCTs) | Study population | ⊕⊕⊝⊝ | Topical antibiotics in addition to systemic antibiotics may increase the number of patients with resolution of ear discharge at 7 to 10 days compared with systemic antibiotics alone. The NNTB is 4 (95% CI 3 to 9). | ||
| 60.0% | 88.2% (72.0 to 100) | 28.2% more (12 more to 48 more) | |||||
| Resolution of ear discharge ‐ measured after 4 weeks | No studies reported this outcome. | ||||||
| Health‐related quality of life | No studies reported this outcome. | ||||||
| Ear pain (otalgia) or discomfort or local irritation | No studies reported this outcome. | ||||||
| Hearing | No studies reported results for this outcome. | ||||||
| Serious complications | No studies reported that any participant died or had any intracranial or extracranial complications. | ||||||
| Suspected ototoxicity | — | 190 (3 RCTs) | Three studies reported that they did not suspect ototoxicity in any participants, but it is unclear how this was measured (de Miguel 1999; Esposito 1990; Ramos 2003). | ⊕⊝⊝⊝ | — | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group | |||||||
| GRADE Working Group grades of evidence | |||||||
| 1Downgraded to low certainty: downgraded by one level due to study limitations (risk of bias) as both studies had unclear randomisation and allocation concealment and were unblinded. Downgraded by one level due to imprecision as there were only two small studies (150 participants) with the confidence interval crossing the line of minimally important benefit. 2Downgraded to very low certainty: downgraded by two levels due to study limitations (risk of bias) as all three studies had unclear randomisation, allocation concealment and were unblinded studies. It was also unclear how the outcome was reported. Downgraded by one level due to imprecision as numeric results were not reported and there were only three small studies (190 participants). | |||||||
| Quinolones versus aminoglycosides for chronic suppurative otitis media | |||||||
| Patient or population: CSOM Settings: secondary care settings in Israel, Turkey, Jordan, Spain and Pakistan Intervention: ciprofloxacin versus tobramycin (2 studies); ciprofloxacin versus gentamycin (3 studies); ofloxacin versus gentamycin (2 studies) Comparison: other antibiotic | |||||||
| Outcomes | Relative effect | Number of participants (studies) | Anticipated absolute effects* (95% CI) | Certainty of the evidence | Comments | ||
| Aminoglycosides | Quinolones | Difference | |||||
| Resolution of ear discharge ‐ measured at 1 to 2 weeks Follow‐up: range 8 days to 2 weeks | RR 1.95 (0.88 to 4.29) | 694 (6 RCTs) | 33.7%1 | 65.7 (29.7% to 100%) | 32.0% more (4.0% lower to 110.9% higher) | ⊕⊝⊝⊝ | We used a random‐effects model due to high heterogeneity. Resolution of ear discharge at 1 to 2 weeks was higher in the quinolones group but the very low certainty of the evidence means that it is very uncertain whether or not one intervention is better or worse than the other. |
| Resolution of ear discharge ‐ Measured after 4 weeks | None of the studies measured this outcome. | ||||||
| Health‐related quality of life | None of the studies measured this outcome. | ||||||
| Ear pain (otalgia) or discomfort or local irritation Follow‐up: 30 days | — | 308 (1 RCT) | One study measured ear pain on a 3‐point scale (Lorente 1995). Results were presented as a mean score. Both groups had a mean score of 0 at 30 days. There was no difference between the groups. | ⊕⊝⊝⊝ | — | ||
| Hearing Follow‐up: 10 days | — | 132 (4 RCTs) | One study presents the hearing levels per group but does not present the data in a way that can be analysed (Tutkun 1995). One study stated in the methods that hearing was measured but only mentioned that neither group showed significant differences (Nawasreh 2001). | ⊕⊝⊝⊝ | — | ||
| Serious complications Follow‐up: 10 to 30 days | None of the studies reported that any participant died or had any intracranial or extracranial complications. | ||||||
| Suspected ototoxicity Follow‐up: 10 to 30 days | — | 352 (2 RCTs) | One study (Lorente 1995) assessed for ototoxicity and did not find any cases. One study (Tutkun 1995) reported assessment of ototoxicity in the methods but did not provide results. None of the studies reported assessment nor any cases of suspected ototoxicity. | ⊕⊝⊝⊝ | — | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group | |||||||
| GRADE Working Group grades of evidence | |||||||
| 1Average event rates in the control group were calculated without the Lorente 1995 study, as this seemed to show a very high rate of resolution (94%) compared to the other studies (range between 28% and 55%). 2Downgraded to very low certainty. Downgraded due to study limitations (risk of bias) as six of seven studies were unblinded and in general the methods were poor. Downgraded due to imprecision as the point estimate shows that more people with quinolones had resolution of discharge compared with aminoglycosides BUT there is a large confidence interval, which includes 'no effect' and a very large effect (four times as many people had resolution with quinolones compared to aminoglycosides). Downgraded due to inconsistency as there was high heterogeneity (I2 = 97%) within the results. 3Downgraded to very low certainty: downgraded by two levels due to study limitations (risk of bias) as all elements of the risk of bias assessment were unclear. Downgraded by one level due to imprecision as the results come from one relatively small study (308 patients). 4Downgraded to very low certainty: downgraded by two levels due to study limitations (risk of bias) as the studies were assessed as either high risk or unclear risk for all elements of the risk of bias assessment. Downgraded by one level due to imprecision as numeric results were not presented and the results came from two small studies (132 patients). 5Downgraded to very low certainty: downgraded by two levels due to study limitations (risk of bias) as many were unblinded and in general the studies had methodological issues and/or were badly reported. In addition, it is not clear how the outcome was measured. Downgraded by one level due to imprecision as numeric results were not reported and there were only two studies (352 participants) that identified ototoxicity as an outcome. | |||||||
Background
This is one of a suite of Cochrane Reviews evaluating the comparative effectiveness of non‐surgical interventions for chronic suppurative otitis media (CSOM) using topical antibiotics, topical antibiotics with corticosteroids, systemic antibiotics, topical antiseptics and aural toileting (ear cleaning) methods (Table 1).
| Topical antibiotics with steroids | Topical antibiotics | Systemic antibiotics | Topical antiseptics | Aural toileting (ear cleaning) | |
| Topical antibiotics with steroids | Review CSOM‐4 | ||||
| Topical antibiotics | Review CSOM‐4 | Review CSOM‐1 | |||
| Systemic antibiotics | Review CSOM‐4 | Review CSOM‐3 | Review CSOM‐2 | ||
| Topical antiseptics | Review CSOM‐4 | Review CSOM‐6 | Review CSOM‐6 | Review CSOM‐5 | |
| Aural toileting | Review CSOM‐4 | Not reviewed | Not reviewed | Not reviewed | Review CSOM‐7 |
| Placebo (or no intervention) | Review CSOM‐4 | Review CSOM‐1 | Review CSOM‐2 | Review CSOM‐5 | Review CSOM‐7 |
CSOM‐1: Topical antibiotics for chronic suppurative otitis media (Brennan‐Jones 2018b).
CSOM‐2: Systemic antibiotics for chronic suppurative otitis media (Chong 2018a).
CSOM‐3: Topical versus systemic antibiotics for chronic suppurative otitis media (Chong 2018b).
CSOM‐4: Topical antibiotics with steroids for chronic suppurative otitis media (Brennan‐Jones 2018a).
CSOM‐5: Topical antiseptics for chronic suppurative otitis media (Head 2018a).
CSOM‐6: Antibiotics versus topical antiseptics for chronic suppurative otitis media (Head 2018b).
CSOM‐7: Aural toilet (ear cleaning) for chronic suppurative otitis media (Bhutta 2018).
This review compares the effectiveness of topical antibiotics (without corticosteroids) against placebo/no treatment, or another topical antibiotic for CSOM.
Description of the condition
Chronic suppurative otitis media (CSOM), which is also often referred to as chronic otitis media (COM), is a chronic inflammation and infection of the middle ear and mastoid cavity, characterised by ear discharge (otorrhoea) through a perforated tympanic membrane.
The predominant symptoms of CSOM are ear discharge and hearing loss. Ear discharge can be persistent or intermittent, and many sufferers find it socially embarrassing as the discharge is often visible and odorous (Orji 2013). Some patients also experience discomfort or earache. Most patients with CSOM experience temporary or permanent hearing loss with average hearing levels typically between 10 and 40 decibels (Jensen 2013). The hearing loss can be disabling, and it can have an impact on speech and language skills, employment prospects, and on children's psychosocial and cognitive development, including academic performance (Elemraid 2010; Olatoke 2008; WHO 2004). Consequently, quality of life can be affected. CSOM can also progress to serious complications in rare cases (and more often when cholesteatoma is present): both extracranial complications (such as mastoid abscess, postauricular fistula and facial palsy) and intracranial complications (such as otitic meningitis, lateral sinus thrombosis and cerebellar abscess) have been reported (Dubey 2007; Yorgancılar 2013).
CSOM is estimated to have a global incidence of 31 million episodes per year, or 4.8 new episodes per 1000 people (all ages), with 22% of cases affecting children under five years of age (Monasta 2012; Schilder 2016). The prevalence of CSOM varies widely between countries, but it disproportionately affects people at socio‐economic disadvantage. It is rare in high‐income countries, but common in many low‐ and middle‐income countries (Mahadevan 2012; Monasta 2012; Schilder 2016; WHO 2004).
Definition of disease
There is no universally accepted definition of CSOM. Some define CSOM in patients with a duration of otorrhoea of more than two weeks but others may consider this an insufficient duration, preferring a minimum duration of six weeks or more than three months (Verhoeff 2006). Some include diseases of the tympanic membrane within the definition of CSOM, such as tympanic perforation without a history of recent ear discharge, or the disease cholesteatoma (a growth of the squamous epithelium of the tympanic membrane).
In accordance with a consensus statement, here we use CSOM only to refer to tympanic membrane perforation, with intermittent or continuous ear discharge (Gates 2002). We have used a duration of otorrhoea of two weeks as an inclusion criterion, in accordance with the definition used by the World Health Organization, but we have used subgroup analyses to explore whether this is a factor that affects observed treatment effectiveness (WHO 2004).
Many people affected by CSOM do not have good access to modern primary health care, let alone specialised ear and hearing care, and in such settings health workers may be unable to view the tympanic membrane to definitively diagnose CSOM. It can also be difficult to view the tympanic membrane when the ear discharge is profuse. Therefore we have also included, as a subset for analysis, studies where participants have had chronic ear discharge for at least two weeks, but where the diagnosis is unknown.
At‐risk populations
Some populations are considered to be at high risk of CSOM. There is a high prevalence of disease among Indigenous people such as the Aboriginal and Torres Strait Islander Australian, Native American and Inuit populations. This is likely due to an interplay of factors, including socio‐economic deprivation and possibly differences resulting from population genetics (Bhutta 2016). Those with primary or secondary immunodeficiency are also susceptible to CSOM. Children with craniofacial malformation (including cleft palate) or chromosomal mutations such as Down syndrome are prone to chronic non‐suppurative otitis media ('glue ear'), and by extrapolation may also be at greater risk of suppurative otitis media. The reasons for this association with craniofacial malformation are not well understood, but may include altered function of the Eustachian tube, coexistent immunodeficiency, or both. These populations may be less responsive to treatment and more likely to develop CSOM, recurrence or complications.
Children who have a grommet (ventilation tube) in the tympanic membrane to treat glue ear or recurrent acute otitis media may be more prone to develop CSOM; however, their pathway to CSOM may differ and therefore they may respond differently to treatment. Children with grommets who have chronic ear discharge meeting the CSOM criteria are therefore considered to be a separate high‐risk subgroup (van der Veen 2006).
Treatment
Treatments for CSOM may include topical antibiotics (administered into the ear) with or without steroids, systemic antibiotics (given either by mouth or by injection), topical antiseptics and ear cleaning (aural toileting), all of which can be used on their own or in various combinations. Whereas primary healthcare workers or patients themselves can deliver some treatments (for example, some aural toileting and antiseptic washouts), in most countries antibiotic therapy requires prescription by a doctor. Surgical interventions to repair the tympanic membrane are an option in cases where complications arise or in patients who have not responded to other treatments; however, there is a range of practice in terms of the type of surgical intervention that should be considered and the timing of the intervention. In addition, access to or availability of surgical interventions is setting‐dependent. This series of Cochrane Reviews therefore focuses on non‐surgical interventions. In addition, most clinicians consider cholesteatoma to be a variant of CSOM, but acknowledge that it will not respond to non‐surgical treatment (or will only respond temporarily) (Bhutta 2011). Therefore, studies in which more than half of the participants were identified as having cholesteatoma are not included in these reviews.
Description of the intervention
Antibiotics are the most commonly used treatment for CSOM. They can be administered topically (as drops, ointments, sprays or creams to the affected area) or systemically (either by mouth or by injection into a vein (intravenous) or muscles (intramuscular)). Topical antibiotics are often used in preference to systemic antibiotics as there may be immediate adverse effects of systemic antibiotics such as gastrointestinal upset. A broader concern is the association of the overuse of systemic antibiotics with increasing bacterial resistance among community‐ and hospital‐acquired pathogens (Costelloe 2010; ECDC 2011; Laxminarayan 2013).
Topical application has the advantage of potentially delivering high concentrations of antibiotic to the affected area, whereas systemic antibiotics are absorbed and distributed throughout the body. However, the penetration of topical antibiotics into the middle ear may be compromised if the perforation in the tympanic membrane is small or there is copious mucopurulent discharge in the ear canal that cannot be cleaned. It may also be difficult to achieve compliance with topical dosing in both children and adults. In these cases, systemic antibiotics may have an advantage.
How the intervention might work
CSOM is a chronic and often polymicrobial (involving more than one micro‐organism) infection of the middle ear. Broad‐spectrum antibiotics such as second‐generation quinolones and aminoglycosides, which are often active against the most frequently cultured micro‐organisms (Pseudomonas aeruginosa andStaphylococcus aureus) are therefore commonly used (Mittal 2015) (Table 2). It is possible that antibiotics for CSOM that target Pseudomonas aeruginosa may have an advantage over antibiotics that do not. Dose and duration of treatment are also important factors but are less likely to affect relative effectiveness if given within the therapeutic range. Generally, treatment for at least five days is necessary and a duration of one to two weeks is sufficient to resolve uncomplicated infections. However, in some cases it may take more than to two weeks for the ear to become dry and therefore longer follow‐up (more than four weeks) may be needed to monitor for recurrence of discharge. Some antibiotics (such as aminoglycosides) may have the potential to be toxic to the inner ear (ototoxicity), which might be experienced as sensorineural hearing loss, dizziness or tinnitus, but this is less likely to be a risk when applied topically in patients with CSOM (Phillips 2007). Local discomfort, ear pain or itching may occur through the action of putting ear drops into the ear or because the topical antibiotics or their excipients cause chemical or allergic irritation of the skin of the outer ear.
| Class of antibiotics | Examples | Route of administration |
| Quinolones | Ciprofloxacin, ofloxacin, levofloxacin | Oral, intravenous, topical |
| Aminoglycosides | Gentamicin, tobramycin | Topical or parenteral |
| Neomycin/framycetin | Only topical | |
| Cephalosporins | Ceftazidime | Parenteral |
| Penicillins | Ticarcillin plus clavulanic acid | Parenteral |
| Monobactams | Aztreonam | Parenteral |
Why it is important to do this review
Topical antibiotics (without steroids) are widely recommended as a first‐line treatment for CSOM. Opinions about the safety of topical antibiotics for treatment of CSOM, particularly the use of aminoglycosides, differ between professional groups and amongst ENT specialists across different countries. There remain concerns about local toxicity to the inner ear (ototoxicity), particularly with the use of topical aminoglycosides (Phillips 2007; Youngs 2016). Quinolone antibiotics are considered by many to have the best overall risk‐benefit profile, but the evidence base contains only a few small trials with high risk of bias. They are also not licensed for the treatment of CSOM (Youngs 2016). In addition, the cost of treatment, especially with quinolones, may be an issue in some settings. Evidence‐based knowledge of their effectiveness and the relative effectiveness of different topical antibiotics could help to optimise their use.
Objectives
To assess the effects of topical antibiotics (without steroids) for people with chronic suppurative otitis media (CSOM).
Methods
Criteria for considering studies for this review
Types of studies
We included studies with the following design characteristics:
-
Randomised controlled trials (including cluster‐randomised trials where the unit of randomisation is the setting or operator) and quasi‐randomised trials.
-
Patients were followed up for at least one week.
We excluded studies with the following design characteristics:
-
Cross‐over trials, because CSOM is not expected to be a stable chronic condition. Unless data from the first phase were available, we excluded such studies.
Types of participants
We included studies with patients (adults and children) who had:
-
chronic ear discharge of unknown cause; or
-
chronic suppurative otitis media.
We defined patients with chronic ear discharge as patients with at least two weeks of ear discharge, where the cause of the discharge was unknown.
We defined patients with chronic suppurative otitis media (CSOM) as patients with:
-
chronic or persistent ear discharge for at least two weeks; and
-
a perforated tympanic membrane.
We did not exclude any populations based on age, risk factors (cleft palate, Down syndrome), ethnicity (e.g. Australian Aboriginal or Torres Strait Islanders) or the presence of ventilation tubes (grommets). Where available, we recorded these factors in the patient characteristics section during data extraction from the studies. If any of the included studies recruited these patients as a majority (80% or more), we analysed them in a subgroup analysis (see Subgroup analysis and investigation of heterogeneity).
We excluded studies where the majority (more than 50%) of participants:
-
had an alternative diagnosis to CSOM (e.g. otitis externa);
-
had underlying cholesteatoma;
-
had ear surgery within the last six weeks.
We did not include studies designed to evaluate interventions in the immediate peri‐surgical period, which were focused on assessing the impact of the intervention on the surgical procedure or outcomes.
Types of interventions
Intervention
We included all (topical) antibiotics applied directly into the ear canal. The most common formulations are ear drops but we also included other formulations such as sprays.
We excluded studies that conducted swabs and tests for antimicrobial sensitivity and then based the choice of antibiotics for each participant on the results of the laboratory test.
Duration
At least five days of treatment with antibiotics was required, except for antibiotics where a shorter duration has been proven to be equivalent.
Dose
There was no limitation on the dose, concentration, volume or frequency of application.
Comparisons
The following were the comparators:
-
Placebo, no intervention (topical antibiotic versus placebo; topical antibiotic versus no intervention).
-
Another topical antibiotic (topical antibiotic A versus topical antibiotic B).
We analysed these as three main scenarios depending on which common therapy was applied in the background:
-
Topical antibiotics as a single treatment (main therapy): this included studies where all participants in both treatment groups either received no other treatment or only received aural toileting. This also included situations where antiseptics were applied only once (e.g. as part of microsuction at the start of treatment).
-
Topical antibiotics as an add‐on therapy to antiseptics: this included studies where all participants in both treatment groups also used a daily antiseptic, with or without aural toileting.
-
Topical antibiotics as an add‐on therapy to systemic or another topical antibiotic: this included studies where all participants in both treatment groups also received a systemic or topical antibiotic, which was a different type to the antibiotic under investigation, with or without aural toileting or antiseptics.
If either one or both intervention and comparison arms also received a topical steroid, we considered the data in an accompanying review in this series ('Topical antibiotics with steroids for chronic suppurative otitis media') (Brennan‐Jones 2018a).
Many comparison pairs were possible in this review. The main comparisons of interest that we summarised and presented in the 'Summary of findings' table are:
-
topical antibiotics as a single treatment (main therapy) versus placebo or no intervention;
-
topical antibiotics as a single treatment (main therapy) versus placebo or no intervention, on top of systemic antibiotics; and
-
topical quinolone versus topical aminoglycoside (both as single treatments).
Types of outcome measures
We analysed the following outcomes in the review, but we did not use them as a basis for including or excluding studies.
We extracted and reported data from the longest available follow‐up for all outcomes.
Primary outcomes
-
Resolution of ear discharge or 'dry ear' (whether otoscopically confirmed or not), measured at:
-
between one week and up to two weeks;
-
two weeks to up to four weeks; and
-
after four weeks.
-
-
Health‐related quality of life using a validated instrument for CSOM (e.g. Chronic Otitis Media Questionnaire (COMQ)‐12 (Phillips 2014a; Phillips 2014b; van Dinther 2015), Chronic Otitis Media Outcome Test (COMOT)‐15 (Baumann 2011), Chronic Ear Survey (CES) (Nadol 2000).
-
Ear pain (otalgia) or discomfort or local irritation.
Secondary outcomes
-
Hearing, measured as the pure‐tone average of air conduction thresholds across four frequencies tested (500 Hz, 1000 Hz, 2000 Hz and 4000 Hz) of the affected ear. If this was not available, we reported the pure‐tone average of the thresholds measured.
-
Serious complications, including intracranial complications (such as otitic meningitis, lateral sinus thrombosis and cerebellar abscess) and extracranial complications (such as mastoid abscess, postauricular fistula and facial palsy) and death.
-
Ototoxicity; this was measured as 'suspected ototoxicity' as reported by the studies where available, and as the number of people with the following symptoms that may be suggestive of ototoxicity:
-
sensorineural hearing loss;
-
balance problems/dizziness/vertigo;
-
tinnitus.
-
Search methods for identification of studies
The Cochrane ENT Information Specialist conducted systematic searches for randomised controlled trials and controlled clinical trials. There were no language, publication year or publication status restrictions. The date of the search was 1 April 2019.
Electronic searches
The Information Specialist searched:
-
the Cochrane ENT Register (searched via the Cochrane Register of Studies to 1 April 2019);
-
the Cochrane Central Register of Controlled Trials (CENTRAL) (searched via the Cochrane Register of Studies Web to 1 April 2019);
-
Ovid MEDLINE(R) Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE(R) Daily and Ovid MEDLINE(R) (1946 to 1 April 2019);
-
Ovid EMBASE (1974 to 1 April 2019);
-
EBSCO CINAHL (1982 to 1 April 2019);
-
LILACS (Latin American and Caribbean Health Science Information database), lilacs.bvsalud.org (search to 1 April 2019);
-
Web of Knowledge, Web of Science (1945 to 1 April 2019);
-
ClinicalTrials.gov, www.clinicaltrials.gov (search via the Cochrane Register of Studies to 1 April 2019);
-
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (search to 1 April 2019).
We also searched:
-
IndMed (search to 22 March 2018);
-
African Index Medicus (search to 22 March 2018).
The search strategies for major databases are detailed in Appendix 1. The Information Specialist modelled subject strategies for databases on the search strategy designed for CENTRAL. The search strategies were designed to identify all relevant studies for a suite of reviews on various interventions for chronic suppurative otitis media (Bhutta 2018; Brennan‐Jones 2018a; Brennan‐Jones 2018b; Chong 2018a; Chong 2018b; Head 2018a; Head 2018b). Where appropriate, they were combined with subject strategy adaptations of the highly sensitive search strategy designed by Cochrane for identifying randomised controlled trials and controlled clinical trials (as described in the Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0, Box 6.4.b. (Handbook 2011)
Searching other resources
We scanned the reference lists of identified publications for additional trials and contacted trial authors where necessary. In addition, the Information Specialist searched Ovid MEDLINE to retrieve existing systematic reviews relevant to this systematic review, so that we could scan their reference lists for additional trials. The Information Specialist also ran non‐systematic searches of Google Scholar to retrieve grey literature and other sources of potential trials.
We did not perform a separate search for adverse effects. We considered the adverse effects described in the included studies only.
We contacted original authors for clarification and further data if trial reports were unclear and we arranged translations of papers where necessary.
Data collection and analysis
Selection of studies
At least two review authors (KH/LYC) independently screened all titles and abstracts of the references obtained from the database searches to identify potentially relevant studies. At least two review authors (KH/LYC) evaluated the full text of each potentially relevant study to determine whether it met the inclusion and exclusion criteria for this review.
We resolved any differences by discussion and consensus, with the involvement of a third author for clinical and methodological input where necessary.
Data extraction and management
At least two review authors (KH/LYC/CBJ/MB) independently extracted data from each study using a standardised data collection form (see Appendix 2). Whenever a study had more than one publication, we retrieved all publications to ensure complete extraction of data. Where there were discrepancies in the data extracted by different review authors, we checked these against the original reports and resolved any differences by discussion and consensus, with the involvement of a third author or a methodologist where appropriate. We contacted the original study authors for clarification or for missing data whenever possible. If differences were found between publications of a study, we contacted the original authors for clarification. We used data from the main paper(s) if no further information was found.
We included key characteristics of the included studies, such as study design, setting (including location), year of study, sample size, age and sex of participants, and how outcomes were defined or collected in the studies. In addition, we also collected baseline information on prognostic factors or effect modifiers (see Appendix 2). For this review, this included the following information whenever available:
-
duration of ear discharge at entry to the study;
-
diagnosis of CSOM (as opposed to patients with chronic ear discharge but without a diagnosis of CSOM);
-
number of participants who may have been at higher risk of CSOM, including those with cleft palate or Down syndrome;
-
ethnicity of participants including the number who were from Indigenous populations;
-
number of participants who had previously had ventilation tubes (grommets) inserted (and, where known, the number who had tubes still in place);
-
number of participants who had previous ear surgery;
-
number of participants who had previous treatments for CSOM (non‐responders, recurrent versus new cases).
We recorded concurrent treatments alongside the details of the interventions used. See the 'Data extraction form' in Appendix 2 for more details.
For the outcomes of interest to the review, we extracted the findings of the studies on an available case analysis basis, i.e. we included data from all participants available at the time points based on the treatment randomised whenever possible, irrespective of compliance or whether participants had received the treatment as planned.
In addition to extracting pre‐specified information about study characteristics and aspects of methodology relevant to risk of bias, we extracted the following summary statistics for each trial and each outcome:
-
For continuous data: the mean values, standard deviations and number of patients for each treatment group. Where endpoint data were not available, we extracted the values for change from baseline. We analysed data from disease‐specific quality of life scales such as COMQ‐12, COMOT‐15 and CES as continuous data.
-
For binary data: the number of participants who experienced an event and the number of patients assessed at the time point.
-
For ordinal scale data: if the data appeared to be approximately normally distributed or if the analysis that the investigators performed suggested parametric tests were appropriate, then we treated the outcome measures as continuous data. Alternatively, if data were available, we converted it into binary data.
-
Time‐to‐event outcomes: we did not expect any outcomes to be measured as time‐to‐event data. However, if outcomes such as resolution of ear discharge were measured in this way, we reported the hazard ratios.
For resolution of ear discharge, we extracted the longest available data within the time frame of interest, defined as from one week up to (and including) two weeks (7 days to 14 days), from two weeks up to (and including) four weeks (15 to 28 days), and after four weeks (28 days or one month).
For other outcomes, we reported the results from the longest available follow‐up period.
Extracting data for pain/discomfort and adverse effects
For these outcomes, there were variations in how studies had reported the outcomes. For example, some studies reported both 'pain' and 'discomfort' separately whereas others did not. Prior to the commencement of data extraction, we agreed and specified a data extraction algorithm for how data should be extracted.
We extracted data for serious complications as a composite outcome. If a study reported more than one complication and we could not distinguish whether these occurred in one or more participants, we extracted the data with the highest incidence to prevent double counting.
Extracting data from figures
Where values for primary or secondary outcomes were shown as figures within the paper, we attempted to contact the study authors to try to obtain the raw values. When the raw values were not provided, we extracted information from the graphs using an online data extraction tool, using the best quality version of the relevant figures available.
Assessment of risk of bias in included studies
At least two review authors (KH/LYC/CBJ/MB) independently assessed the risk of bias of each included study. We followed the guidance in the Cochrane Handbook for Systematic Reviews of Interventions (Handbook 2011), using the Cochrane 'Risk of bias' tool. With this tool we assessed the risk of bias as 'low', 'high' or 'unclear' for each of the following six domains:
-
sequence generation;
-
allocation concealment;
-
blinding of participants, personnel and outcome assessment;
-
incomplete outcome data;
-
selective reporting;
-
other sources of bias.
Measures of treatment effect
We summarised the effects of dichotomous outcomes (e.g. proportion of patients with complete resolution of ear discharge) as risk ratios (RR) with confidence intervals (CIs). For the key outcomes that are presented in the 'Summary of findings' table, we expressed the results as absolute numbers based on the pooled results and compared to the assumed risk. We also calculated the number needed to treat to benefit (NNTB) using the pooled results. The assumed baseline risk was typically either (a) the median of the risks of the control groups in the included studies, this being used to represent a 'medium‐risk population' or, alternatively, (b) the average risk of the control groups in the included studies, which is used as the 'study population' (Handbook 2011). If a large number of studies were available, and where appropriate, we also attempted to present additional data based on the assumed baseline risk in (c) a low‐risk population and (d) a high‐risk population.
For continuous outcomes, we expressed treatment effects as a mean difference (MD) with standard deviation (SD). If different scales were used to measure the same outcome, we used the standardised mean difference (SMD) and provided a clinical interpretation of the SMD values.
Unit of analysis issues
Cross‐over studies
This review did not use data from phase II of cross‐over studies.
The ear as the unit of randomisation: within‐patient randomisation in patients with bilateral ear disease
For data from studies where 'within‐patient' randomisation was used (i.e. studies where both ears (right versus left) were randomised), we adjusted the analyses for the paired nature of the data (Elbourne 2002; Stedman 2011), as outlined in section 16.4 of the Cochrane Handbook for Systematic Reviews of Interventions (Handbook 2011).
The ear as the unit of randomisation: non‐paired randomisation in patients with bilateral ear disease
Some patients with bilateral disease may have received the same treatment in both ears, whereas others received a different treatment in each ear. We did not exclude these studies, but we only reported the data if specific pairwise adjustments were completed or if sufficient data were obtained to be able to make the adjustments.
The patient as the unit of randomisation
Some studies randomised by patient and those with bilateral CSOM received the same intervention for both ears. In some studies the results may be reported as a separate outcome for each ear (the total number of ears is used as the denominator in the analysis). The correlation of response between the left ear and right ear when given the same treatment was expected to be very high, and if both ears were counted in the analysis this was effectively a form of double counting, which may be especially problematic in smaller studies if the number of people with bilateral CSOM was unequal. We did not exclude these studies, but we only reported the results if the paper presented the data in such a way that we could include the data from each participant only once (one data point per participant) or if we had enough information to reliably estimate the effective sample size or inflated standard errors as presented in chapter 16.3 of the Cochrane Handbook for Systematic Reviews of Interventions (Handbook 2011). If this was not possible, we attempted to contact the authors for more information. If there was no response from the authors, then we did not include data from these studies in the analysis.
If we found cluster‐randomised trials by setting or operator, we analysed these according to the methods in section 16.3 of the Cochrane Handbook for Systematic Reviews of Interventions (Handbook 2011).
Dealing with missing data
We attempted to contact the study authors via email whenever the outcome of interest was not reported but the methods of the study suggested that the outcome had been measured. We did the same if not all of the data required for the meta‐analysis were reported, unless the missing data were standard deviations. If standard deviation data were not available, we approximated these using the standard estimation methods from P values, standard errors or 95% CIs if these were reported, as detailed in the Cochrane Handbook for Systematic Reviews of Interventions (Handbook 2011). Where it was impossible to estimate these, we contacted the study authors.
Apart from imputations for missing standard deviations, we did not conduct any other imputations. We extracted and analysed data for all outcomes using the available case analysis method.
Assessment of heterogeneity
We assessed clinical heterogeneity (which may be present even in the absence of statistical heterogeneity) by examining the included studies for potential differences in the types of participants recruited, interventions or controls used, and the outcomes measured. We did not pool studies where the clinical heterogeneity made it unreasonable to do so.
We assessed statistical heterogeneity by visually inspecting the forest plots and by considering the Chi² test (with a significance level set at P value < 0.10) and the I² statistic, which calculated the percentage of variability that is due to heterogeneity rather than chance, with I² values over 50% suggesting substantial heterogeneity (Handbook 2011).
Assessment of reporting biases
We assessed reporting bias as within‐study outcome reporting bias and between‐study publication bias.
Outcome reporting bias (within‐study reporting bias)
We assessed within‐study reporting bias by comparing the outcomes reported in the published report against the study protocol, whenever this could be obtained. If the protocol was not available, we compared the outcomes reported to those listed in the methods section. If results were mentioned but not reported adequately in a way that allowed analysis (e.g. the report only mentioned whether the results were statistically significant or not), bias in a meta‐analysis was likely to occur. We tried to find further information from the study authors, but if no further information could be obtained, we noted this as being a high risk of bias. Where there was insufficient information to judge the risk of bias, we noted this as an unclear risk of bias (Handbook 2011).
Publication bias (between‐study reporting bias)
We intended to create funnel plots if sufficient trials (more than 10) were available for an outcome. If we observed asymmetry of the funnel plot, we would have conducted a more formal investigation using the methods proposed by Egger 1997.
Data synthesis
We conducted all meta‐analyses using Review Manager 5.3 (RevMan 2014). For dichotomous data, we analysed treatment differences as a risk ratio (RR) calculated using the Mantel‐Haenszel methods. We analysed time‐to‐event data using the generic inverse variance method.
For continuous outcomes, if all the data was from the same scale, we pooled the mean values obtained at follow‐up with change outcomes and reported this as a MD. However, if the SMD had to be used as an effect measurement, we did not pool change and endpoint data.
When statistical heterogeneity is low, random‐effects versus fixed‐effect methods yield trivial differences in treatment effects. However, when statistical heterogeneity is high, the random‐effects method provides a more conservative estimate of the difference.
Subgroup analysis and investigation of heterogeneity
We subgrouped studies where most participants (80% or more) met the criteria stated below in order to determine whether the effect of the intervention was different compared to other patients. Due to the risks of reporting and publication bias with unplanned subgroup analyses of trials, we only analysed subgroups reported in studies if these were prespecified and stratified at randomisation.
We planned to conduct subgroup analyses regardless of whether statistical heterogeneity was observed for studies that included patients identified as high risk (i.e. thought to be less responsive to treatment and more likely to develop CSOM, recurrence or complications) and patients with ventilation tubes (grommets). 'High risk' patients include Indigenous populations (e.g. Australian Aboriginal and Torres Strait Islanders, Native Americans and Inuit populations of Alaska, Canada and Greenland), people with craniofacial malformation (e.g. cleft palate), Down syndrome and people with known immunodeficiency.
We planned to present the main analyses of this review in the form of forest plots based on this main subgroup analysis.
-
For the high‐risk group, this applied to the outcomes resolution of ear discharge (dry ear), quality of life, pain/discomfort, development of complications and hearing loss.
For patients with ventilation tubes, this applied to the outcome resolution of ear discharge (dry ear) for the time point of four weeks or more because this group was perceived to be at lower risk of treatment failure and recurrence than other patient groups. If statistical heterogeneity was observed, we also conducted subgroup analysis for the effect modifiers below. If there were statistically significant subgroup effects, we presented these subgroup analysis results as forest plots.
For this review, effect modifiers included:
-
Diagnosis of CSOM: it was likely that some studies would include patients with chronic ear discharge but who had not had a diagnosis of CSOM. Therefore, we subgrouped studies where most patients (80% or more) met the criteria for CSOM diagnosis in order to determine whether the effect of the intervention was different compared to patients where the precise diagnosis was unknown and inclusion into the study was based purely on chronic ear discharge symptoms.
-
Duration of ear discharge: there is uncertainty about whether the duration of ear discharge prior to treatment has an impact on the effectiveness of treatment and whether more established disease (i.e. discharge for more than six weeks) is more refractory to treatment compared with discharge of a shorter duration (i.e. less than six weeks).
-
Patient age: patients who were younger than two years old versus patients up to six years old versus adults. Patients under two years are widely considered to be more difficult to treat.
We presented the results as subgroups regardless of the presence of statistical heterogeneity based on the following two factors:
-
Class of antibiotics: We grouped by pharmacological class, e.g. quinolones, aminoglycosides, penicillins etc. The rationale for this was that different classes may have had different effectiveness and side effect profiles.
-
Spectrum of activity against Pseudomonas aeruginosa (groups with known activity against Pseudomonas aeruginosa versus groups without activity against Pseudomonas aeruginosa). This is the most commonly found bacteria in patients with CSOM and its presence is associated with tissue damage.
When other antibiotics were also used as a common treatment in both the intervention and comparison group, we investigated the class and antipseudomonal activity when statistical heterogeneity was present and could not be explained by the other subgroup analyses.
No other subgroups based on the pharmacological properties of antibiotics were planned, but we considered the method and frequency of aural toileting if there was remaining unexplained heterogeneity despite conducting the other subgroup analyses.
Sensitivity analysis
We planned to carry out sensitivity analyses to determine whether the findings were robust to the decisions made in the course of identifying, screening and analysing the trials. We planned to conduct sensitivity analysis for the following factors, whenever possible:
-
Impact of model chosen: fixed‐effect versus random‐effects model.
-
Risk of bias of included studies: excluding studies with high risk of bias (we defined these as studies that have a high risk of allocation concealment bias and a high risk of attrition bias (overall loss to follow‐up of 20%, differential follow‐up observed)).
-
Where there was statistical heterogeneity, studies that only recruited patients who had previously not responded to one of the treatments under investigation in the RCT. Studies that specifically recruited patients who did not respond to a treatment could potentially have reduced the relative effectiveness of an agent.
If any of these investigations found a difference in the size of the effect or heterogeneity, we mentioned this in the 'Effects of interventions' section and/or presented the findings in a table.
GRADE and 'Summary of findings' table
Using the GRADE approach, at least two review authors (KH/LYC) independently rated the overall certainty of evidence using the GDT tool (http://www.guidelinedevelopment.org/) for the main comparison pairs listed in the Types of interventions section. The certainty of evidence reflects the extent to which we were confident that an estimate of effect was correct and we applied this in the interpretation of results. There were four possible ratings: 'high', 'moderate', 'low' and 'very low' (Handbook 2011). A rating of 'high' certainty evidence implies that we were confident in our estimate of effect and that further research was very unlikely to change our confidence in the estimate of effect. A rating of 'very low' certainty implies that any estimate of effect obtained was very uncertain.
The GRADE approach rates evidence from RCTs that do not have serious limitations as high certainty. However, several factors could lead to the downgrading of the evidence to moderate, low or very low. The degree of downgrading was determined by the seriousness of these factors:
-
study limitations (risk of bias);
-
inconsistency;
-
indirectness of evidence;
-
imprecision;
-
publication bias.
The 'Summary of findings' table presents the following outcomes:
-
resolution of ear discharge or 'dry ear':
-
at between one week and up to two weeks;
-
after four weeks;
-
-
health‐related quality of life;
-
ear pain (otalgia) or discomfort or local irritation;
-
hearing;
-
serious complications;
-
suspected ototoxicity.
Results
Description of studies
Results of the search
The searches retrieved a total of 7256 references and we identified five additional references from other sources. This was reduced to 3147 after removal of duplicates. We screened the titles and abstracts and subsequently removed 2935 references. We assessed 212 full‐text references for eligibility of which we discarded 187; we excluded 150 (including unpublished studies) of these references (122 studies) with reasons recorded in the review (see Excluded studies).
We included 23 references (17 studies). There are two references awaiting classification (Abdul 2005; Abes 1998). See Characteristics of studies awaiting classification. We did not identify any ongoing studies.
A flow chart of study retrieval and selection is provided in Figure 1.

Study flow diagram
Included studies
Seventeen studies were included (Asmatullah 2014; de Miguel 1999; Esposito 1990; Fradis 1997; Gyde 1978; Jamalullah 2016; Kasemsuwan 1997; Kaygusuz 2002; Liu 2003; Lorente 1995; Mira 1993; Nawasreh 2001; Ramos 2003; Siddique 2016; Tutkun 1995; van Hasselt 1997; van Hasselt 1998a). Table 3 provides a summary of the included studies.
| Ref ID (no. participants) | Setting | Population | Intervention 1 | Intervention 2 | Treatment duration | Follow‐up | Background Treatment | Notes |
| Topical antibiotics versus placebo/no treatment (no background or aural toileting) | ||||||||
| (n = 50) | Specialist hospital, Thailand | Mucopurulent otorrhoea with perforated tympanic membrane (CSOM) | Ciprofloxacin 250 mg/mL, 5 drops per 8 hours | Saline, 5 drops per 8 hours | 1 week | 1 week | Aural toilet on day 1, 4 and 7 | Randomised by person |
| Topical antibiotic versus placebo/no treatment (systemic antibiotic as background treatment) | ||||||||
| (n = 50) | General hospital, Spain | Simple chronic otitis media (36%), osteitic chronic otitis media (25.6%), cholesteatomas chronic otitis media (13.6%), post surgery cases (24.8%) | Topical ciprofloxacin 0.2%, 3 drops per 8 hours and oral ciprofloxacin, 500 mg per 12 hours | No treatment | 7 days | 15 days | Aural toileting before beginning treatment, analgesics and antipyretics. Oral ciprofloxacin, 500 mg per 12 hours | Part of 5‐arm trial Randomised by person |
| (n = 40) | University clinic, Italy | Mild or moderate CSOM in acute stage | Ciprofloxacin 250 µg/mL, 3 drops per 12 hours | No treatment | 5 to 10 days | 2 weeks | Oral ciprofloxacin, 250mg per 12 hours | Part of 3‐arm trial Randomised by person |
| (n = 50) | University clinic, Italy | Recurrence of CSOM or suppuration following mastoidectomy or tympanoplasty | Ceftizoxime 500 µg/mL, 2 x 2 mL washes per 12 hours | Saline, 2 x 2 mL washes per 12 hours | 1 week | 3 weeks | Systemic ceftizoxime by intramuscular route every 12 hours Aural toilet at first visit | Randomised by person |
| (n = 100) | ENT departments, Spain | Simple chronic otitis media (42.7%), chronic otitis media with osteolysis (19%), chronic cholesteatoma (14%), chronic otorrhoea in operated ears 24.3%) | Ciprofloxacin 0.2%, 0.5 mL per 8 hours | No treatment | 1 week | 10 days | Oral ciprofloxacin, 500 mg per 12 hours | Part of a 6‐arm trial Randomised by person |
| Quinolones versus aminoglycosides | ||||||||
| (n = 134) | ENT department, Pakistan | Active tubotympanic type CSOM | Ofloxacin 0.3%, 12 drops per day | Gentamycin 0.3%, 12 drops per day | 10 days | 2 weeks | None mentioned | Randomised by person |
| (n = 40) | Outpatient clinic, Israel | Chronic otitis media | Ciprofloxacin (no conc), 15 drops per day | Tobramycin (no conc), 15 drops per day | 3 weeks | 3 weeks | None mentioned | Part of 3‐arm trial Randomised by ear |
| (n = 40) | University ENT clinic, Turkey | CSOM | Ciprofloxacin 0.3%, 6 drops per day | Tobramycin 0.3%, 6 drops per day | 3 weeks | 3 weeks | Daily aspiration | Translated from Turkish Part of 4‐arm trial Randomised by person. |
| (n = 88) | Unclear setting, Jordan | CSOM and intermittent mucopurulent heavy discharge for more than 1 year | Ciprofloxacin 200 µg/mL (0.02%), 15 drops per day | Gentamicin 5 mg/mL, 15 drops per day | 10 days | 2 weeks | None mentioned | Randomised by person |
| (n = 308) | Hospital ENT clinics, Spain | CSOM (purulent discharge > 3 months and perforated membrane) | Ciprofloxacin 0.3%, 15 drops per day | Gentamycin 0.3%, 15 drops per day | 8 days | 30 days | Unclear | Translated from Spanish Assume this is same as Sabater paper Randomised by person |
| (n = 44) | University hospital, Turkey | CSOM and purulent discharge for more than 1 year | Ciprofloxacin 200 µg/mL (0.02%), 15 drops per day | Gentamicin 5 mg/mL, 15 drops per day | 10 days | 10 days | None mentioned | Randomised by person |
| (n = 80) | Otolaryngology department, Pakistan | CSOM (tubotympanic type) | Ofloxacin 0.6%, 12 drops per day | Gentamycin 0.3%, 12 drops per day | 2 weeks | 2 weeks | One aural toilet at start | Randomised by person |
| Quinolones versus others | ||||||||
| (n = 200) | Specialist hospital, Pakistan | Tubotympanic type of CSOM | Ciprofloxacin (no conc), 3 drops per 12 hours | Neomycin/polymixin/gramicidin‐D (no conc), 2 drops per 12 hours | Unclear (probably 4 weeks) | 4 weeks | No information | Randomised by person |
| (n = 50) | Rural setting, Malawi | Children with CSOM | Ofloxacin 0.3%, 3 drops per 8 hours | Neomycin 0.5%/polymixin B 0.1%, 3 drops per 8 hours | 2 weeks | 2 weeks | Aural toilet at start and weekly | Part of a 3‐arm trial Only presented as an internal report Unclear unit of randomisation, results reported by ear |
| (n = unclear) | Rural setting, Malawi | "Mainly children" with CSOM | Ofloxacin 0.3%, 6 drops per 12 hours | Neomycin/polymixin B (no conc), 6 drops per 12 hours | 2 weeks | 8 weeks | Aural toilet at start and weekly | Only a presentation given at a conference available Unclear unit of randomisation, results presented by ear Part of 4‐arm trial ‐ once weekly arms have not been included. |
| Aminoglycosides versus trimethoprim, sulphacetamide and polymixin B (TSP) | ||||||||
| (n = 91) | Outpatient clinic, Canada | Otitis externa (21%), CSOM (51%), subacute otitis (16%), postoperative infection (21%) | Trimethoprim, sulphacetamide and polymyxin B, 16 drops per day | Gentamicin 0.3%, 16 drops per day | Mean: 16 days | 12 months | Not reported | Translated from French Randomised by person but reported by ear Semi cross‐over trial |
| Rifampicin versus chloramphenicol | ||||||||
| (n = 160) | Outpatient department, China | CSOM | Rifampicin 0.1%, 9 drops per day | Chloramphenicol 0.25%, 9 drops per day | 2 weeks | 2 weeks | 3% hydrogen peroxide ear wash daily | Translated from Chinese Randomised by person |
CSOM: chronic suppurative otitis media
Study design
Ten studies were two‐arm trials (Asmatullah 2014; Gyde 1978; Jamalullah 2016; Kasemsuwan 1997; Liu 2003; Lorente 1995; Mira 1993; Nawasreh 2001; Siddique 2016; Tutkun 1995) and three studies were three‐arm trials (Esposito 1990; Fradis 1997; van Hasselt 1997). Two studies were part of a five‐arm trial (de Miguel 1999; Ramos 2003), where only the arms that compared topical antibiotics plus systemic antibiotics with systemic antibiotics alone were used in this review. Two studies were part of a four‐arm trial (Kaygusuz 2002; van Hasselt 1998a), but only two arms are presented in this review. Details of the other study arms for each study can be found in the Characteristics of included studies table.
All studies provided an indication that they were 'randomised controlled trials' and were parallel‐group studies, apart from Gyde 1978, which was a cross‐over RCT.
Sample size
The total number of participants was 2198. Twelve studies reported the sample size in terms of participants (not ears); these had a total of 1797 participants (Asmatullah 2014; de Miguel 1999; Esposito 1990; Jamalullah 2016; Kasemsuwan 1997; Liu 2003; Lorente 1995; Mira 1993; Nawasreh 2001; Ramos 2003; Siddique 2016; Tutkun 1995). The remaining five studies reported both the number of patients and ears, representing 401 participants, or 510 ears (Fradis 1997; Gyde 1978; Kaygusuz 2002; van Hasselt 1997; van Hasselt 1998a).
Unit of randomisation
The individual (rather than the ear) was randomised to treatment group in 14 studies (Asmatullah 2014; de Miguel 1999; Esposito 1990; Gyde 1978; Jamalullah 2016; Kasemsuwan 1997; Kaygusuz 2002; Liu 2003; Lorente 1995; Mira 1993; Nawasreh 2001; Ramos 2003; Siddique 2016; Tutkun 1995). (See Table 4). Of these 14 studies, only one reported the number of patients with bilateral disease (15 patients (8%) had bilateral disease), but as the denominator was by person, it is assumed that no double counting occurred (Siddique 2016). Although Gyde 1978 was randomised by person, the results were reported by ear. The study Gyde 1978 stated that if there was a treatment failure 'ears' were transferred to the alternative treatment group, thus effectively breaking randomisation. These results have not been included in the analysis.
| Reference | Unit of randomisation | Reported | Definition | Otoscopically confirmed? | Time points | Notes |
| Person | Person | "no discharge" | Yes | 1 to 2 weeks (10 days) | — | |
| Person | Person | "global index of clinic microbiological cure" | Yes | 1 to 2 weeks (7 days) | — | |
| Person | Person | "cured" (no definition but assumed to be no discharge) | Unclear, paper states "clinically examined" | 1 week to 2 weeks (6 to 11 days) and 2 to 4 weeks (19 to 24 days) | 1 to 2 weeks examined but not reported | |
| Ear | Ear | "clinical success as defined as cessation of otorrhea and eradication of the microorganisms in the post treatment culture" | Unclear | 2 to 4 weeks (21 days) | Unclear how many patients had bilateral ear disease in each group | |
| Person | Ear | "dry ear" and a negative culture at 3 weeks or a real improvement in at 3 weeks and the cessation of discharge at 6 weeks | Unclear | 2 to 4 weeks (3 weeks) and after 4 weeks (6 weeks) | Semi cross‐over trial. It does not appear that any consideration of the correlation of results between ears has been taken into account. If there was a treatment failure 'ears' were transferred to the alternative groups. These results have not been included in the analysis. If the ear was not dry on review at 6 months, treatment for 3 weeks with the alternative treatment was completed with review after 6 months | |
| Person | Person | "absence" of aural discharge | Yes | 2 to 4 weeks (2 weeks) | — | |
| Person | Person | "cure" | Unclear | 1 to 2 weeks (7 days) | — | |
| Person | Ear | Assessed using 3‐point scale (2 points = no drainage) | Yes | 2 to 4 weeks (day 14 and 21) | Unclear method of allocation, unsure if random selection of study ear | |
| Person | Person | "Cured: otorrhea disappeared, mucosal hyperaemia of the tympanic membrane and tympanic cavity disappeared. Significantly effective: no complaints of otorrhea, no visible purulence in the ear canal and tympanic cavity, and nonvisible or slight hyperaemia of the tympanic membrane and the tympanic canal" | Unclear | 1 to 2 weeks (2 weeks) | — | |
| Person | Person | "Complete resolution of ear discharge" | Yes | 1 to 2 weeks (8 days) and after 4 weeks (30 days) | — | |
| Person | Person | Not reported in a way that could be used in the review | N/A | N/A | Paper plotted the time course of otorrhoea (quantity) on a scale of 0 to 3 at 3, 7 and 21 days | |
| Person | Person | "cessation of otorrhea" | Yes | 1 to 2 weeks (10 days) | — | |
| Person | Person | "cured" according to "indices de curacion" | Unclear | 1 to 2 weeks (10 days) | — | |
| Person | Person | "absence of discharge from middle ear cavity and no inflammation/congestion in middle ear mucosa and tympanic membrane" | Unclear | 2 to 4 weeks (4 weeks) | 15 patients (8%) had bilateral disease but how these cases were handled is not stated. The denominator in the trials is the person so it is assumed that no double counting occurred. | |
| Person | Person | "cessation of otorrhea" | Yes | 1 to 2 weeks (10 days) | — | |
| Unclear, most likely person | Ear | "dry ear" | Unclear | 1 to 2 weeks (1 week) and after 4 weeks (> 2 weeks) | Counting bilateral ears separately. All ears reported separately. Data come from an unpublished report. In the analysis 3/11 (27.27%), 10/30 (33%) and 11/28 (39%) of patients had bilateral disease in the ofloxacin, neomycin and antiseptic acid groups respectively. | |
| Unclear | Ear | "inactive ear" ‐ completely dry middle ear | Unclear | 1 to 2 weeks (1 week), 2 to 4 weeks (2 weeks) and after 4 weeks (8 weeks) | Counting bilateral ears separately |
N/A: not applicable
In the remaining one study randomisation occurred by ear and it appears that the analysis occurred without allowing for correlation/adjustment for the response between paired ears (Fradis 1997). The unit of randomisation for two other studies is unclear: it is most likely by person but the results are reported by ear (van Hasselt 1997; van Hasselt 1998a). In the two studies by van Hasselt, bilateral ears were counted separately, but the number of patients per group was not reported. Data from van Hasselt 1997 come from an unpublished report, with the analysis indicating that 3/11 (27.27%), 10/30 (33%) and 11/28 (39%) of patients had bilateral disease in the ofloxacin, neomycin and antiseptic acid groups respectively. The results of these studies were not included in the analysis due to the risk of double counting.
Location
The studies were conducted in 10 countries including: Israel, Thailand, China, Canada, Jordan, Turkey, Italy, Malawi, Pakistan and Spain (see Table 3).
Setting of trial
With regard to clinical setting, six studies were in outpatient departments of hospitals/medical centres (Fradis 1997; Gyde 1978; Jamalullah 2016; Liu 2003; Mira 1993; Ramos 2003); two studies were based in secondary care from the ENT departments of hospitals (Asmatullah 2014; Lorente 1995); two were in a specialist hospital (Kasemsuwan 1997; Siddique 2016); and one study was in the general hospital (de Miguel 1999). Three studies were undertaken in university clinics/hospitals (Esposito 1990; Kaygusuz 2002; Tutkun 1995), while two studies were community studies taking place in rural settings (van Hasselt 1997; van Hasselt 1998a). The setting of the study was unclear in one trial (Nawasreh 2001).
The years in which the studies were conducted were often not well reported. There was one study from the 1970s (Gyde 1978), and one study in which it is unclear but was most likely to have been conducted in the 1980s (Esposito 1990). Nine studies were conducted in the 1990s (de Miguel 1999; Fradis 1997; Kasemsuwan 1997; Lorente 1995; Mira 1993; Nawasreh 2001; Tutkun 1995; van Hasselt 1997; van Hasselt 1998a), three were published in the 2000s (Kaygusuz 2002; Liu 2003; Ramos 2003), and three were published post 2010 (Asmatullah 2014; Jamalullah 2016; Siddique 2016). See Table 3 for further details.
Population
Age and sex
Two studies did not provide any patient characteristics, with the age only referred to as "mainly children" (van Hasselt 1997; van Hasselt 1998a).
Fifteen studies provided information on the age of participants, with the mean age ranging from 25.8 to 44.4 years old (Asmatullah 2014; de Miguel 1999; Esposito 1990; Fradis 1997; Gyde 1978; Jamalullah 2016; Kasemsuwan 1997; Kaygusuz 2002; Liu 2003; Lorente 1995; Mira 1993; Nawasreh 2001; Ramos 2003; Siddique 2016; Tutkun 1995). The de Miguel 1999 study reported a mean age of 39.6 years, however 17/25 participants were children.
All 15 studies that provided participant characteristics reported that they included both males and females. Of the 2010 participants reported, 860 (43%) were female, with the percentage of females in studies ranging from 18% to 63%.
High‐risk populations
None of the studies reported the inclusion of any of the 'high‐risk' populations as defined by our inclusion criteria (cleft palate, Down syndrome, Indigenous groups, immunocompromised patients). Two studies specifically stated "no diabetes or other comorbidities" (Esposito 1990) or "0% immunocompromised" (Siddique 2016), while the remaining 15 did not report any high‐risk populations (Asmatullah 2014; de Miguel 1999; Fradis 1997; Gyde 1978; Jamalullah 2016; Kasemsuwan 1997; Kaygusuz 2002; Liu 2003; Lorente 1995; Mira 1993; Nawasreh 2001; Ramos 2003; Tutkun 1995; van Hasselt 1997; van Hasselt 1998a). However, the two van Hasselt studies were conducted in rural community areas in Malawi, with the van Hasselt 1997 study noting that hygiene was 'poor'.
Diagnosis
Eight studies provided the diagnosis method for confirmation of tympanic membrane perforation or presence of mucopurulent discharge via otoscopy or microscopic examination (de Miguel 1999; Fradis 1997; Jamalullah 2016; Kasemsuwan 1997; Kaygusuz 2002; Ramos 2003; Tutkun 1995; van Hasselt 1997), while an additional study confirmed perforation of the tympanic membrane but did not provide a method (Lorente 1995). The diagnostic method was not reported in eight studies (Asmatullah 2014; Esposito 1990; Gyde 1978; Liu 2003; Mira 1993; Nawasreh 2001; Siddique 2016; van Hasselt 1998a).
Duration of ear discharge
Nine studies reported the duration of symptoms/mucopurulent discharge for diagnosis, with one study including patients with ear discharge for more than six weeks or sporadically with three or more episodes in a year (Ramos 2003), one study with discharge for more than two months (van Hasselt 1998a), four studies with ear discharge for more than three months (Jamalullah 2016; Kasemsuwan 1997; Kaygusuz 2002; Lorente 1995), and a further three studies longer than one year (Fradis 1997; Nawasreh 2001; Tutkun 1995). Eight studies did not have inclusion criteria or provide details of the average duration of ear discharge at the start of the study (Asmatullah 2014; de Miguel 1999; Esposito 1990; Gyde 1978; Liu 2003; Mira 1993; Siddique 2016; van Hasselt 1997).
Other important effect modifiers
Nine studies did not report on any important effect modifiers (Asmatullah 2014; Kasemsuwan 1997; Liu 2003; Lorente 1995; Nawasreh 2001; Siddique 2016; Tutkun 1995; van Hasselt 1997; van Hasselt 1998a). One study reported the previous use of grommets (Ramos 2003; n = 12; 4%). Five studies provided alternative diagnoses (de Miguel 1999 (n = 17; 13.6%); Gyde 1978 (n = 24; 21%); Mira 1993 (n = 52; 21%); Ramos 2003 (n = 42; 14%)). Five studies reported the number having previous surgery (de Miguel 1999 (n = 31; 24.8%); Fradis 1997 (n = 8; 15.7%); Kaygusuz 2002 (none); Mira 1993 (n = 52; 21%); Ramos 2003 (n = 73; 24.3%)). Four studies reported the number having previous antibiotic treatment for CSOM (de Miguel 1999 (n = 79; 63.2%); Esposito 1990 (n = 38; 63%); Fradis 1997 (n = 46; 90.2%); Ramos 2003 (n = 197; 65.6%)).
Intervention
Intervention details
Details of the interventions, background treatments and treatment durations for each of the included studies are summarised in Table 3.
Topical antibiotics
Fourteen studies used topical quinolones, with four of these using ofloxacin (Asmatullah 2014; Jamalullah 2016; van Hasselt 1997; van Hasselt 1998a) and the remaining 10 using ciprofloxacin (de Miguel 1999; Esposito 1990; Fradis 1997; Kasemsuwan 1997; Kaygusuz 2002; Lorente 1995; Nawasreh 2001; Ramos 2003; Siddique 2016; Tutkun 1995).
Nine studies used aminoglycosides, with six using gentamycin or gentamicin (Asmatullah 2014; Gyde 1978; Jamalullah 2016; Lorente 1995; Nawasreh 2001; Tutkun 1995), two using tobramycin (Fradis 1997; Kaygusuz 2002), and one using neomycin (Siddique 2016).
Two studies used neomycin/polymixin B (van Hasselt 1997; van Hasselt 1998a). The remaining four studies used other topical antibiotics, including rifampicin (Liu 2003), chloramphenicol (Liu 2003), ceftizoxime (Mira 1993) and trimethoprim, sulphacetamide and polymixin B (TSP) combination (Gyde 1978).
Background treatment
Nine studies used aural toileting at different frequencies: two at the start of trial (Jamalullah 2016; Mira 1993), four before each treatment (de Miguel 1999; Gyde 1978; Kaygusuz 2002; Liu 2003), two at the start of trial and at one and two weeks (van Hasselt 1997; van Hasselt 1998a), and one at days one, four and seven (Kasemsuwan 1997).
Three studies used systemic antibiotics as a background treatment, with three via the oral route (de Miguel 1999; Esposito 1990; Ramos 2003) and one via the intramuscular route (Mira 1993). One study also had a background treatment of analgesics and antipyretics (de Miguel 1999).
A further six studies did not mention the use of any background treatments (Esposito 1990; Fradis 1997; Lorente 1995; Nawasreh 2001; Siddique 2016; Tutkun 1995).
Duration of intervention
Nine studies treated for less than two weeks (Asmatullah 2014; de Miguel 1999; Esposito 1990; Kasemsuwan 1997; Lorente 1995; Mira 1993; Nawasreh 2001; Ramos 2003; Tutkun 1995). Seven studies treated for between two to four weeks (Fradis 1997; Gyde 1978; Jamalullah 2016; Kaygusuz 2002; Liu 2003; van Hasselt 1997; van Hasselt 1998a). One study treated for four weeks (Siddique 2016).
Comparison
One study analysed topical antibiotic versus placebo:
-
Kasemsuwan 1997 ‐ ciprofloxacin versus placebo (saline).
Sixteen studies compared different topical antibiotics (topical antibiotic A versus topical antibiotic B).
-
Four studies analysed topical antibiotic versus no treatment/placebo (with systemic antibiotics as background):
-
de Miguel 1999 ‐ ciprofloxacin versus no treatment;
-
Esposito 1990 ‐ ciprofloxacin versus no treatment;
-
Mira 1993 ‐ ceftizoxime versus placebo (saline);
-
Ramos 2003 ‐ ciprofloxacin versus no treatment.
-
-
Seven studies analysed quinolones versus aminoglycosides:
-
Asmatullah 2014 ‐ ofloxacin versus gentamycin;
-
Fradis 1997 ‐ ciprofloxacin versus tobramycin;
-
Jamalullah 2016 ‐ ofloxacin versus gentamycin;
-
Kaygusuz 2002 ‐ ciprofloxacin versus tobramycin;
-
Lorente 1995 ‐ ciprofloxacin versus gentamycin;
-
Nawasreh 2001 ‐ ciprofloxacin versus gentamicin;
-
Tutkun 1995 ‐ ciprofloxacin versus gentamicin.
-
-
Three studies analysed quinolones versus aminoglycosides/polymixin B combination ± gramicidin:
-
Siddique 2016 ‐ ciprofloxacin versus neomycin/polymixin/gramicidin‐D;
-
van Hasselt 1997 ‐ ofloxacin versus neomycin/polymixin B;
-
van Hasselt 1998a ‐ ofloxacin versus neomycin/polymixin B.
-
-
Two studies analysed other antibiotics:
Outcome
Resolution of ear discharge
The definitions, methods and timing of assessment differed between studies, and these are summarised in Table 4.
Health‐related quality of life using a validated instrument
No studies reported health‐related quality of life.
Ear pain (otalgia) or discomfort or local irritation
Four studies measured adverse effects associated with treatment: one described measuring local sensitivity (Gyde 1978), one specified symptoms further, examining ear pain, itching and stinging (measured on a four‐point scale: 0 = none, 3 = severe) (Lorente 1995), whilst the other two broadly mentioned that this was measured in relation to the topical medication (Kasemsuwan 1997; Siddique 2016).
Hearing
Six studies indicated that they measured hearing pre‐ and post‐treatment, however none of these provided details regarding the methods used including whether air or bone conduction methods were used or the frequencies of testing (Fradis 1997; Gyde 1978; Kasemsuwan 1997; Nawasreh 2001; Tutkun 1995; van Hasselt 1997). All results were reported narratively.
Serious complications (including intracranial complications, extracranial complications and death)
Although serious complications, including intracranial complications (such as otitic meningitis, lateral sinus thrombosis and cerebellar abscess) and extracranial complications (such as mastoid abscess, postauricular fistula and facial palsy) and death were not specifically listed in the methods as outcomes that would be recorded, three papers report that no side effects occurred in any participants, which would include the defined serious complications (Gyde 1978; Siddique 2016; Tutkun 1995).
Suspected ototoxicity
One study stated that they tested audiometric and vestibular function to measure suspected ototoxicity outcomes (Esposito 1990). Another study assessed suspected ototoxicity with an audiogram, although a specific definition was not stated, and reported that 0/125 suffered from ototoxicity associated with treatment (Ramos 2003). Three additional studies listed suspected ototoxicity as a potential outcome, but did not describe how it would be assessed (de Miguel 1999; Kasemsuwan 1997; Siddique 2016).
Excluded studies
We excluded 146 papers (150 papers including unpublished studies) (122 studies) after reviewing the full text. Further details for the reasons for exclusion can be found in the Characteristics of excluded studies table. These are the main reasons for the exclusion:
We excluded 40 studies (59 references, 63 including unpublished studies)) because the comparisons were not appropriate for this review, but were relevant to another review in this suite of reviews (Boesorire 2000; Browning 1988; Couzos 2003; Crowther 1991; Eason 1986; Esposito 1992; Fliss 1990; Gendeh 2001; Ghosh 2012; Gupta 2015; Helmi 2000; I‐HEAR‐BETA (in progress study); Indudharan 2005; Jaya 2003; Kiris 1998; Lazo Saenz 1999; Leach 2008; Legent 1994; Loock 2012; Macfadyen 2005; Minja 2006; Miro 2000; Nwokoye 2015; Onali 2018; Panchasara 2015; Papastavros 1989; Picozzi 1983; Picozzi 1984; Povedano 1995; Renukananda 2014; Rotimi 1990; Sanchez Gonzales 2001; Smith 1996; Somekh 2000; Subramaniam 2001; Tong 1996; van der Veen 2007; van Hasselt 1998b; Vishwakarma 2015; Yuen 1994).
We excluded 38 studies (39 references) on the basis of their study design (Baba 1986; Baba 2008; Bluestone 2001; Brook 1979; Brook 1980; Deguchi 1985; Deguchi 1986; Deitmer 2002; Dohar 2002; Esposito 2000; Gehanno 1997; Harris 2016; Hwang 2015; Jahn 1984; Jang 2004; Kadar 2003; Kashiwamura 2004; Kenna 1986; Kothari 1969; Kovacic 1999; Kurilin 1976; Lancaster 1999; Lancaster 2003; Lang 1992; Lautala 1983; Manolidis 2004; Merifield 1993; Morgon 1976; Otwombe 2003; Poliakova 1991; Singhal 1992; Sultan 2017; Sumitsawan 1995; Supiyaphun 1995; Tachibana 1986; Thomsen 1976; Wintermeyer 1997; Wright 2009).
We excluded 22 studies (24 references) due to the population characteristics included in their study (Abbott 2016; Alper 2000; Baba 1982b; Baba 1983; Baba 1983b; Baba 1987; Berman 1990; Block 2000; Bross Soriano 1996; Clayton 1990; Garcia‐Rodriguez 1993; Granath 2007; Gyde 1981; Gyde 1982; Mendelman 1992; Mesure 1973; Principi 1995; Quick 1973; Quick 1975; Saez‐Llorens 2005; Stenstrom 1991; van Dongen 2014).
We excluded 17 studies (18 references) because the interventions were outside of the protocol (Blekher 1967; Browning 1983; Connolly 1997; Dellamonica 1995; Fraysse 1988; ISRCTN12149720; ISRCTN84220089; ISRCTN86106121; Jiang 2016; Khanna 2000; Li 2004; Mora 2012; NCT02592096; NCT02817347; Shkil' 1964; Wilde 1995; Xu 1999).
Five studies (six references) had multiple reasons for exclusion (Baba 1980; Fombeur 1994; Hemlin 1997; Khon 2012; Lorentzen 1978).
Risk of bias in included studies
See Figure 2 for a 'Risk of bias' graph (our judgements about each risk of bias item presented as percentages across all included studies) and Figure 3 for a 'Risk of bias' summary (our judgements about each risk of bias item for each included study).

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Sequence generation
We assessed five studies to be at high risk of selection bias with regards to randomisation (Esposito 1990; Liu 2003; Nawasreh 2001; Siddique 2016; van Hasselt 1997). For Liu 2003, it is not clear whether or not the patients were randomised. Similarly for Siddique 2016 it was unclear what the actual process for the 'non‐probability' sampling was and how to ensure that the allocation was random. Esposito 1990 did not clearly specify the randomisation method and while it was noted that 38/60 patients were previously unsuccessfully treated with at least five days of antibiotics, it was unclear how this was distributed across groups. The abstract for Nawasreh 2001 mentions that randomisation occurred but there was no mention of randomisation or methods for randomisation in the full paper, which only described patients as being "divided" between the two groups. It is unclear if the groups were evenly distributed as there was 40 in one group and 48 in the other, with baseline characteristics between the group not provided. The original report for van Hasselt 1997 indicates this was a "pilot trial", with no reference to blinding or randomisation. However, this was mentioned as a "randomised" trial in the introduction of a 2002 paper by the author. If randomisation was done, there was also no clear ratio for randomisation, with 46, 38 and 12 in the three treatment groups and the cheapest intervention having the most participants.
We assessed one study as low risk (Gyde 1978) and the remaining 11 studies as 'unclear risk' as they did not provide enough information (Asmatullah 2014; de Miguel 1999; Fradis 1997; Jamalullah 2016; Kasemsuwan 1997; Kaygusuz 2002; Lorente 1995; Mira 1993; Ramos 2003; Tutkun 1995; van Hasselt 1998a).
Allocation concealment
We assessed three studies to be at low risk of allocation concealment bias (Fradis 1997; Gyde 1978; Kasemsuwan 1997).
We assessed the remaining 14 studies as at unclear risk as they did not provide enough information with regards to allocation concealment (Asmatullah 2014; de Miguel 1999; Esposito 1990; Jamalullah 2016; Kaygusuz 2002; Liu 2003; Lorente 1995; Mira 1993; Nawasreh 2001; Ramos 2003; Siddique 2016; Tutkun 1995; van Hasselt 1997; van Hasselt 1998a).
Blinding
Performance bias
We assessed 11 studies as high risk for performance bias. Two were at high risk due to a lack of blinding for patients and healthcare practitioners even when it was possible to do (Asmatullah 2014; Jamalullah 2016). Seven were at high risk due to blinding being impossible because of the differences in treatment regimens/administration that mean it is likely to be known to which group they were allocated (de Miguel 1999; Esposito 1990; Liu 2003; Nawasreh 2001; Ramos 2003; Siddique 2016; van Hasselt 1998a). One study was at high risk due to the absence of a clear statement on blinding (Kaygusuz 2002), and one study did not provide details of who was blinded during the "single‐blinded" study (Mira 1993). In this case we assumed that it was the patients who were blinded to treatment, but as the main outcomes were physician‐reported, blinding the patients would not have prevented performance bias. We assessed two studies as low risk because the used sufficient blinding methods (Fradis 1997; Kasemsuwan 1997), while four were at unclear risk due to lack of information (Gyde 1978; Lorente 1995; Tutkun 1995; van Hasselt 1997).
Detection bias
We assessed nine studies to be at high risk of bias. Four of these were due to the subjective nature of the judgement of outcomes (Asmatullah 2014; de Miguel 1999; Esposito 1990; Ramos 2003), whereas three did not mention any attempts to blind assessors even though it would have been feasible (Jamalullah 2016; Nawasreh 2001; Siddique 2016). One study stated that it was double‐blind, although the treatment regimens were not the same and no placebo was used (Kaygusuz 2002). One study did not provide a clear statement on blinding (van Hasselt 1998a). We assessed two studies to be low risk because blinding appeared to be adequate to make detection unlikely (Fradis 1997; Kasemsuwan 1997). We assessed six studies as at unclear risk because there was no mention of blinding or further clarification was necessary but omitted (Gyde 1978; Liu 2003; Lorente 1995; Mira 1993; Tutkun 1995; van Hasselt 1997).
Incomplete outcome data
We assessed four studies to be at high risk of attrition bias (Kasemsuwan 1997; Nawasreh 2001; Tutkun 1995; van Hasselt 1997). Two of these were due to issues regarding participation, with high dropout rates ‐ over 25% in the short time periods within the trials (Kasemsuwan 1997; van Hasselt 1997). It was also noted that Nawasreh 2001 and van Hasselt 1997 had an imbalance of participants between the allocation groups, which could have led to bias. We deemed one study to be at high risk due to a lack of clarity in the statement regarding exclusion of patients ‐ whether this was part of the recruitment criteria or after randomisation (Tutkun 1995).
We assessed eight studies to be low risk, as all or more than 90% of participants appearing in the trial are accounted for in results (Esposito 1990; Gyde 1978; Jamalullah 2016; Kaygusuz 2002; Liu 2003; Mira 1993; Ramos 2003; Siddique 2016). We assessed a further five studies as having unclear risk due to lack of a statement or reasoning (Asmatullah 2014; de Miguel 1999; Fradis 1997; Lorente 1995; van Hasselt 1998a).
Selective reporting
None of the 17 studies had protocols identified through searches of clinical trials registries.
We assessed seven studies to be at high risk of selective reporting bias: three of these studies were due to the methods section not being well presented (Asmatullah 2014; Mira 1993; Nawasreh 2001), three were due to discrepancies in time point outcome reporting (Esposito 1990; Siddique 2016; van Hasselt 1997), and one was due to the study not being published, making it difficult to evaluate the methods fully (van Hasselt 1998a).
We assessed two studies as low risk due to adequate outcome reporting between the methods and results, however no protocols were found (Gyde 1978; Kaygusuz 2002). We assessed the remaining eight studies as having unclear risk of selective reporting (de Miguel 1999; Fradis 1997; Jamalullah 2016; Kasemsuwan 1997; Liu 2003; Lorente 1995; Ramos 2003; Tutkun 1995).
Other potential sources of bias
Funding
Esposito 1990 stated that "the ciprofloxacin tablets and powder used in this study were kindly provided by Bayer Italia Spa, Milan, Italy." Although it was not stated, we assumed one study to have funding from the Christian Blind Mission, as other studies by the same authors were funded through this avenue (van Hasselt 1997). One study specifically stated that "no funding was received from any agency or institution" (Siddique 2016), while the remaining 14 studies provided no information (Asmatullah 2014; de Miguel 1999; Fradis 1997; Gyde 1978; Jamalullah 2016; Kasemsuwan 1997; Kaygusuz 2002; Liu 2003; Lorente 1995; Mira 1993; Nawasreh 2001; Ramos 2003; Tutkun 1995; van Hasselt 1998a).
Declarations of interest
Siddique 2016 stated that "[the] abstract and results of this study were accepted and presented in an oral presentation at the International conference on Medical Education, organised by Association for Excellence in Medical Education (AEME) and held on 07th‐09th March 2014 at University of Health Sciences (UHS) Lahore, Pakistan." The remaining studies did not provide any information about conflicts of interest (Asmatullah 2014; de Miguel 1999; Esposito 1990; Fradis 1997; Gyde 1978; Jamalullah 2016; Kasemsuwan 1997; Kaygusuz 2002; Liu 2003; Lorente 1995; Mira 1993; Nawasreh 2001; Ramos 2003; Tutkun 1995; van Hasselt 1997; van Hasselt 1998a).
Effects of interventions
See: Summary of findings for the main comparison Topical antibiotics versus placebo/no treatment for chronic suppurative otitis media; Summary of findings 2 Topical antibiotics on top of systemic antibiotics for chronic suppurative otitis media; Summary of findings 3 Quinolones versus aminoglycosides for chronic suppurative otitis media
Comparison 1: Topical antibiotics versus placebo or no treatment
One study was included in this comparison: Kasemsuwan 1997 (50 participants), which compared topical ciprofloxacin to saline. See also summary of findings Table for the main comparison.
Primary outcomes
Resolution of ear discharge or 'dry ear'
Between one week and up to two weeks
Kasemsuwan 1997 identified that topical antibiotics appeared to be more effective than saline at one to two weeks follow‐up (risk ratio (RR) 6.74, 95% confidence interval (CI) 1.82 to 24.99; 35 participants; very low‐certainty evidence; Analysis 1.1).
Two weeks to up to four weeks and after four weeks
The study did not report the results per person for this outcome at between two to four weeks or after four weeks.
Health‐related quality of life using a validated instrument
This outcome was not reported.
Ear pain (otalgia) or discomfort or local irritation
Kasemsuwan 1997 reports that "no medical side‐effects and worsening of audiological measurements related to this topical medication were detected" (very low‐certainty evidence).
Secondary outcomes
Hearing
Kasemsuwan 1997 reported "no worsening of audiological outcomes", but specific information relating to hearing levels was not presented (very low‐certainty evidence).
Serious complications (including intracranial complications, extracranial complications and death)
The study did not report that any participant died or had any intracranial or extracranial complications.
Suspected ototoxicity
Kasemsuwan 1997 reported "no suspected ototoxicity" but it is unclear how this was measured.
Subgroup analysis
With only one study included in the quantitative analysis, subgroup analysis was not possible (very low‐certainty evidence).
Comparison 2: Topical antibiotics versus placebo or no treatment (with systemic antibiotics as background treatment)
Four studies (190 participants) were included in this comparison, three of which compared treatment with topical ciprofloxacin drops and systemic ciprofloxacin to systemic ciprofloxacin only (de Miguel 1999; Esposito 1990; Ramos 2003). The remaining study, Mira 1993 (248 participants), compared topical ceftizoxime to placebo ear drops (saline) for seven days with all participants in both treatment arms received intravenous ceftizoxime. None of the primary or secondary outcomes were reported. Two of the studies had reported exactly the same percentages of "cure" across all but one of the intervention arms of the studies (de Miguel 1999; Ramos 2003). We had concerns that these could have been the same set of patients, but the authors clarified that these were entirely different patients (and studies). See summary of findings Table 2 for the main comparison.
Primary outcomes
Resolution of ear discharge or 'dry ear'
Between one week and up to two weeks
Ramos 2003 and de Miguel 1999 found that topical and systemic antibiotics resulted in more dry ears compared to the systemic antibiotics only group (RR 1.47, 95% CI 1.20 to 1.80; 150 participants; 2 studies; I2 = 0%; low‐certainty evidence; Analysis 2.1).
Two weeks to up to four weeks
Esposito 1990 found that topical and systemic antibiotics resulted in more dry ears compared to the systemic antibiotics only group (RR 1.88, 95% CI 1.04 to 3.39; 40 participants; Analysis 2.1).
After four weeks
No studies measured the results at this time point.
Health‐related quality of life using a validated instrument
None of the studies measured this outcome.
Ear pain (otalgia) or discomfort or local irritation
None of the studies measured this outcome although one study reported that "no side effect was recorded in any patient..." (Esposito 1990).
Secondary outcomes
Hearing
Although three studies reported hearing as an outcome in their methods section (de Miguel 1999; Esposito 1990; Ramos 2003), none of the studies reported results for this outcome.
Serious complications (including intracranial complications, extracranial complications and death)
No studies reported that any participant died or had any intracranial or extracranial complications.
Suspected ototoxicity
Three studies reported that they did not suspect ototoxicity in any participants, but it is unclear how this was measured (de Miguel 1999; Esposito 1990; Ramos 2003) (very low‐certainty evidence).
de Miguel 1999 reported that the data did not show cochleovestibular dysfunction during treatment or further follow‐up. They also stated that all post‐treatment audiometries showed a lack of antimicrobial ototoxicity, both for oral and topical routes. However, there was no definition of 'ototoxicity' provided and it may be that they only measured air conduction audiological thresholds rather than bone conduction thresholds as well.
Esposito 1990 stated that "no side effect was recorded in any patient and no worsening of the audiometric function related to the local therapy was observed". Audiometric measurement and vestibular tests were performed before and 24 hours after the end of the therapy in patients receiving topical treatment only.
Ramos 2003 reported a lack of symptoms suggesting vestibular problems, but did not provide details on how this was measured or defined.
Subgroup analysis
No subgroup analysis was completed as there were no differences in any of the identified subgroups.
-
High‐risk populations ‐ none of the studies reported high‐risk populations as defined in our methods.
-
Patients with ventilation tubes ‐ none of the studies reported the inclusion of patients with ventilation tubes.
-
Diagnosis of CSOM ‐ most of the studies included mixed populations of CSOM along with ear discharge from other causes.
-
Duration of ear discharge ‐ only one study reported the duration of discharge.
-
Patient age ‐ none of the studies included participants younger than two years of age. Although two studies included children from five years old stratification by age does not appear to have taken place (de Miguel 1999; Ramos 2003).
Comparison 3: Quinolones versus aminoglycosides
Seven studies (734 participants) were included in this comparison (Asmatullah 2014; Fradis 1997; Jamalullah 2016; Kaygusuz 2002; Lorente 1995; Nawasreh 2001; Tutkun 1995). For details of the interventions and comparisons see Table 3. See also summary of findings Table 3.
Primary outcome
Resolution of ear discharge or 'dry ear'
Between one week and up to two weeks
Six studies (694 participants) reported this outcome and found that more participants given topical quinolones had resolution of ear discharge at between one to two weeks compared with topical aminoglycosides (RR 1.95, 95% CI 0.88 to 4.29; 694 participants; 6 studies; I2 = 97%; very low‐certainty evidence; Analysis 3.1). However, we noted that the heterogeneity was very high. This was driven by the largest study (Lorente 1995), which had an extremely high resolution rate in both arms: 95% and 94% in the quinolone and aminoglycoside arms respectively compared with an average of 75% and 34% in the quinolone and aminoglycoside arms in the remaining studies. After an investigation of the study details, it was not possible to identify why the results were so different, and so we kept the study in the analysis and used a random‐effects model.
Two weeks to up to four weeks
Kaygusuz 2002 found no difference between topical and quinolones compared to aminoglycosides (RR 1.88, 95% CI 1.04 to 3.39; 40 participants; Analysis 3.2).
After four weeks
No studies reported the results per person for this outcome.
Health‐related quality of life using a validated instrument
None of the studies measured this outcome.
Ear pain (otalgia) or discomfort or local irritation
Lorente 1995 (308 participants) measured ear pain on a three‐point scale. Results were presented as a mean score. No differences between the two groups were identified (very low‐certainty evidence).
Secondary outcomes
Hearing
Tutkun 1995 (44 participants) presented mean air and bone conduction hearing levels at 500 Hz, 1000 Hz, 2000 Hz and 4000 Hz pre‐ and post‐treatment by treatment group. No standard deviations were presented although the authors assert that "the difference between these two groups was not statistically significant (p>0.01)". Nawasreh 2001 (88 participants) stated that hearing was measured and that neither group showed significant differences. This evidence is of very low certainty.
Serious complications (including intracranial complications, extracranial complications and death)
No studies reported that any participant died or had any intracranial or extracranial complications.
Suspected ototoxicity
Two studies reported that they had assessed patients for suspected ototoxicity (Lorente 1995; Tutkun 1995). Whilst Lorente 1995 did not present any results, Tutkun 1995 indicated that "There were no side effects, and audiometric evaluation yielded no evidence of ototoxicity as reflected by the pure tone threshold and speech discrimination scores in either group" (very low‐certainty evidence).
Subgroup analysis
With the exception of type of antibiotic, no subgroup analysis could be completed:
-
High‐risk populations ‐ none of the studies reported the inclusion of high‐risk populations as defined in our methods.
-
Patients with ventilation tubes ‐ none of the studies reported the inclusion of patients with ventilation tubes.
-
Diagnosis of CSOM ‐ all of the studies reported patients with 'CSOM'.
-
Duration of ear discharge ‐ where given the duration of ear discharge at the start of the study was between three months and two years. There were no studies using a two‐week or six‐week criteria.
-
Patient age ‐ where given, the youngest patients included were nine years of age and no studies stratified participants by age.
Comparison 4: Quinolones versus aminoglycosides/polymixin B ± gramicidin
Three studies were included in this comparison (van Hasselt 1997 (50 participants), van Hasselt 1998a (unclear number of participants) and Siddique 2016 (200 participants)). van Hasselt 1997 and van Hasselt 1998a both compared topical ofloxacin to topical neomycin/polymyxin B, however the unit of randomisation was unclear in both studies and therefore only Siddique 2016, which compared topical ciprofloxacin to topical neomycin/polymixin B and gramicidin, could be included in the analysis.
Primary outcomes
Resolution of ear discharge or 'dry ear'
Between one week and up to two weeks
No studies reported this outcome.
Two weeks to up to four weeks
Siddique 2016 found that treatment marginally favoured the topical quinolones compared to neomycin/polymyxin B and gramicidin for resolution of discharge (RR 1.12, 95% CI 1.03 to 1.22) at two to four weeks (Analysis 3.2).
After four weeks
No studies reported the results per person for this outcome.
Health‐related quality of life using a validated instrument
None of the studies measured this outcome.
Ear pain (otalgia) or discomfort or local irritation
A "few" patients experienced local irritation upon the first instillation of topical treatment. No information was given regarding the number or treatment arm of the participants.
Secondary outcomes
Hearing
None of the studies measured this outcome.
Serious complications (including intracranial complications, extracranial complications and death)
No studies reported that any participant died or had any intracranial or extracranial complications.
Suspected ototoxicity
None of the studies measured this outcome.
Subgroup analysis
With only one study included in the quantitative analysis, subgroup analysis was not possible.
Comparison 5: Aminoglycosides versus trimethoprim, sulphacetamide and polymyxin B (TSP)
One study was included in this comparison: Gyde 1978 (91 participants) compared a topical aminoglycoside to topical trimethoprim, sulphacetamide and polymyxin B. Participants were randomised by ear and there was no adjustment for the paired nature of this data. Whilst resolution of discharge was assessed at two to four weeks there was no extractable efficacy data.
Primary outcomes
Resolution of ear discharge or 'dry ear'
Between one week and up to two weeks
The study did not report this outcome.
Two weeks to up to four weeks
There were no extractable efficacy data.
After four weeks
The study did not report the results per person for this outcome.
Health‐related quality of life using a validated instrument
The study did not measure this outcome.
Ear pain (otalgia) or discomfort or local irritation
The study reported no signs of local sensitivity or fungal proliferation.
Secondary outcomes
Hearing
The study did not measure this outcome.
Serious complications (including intracranial complications, extracranial complications and death)
No side effects were reported.
Suspected ototoxicity
The study reported no signs of ototoxicity. It is unclear how this outcome was measured.
Subgroup analysis
With only one study included in the quantitative analysis, subgroup analysis was not possible.
Comparison 6: Rifampicin versus chloramphenicol
One study was included in this comparison: Liu 2003 (160 participants) compared topical rifampicin to topical chloramphenicol. While resolution of discharge was assessed at one to two weeks there were no extractable efficacy data.
Primary outcomes
Resolution of ear discharge or 'dry ear'
Between one week and up to two weeks
Liu 2003 found that rifampicin resulted in more dry ears compared to chloramphenicol (RR 1.78, 95% CI 1.35 to 2.34) (Analysis 4.1).
Two weeks to up to four weeks
The study did not measure this outcome.
After four weeks
The study did not measure this outcome.
Health‐related quality of life using a validated instrument
The study did not measure this outcome.
Ear pain (otalgia) or discomfort or local irritation
The study reported no signs of local sensitivity or fungal proliferation.
Secondary outcomes
Hearing
The study did not measure this outcome.
Serious complications (including intracranial complications, extracranial complications and death)
The study did not report that any participant died or had any intracranial or extracranial complications.
Suspected ototoxicity
The study reported no signs of ototoxicity. It is unclear how this outcome was measured.
Subgroup analysis
With only one study included in the quantitative analysis, subgroup analysis was not possible.
Discussion
Summary of main results
We found 17 studies reporting on six different comparisons (Asmatullah 2014; de Miguel 1999; Esposito 1990; Fradis 1997; Gyde 1978; Jamalullah 2016; Kasemsuwan 1997; Kaygusuz 2002; Liu 2003; Lorente 1995; Mira 1993; Nawasreh 2001; Ramos 2003; Siddique 2016; Tutkun 1995; van Hasselt 1997; van Hasselt 1998a). Due to the choice of outcome measures used in these studies and the incomplete reporting of results, for many of the comparisons we were not able to find a substantial amount of evidence. Of the 17 studies, one examined the effectiveness of topical antibiotics compared to no treatment (Comparison 1), four examined topical antibiotics compared to no treatment with systemic antibiotics as a background treatment (Comparison 2), and 12 studies directly compared different topical antibiotics (Comparisons 3 to 6).
The following is a summary of the key findings for the comparisons:
Comparison 1: Topical antibiotics versus placebo or no treatment (without background treatment)
We included one study examining topical antibiotics versus no treatment, with no background treatment (Kasemsuwan 1997; 50 participants). This study compared ciprofloxacin drops to saline with resolution of discharge measured at one to two weeks. It was unclear if the resolution of discharge was otoscopically confirmed. Topical antibiotics appeared to be more effective than saline (risk ratio (RR) 6.74, 95% confidence interval (CI) 1.82 to 24.99). However, this study was too small to provide any certainty of the findings (GRADE assessment: very low‐certainty evidence). No adverse events, suspected ototoxicity or worsening of audiological measurements were reported and the other outcomes were either not measured or not reported. See summary of findings Table for the main comparison for the comparison.
Comparison 2: Topical antibiotics versus placebo or no treatment (with background treatment)
We included two studies examining topical antibiotics versus no treatment, with background treatment (de Miguel 1999; Esposito 1990; 90 participants). Both compared treatment with topical ciprofloxacin drops and systemic ciprofloxacin to systemic ciprofloxacin only. Only one study reported that the resolution of discharge was otoscopically confirmed (de Miguel 1999). Resolution of discharge was measured at one to two weeks and two to four weeks. Treatment marginally favoured the topical and systemic antibiotics group compared to the systemic antibiotics only group for resolution of discharge (RR 1.47, 95% CI 1.20 to 1.80 at one to two weeks and RR 1.88. 95% CI 1.04 to 3.39 at two to four weeks). These studies were too small to provide any certainty of the findings (GRADE assessment: low‐certainty evidence). The other outcomes were either not measured or poorly reported. See summary of findings Table 2 for the comparison.
Comparison 3: Aminoglycoside versus quinolones
We found seven studies (Asmatullah 2014; Fradis 1997; Jamalullah 2016; Kaygusuz 2002; Lorente 1995; Nawasreh 2001; Tutkun 1995; 734 participants). Five studies used the quinolone ciprofloxacin (Fradis 1997; Kaygusuz 2002; Lorente 1995; Nawasreh 2001; Tutkun 1995). The studies used varying dosages of six (Kaygusuz 2002) or 15 (Fradis 1997; Lorente 1995; Nawasreh 2001; Tutkun 1995) drops per day, and variable concentrations including 0.3% (Kaygusuz 2002; Lorente 1995) and 0.6%, 200 µg/mL (Nawasreh 2001; Tutkun 1995), or did not report the concentration (Fradis 1997). Two studies used the quinolone ofloxacin (Asmatullah 2014; Jamalullah 2016). Both studies had the same dosage of 12 drops per day, whilst Jamalullah 2016 used a 0.6% concentration and Asmatullah 2014 used a 0.3% concentration. Five studies reported that the resolution of discharge was otoscopically confirmed (Asmatullah 2014; Jamalullah 2016; Kaygusuz 2002; Lorente 1995; Tutkun 1995).
We found that resolution of discharge at one to two weeks was almost twice as likely in the quinolones group, although this was not statistically significant (GRADE assessment: very low‐certainty evidence). See summary of findings Table 3 for the comparison.
Tutkun 1995 (44 participants) presented mean air and bone conduction hearing levels at 500 Hz, 1000 Hz, 2000 Hz and 4000 Hz pre‐ and post‐treatment by treatment group. No standard deviations were presented although the authors assert that "the difference between these two groups was not statistically significant (p>0.01)". Nawasreh 2001 (88 participants) stated that hearing was measured and that neither group showed significant differences. Lorente 1995 (308 participants) measured ear pain on a three‐point scale. Results were presented as a mean score. There was no difference in hearing levels between the groups.
Comparison 4: Quinolones versus aminoglycosides/polymixin B ± gramicidin
We found three studies for this comparison (van Hasselt 1997 (50 participants); van Hasselt 1998a (unknown number of participants); Siddique 2016 (186 participants). Both van Hasselt 1997 and van Hasselt 1998a used ofloxacin at 0.3% with dosages of three drops per eight hours and six drops per 12 hours respectively and compared this to a combination of neomycin and polymixin B of varying or unclear concentration and dosages. None of the studies reported that the resolution of discharge was otoscopically confirmed. Treatment duration was two weeks for both studies. Siddique 2016 compared ciprofloxacin of unknown concentration with a dosage of three drops per 12 hours with neomycin and polymixin B with gramicidin D of unknown concentration with a dosage of two drops per 12 hours. Treatment duration was unclear but likely lasted four weeks.
Only Siddique 2016 reported resolution of discharge and this was at four weeks post‐treatment. More patients experienced resolution of discharge with quinolones at four weeks compared with neomycin and polymixin B with gramicidin D (RR 1.12, 95% CI 1.03 to 1.22).
Comparison 5: Aminoglycosides versus trimethoprim sulphacetamide and polymyxin B
We included only one study examining topical aminoglycosides (gentamicin) versus a combination of trimethoprim sulphacetamide and polymyxin B (Gyde 1978; 91 participants, 100 ears). The study was translated from French and 51% of participants had CSOM (other diagnoses were otitis externa (21%), 'subacute otitis' (16%) and postoperative discharge (12%)). It was not reported whether the resolution of discharge was otoscopically confirmed. Participants were randomised by ear, with no adjustment for the paired nature of the data. Whilst resolution of discharge was assessed at two to four weeks there were no extractable efficacy data.
The study authors reported no signs of ototoxicity, excessive fungal proliferation or any local sensitivity to the ear drops. Health‐related quality of life, hearing level and serious complications were not reported.
Comparison 6: Rifampicin versus chloramphenicol
We found one study examining this comparison (Liu 2003; 160 participants), which was translated from Chinese. This study compared topical rifampicin (0.1%, nine drops per day, treatment duration of two weeks) with topical chloramphenicol (0.25%, nine drops per day, treatment duration of two weeks) with a background treatment of 3% hydrogen peroxide ear wash daily in both arms. It was not reported whether the resolution of discharge was otoscopically confirmed. Topical rifampicin appeared to be more effective than chloramphenicol for resolving ear discharge one to two weeks after completion of treatment (RR 1.78, 95% CI 1.35 to 2.34). However, this study was too small to provide any certainty of the findings (GRADE assessment: very low‐certainty evidence). No adverse events, suspected ototoxicity or worsening of audiological measurements were reported and the other outcomes were either not measured or not reported.
Overall completeness and applicability of evidence
The doses used in the studies were in keeping with manufacturers' recommendations and are applicable to the population being studied. The population of patients with CSOM are likely to receive treatment in both primary and secondary care settings.
Whilst the inclusion criteria was ear discharge for more than two weeks, reflecting the World Health Organization (WHO) guidelines for CSOM diagnosis, the majority of studies included patients who had ear discharge for more than six weeks before intervention, which is in keeping with a number of local treatment protocols and the practice of many tertiary‐based otolaryngologists. The length of follow‐up in most studies was between one to four weeks, meaning that there was limited evidence regarding the long‐term effectiveness of topical antibiotics for the resolution of discharge for people with CSOM.
No studies examined children under two years of age. As the peak prevalence of otitis media is in children under two years of age this leaves us with no information on this important patient group. Similarly, no studies included participants classed as 'high‐risk' in our protocol, including Indigenous populations and immunocompromised patients. Patients in these high‐risk groups can be a challenge for clinicians to treat effectively and evidence to support best‐practice interventions for these people is needed. The effectiveness of topical antibiotics is likely to be influenced by the sensitivity of the antibiotic to the micro‐organisms present. We were unable to carry out a subgroup analysis of the spectrum of antibiotic activity as the data were either not in the included studies or heterogeneity was not observed, which leaves us with no information on this aspect of antibiotic treatment.
Disease‐specific health‐related quality of life, which is both specific to the disease and important to patients, was not used in the included studies as an outcome measure. There is therefore no information at all on whether the different types of antibiotics used have an impact on patients' quality of life.
Quality of the evidence
The certainty of the evidence for all outcomes in these comparisons was very low (GRADE assessment), due to the small number of participants available for analysis (resulting in large confidence intervals) and limitations in the methods of study conduct and reporting. Accuracy of the diagnosis was also a potential issue throughout the studies included in this review. Of the 17 included studies, only six described the use of otoscopic confirmation of resolution of discharge. This may have impacted on the accuracy of the diagnostic outcome and therefore the response to treatment.
Potential biases in the review process
In most cases the studies did not report enough information for us to further analyse the results. We have had to take readings from graphs using a digital graph reader and impute standard deviations based on the P values reported. They were often only reported as 'P value < 0.05' or 'P value < 0.01' in comparisons where the studies found statistical significance. Our imputations are based on these values (using P value = 0.01 or P value = 0.05) and we are therefore conservative in our estimation of the standard deviations. However, this lack of information about non‐significant results could have prevented us from drawing more conclusive results about the lack of difference between groups.
Agreements and disagreements with other studies or reviews
This review is part of a series of reviews on CSOM (Bhutta 2018; Brennan‐Jones 2018a; Brennan‐Jones 2018b; Chong 2018a; Chong 2018b; Head 2018a; Head 2018b). A companion review looks at the effectiveness of topical antibiotics with steroids for the treatment of CSOM (Brennan‐Jones 2018a).
There are few previous reviews or guidelines for CSOM. The WHO in 2004 suggested that first‐line treatment of CSOM should comprise aural toilet and topical antibiotic drops, with second‐line treatment comprising an alternative topical antibiotic (guided by results of microbiological culture) or parenteral antibiotics (WHO 2004). The Australian government recommendations from 2010 for the treatment of Aboriginal and Torres Strait islanders gave similar recommendations, with first‐line treatment comprising aural toilet (or antiseptic washout) followed by topical antibiotics, and second‐line treatment with parenteral antibiotics (Morris 2010). An expert panel of the American Academy of Otolaryngologists in 2000 came to a similar conclusion (Hannley 2000).
These reviews supersede a pair of previous Cochrane Reviews examining topical antibiotics for CSOM (Macfadyen 2005a; Macfadyen 2005b).
Although we planned subgroup analyses for different participant characteristics (age, high‐risk, ventilation tubes), treatment duration and spectrum of antibiotic activity these were not carried out either because the data were not available or heterogeneity was not observed.

Study flow diagram
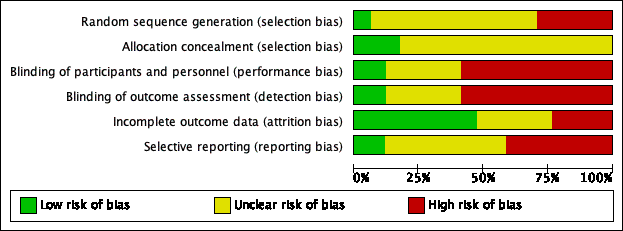
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
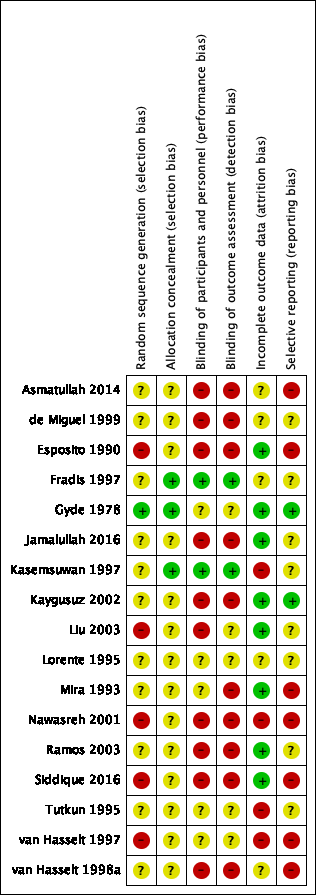
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
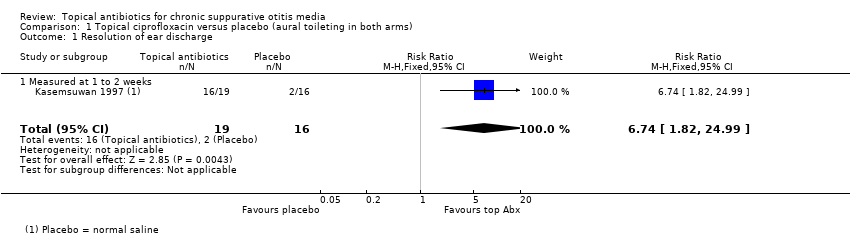
Comparison 1 Topical ciprofloxacin versus placebo (aural toileting in both arms), Outcome 1 Resolution of ear discharge.
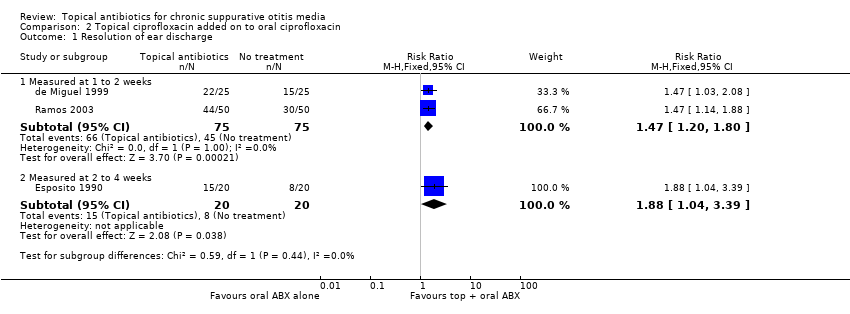
Comparison 2 Topical ciprofloxacin added on to oral ciprofloxacin, Outcome 1 Resolution of ear discharge.
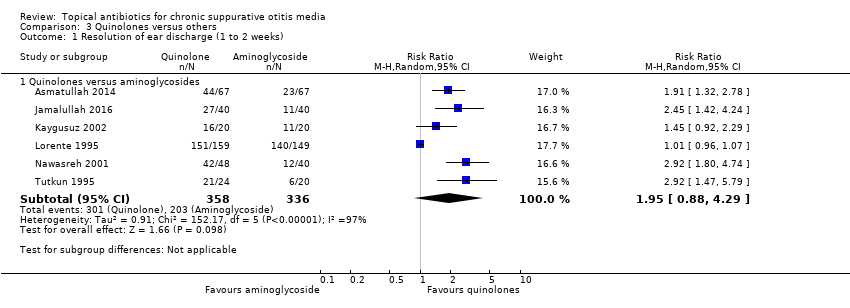
Comparison 3 Quinolones versus others, Outcome 1 Resolution of ear discharge (1 to 2 weeks).
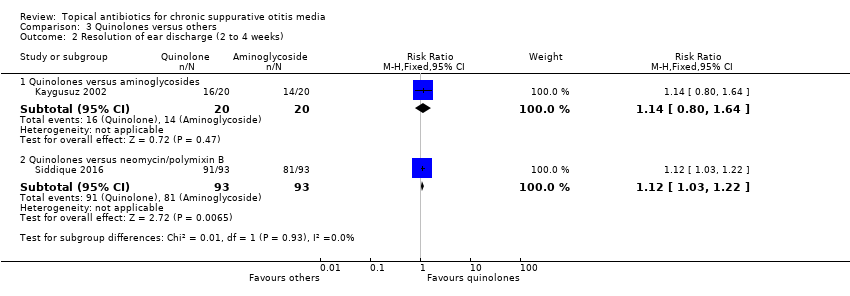
Comparison 3 Quinolones versus others, Outcome 2 Resolution of ear discharge (2 to 4 weeks).

Comparison 4 Rifampicin versus chloramphenicol, Outcome 1 Resolution of ear discharge (1 to 2 weeks).
| Topical antibiotics (ciprofloxacin) versus placebo/no treatment for chronic suppurative otitis media | |||||||
| Patient or population: patients with mucopurulent otorrhoea Settings: specialist hospital in Thailand Intervention: ciprofloxacin ear drops Comparison: saline | |||||||
| Outcomes | Relative effect | Number of participants (studies) | Anticipated absolute effects* (95% CI) | Certainty of the evidence | Comments | ||
| Without topical antibiotic | With topical antibiotic | Difference | |||||
| Resolution of ear discharge ‐ measured at 1 to 2 weeks Follow‐up: 7 days | RR 6.74 (1.82 to 24.99) | 35 (1 RCT) | Study population | ⊕⊝⊝⊝ | Topical antibiotics may increase the number of patients with resolution of ear discharge at 7 days compared with placebo, but we are very uncertain about the results. | ||
| 12.5% | 84.2% (22.8 to 100.0) | 71.8% more (10.3 to 299.9) | |||||
| Resolution of ear discharge ‐ measured after 4 weeks | No study measured this outcome. | ||||||
| Health‐related quality of life | No study measured this outcome. | ||||||
| Ear pain (otalgia) or discomfort or local irritation Follow‐up: 7 days | — | 35 (1 RCT) | Authors reported "no medical side‐effects and worsening of audiological measurements related to this topical medication were detected". | ⊕⊝⊝⊝ | — | ||
| Hearing Follow‐up: 7 days | — | 35 (1 RCT) | Authors reported "no ... worsening of audiological measurements related to this topical medication were detected." | ⊕⊝⊝⊝ | — | ||
| Serious complications | No studies reported that any participant died or had any intracranial or extracranial complications. | ||||||
| Suspected ototoxicity Follow‐up: 7 days | — | 35 (1 RCT) | Authors report "no suspected ototoxicity" but it is unclear how this was measured. | ⊕⊝⊝⊝ | — | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group | |||||||
| GRADE Working Group grades of evidence | |||||||
| 1Downgraded to very low certainty: downgraded by one level due to study limitations (risk of bias) because there were concerns about incomplete data (50 people entered the study but results are only reported for 35). Downgraded by two levels due to imprecision as there was one very small study (35 participants) with wide confidence intervals. 2Downgraded to very low certainty: downgraded by two levels due to study limitations (risk of bias) because there were concerns about incomplete data (50 people entered the study but results are only reported for 35) and it is unclear how this outcome was measured as the paper just reports "no medical side effects". Downgraded by one level due to imprecision as numeric results were not provided and it was only one very small study (35 participants). 3Downgraded to very low certainty: downgraded by two levels due to study limitations (risk of bias) because of concerns about incomplete data (50 people entered the study but results are only reported for 35) and the methods used for measuring hearing were not provided in the paper. Downgraded by one level due to imprecision as numeric results were not reported and it was only one very small study (35 participants). | |||||||
| Topical antibiotics (ciprofloxacin) on top of systemic antibiotics (ciprofloxacin) for chronic suppurative otitis media | |||||||
| Patient or population: CSOM, recurrence of CSOM or suppuration following mastoidectomy or tympanoplasty Settings: secondary care clinics in Spain and Italy Intervention: ciprofloxacin (topical) plus ciprofloxacin (systemic) Comparison: ciprofloxacin (systemic) | |||||||
| Outcomes | Relative effect | Number of participants (studies) | Anticipated absolute effects* (95% CI) | Certainty of the evidence | Comments | ||
| Without topical antibiotics | With topical antibiotics | Difference | |||||
| Resolution of ear discharge‐ measured at 1 to 2 weeks Follow‐up: 7 to 10 days | RR 1.47 (1.20 to 1.80) | 150 (2 RCTs) | Study population | ⊕⊕⊝⊝ | Topical antibiotics in addition to systemic antibiotics may increase the number of patients with resolution of ear discharge at 7 to 10 days compared with systemic antibiotics alone. The NNTB is 4 (95% CI 3 to 9). | ||
| 60.0% | 88.2% (72.0 to 100) | 28.2% more (12 more to 48 more) | |||||
| Resolution of ear discharge ‐ measured after 4 weeks | No studies reported this outcome. | ||||||
| Health‐related quality of life | No studies reported this outcome. | ||||||
| Ear pain (otalgia) or discomfort or local irritation | No studies reported this outcome. | ||||||
| Hearing | No studies reported results for this outcome. | ||||||
| Serious complications | No studies reported that any participant died or had any intracranial or extracranial complications. | ||||||
| Suspected ototoxicity | — | 190 (3 RCTs) | Three studies reported that they did not suspect ototoxicity in any participants, but it is unclear how this was measured (de Miguel 1999; Esposito 1990; Ramos 2003). | ⊕⊝⊝⊝ | — | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group | |||||||
| GRADE Working Group grades of evidence | |||||||
| 1Downgraded to low certainty: downgraded by one level due to study limitations (risk of bias) as both studies had unclear randomisation and allocation concealment and were unblinded. Downgraded by one level due to imprecision as there were only two small studies (150 participants) with the confidence interval crossing the line of minimally important benefit. 2Downgraded to very low certainty: downgraded by two levels due to study limitations (risk of bias) as all three studies had unclear randomisation, allocation concealment and were unblinded studies. It was also unclear how the outcome was reported. Downgraded by one level due to imprecision as numeric results were not reported and there were only three small studies (190 participants). | |||||||
| Quinolones versus aminoglycosides for chronic suppurative otitis media | |||||||
| Patient or population: CSOM Settings: secondary care settings in Israel, Turkey, Jordan, Spain and Pakistan Intervention: ciprofloxacin versus tobramycin (2 studies); ciprofloxacin versus gentamycin (3 studies); ofloxacin versus gentamycin (2 studies) Comparison: other antibiotic | |||||||
| Outcomes | Relative effect | Number of participants (studies) | Anticipated absolute effects* (95% CI) | Certainty of the evidence | Comments | ||
| Aminoglycosides | Quinolones | Difference | |||||
| Resolution of ear discharge ‐ measured at 1 to 2 weeks Follow‐up: range 8 days to 2 weeks | RR 1.95 (0.88 to 4.29) | 694 (6 RCTs) | 33.7%1 | 65.7 (29.7% to 100%) | 32.0% more (4.0% lower to 110.9% higher) | ⊕⊝⊝⊝ | We used a random‐effects model due to high heterogeneity. Resolution of ear discharge at 1 to 2 weeks was higher in the quinolones group but the very low certainty of the evidence means that it is very uncertain whether or not one intervention is better or worse than the other. |
| Resolution of ear discharge ‐ Measured after 4 weeks | None of the studies measured this outcome. | ||||||
| Health‐related quality of life | None of the studies measured this outcome. | ||||||
| Ear pain (otalgia) or discomfort or local irritation Follow‐up: 30 days | — | 308 (1 RCT) | One study measured ear pain on a 3‐point scale (Lorente 1995). Results were presented as a mean score. Both groups had a mean score of 0 at 30 days. There was no difference between the groups. | ⊕⊝⊝⊝ | — | ||
| Hearing Follow‐up: 10 days | — | 132 (4 RCTs) | One study presents the hearing levels per group but does not present the data in a way that can be analysed (Tutkun 1995). One study stated in the methods that hearing was measured but only mentioned that neither group showed significant differences (Nawasreh 2001). | ⊕⊝⊝⊝ | — | ||
| Serious complications Follow‐up: 10 to 30 days | None of the studies reported that any participant died or had any intracranial or extracranial complications. | ||||||
| Suspected ototoxicity Follow‐up: 10 to 30 days | — | 352 (2 RCTs) | One study (Lorente 1995) assessed for ototoxicity and did not find any cases. One study (Tutkun 1995) reported assessment of ototoxicity in the methods but did not provide results. None of the studies reported assessment nor any cases of suspected ototoxicity. | ⊕⊝⊝⊝ | — | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group | |||||||
| GRADE Working Group grades of evidence | |||||||
| 1Average event rates in the control group were calculated without the Lorente 1995 study, as this seemed to show a very high rate of resolution (94%) compared to the other studies (range between 28% and 55%). 2Downgraded to very low certainty. Downgraded due to study limitations (risk of bias) as six of seven studies were unblinded and in general the methods were poor. Downgraded due to imprecision as the point estimate shows that more people with quinolones had resolution of discharge compared with aminoglycosides BUT there is a large confidence interval, which includes 'no effect' and a very large effect (four times as many people had resolution with quinolones compared to aminoglycosides). Downgraded due to inconsistency as there was high heterogeneity (I2 = 97%) within the results. 3Downgraded to very low certainty: downgraded by two levels due to study limitations (risk of bias) as all elements of the risk of bias assessment were unclear. Downgraded by one level due to imprecision as the results come from one relatively small study (308 patients). 4Downgraded to very low certainty: downgraded by two levels due to study limitations (risk of bias) as the studies were assessed as either high risk or unclear risk for all elements of the risk of bias assessment. Downgraded by one level due to imprecision as numeric results were not presented and the results came from two small studies (132 patients). 5Downgraded to very low certainty: downgraded by two levels due to study limitations (risk of bias) as many were unblinded and in general the studies had methodological issues and/or were badly reported. In addition, it is not clear how the outcome was measured. Downgraded by one level due to imprecision as numeric results were not reported and there were only two studies (352 participants) that identified ototoxicity as an outcome. | |||||||
| Topical antibiotics with steroids | Topical antibiotics | Systemic antibiotics | Topical antiseptics | Aural toileting (ear cleaning) | |
| Topical antibiotics with steroids | Review CSOM‐4 | ||||
| Topical antibiotics | Review CSOM‐4 | Review CSOM‐1 | |||
| Systemic antibiotics | Review CSOM‐4 | Review CSOM‐3 | Review CSOM‐2 | ||
| Topical antiseptics | Review CSOM‐4 | Review CSOM‐6 | Review CSOM‐6 | Review CSOM‐5 | |
| Aural toileting | Review CSOM‐4 | Not reviewed | Not reviewed | Not reviewed | Review CSOM‐7 |
| Placebo (or no intervention) | Review CSOM‐4 | Review CSOM‐1 | Review CSOM‐2 | Review CSOM‐5 | Review CSOM‐7 |
| CSOM‐1: Topical antibiotics for chronic suppurative otitis media (Brennan‐Jones 2018b). CSOM‐2: Systemic antibiotics for chronic suppurative otitis media (Chong 2018a). CSOM‐3: Topical versus systemic antibiotics for chronic suppurative otitis media (Chong 2018b). CSOM‐4: Topical antibiotics with steroids for chronic suppurative otitis media (Brennan‐Jones 2018a). CSOM‐5: Topical antiseptics for chronic suppurative otitis media (Head 2018a). CSOM‐6: Antibiotics versus topical antiseptics for chronic suppurative otitis media (Head 2018b). CSOM‐7: Aural toilet (ear cleaning) for chronic suppurative otitis media (Bhutta 2018). | |||||
| Class of antibiotics | Examples | Route of administration |
| Quinolones | Ciprofloxacin, ofloxacin, levofloxacin | Oral, intravenous, topical |
| Aminoglycosides | Gentamicin, tobramycin | Topical or parenteral |
| Neomycin/framycetin | Only topical | |
| Cephalosporins | Ceftazidime | Parenteral |
| Penicillins | Ticarcillin plus clavulanic acid | Parenteral |
| Monobactams | Aztreonam | Parenteral |
| Ref ID (no. participants) | Setting | Population | Intervention 1 | Intervention 2 | Treatment duration | Follow‐up | Background Treatment | Notes |
| Topical antibiotics versus placebo/no treatment (no background or aural toileting) | ||||||||
| (n = 50) | Specialist hospital, Thailand | Mucopurulent otorrhoea with perforated tympanic membrane (CSOM) | Ciprofloxacin 250 mg/mL, 5 drops per 8 hours | Saline, 5 drops per 8 hours | 1 week | 1 week | Aural toilet on day 1, 4 and 7 | Randomised by person |
| Topical antibiotic versus placebo/no treatment (systemic antibiotic as background treatment) | ||||||||
| (n = 50) | General hospital, Spain | Simple chronic otitis media (36%), osteitic chronic otitis media (25.6%), cholesteatomas chronic otitis media (13.6%), post surgery cases (24.8%) | Topical ciprofloxacin 0.2%, 3 drops per 8 hours and oral ciprofloxacin, 500 mg per 12 hours | No treatment | 7 days | 15 days | Aural toileting before beginning treatment, analgesics and antipyretics. Oral ciprofloxacin, 500 mg per 12 hours | Part of 5‐arm trial Randomised by person |
| (n = 40) | University clinic, Italy | Mild or moderate CSOM in acute stage | Ciprofloxacin 250 µg/mL, 3 drops per 12 hours | No treatment | 5 to 10 days | 2 weeks | Oral ciprofloxacin, 250mg per 12 hours | Part of 3‐arm trial Randomised by person |
| (n = 50) | University clinic, Italy | Recurrence of CSOM or suppuration following mastoidectomy or tympanoplasty | Ceftizoxime 500 µg/mL, 2 x 2 mL washes per 12 hours | Saline, 2 x 2 mL washes per 12 hours | 1 week | 3 weeks | Systemic ceftizoxime by intramuscular route every 12 hours Aural toilet at first visit | Randomised by person |
| (n = 100) | ENT departments, Spain | Simple chronic otitis media (42.7%), chronic otitis media with osteolysis (19%), chronic cholesteatoma (14%), chronic otorrhoea in operated ears 24.3%) | Ciprofloxacin 0.2%, 0.5 mL per 8 hours | No treatment | 1 week | 10 days | Oral ciprofloxacin, 500 mg per 12 hours | Part of a 6‐arm trial Randomised by person |
| Quinolones versus aminoglycosides | ||||||||
| (n = 134) | ENT department, Pakistan | Active tubotympanic type CSOM | Ofloxacin 0.3%, 12 drops per day | Gentamycin 0.3%, 12 drops per day | 10 days | 2 weeks | None mentioned | Randomised by person |
| (n = 40) | Outpatient clinic, Israel | Chronic otitis media | Ciprofloxacin (no conc), 15 drops per day | Tobramycin (no conc), 15 drops per day | 3 weeks | 3 weeks | None mentioned | Part of 3‐arm trial Randomised by ear |
| (n = 40) | University ENT clinic, Turkey | CSOM | Ciprofloxacin 0.3%, 6 drops per day | Tobramycin 0.3%, 6 drops per day | 3 weeks | 3 weeks | Daily aspiration | Translated from Turkish Part of 4‐arm trial Randomised by person. |
| (n = 88) | Unclear setting, Jordan | CSOM and intermittent mucopurulent heavy discharge for more than 1 year | Ciprofloxacin 200 µg/mL (0.02%), 15 drops per day | Gentamicin 5 mg/mL, 15 drops per day | 10 days | 2 weeks | None mentioned | Randomised by person |
| (n = 308) | Hospital ENT clinics, Spain | CSOM (purulent discharge > 3 months and perforated membrane) | Ciprofloxacin 0.3%, 15 drops per day | Gentamycin 0.3%, 15 drops per day | 8 days | 30 days | Unclear | Translated from Spanish Assume this is same as Sabater paper Randomised by person |
| (n = 44) | University hospital, Turkey | CSOM and purulent discharge for more than 1 year | Ciprofloxacin 200 µg/mL (0.02%), 15 drops per day | Gentamicin 5 mg/mL, 15 drops per day | 10 days | 10 days | None mentioned | Randomised by person |
| (n = 80) | Otolaryngology department, Pakistan | CSOM (tubotympanic type) | Ofloxacin 0.6%, 12 drops per day | Gentamycin 0.3%, 12 drops per day | 2 weeks | 2 weeks | One aural toilet at start | Randomised by person |
| Quinolones versus others | ||||||||
| (n = 200) | Specialist hospital, Pakistan | Tubotympanic type of CSOM | Ciprofloxacin (no conc), 3 drops per 12 hours | Neomycin/polymixin/gramicidin‐D (no conc), 2 drops per 12 hours | Unclear (probably 4 weeks) | 4 weeks | No information | Randomised by person |
| (n = 50) | Rural setting, Malawi | Children with CSOM | Ofloxacin 0.3%, 3 drops per 8 hours | Neomycin 0.5%/polymixin B 0.1%, 3 drops per 8 hours | 2 weeks | 2 weeks | Aural toilet at start and weekly | Part of a 3‐arm trial Only presented as an internal report Unclear unit of randomisation, results reported by ear |
| (n = unclear) | Rural setting, Malawi | "Mainly children" with CSOM | Ofloxacin 0.3%, 6 drops per 12 hours | Neomycin/polymixin B (no conc), 6 drops per 12 hours | 2 weeks | 8 weeks | Aural toilet at start and weekly | Only a presentation given at a conference available Unclear unit of randomisation, results presented by ear Part of 4‐arm trial ‐ once weekly arms have not been included. |
| Aminoglycosides versus trimethoprim, sulphacetamide and polymixin B (TSP) | ||||||||
| (n = 91) | Outpatient clinic, Canada | Otitis externa (21%), CSOM (51%), subacute otitis (16%), postoperative infection (21%) | Trimethoprim, sulphacetamide and polymyxin B, 16 drops per day | Gentamicin 0.3%, 16 drops per day | Mean: 16 days | 12 months | Not reported | Translated from French Randomised by person but reported by ear Semi cross‐over trial |
| Rifampicin versus chloramphenicol | ||||||||
| (n = 160) | Outpatient department, China | CSOM | Rifampicin 0.1%, 9 drops per day | Chloramphenicol 0.25%, 9 drops per day | 2 weeks | 2 weeks | 3% hydrogen peroxide ear wash daily | Translated from Chinese Randomised by person |
| CSOM: chronic suppurative otitis media | ||||||||
| Reference | Unit of randomisation | Reported | Definition | Otoscopically confirmed? | Time points | Notes |
| Person | Person | "no discharge" | Yes | 1 to 2 weeks (10 days) | — | |
| Person | Person | "global index of clinic microbiological cure" | Yes | 1 to 2 weeks (7 days) | — | |
| Person | Person | "cured" (no definition but assumed to be no discharge) | Unclear, paper states "clinically examined" | 1 week to 2 weeks (6 to 11 days) and 2 to 4 weeks (19 to 24 days) | 1 to 2 weeks examined but not reported | |
| Ear | Ear | "clinical success as defined as cessation of otorrhea and eradication of the microorganisms in the post treatment culture" | Unclear | 2 to 4 weeks (21 days) | Unclear how many patients had bilateral ear disease in each group | |
| Person | Ear | "dry ear" and a negative culture at 3 weeks or a real improvement in at 3 weeks and the cessation of discharge at 6 weeks | Unclear | 2 to 4 weeks (3 weeks) and after 4 weeks (6 weeks) | Semi cross‐over trial. It does not appear that any consideration of the correlation of results between ears has been taken into account. If there was a treatment failure 'ears' were transferred to the alternative groups. These results have not been included in the analysis. If the ear was not dry on review at 6 months, treatment for 3 weeks with the alternative treatment was completed with review after 6 months | |
| Person | Person | "absence" of aural discharge | Yes | 2 to 4 weeks (2 weeks) | — | |
| Person | Person | "cure" | Unclear | 1 to 2 weeks (7 days) | — | |
| Person | Ear | Assessed using 3‐point scale (2 points = no drainage) | Yes | 2 to 4 weeks (day 14 and 21) | Unclear method of allocation, unsure if random selection of study ear | |
| Person | Person | "Cured: otorrhea disappeared, mucosal hyperaemia of the tympanic membrane and tympanic cavity disappeared. Significantly effective: no complaints of otorrhea, no visible purulence in the ear canal and tympanic cavity, and nonvisible or slight hyperaemia of the tympanic membrane and the tympanic canal" | Unclear | 1 to 2 weeks (2 weeks) | — | |
| Person | Person | "Complete resolution of ear discharge" | Yes | 1 to 2 weeks (8 days) and after 4 weeks (30 days) | — | |
| Person | Person | Not reported in a way that could be used in the review | N/A | N/A | Paper plotted the time course of otorrhoea (quantity) on a scale of 0 to 3 at 3, 7 and 21 days | |
| Person | Person | "cessation of otorrhea" | Yes | 1 to 2 weeks (10 days) | — | |
| Person | Person | "cured" according to "indices de curacion" | Unclear | 1 to 2 weeks (10 days) | — | |
| Person | Person | "absence of discharge from middle ear cavity and no inflammation/congestion in middle ear mucosa and tympanic membrane" | Unclear | 2 to 4 weeks (4 weeks) | 15 patients (8%) had bilateral disease but how these cases were handled is not stated. The denominator in the trials is the person so it is assumed that no double counting occurred. | |
| Person | Person | "cessation of otorrhea" | Yes | 1 to 2 weeks (10 days) | — | |
| Unclear, most likely person | Ear | "dry ear" | Unclear | 1 to 2 weeks (1 week) and after 4 weeks (> 2 weeks) | Counting bilateral ears separately. All ears reported separately. Data come from an unpublished report. In the analysis 3/11 (27.27%), 10/30 (33%) and 11/28 (39%) of patients had bilateral disease in the ofloxacin, neomycin and antiseptic acid groups respectively. | |
| Unclear | Ear | "inactive ear" ‐ completely dry middle ear | Unclear | 1 to 2 weeks (1 week), 2 to 4 weeks (2 weeks) and after 4 weeks (8 weeks) | Counting bilateral ears separately | |
| N/A: not applicable | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Resolution of ear discharge Show forest plot | 1 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.74 [1.82, 24.99] |
| 1.1 Measured at 1 to 2 weeks | 1 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.74 [1.82, 24.99] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Resolution of ear discharge Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Measured at 1 to 2 weeks | 2 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.47 [1.20, 1.80] |
| 1.2 Measured at 2 to 4 weeks | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.88 [1.04, 3.39] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Resolution of ear discharge (1 to 2 weeks) Show forest plot | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Quinolones versus aminoglycosides | 6 | 694 | Risk Ratio (M‐H, Random, 95% CI) | 1.95 [0.88, 4.29] |
| 2 Resolution of ear discharge (2 to 4 weeks) Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Quinolones versus aminoglycosides | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.80, 1.64] |
| 2.2 Quinolones versus neomycin/polymixin B | 1 | 186 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [1.03, 1.22] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Resolution of ear discharge (1 to 2 weeks) Show forest plot | 1 | 160 | Risk Ratio (M‐H, Random, 95% CI) | 1.78 [1.35, 2.34] |

