Antibióticos tópicos para la otitis media supurativa crónica
Appendices
Appendix 1. Search strategies
| CENTRAL (the Cochrane Register of Studies) | MEDLINE (Ovid) | Embase (Ovid) |
| 1 MESH DESCRIPTOR Otitis Media EXPLODE ALL AND CENTRAL:TARGET | 1 exp Otitis Media/ 2 ("otitis media" or OME).ab,ti. 3 exp Tympanic Membrane Perforation/ 4 exp Tympanic Membrane/ 5 ("ear drum*" or eardrum* or tympanic).ab,ti. 6 4 or 5 7 (perforat* or hole or ruptur*).ab,ti. 8 6 and 7 9 1 or 2 or 3 or 4 or 8 10 exp Suppuration/ n 11 (suppurat* or pus or purulen* or discharg* or mucosal or otorrh* or otorh* or otoliquor* or active or weep* or moist or wet or mucopurulen* or discomfort or pain* or earach*).ab,ti. 12 10 or 11 13 exp Chronic Disease/ 14 exp Recurrence/ 15 (chronic* or persist* or recurr* or repeat*).ab,ti. 16 13 or 14 or 15 17 9 and 12 and 16 18 ((chronic or persist*) adj3 (ear or ears or aural) adj3 (suppurat* or pus or purulen* or discharg* or mucosal or otorrh* or otorh* or otoliquor* or active or weep* or wet or moist or mucopurulen* or pain* or discomfort)).ab,ti. 19 CSOM.ab,ti. 20 exp Otitis Media, Suppurative/ 21 (earach* adj6 (chronic or persist* or recurr* or repeat*)).ab,ti. 22 17 or 18 or 19 or 20 or 21 | 1 exp otitis media/ 2 ("otitis media" or OME).ab,ti. 3 exp eardrum perforation/ 4 exp eardrum/ 5 ("ear drum*" or eardrum* or tympanic).ab,ti. 6 4 or 5 7 (perforat* or hole or ruptur*).ab,ti. 8 6 and 7 9 1 or 2 or 3 or 8 10 exp suppuration/ 11 (suppurat* or pus or purulen* or discharg* or mucosal or otorrh* or otorh* or otoliquor* or active or weep* or moist or wet or mucopurulen* or discomfort or pain* or earach*).ab,ti. 12 10 or 11 13 exp chronic disease/ 14 exp recurrent disease/ 15 (chronic* or persist* or recurr* or repeat*).ab,ti. 16 13 or 14 or 15 17 9 and 12 and 16 18 exp suppurative otitis media/ 19 CSOM.ab,ti. 20 ((chronic or persist*) adj3 (ear or ears or aural) adj3 (suppurat* or pus or purulen* or discharg* or mucosal or otorrh* or otorh* or otoliquor* or active or weep* or wet or moist or mucopurulen* or pain* or discomfort or disease*)).ab,ti. 21 (earach* adj3 (chronic or persist* or recurr* or repeat*)).ab,ti. 22 17 or 18 or 19 or 20 or 21 |
| Web of Science (Web of Knowledge) | CINAHL (EBSCO) | Cochrane ENT Register (the Cochrane Register of Studies) |
| #1 TOPIC: ("otitis media" or OME) Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, BKCI‐S, BKCI‐SSH, ESCI, CCR‐EXPANDED, IC Timespan=All years #2 TOPIC: (("ear drum*" or eardrum* or tympanic) AND (perforat* or hole or ruptur*)) Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, BKCI‐S, BKCI‐SSH, ESCI, CCR‐EXPANDED, IC Timespan=All years #3 #2 OR #1 Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, BKCI‐S, BKCI‐SSH, ESCI, CCR‐EXPANDED, IC Timespan=All years #4 TOPIC: ((suppurat* or pus or purulen* or discharg* or mucosal or otorrh* or otorh* or otoliquor* or active or weep* or moist or wet or mucopurulen* or discomfort or pain* or earach*) AND (chronic* or persist* or recurr* or repeat*)) Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, BKCI‐S, BKCI‐SSH, ESCI, CCR‐EXPANDED, IC Timespan=All years #5 #4 AND #3 Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, BKCI‐S, BKCI‐SSH, ESCI, CCR‐EXPANDED, IC Timespan=All years #6 TOPIC: (((chronic or persist*) NEAR/3 (ear or ears or aural) NEAR/3 (suppurat* or pus or purulen* or discharg* or mucosal or otorrh* or otorh* or otoliquor* or active or weep* or wet or moist or mucopurulen* or pain* or discomfort))) Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, BKCI‐S, BKCI‐SSH, ESCI, CCR‐EXPANDED, IC Timespan=All years #7 TOPIC: ((earach* NEAR/3 (chronic or persist* or recurr* or repeat*))) Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, BKCI‐S, BKCI‐SSH, ESCI, CCR‐EXPANDED, IC Timespan=All years #8 #7 OR #6 OR #5 Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, BKCI‐S, BKCI‐SSH, ESCI, CCR‐EXPANDED, IC Timespan=All years | S21 S17 OR S18 OR S19 OR S20 S20 TX ((chronic or persist*) N3 (ear or ears or aural) N3 (suppurat* or pus or purulen* or discharg* or mucosal or otorrh* or otorh* or otoliquor* or active or weep* or wet or moist or mucopurulen* or pain* or discomfort)) S19 TX (earach* N3 (chronic or persist* or recurr* or repeat*)) S18 TX csom S17 S9 AND S12 AND S16 S16 S13 OR S14 OR S15 S15 TX chronic* or persist* or recurr* or repeat* S14 (MH "Recurrence") S13 (MH "Chronic Disease") S12 S10 OR S11 S11 TX suppurat* or pus or purulen* or discharg* or mucosal or otorrh* or otorh* or otoliquor* or active or weep* or moist or wet or mucopurulen* or discomfort or pain* or earach*) S10 (MH "Suppuration+") S9 S1 OR S2 OR S3 OR S8 S8 S6 AND S7 S7 TX perforat* or hole or ruptur* S6 S4 OR S5 S5 TX "ear drum*" or eardrum* or tympanic S4 (MH "Tympanic Membrane") S3 (MH "Tympanic Membrane Perforation") S2 TX "otitis media" or OME S1 (MH "Otitis Media+") | 1 ("otitis media" or OME):AB,EH,KW,KY,MC,MH,TI,TO AND INREGISTER 2 (("ear drum*" or eardrum* or tympanic)):AB,EH,KW,KY,MC,MH,TI,TO AND INREGISTER 3 (perforat* or hole or ruptur*):AB,EH,KW,KY,MC,MH,TI,TO AND INREGISTER 4 #2 AND #3 AND INREGISTER 5 #4 OR #1 AND INREGISTER 6 (suppurat* or pus or purulen* or discharg* or mucosal or otorrh* or otorh* or otoliquor* or active or weep* or wet or moist or discomfort or earach* or mucopurulen*):AB,EH,KW,KY,MC,MH,TI,TO AND INREGISTER 7 (pain):AB,TI,TO AND INREGISTER 8 #6 OR #7 AND INREGISTER 9 (chronic* or persist* or recurr* or repeat*):AB,EH,KW,KY,MC,MH,TI,TO AND INREGISTER 10 #5 AND #8 AND #9 AND INREGISTER 11 (csom):AB,EH,KW,KY,MC,MH,TI,TO AND INREGISTER 12 (((chronic* or persist* or recurr* or repeat*) and (ear or ears or aural) and (suppurat* or pus or purulen* or discharg* or mucosal or otorrh* or otorh* or otoliquor* or active or weep* or wet or moist or mucopurulen* or pain* or discomfort or disease*))):AB,EH,KW,KY,MC,MH,TI,TO AND INREGISTER 13 ((earach* and (chronic or persist* or recurr* or repeat*))):AB,EH,KW,KY,MC,MH,TI,TO AND INREGISTER 14 #10 OR #11 OR #12 OR #13 AND INREGISTER |
| ClinicalTrials.gov | ICTRP (WHO Portal) | Other |
| Search 1 (clinicaltrials.gov): (chronic OR persistent OR recurrence OR recurrent) AND (suppuration OR pus OR discharge OR otorrhea or active OR mucopurulent) AND Condition: "Otitis Media" OR OME AND Study type: interventional Search 2 (clinicaltrials.gov): (chronic OR persistent OR recurrence OR recurrent) AND (earache OR "ear ache" OR "ear pain" OR "ear discharge" OR "wet ear" OR "moist ear" OR "weeping ear") AND Study type: interventional Search 3 (clinicaltrials.gov): ("ear drum" OR eardrum OR "tympanic membrane") AND (hole OR perforation OR rupture) AND Study type: interventional Search 4 (the Cochrane Register of Studies): 1 ("otitis media" or OME):AB,EH,KW,KY,MC,MH,TI,TO AND INSEGMENT 2 (("ear drum*" or eardrum* or tympanic)):AB,EH,KW,KY,MC,MH,TI,TO AND INSEGMENT 3 (perforat* or hole or ruptur*):AB,EH,KW,KY,MC,MH,TI,TO AND INSEGMENT 4 #2 AND #3 AND INSEGMENT 5 #4 OR #1 AND INSEGMENT 6 (suppurat* or pus or purulen* or discharg* or mucosal or otorrh* or otorh* or otoliquor* or active or weep* or wet or moist or discomfort or earach* or Mucopurulen*):AB,EH,KW,KY,MC,MH,TI,TO AND INSEGMENT 7 (pain):AB,TI,TO AND INSEGMENT 8 #6 OR #7 AND INSEGMENT 9 (chronic* or persist* or recurr* or repeat*):AB,EH,KW,KY,MC,MH,TI,TO AND INSEGMENT 10 #5 AND #8 AND #9 AND INSEGMENT 11 (csom):AB,EH,KW,KY,MC,MH,TI,TO AND INSEGMENT 12 (((chronic* or persist* or recurr* or repeat*) and (ear or ears or aural) and (suppurat* or pus or purulen* or discharg* or mucosal or otorrh* or otorh* or otoliquor* or active or weep* or wet or moist or Mucopurulen* or pain* or discomfort or disease*))):AB,EH,KW,KY,MC,MH,TI,TO AND INSEGMENT 13 ((earach* and (chronic or persist* or recurr* or repeat*))):AB,EH,KW,KY,MC,MH,TI,TO AND INSEGMENT 14 #10 OR #11 OR #12 OR #13 AND INSEGMENT 15 (nct*):AU AND INSEGMENT 16 #14 AND #15 | otitis media AND chronic OR ear discharge OR earache OR wet ear OR weeping ear OR moist ear OR CSOM OR OME AND chronic OR tympanic membrane AND perforation OR eardrum AND hole OR eardrum AND perforation | LILACS TW:"otitis media" OR "TW:"ear discharge" OR TW:earache OR ((TW:eardrum OR TW:tympanic) AND (TW:perforation OR hole)) OR ((TW:wet OR moist OR weeping) AND TW:ear) AND: Filter: Controlled Clinical Trial IndMed Chronic Suppurative Otitis Media OR Chronic Otitis Media OR CSOM African Index Medicus “chronic suppurative otitis media" OR "chronic otitis media“ OR CSOM |
Appendix 2. Data extraction form
| REF ID: | Study title: |
| Date of extraction: | Extracted by: |
| Name and email address of correspondence authors: |
| General comments/notes (internal for discussion): |
FLOW CHART OF TRIAL:
| Intervention ( name the intervention) | Comparison ( name the intervention) | |
| No. of people screened | ||
| No. of participants randomised ‐ all | ||
| No. randomised to each group | ||
| No. receiving treatment as allocated | ||
| No. not receiving treatment as allocated ‐ Reason 1 ‐ Reason 2 | ||
| No. that dropped out1 (no follow‐up data for any outcome available) | ||
| No. excluded from analysis2 (for all outcomes) ‐ Reason 1 ‐ Reason 2 | ||
1This includes patients who withdrew and provided no data, or did not turn up for follow‐up.
2This should be the people who were excluded from all analyses (e.g. because the data could not be interpreted or the outcome was not recorded for some reason). This is the number of people who dropped out, plus the people who were excluded by the authors for some reason (e.g. non‐compliant).
INFORMATION TO GO INTO THE 'CHARACTERISTICS OF INCLUDED STUDIES' TABLE:
| Methods | X arm, double‐/single‐/non‐blinded, [multicentre] parallel‐group/cross‐over/cluster RCT, with x duration of treatment and x duration of follow‐up |
| Participants | Location: [country, rural?, no. of sites etc.] Setting of recruitment and treatment: [specialist hospital? general practice? school? state YEAR] Sample size:
Participant (baseline) characteristics:
Inclusion criteria:
Exclusion criteria: |
| Interventions | Intervention (n = x): drug name, method of administration, dose per day/frequency of administration, duration of treatment For aural toileting: who does it, methods or tools used, frequency, duration Comparator group (n = y): Concurrent treatment: Use of additional interventions (common to both treatment arms): |
| Outcomes | Outcomes of interest in the review: Primary outcomes:
Secondary outcomes
|
| Funding sources | "No information provided"/"None declared"/State source of funding |
| Declarations of interest | "No information provided"/"None declared"/State conflict |
| Notes | Clinical trial registry no: (if available) Unit of randomisation: person/ears/other (e.g. cluster‐randomised by hospital/school) [In the case of randomisation by person]: Methods for including patients with bilateral disease, for example:
|
RISK OF BIAS TABLE:
(See table 8.5d in the Cochrane Handbook for Systematic Reviews of Interventions: http://handbook.cochrane.org/).
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High/low/unclear risk | Quote: "…" Comment: |
| Allocation concealment (selection bias) | High/low/unclear risk | Quote: "…" Comment: |
| Blinding of participants and personnel (performance bias) | High/low/unclear risk | Quote: "…" Comment: |
| Blinding of outcome assessment (detection bias) | High/low/unclear risk | Quote: "…" Comment: |
| Incomplete outcome data (attrition bias) | High/low/unclear risk | Quote: "…" Comment: |
| Selective reporting (reporting bias) | High/low/unclear risk | Quote: "…" Comment: |
FINDINGS OF STUDY
CONTINUOUS OUTCOMES
| Results (continuous data table) | |||||||
| Outcome | Intervention (name the intervention) | Comparison (name the intervention) | Other summary statistics/Notes | ||||
| Mean | SD | N | Mean | SD | N | Mean difference (95% CI), P values etc. | |
| Disease‐specific health‐related quality of life (COMQ‐12, COMOT‐15, CES)1 Time point: (state) | |||||||
| Hearing: [Measurement method: include frequencies and report results separately if they are presented in the paper] Time point: [xx] | |||||||
| Comments: [If there is no information apart from (vague) narration, quote here] [If information is in the form of graphs, used this software to read it: http://arohatgi.info/WebPlotDigitizer/app/, and save a copy of your charts in a folder] | |||||||
1State the measurement method: this will be instrument name/range for patient‐reported outcomes.
DICHOTOMOUS OUTCOMES
| Results (dichotomous data table) | ||||||
| Outcome | Applicable review/ Intervention1 | Group A ‐ intervention arm | Group B – control | Other summary statistics/Notes | ||
| No. of people with events | No. of people analysed | No. of people with events | No. of people analysed | P values, RR (95% CI), OR (95% CI) | ||
| Resolution of ear discharge or 'dry ear' at 1 to 2 weeks [Measurement method or definition used: not/unclear if/otoscopically confirmed]1 Time point: [State actual time point] | ||||||
| Resolution of ear discharge or 'dry ear' at 2 to 4 weeks [Measurement method or definition used: not/unclear if/otoscopically confirmed] Time point: [xx] | ||||||
| Resolution of ear discharge or 'dry ear' after 4 weeks [Measurement method or definition used: not/unclear if/otoscopically confirmed] Time point: [xx] | ||||||
| Ear pain/discomfort/local irritation Time point: [xx] | ||||||
| Suspected ototoxicity [Measurement method or definition used] Time point: [xx] | ||||||
| Sensorineural hearing loss [Measurement method or definition used] Time point: [xx] | ||||||
| Tinnitus [Measurement method or definition used] Time point: [xx] | ||||||
| Dizziness/vertigo/balance [Measurement method or definition used] Time point: [xx] | ||||||
| Serious complications: Time point: state length of follow‐up of the trial | Note down the page number/table where info was found for ease of checking | |||||
| Otitic meningitis [How was this diagnosed?] | ||||||
| Lateral sinus thrombosis [How was this diagnosed?] | ||||||
| Cerebellar abscess [How was this diagnosed?] | ||||||
| Mastoid abscess/mastoiditis [How was this diagnosed?] | ||||||
| Postauricular fistula [How was this diagnosed?] | ||||||
| Facial palsy [How was this diagnosed?] | ||||||
| Other complications [How was this diagnosed?] | ||||||
| Death [How was this diagnosed?] | ||||||
| Multiple serious complications [How was this diagnosed?] | ||||||
| Comment/additional notes: If any calculations are needed to arrive at the data above, note this down here. | ||||||
1State briefly how this was measured in the study, especially whether there was deviation from what was expected in the protocol.
For adverse events, note down how these were collected, e.g. whether the adverse event was one of the prespecified events that the study planned to collect, when it was collected and how/who measured it (e.g. as reported by patients, during examination and whether any scoring system was used).

Study flow diagram
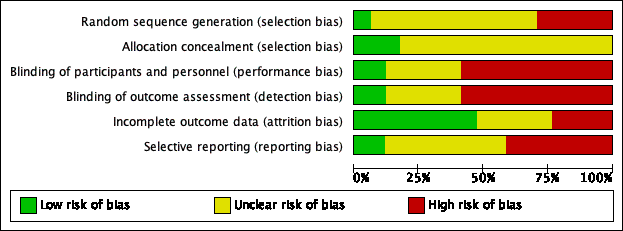
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
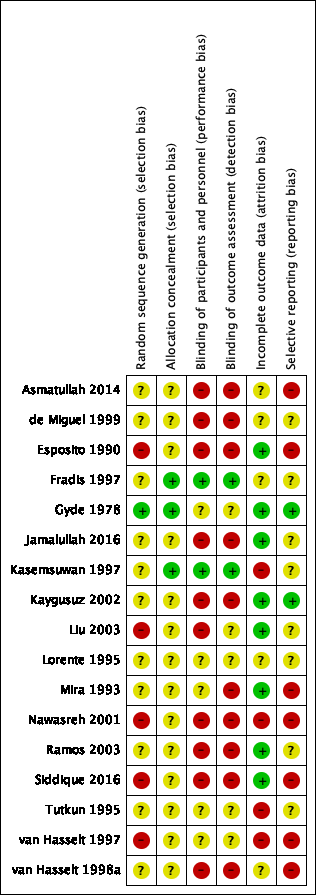
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
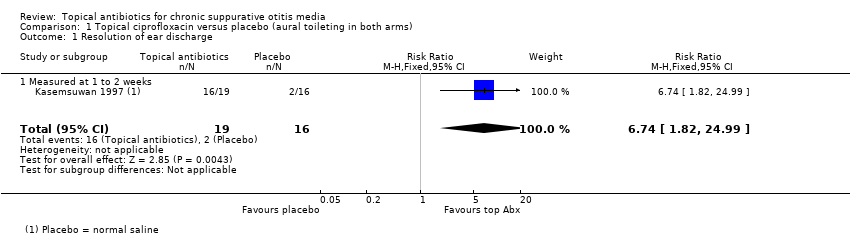
Comparison 1 Topical ciprofloxacin versus placebo (aural toileting in both arms), Outcome 1 Resolution of ear discharge.
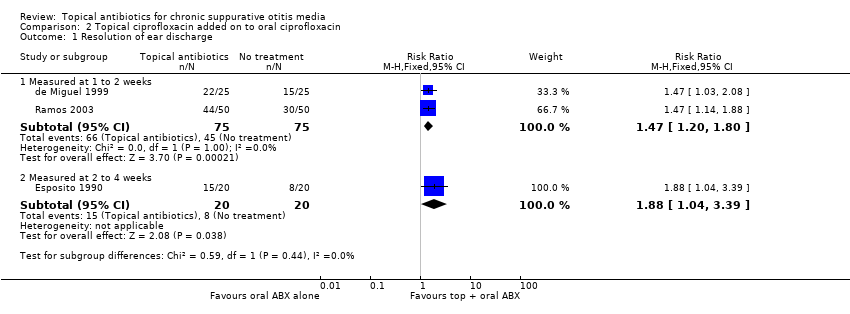
Comparison 2 Topical ciprofloxacin added on to oral ciprofloxacin, Outcome 1 Resolution of ear discharge.
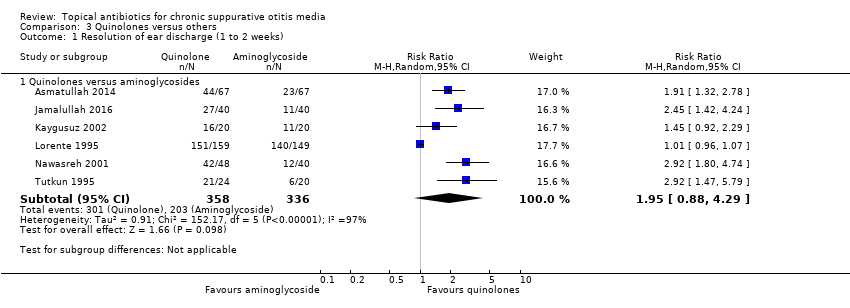
Comparison 3 Quinolones versus others, Outcome 1 Resolution of ear discharge (1 to 2 weeks).
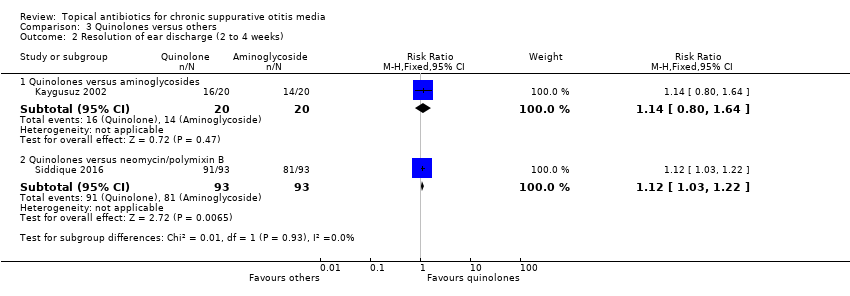
Comparison 3 Quinolones versus others, Outcome 2 Resolution of ear discharge (2 to 4 weeks).

Comparison 4 Rifampicin versus chloramphenicol, Outcome 1 Resolution of ear discharge (1 to 2 weeks).
| Topical antibiotics (ciprofloxacin) versus placebo/no treatment for chronic suppurative otitis media | |||||||
| Patient or population: patients with mucopurulent otorrhoea Settings: specialist hospital in Thailand Intervention: ciprofloxacin ear drops Comparison: saline | |||||||
| Outcomes | Relative effect | Number of participants (studies) | Anticipated absolute effects* (95% CI) | Certainty of the evidence | Comments | ||
| Without topical antibiotic | With topical antibiotic | Difference | |||||
| Resolution of ear discharge ‐ measured at 1 to 2 weeks Follow‐up: 7 days | RR 6.74 (1.82 to 24.99) | 35 (1 RCT) | Study population | ⊕⊝⊝⊝ | Topical antibiotics may increase the number of patients with resolution of ear discharge at 7 days compared with placebo, but we are very uncertain about the results. | ||
| 12.5% | 84.2% (22.8 to 100.0) | 71.8% more (10.3 to 299.9) | |||||
| Resolution of ear discharge ‐ measured after 4 weeks | No study measured this outcome. | ||||||
| Health‐related quality of life | No study measured this outcome. | ||||||
| Ear pain (otalgia) or discomfort or local irritation Follow‐up: 7 days | — | 35 (1 RCT) | Authors reported "no medical side‐effects and worsening of audiological measurements related to this topical medication were detected". | ⊕⊝⊝⊝ | — | ||
| Hearing Follow‐up: 7 days | — | 35 (1 RCT) | Authors reported "no ... worsening of audiological measurements related to this topical medication were detected." | ⊕⊝⊝⊝ | — | ||
| Serious complications | No studies reported that any participant died or had any intracranial or extracranial complications. | ||||||
| Suspected ototoxicity Follow‐up: 7 days | — | 35 (1 RCT) | Authors report "no suspected ototoxicity" but it is unclear how this was measured. | ⊕⊝⊝⊝ | — | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group | |||||||
| GRADE Working Group grades of evidence | |||||||
| 1Downgraded to very low certainty: downgraded by one level due to study limitations (risk of bias) because there were concerns about incomplete data (50 people entered the study but results are only reported for 35). Downgraded by two levels due to imprecision as there was one very small study (35 participants) with wide confidence intervals. 2Downgraded to very low certainty: downgraded by two levels due to study limitations (risk of bias) because there were concerns about incomplete data (50 people entered the study but results are only reported for 35) and it is unclear how this outcome was measured as the paper just reports "no medical side effects". Downgraded by one level due to imprecision as numeric results were not provided and it was only one very small study (35 participants). 3Downgraded to very low certainty: downgraded by two levels due to study limitations (risk of bias) because of concerns about incomplete data (50 people entered the study but results are only reported for 35) and the methods used for measuring hearing were not provided in the paper. Downgraded by one level due to imprecision as numeric results were not reported and it was only one very small study (35 participants). | |||||||
| Topical antibiotics (ciprofloxacin) on top of systemic antibiotics (ciprofloxacin) for chronic suppurative otitis media | |||||||
| Patient or population: CSOM, recurrence of CSOM or suppuration following mastoidectomy or tympanoplasty Settings: secondary care clinics in Spain and Italy Intervention: ciprofloxacin (topical) plus ciprofloxacin (systemic) Comparison: ciprofloxacin (systemic) | |||||||
| Outcomes | Relative effect | Number of participants (studies) | Anticipated absolute effects* (95% CI) | Certainty of the evidence | Comments | ||
| Without topical antibiotics | With topical antibiotics | Difference | |||||
| Resolution of ear discharge‐ measured at 1 to 2 weeks Follow‐up: 7 to 10 days | RR 1.47 (1.20 to 1.80) | 150 (2 RCTs) | Study population | ⊕⊕⊝⊝ | Topical antibiotics in addition to systemic antibiotics may increase the number of patients with resolution of ear discharge at 7 to 10 days compared with systemic antibiotics alone. The NNTB is 4 (95% CI 3 to 9). | ||
| 60.0% | 88.2% (72.0 to 100) | 28.2% more (12 more to 48 more) | |||||
| Resolution of ear discharge ‐ measured after 4 weeks | No studies reported this outcome. | ||||||
| Health‐related quality of life | No studies reported this outcome. | ||||||
| Ear pain (otalgia) or discomfort or local irritation | No studies reported this outcome. | ||||||
| Hearing | No studies reported results for this outcome. | ||||||
| Serious complications | No studies reported that any participant died or had any intracranial or extracranial complications. | ||||||
| Suspected ototoxicity | — | 190 (3 RCTs) | Three studies reported that they did not suspect ototoxicity in any participants, but it is unclear how this was measured (de Miguel 1999; Esposito 1990; Ramos 2003). | ⊕⊝⊝⊝ | — | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group | |||||||
| GRADE Working Group grades of evidence | |||||||
| 1Downgraded to low certainty: downgraded by one level due to study limitations (risk of bias) as both studies had unclear randomisation and allocation concealment and were unblinded. Downgraded by one level due to imprecision as there were only two small studies (150 participants) with the confidence interval crossing the line of minimally important benefit. 2Downgraded to very low certainty: downgraded by two levels due to study limitations (risk of bias) as all three studies had unclear randomisation, allocation concealment and were unblinded studies. It was also unclear how the outcome was reported. Downgraded by one level due to imprecision as numeric results were not reported and there were only three small studies (190 participants). | |||||||
| Quinolones versus aminoglycosides for chronic suppurative otitis media | |||||||
| Patient or population: CSOM Settings: secondary care settings in Israel, Turkey, Jordan, Spain and Pakistan Intervention: ciprofloxacin versus tobramycin (2 studies); ciprofloxacin versus gentamycin (3 studies); ofloxacin versus gentamycin (2 studies) Comparison: other antibiotic | |||||||
| Outcomes | Relative effect | Number of participants (studies) | Anticipated absolute effects* (95% CI) | Certainty of the evidence | Comments | ||
| Aminoglycosides | Quinolones | Difference | |||||
| Resolution of ear discharge ‐ measured at 1 to 2 weeks Follow‐up: range 8 days to 2 weeks | RR 1.95 (0.88 to 4.29) | 694 (6 RCTs) | 33.7%1 | 65.7 (29.7% to 100%) | 32.0% more (4.0% lower to 110.9% higher) | ⊕⊝⊝⊝ | We used a random‐effects model due to high heterogeneity. Resolution of ear discharge at 1 to 2 weeks was higher in the quinolones group but the very low certainty of the evidence means that it is very uncertain whether or not one intervention is better or worse than the other. |
| Resolution of ear discharge ‐ Measured after 4 weeks | None of the studies measured this outcome. | ||||||
| Health‐related quality of life | None of the studies measured this outcome. | ||||||
| Ear pain (otalgia) or discomfort or local irritation Follow‐up: 30 days | — | 308 (1 RCT) | One study measured ear pain on a 3‐point scale (Lorente 1995). Results were presented as a mean score. Both groups had a mean score of 0 at 30 days. There was no difference between the groups. | ⊕⊝⊝⊝ | — | ||
| Hearing Follow‐up: 10 days | — | 132 (4 RCTs) | One study presents the hearing levels per group but does not present the data in a way that can be analysed (Tutkun 1995). One study stated in the methods that hearing was measured but only mentioned that neither group showed significant differences (Nawasreh 2001). | ⊕⊝⊝⊝ | — | ||
| Serious complications Follow‐up: 10 to 30 days | None of the studies reported that any participant died or had any intracranial or extracranial complications. | ||||||
| Suspected ototoxicity Follow‐up: 10 to 30 days | — | 352 (2 RCTs) | One study (Lorente 1995) assessed for ototoxicity and did not find any cases. One study (Tutkun 1995) reported assessment of ototoxicity in the methods but did not provide results. None of the studies reported assessment nor any cases of suspected ototoxicity. | ⊕⊝⊝⊝ | — | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group | |||||||
| GRADE Working Group grades of evidence | |||||||
| 1Average event rates in the control group were calculated without the Lorente 1995 study, as this seemed to show a very high rate of resolution (94%) compared to the other studies (range between 28% and 55%). 2Downgraded to very low certainty. Downgraded due to study limitations (risk of bias) as six of seven studies were unblinded and in general the methods were poor. Downgraded due to imprecision as the point estimate shows that more people with quinolones had resolution of discharge compared with aminoglycosides BUT there is a large confidence interval, which includes 'no effect' and a very large effect (four times as many people had resolution with quinolones compared to aminoglycosides). Downgraded due to inconsistency as there was high heterogeneity (I2 = 97%) within the results. 3Downgraded to very low certainty: downgraded by two levels due to study limitations (risk of bias) as all elements of the risk of bias assessment were unclear. Downgraded by one level due to imprecision as the results come from one relatively small study (308 patients). 4Downgraded to very low certainty: downgraded by two levels due to study limitations (risk of bias) as the studies were assessed as either high risk or unclear risk for all elements of the risk of bias assessment. Downgraded by one level due to imprecision as numeric results were not presented and the results came from two small studies (132 patients). 5Downgraded to very low certainty: downgraded by two levels due to study limitations (risk of bias) as many were unblinded and in general the studies had methodological issues and/or were badly reported. In addition, it is not clear how the outcome was measured. Downgraded by one level due to imprecision as numeric results were not reported and there were only two studies (352 participants) that identified ototoxicity as an outcome. | |||||||
| Topical antibiotics with steroids | Topical antibiotics | Systemic antibiotics | Topical antiseptics | Aural toileting (ear cleaning) | |
| Topical antibiotics with steroids | Review CSOM‐4 | ||||
| Topical antibiotics | Review CSOM‐4 | Review CSOM‐1 | |||
| Systemic antibiotics | Review CSOM‐4 | Review CSOM‐3 | Review CSOM‐2 | ||
| Topical antiseptics | Review CSOM‐4 | Review CSOM‐6 | Review CSOM‐6 | Review CSOM‐5 | |
| Aural toileting | Review CSOM‐4 | Not reviewed | Not reviewed | Not reviewed | Review CSOM‐7 |
| Placebo (or no intervention) | Review CSOM‐4 | Review CSOM‐1 | Review CSOM‐2 | Review CSOM‐5 | Review CSOM‐7 |
| CSOM‐1: Topical antibiotics for chronic suppurative otitis media (Brennan‐Jones 2018b). CSOM‐2: Systemic antibiotics for chronic suppurative otitis media (Chong 2018a). CSOM‐3: Topical versus systemic antibiotics for chronic suppurative otitis media (Chong 2018b). CSOM‐4: Topical antibiotics with steroids for chronic suppurative otitis media (Brennan‐Jones 2018a). CSOM‐5: Topical antiseptics for chronic suppurative otitis media (Head 2018a). CSOM‐6: Antibiotics versus topical antiseptics for chronic suppurative otitis media (Head 2018b). CSOM‐7: Aural toilet (ear cleaning) for chronic suppurative otitis media (Bhutta 2018). | |||||
| Class of antibiotics | Examples | Route of administration |
| Quinolones | Ciprofloxacin, ofloxacin, levofloxacin | Oral, intravenous, topical |
| Aminoglycosides | Gentamicin, tobramycin | Topical or parenteral |
| Neomycin/framycetin | Only topical | |
| Cephalosporins | Ceftazidime | Parenteral |
| Penicillins | Ticarcillin plus clavulanic acid | Parenteral |
| Monobactams | Aztreonam | Parenteral |
| Ref ID (no. participants) | Setting | Population | Intervention 1 | Intervention 2 | Treatment duration | Follow‐up | Background Treatment | Notes |
| Topical antibiotics versus placebo/no treatment (no background or aural toileting) | ||||||||
| (n = 50) | Specialist hospital, Thailand | Mucopurulent otorrhoea with perforated tympanic membrane (CSOM) | Ciprofloxacin 250 mg/mL, 5 drops per 8 hours | Saline, 5 drops per 8 hours | 1 week | 1 week | Aural toilet on day 1, 4 and 7 | Randomised by person |
| Topical antibiotic versus placebo/no treatment (systemic antibiotic as background treatment) | ||||||||
| (n = 50) | General hospital, Spain | Simple chronic otitis media (36%), osteitic chronic otitis media (25.6%), cholesteatomas chronic otitis media (13.6%), post surgery cases (24.8%) | Topical ciprofloxacin 0.2%, 3 drops per 8 hours and oral ciprofloxacin, 500 mg per 12 hours | No treatment | 7 days | 15 days | Aural toileting before beginning treatment, analgesics and antipyretics. Oral ciprofloxacin, 500 mg per 12 hours | Part of 5‐arm trial Randomised by person |
| (n = 40) | University clinic, Italy | Mild or moderate CSOM in acute stage | Ciprofloxacin 250 µg/mL, 3 drops per 12 hours | No treatment | 5 to 10 days | 2 weeks | Oral ciprofloxacin, 250mg per 12 hours | Part of 3‐arm trial Randomised by person |
| (n = 50) | University clinic, Italy | Recurrence of CSOM or suppuration following mastoidectomy or tympanoplasty | Ceftizoxime 500 µg/mL, 2 x 2 mL washes per 12 hours | Saline, 2 x 2 mL washes per 12 hours | 1 week | 3 weeks | Systemic ceftizoxime by intramuscular route every 12 hours Aural toilet at first visit | Randomised by person |
| (n = 100) | ENT departments, Spain | Simple chronic otitis media (42.7%), chronic otitis media with osteolysis (19%), chronic cholesteatoma (14%), chronic otorrhoea in operated ears 24.3%) | Ciprofloxacin 0.2%, 0.5 mL per 8 hours | No treatment | 1 week | 10 days | Oral ciprofloxacin, 500 mg per 12 hours | Part of a 6‐arm trial Randomised by person |
| Quinolones versus aminoglycosides | ||||||||
| (n = 134) | ENT department, Pakistan | Active tubotympanic type CSOM | Ofloxacin 0.3%, 12 drops per day | Gentamycin 0.3%, 12 drops per day | 10 days | 2 weeks | None mentioned | Randomised by person |
| (n = 40) | Outpatient clinic, Israel | Chronic otitis media | Ciprofloxacin (no conc), 15 drops per day | Tobramycin (no conc), 15 drops per day | 3 weeks | 3 weeks | None mentioned | Part of 3‐arm trial Randomised by ear |
| (n = 40) | University ENT clinic, Turkey | CSOM | Ciprofloxacin 0.3%, 6 drops per day | Tobramycin 0.3%, 6 drops per day | 3 weeks | 3 weeks | Daily aspiration | Translated from Turkish Part of 4‐arm trial Randomised by person. |
| (n = 88) | Unclear setting, Jordan | CSOM and intermittent mucopurulent heavy discharge for more than 1 year | Ciprofloxacin 200 µg/mL (0.02%), 15 drops per day | Gentamicin 5 mg/mL, 15 drops per day | 10 days | 2 weeks | None mentioned | Randomised by person |
| (n = 308) | Hospital ENT clinics, Spain | CSOM (purulent discharge > 3 months and perforated membrane) | Ciprofloxacin 0.3%, 15 drops per day | Gentamycin 0.3%, 15 drops per day | 8 days | 30 days | Unclear | Translated from Spanish Assume this is same as Sabater paper Randomised by person |
| (n = 44) | University hospital, Turkey | CSOM and purulent discharge for more than 1 year | Ciprofloxacin 200 µg/mL (0.02%), 15 drops per day | Gentamicin 5 mg/mL, 15 drops per day | 10 days | 10 days | None mentioned | Randomised by person |
| (n = 80) | Otolaryngology department, Pakistan | CSOM (tubotympanic type) | Ofloxacin 0.6%, 12 drops per day | Gentamycin 0.3%, 12 drops per day | 2 weeks | 2 weeks | One aural toilet at start | Randomised by person |
| Quinolones versus others | ||||||||
| (n = 200) | Specialist hospital, Pakistan | Tubotympanic type of CSOM | Ciprofloxacin (no conc), 3 drops per 12 hours | Neomycin/polymixin/gramicidin‐D (no conc), 2 drops per 12 hours | Unclear (probably 4 weeks) | 4 weeks | No information | Randomised by person |
| (n = 50) | Rural setting, Malawi | Children with CSOM | Ofloxacin 0.3%, 3 drops per 8 hours | Neomycin 0.5%/polymixin B 0.1%, 3 drops per 8 hours | 2 weeks | 2 weeks | Aural toilet at start and weekly | Part of a 3‐arm trial Only presented as an internal report Unclear unit of randomisation, results reported by ear |
| (n = unclear) | Rural setting, Malawi | "Mainly children" with CSOM | Ofloxacin 0.3%, 6 drops per 12 hours | Neomycin/polymixin B (no conc), 6 drops per 12 hours | 2 weeks | 8 weeks | Aural toilet at start and weekly | Only a presentation given at a conference available Unclear unit of randomisation, results presented by ear Part of 4‐arm trial ‐ once weekly arms have not been included. |
| Aminoglycosides versus trimethoprim, sulphacetamide and polymixin B (TSP) | ||||||||
| (n = 91) | Outpatient clinic, Canada | Otitis externa (21%), CSOM (51%), subacute otitis (16%), postoperative infection (21%) | Trimethoprim, sulphacetamide and polymyxin B, 16 drops per day | Gentamicin 0.3%, 16 drops per day | Mean: 16 days | 12 months | Not reported | Translated from French Randomised by person but reported by ear Semi cross‐over trial |
| Rifampicin versus chloramphenicol | ||||||||
| (n = 160) | Outpatient department, China | CSOM | Rifampicin 0.1%, 9 drops per day | Chloramphenicol 0.25%, 9 drops per day | 2 weeks | 2 weeks | 3% hydrogen peroxide ear wash daily | Translated from Chinese Randomised by person |
| CSOM: chronic suppurative otitis media | ||||||||
| Reference | Unit of randomisation | Reported | Definition | Otoscopically confirmed? | Time points | Notes |
| Person | Person | "no discharge" | Yes | 1 to 2 weeks (10 days) | — | |
| Person | Person | "global index of clinic microbiological cure" | Yes | 1 to 2 weeks (7 days) | — | |
| Person | Person | "cured" (no definition but assumed to be no discharge) | Unclear, paper states "clinically examined" | 1 week to 2 weeks (6 to 11 days) and 2 to 4 weeks (19 to 24 days) | 1 to 2 weeks examined but not reported | |
| Ear | Ear | "clinical success as defined as cessation of otorrhea and eradication of the microorganisms in the post treatment culture" | Unclear | 2 to 4 weeks (21 days) | Unclear how many patients had bilateral ear disease in each group | |
| Person | Ear | "dry ear" and a negative culture at 3 weeks or a real improvement in at 3 weeks and the cessation of discharge at 6 weeks | Unclear | 2 to 4 weeks (3 weeks) and after 4 weeks (6 weeks) | Semi cross‐over trial. It does not appear that any consideration of the correlation of results between ears has been taken into account. If there was a treatment failure 'ears' were transferred to the alternative groups. These results have not been included in the analysis. If the ear was not dry on review at 6 months, treatment for 3 weeks with the alternative treatment was completed with review after 6 months | |
| Person | Person | "absence" of aural discharge | Yes | 2 to 4 weeks (2 weeks) | — | |
| Person | Person | "cure" | Unclear | 1 to 2 weeks (7 days) | — | |
| Person | Ear | Assessed using 3‐point scale (2 points = no drainage) | Yes | 2 to 4 weeks (day 14 and 21) | Unclear method of allocation, unsure if random selection of study ear | |
| Person | Person | "Cured: otorrhea disappeared, mucosal hyperaemia of the tympanic membrane and tympanic cavity disappeared. Significantly effective: no complaints of otorrhea, no visible purulence in the ear canal and tympanic cavity, and nonvisible or slight hyperaemia of the tympanic membrane and the tympanic canal" | Unclear | 1 to 2 weeks (2 weeks) | — | |
| Person | Person | "Complete resolution of ear discharge" | Yes | 1 to 2 weeks (8 days) and after 4 weeks (30 days) | — | |
| Person | Person | Not reported in a way that could be used in the review | N/A | N/A | Paper plotted the time course of otorrhoea (quantity) on a scale of 0 to 3 at 3, 7 and 21 days | |
| Person | Person | "cessation of otorrhea" | Yes | 1 to 2 weeks (10 days) | — | |
| Person | Person | "cured" according to "indices de curacion" | Unclear | 1 to 2 weeks (10 days) | — | |
| Person | Person | "absence of discharge from middle ear cavity and no inflammation/congestion in middle ear mucosa and tympanic membrane" | Unclear | 2 to 4 weeks (4 weeks) | 15 patients (8%) had bilateral disease but how these cases were handled is not stated. The denominator in the trials is the person so it is assumed that no double counting occurred. | |
| Person | Person | "cessation of otorrhea" | Yes | 1 to 2 weeks (10 days) | — | |
| Unclear, most likely person | Ear | "dry ear" | Unclear | 1 to 2 weeks (1 week) and after 4 weeks (> 2 weeks) | Counting bilateral ears separately. All ears reported separately. Data come from an unpublished report. In the analysis 3/11 (27.27%), 10/30 (33%) and 11/28 (39%) of patients had bilateral disease in the ofloxacin, neomycin and antiseptic acid groups respectively. | |
| Unclear | Ear | "inactive ear" ‐ completely dry middle ear | Unclear | 1 to 2 weeks (1 week), 2 to 4 weeks (2 weeks) and after 4 weeks (8 weeks) | Counting bilateral ears separately | |
| N/A: not applicable | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Resolution of ear discharge Show forest plot | 1 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.74 [1.82, 24.99] |
| 1.1 Measured at 1 to 2 weeks | 1 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.74 [1.82, 24.99] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Resolution of ear discharge Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Measured at 1 to 2 weeks | 2 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.47 [1.20, 1.80] |
| 1.2 Measured at 2 to 4 weeks | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.88 [1.04, 3.39] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Resolution of ear discharge (1 to 2 weeks) Show forest plot | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Quinolones versus aminoglycosides | 6 | 694 | Risk Ratio (M‐H, Random, 95% CI) | 1.95 [0.88, 4.29] |
| 2 Resolution of ear discharge (2 to 4 weeks) Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Quinolones versus aminoglycosides | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.80, 1.64] |
| 2.2 Quinolones versus neomycin/polymixin B | 1 | 186 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [1.03, 1.22] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Resolution of ear discharge (1 to 2 weeks) Show forest plot | 1 | 160 | Risk Ratio (M‐H, Random, 95% CI) | 1.78 [1.35, 2.34] |

