Antibióticos tópicos para la otitis media supurativa crónica
Información
- DOI:
- https://doi.org/10.1002/14651858.CD013051.pub2Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 02 enero 2020see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Enfermedades de oído, nariz y garganta
- Copyright:
-
- Copyright © 2020 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
Christopher G Brennan‐Jones: clinical guidance at all stages of the review; reviewed the analyses; wrote, reviewed and edited the text of the review.
Lee Yee Chong: scoped the review, designed and wrote the protocol. Screened the search results and selected studies, carried out data extraction, 'Risk of bias' assessment and statistical analyses, reviewed and edited the text of the review.
Karen Head: scoped the review, designed and wrote the protocol. Screened the search results and selected studies, carried out data extraction, 'Risk of bias' assessment and statistical analyses, wrote the text of the review.
Martin J Burton: clinical guidance at all stages of the review; reviewed the analyses and reviewed and edited the text of the review. Wrote the abstract for the review.
Anne GM Schilder: clinical guidance at all stages of the review; reviewed the analyses and reviewed and edited the text of the review.
Mahmood F Bhutta: helped to scope, design and write the protocol; reviewed the analyses of results and provided clinical guidance at all stages of the review. Reviewed and edited the text of the review.
Sources of support
Internal sources
-
No sources of support supplied
External sources
-
National Institute for Health Research, UK.
Infrastructure funding for Cochrane ENT
-
NHMRC Centre of Research Excellence in Ear and Hearing Health of Aboriginal and Torres Strait Islander Children, Australia.
-
Department of Health, Western Australia, Australia.
Infrastructure funding
-
NHMRC Fellowship for CG Brennan‐Jones #1142897, Australia.
Personnel support
-
WA Department of Health, Australia.
Future Health Merit Award
Declarations of interest
Christopher G Brennan‐Jones: Dr Brennan‐Jones's research team is primarily funded by the Australian NHMRC and the WA Department of Health. He sits on the national Technical Advisory Group responsible for developing treatment guidelines for otitis media in Australia.
Karen Head: none known.
Lee Yee Chong: none known.
Martin J Burton: Professor Martin Burton is joint Co‐ordinating Editor of Cochrane ENT, but had no role in the editorial process for this review.
Anne GM Schilder: Professor Anne Schilder is joint Co‐ordinating Editor of Cochrane ENT, but had no role in the editorial process for this review. Her evidENT team at UCL is supported in part by the National Institute of Health Research (NIHR) University College London Hospitals Biomedical Research Centre. The research is funded by the NIHR and EU Horizon2020. She is the national chair of the NIHR Clinical Research Network ENT Specialty. She is the Surgical Specialty Lead for ENT for the Royal College of Surgeons of England's Clinical Trials Initiative. In her role as director of the NIHR UCLH BRC Deafness and Hearing Problems Theme, she acts as an advisor on clinical trial design and delivery to a range of biotech companies, most currently Novus Therapeutics.
Mahmood F Bhutta: Dr Mahmood Bhutta has received an honorarium from Novus Therapeutics for advice on an experimental treatment for otitis media (not related to any treatment in this review).
Acknowledgements
This project was funded by the NHMRC Centre of Research Excellence for Ear and Hearing Health of Aboriginal and Torres Strait Islander Children (NHMRC CRE_ICHEAR) and the Western Australian Department of Health through a Future Health Merit Award. The contents of the publications arising from this work are solely the responsibility of the authors and do not reflect the views of NHMRC or the Department of Health.
We are grateful to Professor Amanda Leach for peer reviewing the protocol for this review, to Mr Iain Swan for peer reviewing the protocol and review, and to consumer referee Joan Blakely for her helpful comments at all stages. We would also like to thank Dr Adrian James, as Acting Co‐ordinating Editor for Cochrane ENT, for his insightful comments and advice, and the other members of the Cochrane ENT editorial board for their input and encouragement.
We would like to sincerely thank Jenny Bellorini and Samantha Cox from the Cochrane ENT team for their invaluable help, which has enabled the completion of this suite of reviews, and Jessica Daw for assisting with preparation and collation of the final reviews.
We would also like to thank the following clinicians, scientists and consumers who provided comments on the initial scoping review and prioritisation exercise for this suite of reviews into CSOM: Amanda Leach, Chris Perry, Courtney McMahen, De Wet Swanepoel, Deborah Lehmann, Eka Dian Safitri, Francis Lannigan, Harvey Coates, Has Gunasekera, Ian Williamson, Jenny Reath, Kathy Brooker, Kathy Currie, Kelvin Kong, Matthew Brown, Pavanee Intakorn, Penny Abbot, Samantha Harkus, Sharon Weeks, Shelly Chadha, Stephen O'Leary, Victoria Stroud and Yupitri Pitoyo.
We are indebted to Therese Dalsbø, Artur Gevorgyan, Nathan Gonik, Anna Kashchuk, Esther Martin, Stefano Morettini, Jussi Mustonen, Irina Telegina, Yu‐Tian Xiao, Ibrahim Ethem Yayali, Francine Choi, Chiara Arienti, Maria Paula Garcia, Karen Sagomonyants and Elizabeth Weeda for translating and identifying primary studies for inclusion or exclusion for this suite of reviews.
We are also indebted to Erika Ota from Cochrane Japan for organising a group of MSc students, Shunka Cho, Kiriko Sasayama, Asuka Ohashi, Noyuri Yamaji and Mika Kato, to help with translating and identifying primary studies for inclusion or exclusion for this suite of reviews.
We thank Carolyn McFadyen for her help and support in providing documents from the previous Cochrane Reviews.
We thank Dr Nathan Tu for his contributions and clinical guidance at the protocol stage of this review.
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure, Cochrane Programme Grant or Cochrane Incentive funding to Cochrane ENT. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Version history
| Published | Title | Stage | Authors | Version |
| 2020 Jan 02 | Topical antibiotics for chronic suppurative otitis media | Review | Christopher G Brennan‐Jones, Karen Head, Lee‐Yee Chong, Martin J Burton, Anne GM Schilder, Mahmood F Bhutta | |
| 2018 Jun 18 | Topical antibiotics for chronic suppurative otitis media | Protocol | Christopher G Brennan‐Jones, Karen Head, Lee‐Yee Chong, Nathan Tu, Martin J Burton, Anne GM Schilder, Mahmood F Bhutta | |
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
Medical Subject Headings Check Words
Humans;
PICO

Study flow diagram
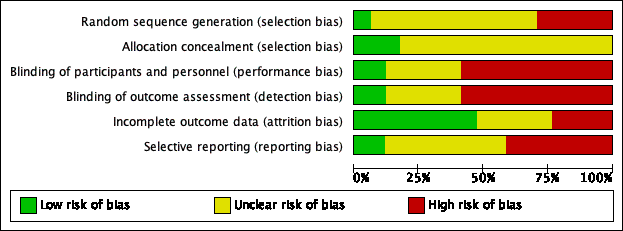
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
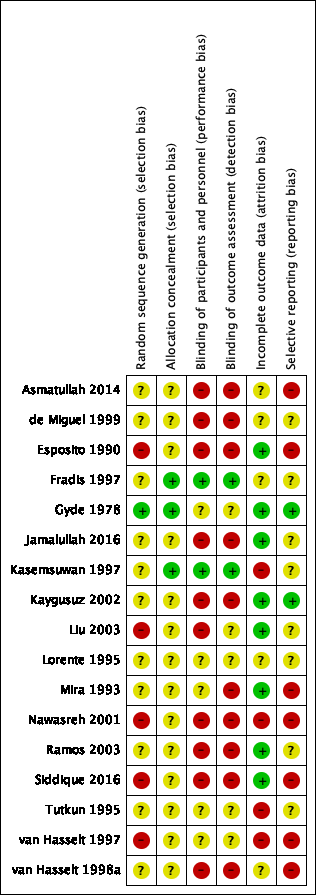
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
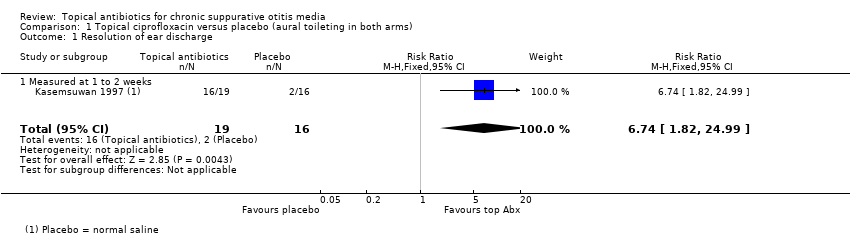
Comparison 1 Topical ciprofloxacin versus placebo (aural toileting in both arms), Outcome 1 Resolution of ear discharge.
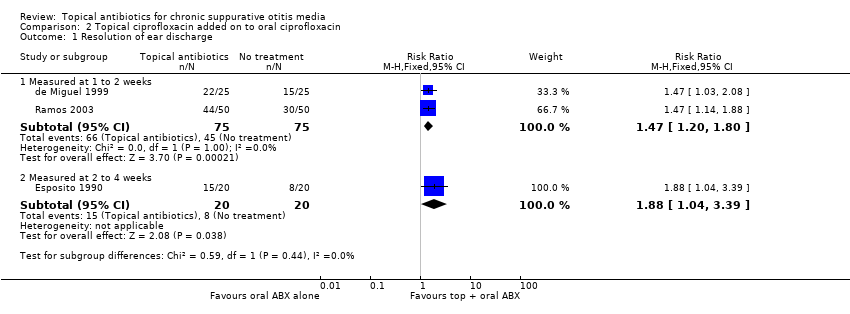
Comparison 2 Topical ciprofloxacin added on to oral ciprofloxacin, Outcome 1 Resolution of ear discharge.
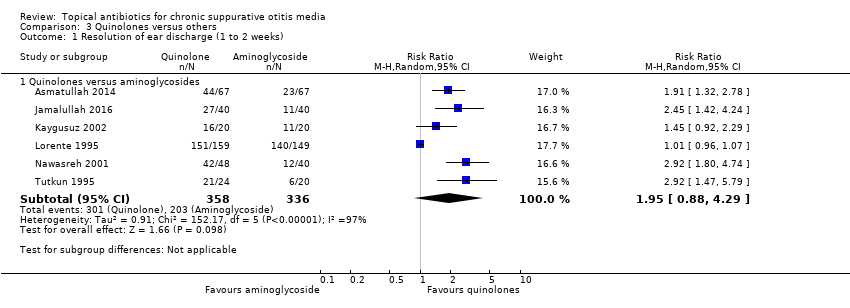
Comparison 3 Quinolones versus others, Outcome 1 Resolution of ear discharge (1 to 2 weeks).
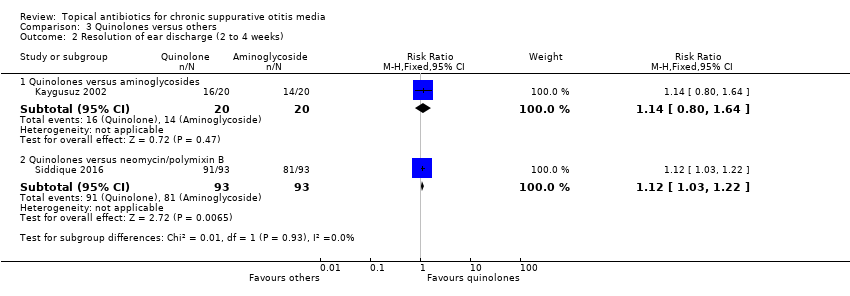
Comparison 3 Quinolones versus others, Outcome 2 Resolution of ear discharge (2 to 4 weeks).

Comparison 4 Rifampicin versus chloramphenicol, Outcome 1 Resolution of ear discharge (1 to 2 weeks).
| Topical antibiotics (ciprofloxacin) versus placebo/no treatment for chronic suppurative otitis media | |||||||
| Patient or population: patients with mucopurulent otorrhoea Settings: specialist hospital in Thailand Intervention: ciprofloxacin ear drops Comparison: saline | |||||||
| Outcomes | Relative effect | Number of participants (studies) | Anticipated absolute effects* (95% CI) | Certainty of the evidence | Comments | ||
| Without topical antibiotic | With topical antibiotic | Difference | |||||
| Resolution of ear discharge ‐ measured at 1 to 2 weeks Follow‐up: 7 days | RR 6.74 (1.82 to 24.99) | 35 (1 RCT) | Study population | ⊕⊝⊝⊝ | Topical antibiotics may increase the number of patients with resolution of ear discharge at 7 days compared with placebo, but we are very uncertain about the results. | ||
| 12.5% | 84.2% (22.8 to 100.0) | 71.8% more (10.3 to 299.9) | |||||
| Resolution of ear discharge ‐ measured after 4 weeks | No study measured this outcome. | ||||||
| Health‐related quality of life | No study measured this outcome. | ||||||
| Ear pain (otalgia) or discomfort or local irritation Follow‐up: 7 days | — | 35 (1 RCT) | Authors reported "no medical side‐effects and worsening of audiological measurements related to this topical medication were detected". | ⊕⊝⊝⊝ | — | ||
| Hearing Follow‐up: 7 days | — | 35 (1 RCT) | Authors reported "no ... worsening of audiological measurements related to this topical medication were detected." | ⊕⊝⊝⊝ | — | ||
| Serious complications | No studies reported that any participant died or had any intracranial or extracranial complications. | ||||||
| Suspected ototoxicity Follow‐up: 7 days | — | 35 (1 RCT) | Authors report "no suspected ototoxicity" but it is unclear how this was measured. | ⊕⊝⊝⊝ | — | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group | |||||||
| GRADE Working Group grades of evidence | |||||||
| 1Downgraded to very low certainty: downgraded by one level due to study limitations (risk of bias) because there were concerns about incomplete data (50 people entered the study but results are only reported for 35). Downgraded by two levels due to imprecision as there was one very small study (35 participants) with wide confidence intervals. 2Downgraded to very low certainty: downgraded by two levels due to study limitations (risk of bias) because there were concerns about incomplete data (50 people entered the study but results are only reported for 35) and it is unclear how this outcome was measured as the paper just reports "no medical side effects". Downgraded by one level due to imprecision as numeric results were not provided and it was only one very small study (35 participants). 3Downgraded to very low certainty: downgraded by two levels due to study limitations (risk of bias) because of concerns about incomplete data (50 people entered the study but results are only reported for 35) and the methods used for measuring hearing were not provided in the paper. Downgraded by one level due to imprecision as numeric results were not reported and it was only one very small study (35 participants). | |||||||
| Topical antibiotics (ciprofloxacin) on top of systemic antibiotics (ciprofloxacin) for chronic suppurative otitis media | |||||||
| Patient or population: CSOM, recurrence of CSOM or suppuration following mastoidectomy or tympanoplasty Settings: secondary care clinics in Spain and Italy Intervention: ciprofloxacin (topical) plus ciprofloxacin (systemic) Comparison: ciprofloxacin (systemic) | |||||||
| Outcomes | Relative effect | Number of participants (studies) | Anticipated absolute effects* (95% CI) | Certainty of the evidence | Comments | ||
| Without topical antibiotics | With topical antibiotics | Difference | |||||
| Resolution of ear discharge‐ measured at 1 to 2 weeks Follow‐up: 7 to 10 days | RR 1.47 (1.20 to 1.80) | 150 (2 RCTs) | Study population | ⊕⊕⊝⊝ | Topical antibiotics in addition to systemic antibiotics may increase the number of patients with resolution of ear discharge at 7 to 10 days compared with systemic antibiotics alone. The NNTB is 4 (95% CI 3 to 9). | ||
| 60.0% | 88.2% (72.0 to 100) | 28.2% more (12 more to 48 more) | |||||
| Resolution of ear discharge ‐ measured after 4 weeks | No studies reported this outcome. | ||||||
| Health‐related quality of life | No studies reported this outcome. | ||||||
| Ear pain (otalgia) or discomfort or local irritation | No studies reported this outcome. | ||||||
| Hearing | No studies reported results for this outcome. | ||||||
| Serious complications | No studies reported that any participant died or had any intracranial or extracranial complications. | ||||||
| Suspected ototoxicity | — | 190 (3 RCTs) | Three studies reported that they did not suspect ototoxicity in any participants, but it is unclear how this was measured (de Miguel 1999; Esposito 1990; Ramos 2003). | ⊕⊝⊝⊝ | — | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group | |||||||
| GRADE Working Group grades of evidence | |||||||
| 1Downgraded to low certainty: downgraded by one level due to study limitations (risk of bias) as both studies had unclear randomisation and allocation concealment and were unblinded. Downgraded by one level due to imprecision as there were only two small studies (150 participants) with the confidence interval crossing the line of minimally important benefit. 2Downgraded to very low certainty: downgraded by two levels due to study limitations (risk of bias) as all three studies had unclear randomisation, allocation concealment and were unblinded studies. It was also unclear how the outcome was reported. Downgraded by one level due to imprecision as numeric results were not reported and there were only three small studies (190 participants). | |||||||
| Quinolones versus aminoglycosides for chronic suppurative otitis media | |||||||
| Patient or population: CSOM Settings: secondary care settings in Israel, Turkey, Jordan, Spain and Pakistan Intervention: ciprofloxacin versus tobramycin (2 studies); ciprofloxacin versus gentamycin (3 studies); ofloxacin versus gentamycin (2 studies) Comparison: other antibiotic | |||||||
| Outcomes | Relative effect | Number of participants (studies) | Anticipated absolute effects* (95% CI) | Certainty of the evidence | Comments | ||
| Aminoglycosides | Quinolones | Difference | |||||
| Resolution of ear discharge ‐ measured at 1 to 2 weeks Follow‐up: range 8 days to 2 weeks | RR 1.95 (0.88 to 4.29) | 694 (6 RCTs) | 33.7%1 | 65.7 (29.7% to 100%) | 32.0% more (4.0% lower to 110.9% higher) | ⊕⊝⊝⊝ | We used a random‐effects model due to high heterogeneity. Resolution of ear discharge at 1 to 2 weeks was higher in the quinolones group but the very low certainty of the evidence means that it is very uncertain whether or not one intervention is better or worse than the other. |
| Resolution of ear discharge ‐ Measured after 4 weeks | None of the studies measured this outcome. | ||||||
| Health‐related quality of life | None of the studies measured this outcome. | ||||||
| Ear pain (otalgia) or discomfort or local irritation Follow‐up: 30 days | — | 308 (1 RCT) | One study measured ear pain on a 3‐point scale (Lorente 1995). Results were presented as a mean score. Both groups had a mean score of 0 at 30 days. There was no difference between the groups. | ⊕⊝⊝⊝ | — | ||
| Hearing Follow‐up: 10 days | — | 132 (4 RCTs) | One study presents the hearing levels per group but does not present the data in a way that can be analysed (Tutkun 1995). One study stated in the methods that hearing was measured but only mentioned that neither group showed significant differences (Nawasreh 2001). | ⊕⊝⊝⊝ | — | ||
| Serious complications Follow‐up: 10 to 30 days | None of the studies reported that any participant died or had any intracranial or extracranial complications. | ||||||
| Suspected ototoxicity Follow‐up: 10 to 30 days | — | 352 (2 RCTs) | One study (Lorente 1995) assessed for ototoxicity and did not find any cases. One study (Tutkun 1995) reported assessment of ototoxicity in the methods but did not provide results. None of the studies reported assessment nor any cases of suspected ototoxicity. | ⊕⊝⊝⊝ | — | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group | |||||||
| GRADE Working Group grades of evidence | |||||||
| 1Average event rates in the control group were calculated without the Lorente 1995 study, as this seemed to show a very high rate of resolution (94%) compared to the other studies (range between 28% and 55%). 2Downgraded to very low certainty. Downgraded due to study limitations (risk of bias) as six of seven studies were unblinded and in general the methods were poor. Downgraded due to imprecision as the point estimate shows that more people with quinolones had resolution of discharge compared with aminoglycosides BUT there is a large confidence interval, which includes 'no effect' and a very large effect (four times as many people had resolution with quinolones compared to aminoglycosides). Downgraded due to inconsistency as there was high heterogeneity (I2 = 97%) within the results. 3Downgraded to very low certainty: downgraded by two levels due to study limitations (risk of bias) as all elements of the risk of bias assessment were unclear. Downgraded by one level due to imprecision as the results come from one relatively small study (308 patients). 4Downgraded to very low certainty: downgraded by two levels due to study limitations (risk of bias) as the studies were assessed as either high risk or unclear risk for all elements of the risk of bias assessment. Downgraded by one level due to imprecision as numeric results were not presented and the results came from two small studies (132 patients). 5Downgraded to very low certainty: downgraded by two levels due to study limitations (risk of bias) as many were unblinded and in general the studies had methodological issues and/or were badly reported. In addition, it is not clear how the outcome was measured. Downgraded by one level due to imprecision as numeric results were not reported and there were only two studies (352 participants) that identified ototoxicity as an outcome. | |||||||
| Topical antibiotics with steroids | Topical antibiotics | Systemic antibiotics | Topical antiseptics | Aural toileting (ear cleaning) | |
| Topical antibiotics with steroids | Review CSOM‐4 | ||||
| Topical antibiotics | Review CSOM‐4 | Review CSOM‐1 | |||
| Systemic antibiotics | Review CSOM‐4 | Review CSOM‐3 | Review CSOM‐2 | ||
| Topical antiseptics | Review CSOM‐4 | Review CSOM‐6 | Review CSOM‐6 | Review CSOM‐5 | |
| Aural toileting | Review CSOM‐4 | Not reviewed | Not reviewed | Not reviewed | Review CSOM‐7 |
| Placebo (or no intervention) | Review CSOM‐4 | Review CSOM‐1 | Review CSOM‐2 | Review CSOM‐5 | Review CSOM‐7 |
| CSOM‐1: Topical antibiotics for chronic suppurative otitis media (Brennan‐Jones 2018b). CSOM‐2: Systemic antibiotics for chronic suppurative otitis media (Chong 2018a). CSOM‐3: Topical versus systemic antibiotics for chronic suppurative otitis media (Chong 2018b). CSOM‐4: Topical antibiotics with steroids for chronic suppurative otitis media (Brennan‐Jones 2018a). CSOM‐5: Topical antiseptics for chronic suppurative otitis media (Head 2018a). CSOM‐6: Antibiotics versus topical antiseptics for chronic suppurative otitis media (Head 2018b). CSOM‐7: Aural toilet (ear cleaning) for chronic suppurative otitis media (Bhutta 2018). | |||||
| Class of antibiotics | Examples | Route of administration |
| Quinolones | Ciprofloxacin, ofloxacin, levofloxacin | Oral, intravenous, topical |
| Aminoglycosides | Gentamicin, tobramycin | Topical or parenteral |
| Neomycin/framycetin | Only topical | |
| Cephalosporins | Ceftazidime | Parenteral |
| Penicillins | Ticarcillin plus clavulanic acid | Parenteral |
| Monobactams | Aztreonam | Parenteral |
| Ref ID (no. participants) | Setting | Population | Intervention 1 | Intervention 2 | Treatment duration | Follow‐up | Background Treatment | Notes |
| Topical antibiotics versus placebo/no treatment (no background or aural toileting) | ||||||||
| (n = 50) | Specialist hospital, Thailand | Mucopurulent otorrhoea with perforated tympanic membrane (CSOM) | Ciprofloxacin 250 mg/mL, 5 drops per 8 hours | Saline, 5 drops per 8 hours | 1 week | 1 week | Aural toilet on day 1, 4 and 7 | Randomised by person |
| Topical antibiotic versus placebo/no treatment (systemic antibiotic as background treatment) | ||||||||
| (n = 50) | General hospital, Spain | Simple chronic otitis media (36%), osteitic chronic otitis media (25.6%), cholesteatomas chronic otitis media (13.6%), post surgery cases (24.8%) | Topical ciprofloxacin 0.2%, 3 drops per 8 hours and oral ciprofloxacin, 500 mg per 12 hours | No treatment | 7 days | 15 days | Aural toileting before beginning treatment, analgesics and antipyretics. Oral ciprofloxacin, 500 mg per 12 hours | Part of 5‐arm trial Randomised by person |
| (n = 40) | University clinic, Italy | Mild or moderate CSOM in acute stage | Ciprofloxacin 250 µg/mL, 3 drops per 12 hours | No treatment | 5 to 10 days | 2 weeks | Oral ciprofloxacin, 250mg per 12 hours | Part of 3‐arm trial Randomised by person |
| (n = 50) | University clinic, Italy | Recurrence of CSOM or suppuration following mastoidectomy or tympanoplasty | Ceftizoxime 500 µg/mL, 2 x 2 mL washes per 12 hours | Saline, 2 x 2 mL washes per 12 hours | 1 week | 3 weeks | Systemic ceftizoxime by intramuscular route every 12 hours Aural toilet at first visit | Randomised by person |
| (n = 100) | ENT departments, Spain | Simple chronic otitis media (42.7%), chronic otitis media with osteolysis (19%), chronic cholesteatoma (14%), chronic otorrhoea in operated ears 24.3%) | Ciprofloxacin 0.2%, 0.5 mL per 8 hours | No treatment | 1 week | 10 days | Oral ciprofloxacin, 500 mg per 12 hours | Part of a 6‐arm trial Randomised by person |
| Quinolones versus aminoglycosides | ||||||||
| (n = 134) | ENT department, Pakistan | Active tubotympanic type CSOM | Ofloxacin 0.3%, 12 drops per day | Gentamycin 0.3%, 12 drops per day | 10 days | 2 weeks | None mentioned | Randomised by person |
| (n = 40) | Outpatient clinic, Israel | Chronic otitis media | Ciprofloxacin (no conc), 15 drops per day | Tobramycin (no conc), 15 drops per day | 3 weeks | 3 weeks | None mentioned | Part of 3‐arm trial Randomised by ear |
| (n = 40) | University ENT clinic, Turkey | CSOM | Ciprofloxacin 0.3%, 6 drops per day | Tobramycin 0.3%, 6 drops per day | 3 weeks | 3 weeks | Daily aspiration | Translated from Turkish Part of 4‐arm trial Randomised by person. |
| (n = 88) | Unclear setting, Jordan | CSOM and intermittent mucopurulent heavy discharge for more than 1 year | Ciprofloxacin 200 µg/mL (0.02%), 15 drops per day | Gentamicin 5 mg/mL, 15 drops per day | 10 days | 2 weeks | None mentioned | Randomised by person |
| (n = 308) | Hospital ENT clinics, Spain | CSOM (purulent discharge > 3 months and perforated membrane) | Ciprofloxacin 0.3%, 15 drops per day | Gentamycin 0.3%, 15 drops per day | 8 days | 30 days | Unclear | Translated from Spanish Assume this is same as Sabater paper Randomised by person |
| (n = 44) | University hospital, Turkey | CSOM and purulent discharge for more than 1 year | Ciprofloxacin 200 µg/mL (0.02%), 15 drops per day | Gentamicin 5 mg/mL, 15 drops per day | 10 days | 10 days | None mentioned | Randomised by person |
| (n = 80) | Otolaryngology department, Pakistan | CSOM (tubotympanic type) | Ofloxacin 0.6%, 12 drops per day | Gentamycin 0.3%, 12 drops per day | 2 weeks | 2 weeks | One aural toilet at start | Randomised by person |
| Quinolones versus others | ||||||||
| (n = 200) | Specialist hospital, Pakistan | Tubotympanic type of CSOM | Ciprofloxacin (no conc), 3 drops per 12 hours | Neomycin/polymixin/gramicidin‐D (no conc), 2 drops per 12 hours | Unclear (probably 4 weeks) | 4 weeks | No information | Randomised by person |
| (n = 50) | Rural setting, Malawi | Children with CSOM | Ofloxacin 0.3%, 3 drops per 8 hours | Neomycin 0.5%/polymixin B 0.1%, 3 drops per 8 hours | 2 weeks | 2 weeks | Aural toilet at start and weekly | Part of a 3‐arm trial Only presented as an internal report Unclear unit of randomisation, results reported by ear |
| (n = unclear) | Rural setting, Malawi | "Mainly children" with CSOM | Ofloxacin 0.3%, 6 drops per 12 hours | Neomycin/polymixin B (no conc), 6 drops per 12 hours | 2 weeks | 8 weeks | Aural toilet at start and weekly | Only a presentation given at a conference available Unclear unit of randomisation, results presented by ear Part of 4‐arm trial ‐ once weekly arms have not been included. |
| Aminoglycosides versus trimethoprim, sulphacetamide and polymixin B (TSP) | ||||||||
| (n = 91) | Outpatient clinic, Canada | Otitis externa (21%), CSOM (51%), subacute otitis (16%), postoperative infection (21%) | Trimethoprim, sulphacetamide and polymyxin B, 16 drops per day | Gentamicin 0.3%, 16 drops per day | Mean: 16 days | 12 months | Not reported | Translated from French Randomised by person but reported by ear Semi cross‐over trial |
| Rifampicin versus chloramphenicol | ||||||||
| (n = 160) | Outpatient department, China | CSOM | Rifampicin 0.1%, 9 drops per day | Chloramphenicol 0.25%, 9 drops per day | 2 weeks | 2 weeks | 3% hydrogen peroxide ear wash daily | Translated from Chinese Randomised by person |
| CSOM: chronic suppurative otitis media | ||||||||
| Reference | Unit of randomisation | Reported | Definition | Otoscopically confirmed? | Time points | Notes |
| Person | Person | "no discharge" | Yes | 1 to 2 weeks (10 days) | — | |
| Person | Person | "global index of clinic microbiological cure" | Yes | 1 to 2 weeks (7 days) | — | |
| Person | Person | "cured" (no definition but assumed to be no discharge) | Unclear, paper states "clinically examined" | 1 week to 2 weeks (6 to 11 days) and 2 to 4 weeks (19 to 24 days) | 1 to 2 weeks examined but not reported | |
| Ear | Ear | "clinical success as defined as cessation of otorrhea and eradication of the microorganisms in the post treatment culture" | Unclear | 2 to 4 weeks (21 days) | Unclear how many patients had bilateral ear disease in each group | |
| Person | Ear | "dry ear" and a negative culture at 3 weeks or a real improvement in at 3 weeks and the cessation of discharge at 6 weeks | Unclear | 2 to 4 weeks (3 weeks) and after 4 weeks (6 weeks) | Semi cross‐over trial. It does not appear that any consideration of the correlation of results between ears has been taken into account. If there was a treatment failure 'ears' were transferred to the alternative groups. These results have not been included in the analysis. If the ear was not dry on review at 6 months, treatment for 3 weeks with the alternative treatment was completed with review after 6 months | |
| Person | Person | "absence" of aural discharge | Yes | 2 to 4 weeks (2 weeks) | — | |
| Person | Person | "cure" | Unclear | 1 to 2 weeks (7 days) | — | |
| Person | Ear | Assessed using 3‐point scale (2 points = no drainage) | Yes | 2 to 4 weeks (day 14 and 21) | Unclear method of allocation, unsure if random selection of study ear | |
| Person | Person | "Cured: otorrhea disappeared, mucosal hyperaemia of the tympanic membrane and tympanic cavity disappeared. Significantly effective: no complaints of otorrhea, no visible purulence in the ear canal and tympanic cavity, and nonvisible or slight hyperaemia of the tympanic membrane and the tympanic canal" | Unclear | 1 to 2 weeks (2 weeks) | — | |
| Person | Person | "Complete resolution of ear discharge" | Yes | 1 to 2 weeks (8 days) and after 4 weeks (30 days) | — | |
| Person | Person | Not reported in a way that could be used in the review | N/A | N/A | Paper plotted the time course of otorrhoea (quantity) on a scale of 0 to 3 at 3, 7 and 21 days | |
| Person | Person | "cessation of otorrhea" | Yes | 1 to 2 weeks (10 days) | — | |
| Person | Person | "cured" according to "indices de curacion" | Unclear | 1 to 2 weeks (10 days) | — | |
| Person | Person | "absence of discharge from middle ear cavity and no inflammation/congestion in middle ear mucosa and tympanic membrane" | Unclear | 2 to 4 weeks (4 weeks) | 15 patients (8%) had bilateral disease but how these cases were handled is not stated. The denominator in the trials is the person so it is assumed that no double counting occurred. | |
| Person | Person | "cessation of otorrhea" | Yes | 1 to 2 weeks (10 days) | — | |
| Unclear, most likely person | Ear | "dry ear" | Unclear | 1 to 2 weeks (1 week) and after 4 weeks (> 2 weeks) | Counting bilateral ears separately. All ears reported separately. Data come from an unpublished report. In the analysis 3/11 (27.27%), 10/30 (33%) and 11/28 (39%) of patients had bilateral disease in the ofloxacin, neomycin and antiseptic acid groups respectively. | |
| Unclear | Ear | "inactive ear" ‐ completely dry middle ear | Unclear | 1 to 2 weeks (1 week), 2 to 4 weeks (2 weeks) and after 4 weeks (8 weeks) | Counting bilateral ears separately | |
| N/A: not applicable | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Resolution of ear discharge Show forest plot | 1 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.74 [1.82, 24.99] |
| 1.1 Measured at 1 to 2 weeks | 1 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.74 [1.82, 24.99] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Resolution of ear discharge Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Measured at 1 to 2 weeks | 2 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.47 [1.20, 1.80] |
| 1.2 Measured at 2 to 4 weeks | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.88 [1.04, 3.39] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Resolution of ear discharge (1 to 2 weeks) Show forest plot | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Quinolones versus aminoglycosides | 6 | 694 | Risk Ratio (M‐H, Random, 95% CI) | 1.95 [0.88, 4.29] |
| 2 Resolution of ear discharge (2 to 4 weeks) Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Quinolones versus aminoglycosides | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.80, 1.64] |
| 2.2 Quinolones versus neomycin/polymixin B | 1 | 186 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [1.03, 1.22] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Resolution of ear discharge (1 to 2 weeks) Show forest plot | 1 | 160 | Risk Ratio (M‐H, Random, 95% CI) | 1.78 [1.35, 2.34] |

