Non‐contact methods for the detection of people at risk of primary angle closure glaucoma
Abstract
This is a protocol for a Cochrane Review (Diagnostic test accuracy). The objectives are as follows:
To determine the diagnostic accuracy of non‐contact screening methods for identifying eyes with a narrow angle.
Background
Clinical problem
Primary angle closure (PAC) is characterised by appositional or adhesional (synechial) narrowing (and eventually occlusion) of the drainage angle in the anterior chamber of the eye, resulting in elevated intraocular pressure (IOP) and subsequent glaucomatous optic neuropathy, a condition known as primary angle closure glaucoma (PACG). The occlusion of the drainage angle may occur rapidly or slowly. Rapid occlusion results in symptomatic IOP elevation that requires emergency medical treatment (known as acute angle closure). Individuals presenting with acute angle closure, characterised by eye pain, headache, corneal oedema and vascular congestion, are treated initially with topical and oral medications to lower the IOP. This is followed by laser peripheral iridotomy as soon as possible after angle closure, usually with prophylactic treatment of the fellow eye (Emanuel 2014). An occlusion that develops insidiously results in chronically raised IOP, which is often asymptomatic. Management for chronic angle closure involves: medical (topical hypotensives); laser peripheral iridotomy; filtration surgery or a combination of these to lower the IOP and open up the drainage angle. A recently published multicentred randomised controlled trial has provided evidence that clear lens extraction is associated with better clinical and patient‐reported outcomes than peripheral iridotomy and may therefore be a better first‐line treatment option (Azuara‐Blanco 2016).
A recent systematic review found the global prevalence of PACG to be 0.5% of individuals aged 40 to 80 years, and estimated that the number of people with the disease will reach 23.4 million by 2020 and 32 million by 2040 (Tham 2014). Although, globally, open‐angle glaucoma is more common (3%) (Tham 2014), PACG is more likely to result in bilateral blindness (Quigley 1996; Resnikoff 2004). PACG accounts for approximately 50% of glaucoma blindness, and it has been estimated that by 2020, 5.3 million people worldwide will be bilaterally blind (Quigley 2006).
A classification scheme for PAC designed for use in prevalence surveys and epidemiological research has been published by Foster and colleagues (Foster 2002). This identifies three stages in the natural history of angle closure from initial irido‐trabecular contact (ITC) to anterior segment signs of disease (raised IOP, peripheral anterior synechiae (PAS), or both), culminating in glaucomatous optic neuropathy.
-
PAC suspect (PACS): an eye in which appositional contact between the peripheral iris and posterior trabecular meshwork is considered in two or more quadrants, in dark room conditions using static gonioscopy,
-
PAC: an eye with an occludable drainage angle and features indicating that trabecular obstruction by the peripheral iris has occurred, such as PAS, elevated IOP (> 21 mmHg), iris whorling (distortion of the radially orientated iris fibres), “glaucomfleken” lens opacities or excessive pigment deposition on the trabecular surface. There is no evidence of glaucomatous optic neuropathy or associated glaucomatous field loss.
-
PAC glaucoma (PACG): signs of PAC, as described above, and evidence of glaucomatous optic neuropathy.
It has been estimated that the proportion of PACS that converts to PAC ranges from 10% to 40% per decade (Alsbirk 1992; Thomas 2003; Yip 2008), and the five year risk of progression from PAC to PACG has been reported to be 28% to 30% (Thomas 2003; Wilensky 1993).
There are various anatomical and demographic risk factors for PAC (Congdon 1996; Lowe 1970). Anatomical risk factors include: a shallow anterior chamber depth (ACD), thickening of the crystalline lens, small corneal diameter and a short axial length (Nolan 2006). The risk of PACG increases with age (Day 2012) and the prevalence also varies with ethnicity, with higher rates occurring in Inuit and Asian populations (Clemmesen 1971; Drance 1973; Tham 2014).
Target condition being diagnosed
For this review we will use a narrow angle as the target condition indicative of an anatomical predisposition to angle closure as identified by gonioscopy (Weinreb 2006). In this review we define a narrow angle as either:
-
an eye which has appositional contact between the peripheral iris and posterior trabecular meshwork in two or more quadrants (≥180°); or
-
an eye with or at risk of angle closure as judged by a trained and experienced eye care professional using gonioscopy with or without indentation.
Conditions that are similar to the target condition include secondary angle closure glaucoma, such as aqueous misdirection, neovascular glaucoma and ciliary body swelling. The clinical features and management of conditions that cause secondary angle closure glaucoma have been reviewed by Parivadhini 2014 and will not be investigated in this review.
Index test(s)
Targeted screening for PAC/PACG has established the effectiveness of measuring anterior chamber dimensions to identify occludable angles (Congdon 1996; Devereux 2000; Kurita 2009). A variety of non‐contact methods are available for the assessment of the ACD, anterior chamber angle (ACA), or both.
Flashlight/pen torch/oblique handlight technique
The flashlight test is an accessible screening method if no other equipment is available. The test can be carried out in a primary‐ or secondary‐care setting and involves shining a pen torch into the eye from the temporal limbus parallel to the iris to assess the ACD. Quantitative grading uses a four‐point scale, derived from how much the iris is illuminated by the light of the pen torch (grade 4 = iris is fully illuminated; grade 1 = less than one‐third of the iris is illuminated) (Van Herick 1969; Vargas 1973)); grade 1 is associated with a high risk of angle closure. Qualitative grading can be used to describe the amount of shadow falling on the iris as shallow, medium or deep, and is further described by He 2007.
Limbal anterior chamber depth assessment (van Herick technique)
The van Herick technique is used to assess the ACD at the limbus using a slit lamp biomicroscope (Van Herick 1969). The illumination system is set at 60° from the observation system. A focused vertical slit‐beam is positioned at the limbus and moved just onto the cornea until the beam separates into a corneal section and reflection of the beam onto the iris. An estimate of the thickness of the dark space between the beams (which corresponds to the limbal anterior chamber depth (LACD)) is recorded as a fraction (or percentage) of the corneal section thickness over the central portion of the beam. Van Herick 1969 originally described a four‐point grading scheme, which was extended to a seven‐point scale by Foster 2000. Foster 2000 used an intuitive percentage scale, in an effort to improve the precision of the measurement. Van Herick 1969 considered that an eye with a LACD of grade 2 or less required gonioscopy and that a grade 1 angle was at a high risk of angle closure. Foster 2000 further subdivided grade 1 into 5% and 15% cut‐off values and found that the augmented scale was associated with an improved test accuracy.
Scanning peripheral anterior chamber depth analysis
Scanning peripheral anterior chamber depth analysis (SPAC) is an objective method for measuring the peripheral and central ACD by automatically taking 21 slit lamp images of the anterior chamber using a 1 mm‐wide slit at 0.4‐mm intervals from the optical axis towards the limbus (Kashiwagi 2006). These measurements are compared to a normative database and converted into a numerical scale ranging from 1 to 12, with 12 representing the deepest ACD. In addition, the instrument provides a categorical grading of the risk of angle closure, with suspect angle closure indicated by ≥ 4 measured points exceeding the 95% confidence interval (CI), potential angle closure indicated by ≥ 4 points exceeding the 72% CI, and normal. The device has been shown to be reproducible and easy to operate, therefore making it suitable for use by non‐clinicians (Kashiwagi 2004).
Scheimpflug photography
The Scheimpflug principle is used to correct perspective distortion in aerial photographs and has been adapted for ocular imaging. The Oculus Pentacam (Oculus, Wetzlar, Germany) device employs this principle using monochromatic blue light at a wavelength of 475 nm. By rotating the apparatus around the optical axis of the eye, a series of radially oriented images is generated in three dimensions around the 360° extent of the anterior segment. Between 12 and 50 real‐time sections from the anterior surface of the cornea to the posterior vertex of the lens are acquired within a 2‐s acquisition frame. This generates a set of measurements that provide a detailed description of the biometric configuration of the anterior segment, which includes the ACA, ACD and the anterior chamber volume (ACV). When calculating the ACA, it should be noted that this is not a direct measurement of the ACA, but is extrapolated from the measurements taken by the Pentacam. Some studies have found the ACD to be an effective indicator for the detection of narrow angles using various cut‐off ACD values (2.6 mm, 1.93 mm, 2.27 mm) (Hong 2009; Kurita 2009; Rossi 2012). Another study found ACV to partition normal eyes from those at risk of angle closure (Grewal 2011). Currently there is no consensus on which parameter or cut‐off value to use in the determination of a narrow angle.
Anterior segment‐ocular coherence tomography
Anterior segment‐ocular coherence tomography (AS‐OCT) allows both qualitative and quantitative analysis of the angle. The technique is based on low‐coherence interferometry whereby the delay and intensity of light reflected from the ocular tissue structures is measured. There are currently several AS‐OCT devices available on the market; depending on the device, they use one of the following methods to obtain clinical data: time domain, spectral domain or the more recent swept source domain method. Spectral and swept source domain methods have a higher scan speed and resolution than time domain methods. A wavelength of 1310 nm is used to image the anterior segment and inbuilt software is used to quantitatively assess in detail angle parameters, which include: the trabeculo‐iris space area (TISA), angle recess area (ARA) and angle opening distance (AOD) (Quek 2011). Qualitative interpretation has been typically defined by contact between the peripheral iris and any part of the angle wall anterior to the scleral spur. Studies state different AODs of 500 or 750 microns in the detection or diagnosis of narrow angles or an ARA of less than 20° (Smith 2013). There is no current consensus on which values to use with any of the parameters mentioned to identify a narrow angle.
Clinical pathway
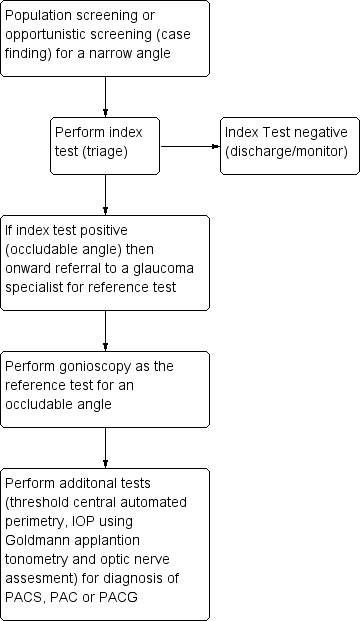
A variety of non‐contact devices with varying degrees of sophistication have been developed to evaluate the risk of angle closure . The high prevalence of PAC and the burden of blindness attributable to PACG in high‐risk populations open up the possibility of using such techniques for population screening (see Figure 1) (Nolan 2003; Nolan 2006). More commonly, non‐invasive assessment of the dimensions of the ACD, angle, or both are part of a standard ophthalmic examination in individuals who are asymptomatic or those presenting with symptoms of angle closure. If the index test(s) is positive, such individuals are identified as being 'at risk' of PACG and are referred for further assessment, usually to a glaucoma subspecialist ophthalmologist. The ophthalmologist will carry out gonioscopy (the reference standard for qualitative and quantitative assessment of the ACA). If a narrow angle is diagnosed, additional tests are then performed, such as IOP measurement using Goldmann applanation tonometry, optic nerve head examination and automated threshold visual field testing, to further diagnose the narrow angle as PACS/PAC/PACG. Depending on the clinical presentation, the affected individual may be closely monitored or undergo prophylactic treatment with laser iridotomy or lens extraction, possibly in conjunction with IOP‐lowering eye drops.
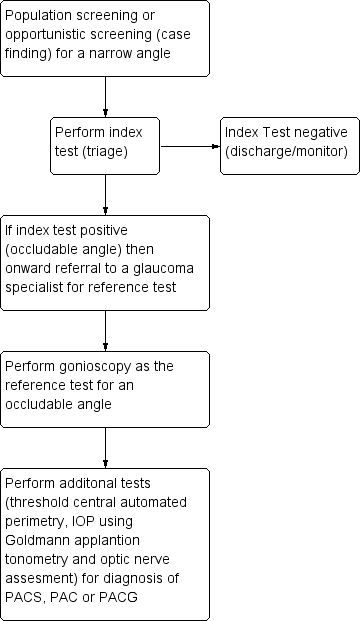
Clinical Pathway
Role of index test(s)
The gold standard test to detect a narrow angle is gonioscopy; however, this is not routinely performed outside the specialist setting since it requires a high level of skill, which may lead to missed diagnoses. Non‐contact screening tests are relatively quick and can be carried out by appropriately trained healthcare professionals or technicians as a triage test to identify eyes at risk of angle closure. These non‐contact tests cannot replace gonioscopy as they do not provide sufficient information on the ACA anatomy (Smith 2013). It should be noted that in some cases, when gonioscopy fails to visualise the anterior chamber configuration and depth, typically in secondary causes of angle closure, AS‐OCT and Pentacam imaging can be used to provide objective measurements (Kang 2013). In addition, AS‐OCT and Pentacam imaging can be used to supplement existing clinical documentation by providing objective measurements (Smith 2013).
Alternative test(s)
Tests that use contact methods, such as ultrasound biomicroscopy, have been reviewed by Smith 2013, and will not be included in the current review.
Rationale
A systematic review published in 2013 evaluated whether anterior segment imaging (using ultrasound biomicroscopy, ocular coherence tomography (OCT), Scheimpflug photography or SPAC) aided the diagnosis of PAC (Smith 2013). This review included 79 studies and concluded that although anterior segment imaging provided useful information, none of the methods provided sufficient information about the anatomy of ACA to be considered a substitute for gonioscopy. However, no meta‐analysis of accuracy data was conducted. The current review will update and extend this review by considering the following non‐contact methods of anterior chamber assessment (flashlight test, slit‐lamp techniques for limbal and central ACD assessment, AS‐OCT, Scheimpflug photography and SPAC).
Objectives
To determine the diagnostic accuracy of non‐contact screening methods for identifying eyes with a narrow angle.
Secondary objectives
-
To asssess and compare the accuracy of index non‐contact screening tests for identifying eyes with a narrow angle
-
To investigate the accuracy of each non‐contact screening method for detecting the most severe referable condition or PACG (versus PAC, PACS or a non‐occludable angle)
-
To explore potential causes of heterogeneity in diagnostic performance
Methods
Criteria for considering studies for this review
Types of studies
We will include all prospective and retrospective cohort studies ('single‐gate' design) and case‐control studies ('two‐gate' design) that have evaluated the accuracy of non‐contact methods for diagnosing narrow angles compared to a gonioscopy reference standard. We will include studies comparing each method separately, and studies comparing more than one method, to the reference standard in the same population. This will include studies in which participants receive all the tests or are randomised to receive different tests. We will include only studies that provide sufficient data to allow the calculation of sensitivity and specificity.
Non‐contact methods for the detection of narrow angles are mainly of interest in screening and primary‐care settings as a triage test aiming to guide referrals to ophthalmologists. However, since the relative accuracy of these tests in these settings is not well known, we will include studies investigating these tests in any setting, and will assess the effect of this on accuracy in subgroup analyses.
Participants
We will include all participants who meet the inclusion criteria for studies conducted in any setting (including population screening, and primary or secondary care), which evaluated any of the index tests against the reference standard.
Index tests
We will assess non‐contact methods including: the flashlight/pen torch/oblique handlight technique, LACD using the van Herick technique, SPAC, Scheimpflug photography and AS‐OCT.
Target conditions
A narrow angle, as a referable condition that can include PACS, PAC or PACG, as described above, will be the target condition of interest.
As a secondary objective, we will also extract data to investigate the accuracy of the test for detecting the most severe referable condition or PACG (versus PAC, PACS or non‐occludable angle).
Reference standards
Gonioscopy will be the reference standard for the diagnosis of a narrow angle. We will further classify a narrow angle into one of three subgroups PACS, PAC, PACG, if the following measurements have been taken; IOP measurement, visual field assessment and optic disc examination.
Gonioscopy
Gonioscopy is the acknowledged reference standard for the evaluation of eyes with and at risk of angle closure, and should be performed on both eyes in any individual with suspected angle closure. The technique should be performed under dark‐room conditions and used in the primary position to visualise angle structures, the presence of ITC, PAS, or both (Bhargava 1973). Dynamic assessment is helpful in distinguishing ITC from PAS using a four‐mirror lens, which is applied to the cornea creating pressure with the goniolens. The Shaffer grading system, which records the ACA width in four quadrants, from grade 0 (closed) to grade 4 (wide open), is the most widely adopted ACA classification scheme (Shaffer 1960). Angle morphology can be further described using the Scheie grading system (Scheie 1957). This scheme describes the angle according to the anatomical structures observed (grade IV: Schwalbe’s line not visible; grade III: Schwalbe’s line visible; grade II: anterior trabecular meshwork visible; grade I: visible scleral spur; and grade 0: ciliary body band visible).
Search methods for identification of studies
Electronic searches
The Cochrane Eyes and Vision Information Specialist will search the following electronic databases. We will impose no language or publication year restrictions.
-
Cochrane Central Register of Controlled Trials (CENTRAL; latest issue) (which contains the Cochrane Eyes and Vision Trials Register) in the Cochrane Library (Appendix 1);
-
Health Technology Assessment Database (HTAD; latest issue) in the Cochrane Library (Appendix 1);
-
MEDLINE Ovid (1946 to present) (Appendix 2);
-
Embase Ovid (1980 to present) (Appendix 3);
-
BIOSIS (January 1969 to present) (Appendix 4);
-
System for Information on Grey Literature in Europe (OpenGrey) (1995 to present) (Appendix 5);
-
Aggressive Research Intelligence Facility database (ARIF) (www.birmingham.ac.uk/research/activity/mds/projects/HaPS/PHEB/ARIF/index.aspx) (Appendix 6);
-
ISRCTN registry (www.isrctn.com/editAdvancedSearch) (Appendix 7);
-
US National Institutes of Health Ongoing Trials Register ‐ ClinicalTrials.gov (www.clinicaltrials.gov) (Appendix 8);
-
World Health Organization International Clinical Trials Registry Platform (www.who.int/ictrp) (Appendix 9).
Searching other resources
We will search the references of included studies for information about further studies. We do not intend to handsearch journals and conference proceedings.
Data collection and analysis
Selection of studies
Two review authors (AJ and IC) will independently assess the titles and abstracts of all studies identified by the electronic searches. We will label each record at this stage as "definitely relevant", "possibly relevant" or "definitely not relevant". We will exclude records labelled as "definitely not relevant" by both review authors. We will retrieve full‐text reports of records labelled as "definitely relevant" or "possibly relevant" and the two review authors will independently assess whether these meet the inclusion criteria. We will resolve any disagreement when present at any stage through discussion. When necessary, we will consult a third review author or contact the study investigators for more information to determine eligibility.
Data extraction and management
Two review authors (AJ and JL) will independently extract the following data, where possible, from the included studies: the number of true positives (TP), false positives (FP), true negatives (TN) and false negatives (FN) using 2 x 2 contingency tables. From the 2 X 2 tables we will calculate sensitivity (the proportion of diseased people correctly diagnosed) and specificity (the proportion of non‐diseased people correctly diagnosed) with 95% CIs.
One review author will enter data into Review Manager 5 (RevMan 5) (Review Manager 2014) and a second review author will verify the entered data. We will resolve any disagreement when present at any stage through discussion. We will contact study investigators to provide missing information or to clarify data, and we will allow two weeks for a response. If we do not receive a response during this time, we will proceed using the information available, as provided in the published reports. We will summarise the characteristics of included studies in a 'Characteristics of included studies' table, as shown below. See Appendix 10 for abbreviations.
| Study identification | First author, year of publication. |
| Clinical features and settings | Previous testing and clinical setting including country where the study was conducted. Presentation at recruitment, prior treatment that would affect the ACD (i.e. peripheral iridotomy, iridoplasty, etc.) |
| Participants | Sample size, age, sex, ethnicity and country |
| Study design | Whether the sample was selected as a single group (consecutive series) or as separate groups with and without the target condition (case‐control). Whether participants were consecutively enrolled in the study and were identified retrospectively or prospectively. Training involved for index tests, both eyes included in the study |
| Target condition | A narrow angle as a referable condition, which includes PACS, PAC and PACG |
| Reference standard | The reference standard test used: gonioscopy for diagnosing a narrow angle; this is acceptable if this is the only target condition in large‐scale screening or primary‐care settings. Gonioscopy combined with tonometry, visual fields investigation and optic disc assessment for distinguishing the relative subgroup of participants with a narrow angle PACS/PAC/PACG |
| Index tests | Flashlight/pen torch/oblique handlight technique: grade recorded LACD using the van Herick technique: van Herick grade, or percentage, or both SPAC: numerical or categorical grade, or both Pentacam Scheimpflug photography: ACA, ACV and ACD AS‐OCT: model of OCT device, manufacturer and any technical characteristics (e.g. software analyses). TISA, ARA, AOD 500 microns and 750 microns for each parameter |
| Follow up | Numbers of participants lost to follow‐up or who had uninterpretable test results |
| Notes | Source of funding, anything else of relevance |
Assessment of methodological quality
Two review authors will independently assess each included study for risk of bias using the QUADAS 2 tool to assess the susceptibility to bias of the included studies, based on guidance presented in Table 1 (Whiting 2011). We will assess each study and judge each bias criterion to be at 'high', 'low' or 'unclear' risk of bias (lack of information or uncertainty over the potential for bias). Concerns regarding applicability will be rated as 'high', 'low' or 'unclear' concerns.
| DOMAIN | LOW | HIGH | UNCLEAR |
| PARTICIPANT SELECTION | Describe methods of participant selection; describe included participants (prior testing, presentation, intended use of index test and setting) | ||
| Was a consecutive or random sample of participants enrolled? | Consecutive sampling or random sampling of people according to inclusion criteria | Non‐consecutive cohort of referrals (from primary care) or (in screening setting) sampling based on volunteering or referral | Unclear whether consecutive or random sampling used |
| Was a case‐control design avoided? | No selective recruitment of people with or without narrow angles, or nested case‐control designs (systematically and randomly selected from a defined population cohort) | Selection of either cases or controls in a predetermined, non‐random fashion; or enrichment of the cases from a selected population | Unclear selection mechanism |
| Did the study avoid inappropriate exclusions? | Exclusions are detailed and felt to be appropriate (e.g. people with corneal opacities, known ocular malformation or disease causing bulbar derangement) | Inappropriate exclusions are reported (e.g. of people with borderline index test results) | Exclusions are not detailed (pending contact with study authors) |
| Risk of bias: could the selection of participants have introduced bias? | All signalling questions = ‘Yes’ | Any signalling question = ‘No’ | Unclear |
| Concerns regarding applicability: are there concerns that the included participants do not match the review question? | Inclusion of participants without a previous diagnosis of a narrow angle | Inclusion of participants with a previous diagnosis of a narrow angle | Unclear inclusion criteria |
| INDEX TEST | Describe the index test and how it was conducted and interpreted | ||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Test performed “blinded” or “independently and without knowledge of” reference standard results are sufficient and full details of the blinding procedure are not required; or clear temporal pattern to the order of testing that precludes the need for formal blinding | Reference standard results were available to those who conducted or interpreted the index tests | Unclear whether results are interpreted independently |
| If a threshold was used, was it prespecified? | The study authors declare that the selected cut‐off used to dichotomise data was specified a priori; or a protocol is available with this information | A study is classified at higher risk of bias if the authors define the optimal cut‐off post hoc, based on their own study data | No information on preselection of index test cut‐off values |
| Risk of bias: could the conduct or interpretation of the index test have introduced bias? | All signalling questions = ‘Yes’ | Any signalling question = ‘No’ | Unclear |
| Concerns regarding applicability: are there concerns that the index test, its conduct or interpretation differ from the review question? | Tests used and testing procedure clearly reported and tests executed by personnel with sufficient training | Tests used are not validated or study personnel was insufficiently trained | Unclear execution of the tests or unclear study personnel profile, background and training |
| REFERENCE STANDARD | Describe the reference standard and how it was conducted and interpreted | ||
| Is the reference standard likely to correctly classify the target condition? | Not applicable. Score ‘Yes’ for all studies | ||
| Were the reference standard results interpreted without knowledge of the results of the index test? | Reference standard performed “blinded” or “independently and without knowledge of” index test results are sufficient and full details of the blinding procedure are not required; or clear temporal pattern to the order of testing that precludes the need for formal blinding | Index test results were available to those who conducted the reference standard | Unclear whether results were interpreted independently |
| Risk of bias: could the reference standard, its conduct or its interpretation have introduced bias? | All signalling questions = ‘Yes’ | Any signalling question = ‘No’ | Unclear |
| Concerns regarding applicability: are there concerns that the target condition as defined by the reference standard does not match the review question? | Not applicable. Score ‘Yes’ for all studies | ||
| FLOW AND TIMING | Describe any participants who did not receive the index test(s) or reference standard, or either, or who were excluded from the 2 x 2 table (refer to study flow diagram); describe the time interval and any interventions between index test(s) and reference standard | ||
| Was there an appropriate interval between index test(s) and reference standard? | No more than three months between index and reference test execution | More than three months between index and reference test execution | Unclear whether test results were executed within three months |
| Did all participants receive a reference standard? | All participants receiving the index test were verified with the reference standard | Not all participants receiving the index test were verified with the reference standard | Unclear whether all participants receiving the index test were verified with the reference standard |
| Did all participants receive the same reference standard? | Not applicable. Score ‘Yes’ for all studies | ||
| Were all participants included in the analysis? | The number of participants included in the study match the number in analysis | The number of participants included in the study does not match the number in analysis | Insufficient information on whether the number of participants included in the study matches the number in analysis |
| Risk of bias: could the participants' flow through the study have introduced bias? | All signalling questions = ‘Yes’ | Any signalling question = ‘No’ | Unclear |
Statistical analysis and data synthesis
We aim to extract and analyse the data available at fixed thresholds for each index test, in order to ease the interpretability of our summary measures of accuracy. Our preferred thresholds will be:
-
flashlight/pen torch/oblique handlight technique: grades 1 and 2;
-
LACD using the van Herick technique: van Herick grades 1 and 2 (percentages will be converted to grades as appropriate);
-
SPAC: categorical grading of suspect angle closure or potential angle closure, as provided by the device.
As there is no current consensus regarding thresholds for Pentacam Scheimpflug photography and AS‐OCT, we will extract these data, if available, from the included studies.
If we identify sufficient studies providing data at fixed thresholds for each test, we will fit a bivariate model using the METADAS macro in SAS. If fixed thresholds are sparsely or incompletely reported in studies we will fit hierarchical summary receiver operating characteristic (HSROC) curve models using the same software. For comparisons between index tests, we will use a covariate coding for each test in the bivariate or HSROC model. If the HSROC model is appropriate, we will assume the same shape for a summary receiver operating characteristic (sROC) curve for all index tests and we will compare them using relative diagnostic odds ratio (DOR). We will also report estimates of test accuracy, such as sensitivity values at 90% and 95% specificity, which are useful measures of the performance screening test.
We will assess and compare the accuracy of different index tests using all available studies, thus allowing for indirect comparisons. As Takwoingi 2013 showed that direct comparisons conducted within each study are more reliable than indirect comparisons, we will also present such within‐study comparisons graphically in ROC plots. We will plot data points and join the two estimates (one for each test) from each study by a line to show the difference in accuracy between tests. If a sufficient number of such paired studies are available, we will pool them in bivariate or HSROC meta‐analyses, as appropriate, and test their relative accuracy with a covariate coding for each test using the methods described above.
Since narrow angles are often bilateral, this complication may result in unit of analysis issues. We will include studies that evaluated only one eye of each participant or, in participants with two affected eyes, studies that randomly selected only one eye. We will also include studies that included both eyes in our review, but we will acknowledge the unit of analysis issue when formulating our conclusions (i.e. acknowledging the overestimate of the precision in accuracy).
Investigations of heterogeneity
We will initially investigate any heterogeneity in sensitivity and specificity through the visual inspection of forest plots and the degree to which individual study results lie close to the summary ROC curve. For diagnostic tests with a sufficient number of eligible studies, we plan to formally explore heterogeneity by using the following study‐level covariates:
-
study design (e.g. single‐gate and two‐gate designs);
-
diagnostic reference thresholds (gonioscopy grading (e.g. number of quadrants occluded));
-
characteristics of the study population (e.g. high versus low prevalence, ethnicity).
Sensitivity analyses
If we identify sufficient studies, we will perform a sensitivity analysis to assess the impact of risk of bias on test accuracy by repeating the analysis after removing studies at high risk of bias.
| DOMAIN | LOW | HIGH | UNCLEAR |
| PARTICIPANT SELECTION | Describe methods of participant selection; describe included participants (prior testing, presentation, intended use of index test and setting) | ||
| Was a consecutive or random sample of participants enrolled? | Consecutive sampling or random sampling of people according to inclusion criteria | Non‐consecutive cohort of referrals (from primary care) or (in screening setting) sampling based on volunteering or referral | Unclear whether consecutive or random sampling used |
| Was a case‐control design avoided? | No selective recruitment of people with or without narrow angles, or nested case‐control designs (systematically and randomly selected from a defined population cohort) | Selection of either cases or controls in a predetermined, non‐random fashion; or enrichment of the cases from a selected population | Unclear selection mechanism |
| Did the study avoid inappropriate exclusions? | Exclusions are detailed and felt to be appropriate (e.g. people with corneal opacities, known ocular malformation or disease causing bulbar derangement) | Inappropriate exclusions are reported (e.g. of people with borderline index test results) | Exclusions are not detailed (pending contact with study authors) |
| Risk of bias: could the selection of participants have introduced bias? | All signalling questions = ‘Yes’ | Any signalling question = ‘No’ | Unclear |
| Concerns regarding applicability: are there concerns that the included participants do not match the review question? | Inclusion of participants without a previous diagnosis of a narrow angle | Inclusion of participants with a previous diagnosis of a narrow angle | Unclear inclusion criteria |
| INDEX TEST | Describe the index test and how it was conducted and interpreted | ||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Test performed “blinded” or “independently and without knowledge of” reference standard results are sufficient and full details of the blinding procedure are not required; or clear temporal pattern to the order of testing that precludes the need for formal blinding | Reference standard results were available to those who conducted or interpreted the index tests | Unclear whether results are interpreted independently |
| If a threshold was used, was it prespecified? | The study authors declare that the selected cut‐off used to dichotomise data was specified a priori; or a protocol is available with this information | A study is classified at higher risk of bias if the authors define the optimal cut‐off post hoc, based on their own study data | No information on preselection of index test cut‐off values |
| Risk of bias: could the conduct or interpretation of the index test have introduced bias? | All signalling questions = ‘Yes’ | Any signalling question = ‘No’ | Unclear |
| Concerns regarding applicability: are there concerns that the index test, its conduct or interpretation differ from the review question? | Tests used and testing procedure clearly reported and tests executed by personnel with sufficient training | Tests used are not validated or study personnel was insufficiently trained | Unclear execution of the tests or unclear study personnel profile, background and training |
| REFERENCE STANDARD | Describe the reference standard and how it was conducted and interpreted | ||
| Is the reference standard likely to correctly classify the target condition? | Not applicable. Score ‘Yes’ for all studies | ||
| Were the reference standard results interpreted without knowledge of the results of the index test? | Reference standard performed “blinded” or “independently and without knowledge of” index test results are sufficient and full details of the blinding procedure are not required; or clear temporal pattern to the order of testing that precludes the need for formal blinding | Index test results were available to those who conducted the reference standard | Unclear whether results were interpreted independently |
| Risk of bias: could the reference standard, its conduct or its interpretation have introduced bias? | All signalling questions = ‘Yes’ | Any signalling question = ‘No’ | Unclear |
| Concerns regarding applicability: are there concerns that the target condition as defined by the reference standard does not match the review question? | Not applicable. Score ‘Yes’ for all studies | ||
| FLOW AND TIMING | Describe any participants who did not receive the index test(s) or reference standard, or either, or who were excluded from the 2 x 2 table (refer to study flow diagram); describe the time interval and any interventions between index test(s) and reference standard | ||
| Was there an appropriate interval between index test(s) and reference standard? | No more than three months between index and reference test execution | More than three months between index and reference test execution | Unclear whether test results were executed within three months |
| Did all participants receive a reference standard? | All participants receiving the index test were verified with the reference standard | Not all participants receiving the index test were verified with the reference standard | Unclear whether all participants receiving the index test were verified with the reference standard |
| Did all participants receive the same reference standard? | Not applicable. Score ‘Yes’ for all studies | ||
| Were all participants included in the analysis? | The number of participants included in the study match the number in analysis | The number of participants included in the study does not match the number in analysis | Insufficient information on whether the number of participants included in the study matches the number in analysis |
| Risk of bias: could the participants' flow through the study have introduced bias? | All signalling questions = ‘Yes’ | Any signalling question = ‘No’ | Unclear |