Abdominal ultrasound for diagnosing abdominal tuberculosis or disseminated tuberculosis with abdominal involvement in HIV‐positive adults
Abstract
This is a protocol for a Cochrane Review (Diagnostic test accuracy). The objectives are as follows:
To determine the diagnostic accuracy of abdominal ultrasound as a standalone test for detecting abdominal TB or disseminated TB with abdominal involvement in HIV‐positive adults.
Background
Target condition being diagnosed
Tuberculosis (TB) is caused by the bacillus Mycobacterium tuberculosis. Although it usually affects the lungs (pulmonary TB), it can also spread to other body sites (extrapulmonary TB) (WHO 2013a).
An estimated 10.4 million people were diagnosed with TB in 2015 and 1.8 million people died due to TB (WHO 2016b). Resource‐limited countries are the most affected, for example, the African region of the World Health Organization (WHO) had the second highest number of incident cases (2.7 million), but the highest incidence rate (275 versus 142 globally) and mortality rate (HIV‐positive: 30 versus 5.3 globally; HIV‐negative: 45 versus 19 globally) per 100,000 people (WHO 2016b).
The probability of developing TB is higher among people living with HIV. Approximately 1.1 million people diagnosed worldwide with TB in 2015 were HIV‐positive (WHO 2016b), while the HIV prevalence among incident TB cases in the African region was 31% (WHO 2016b).
The worldwide case detection rate in 2015 was only an estimated 59% (WHO 2016b). This low rate of detection relates to problems with TB diagnostic tests (sensitivity, specificity, and availability), the negative influence of HIV infection on the performance of diagnostic tests, and HIV co‐infection and the opportunistic conditions that complicate it (Palmieri 2002; Dawson 2010; Padmapriyadarsini 2011; Steingart 2014; WHO 2016a). The diagnosis of active TB in HIV‐positive patients with advanced immunosuppression is challenging due to more atypical clinical presentations; other opportunistic pulmonary infections with similar presentations; high proportion negative sputum smears; and high rates of extrapulmonary TB (Sharma 2005). This is illustrated by autopsy studies, which indicate a very high proportion of TB in HIV‐positive adults (32% to 47%); almost half (46%) of adult TB cases remained undiagnosed premortem (Gupta 2015).
In people with HIV‐associated TB, extrapulmonary TB accounts for up to 50% of all TB cases (Sharma 2004b; Kingkaew 2009; Namme 2013), and is often disseminated (two or more non‐contiguous sites simultaneously infected) (Sharma 2005). Any anatomical site can be involved, but the commonest sites are the lymph nodes, pleura, meninges, and the abdominal cavity (Sharma 2005). Many terms are used in the literature to describe TB in the abdominal cavity. For the purpose of this Cochrane Review, we will use the terms abdominal TB or disseminated TB with abdominal involvement — excluding genitourinary TB. Many abdominal structures can be affected in abdominal TB or disseminated TB with abdominal involvement, including involvement of the gastrointestinal tract, peritoneum, omentum, mesentery, intra‐abdominal lymph nodes, and solid organs (liver, spleen, pancreas) (Sharma 2004b). Patients often present with non‐specific symptoms and signs, and a high index of suspicion is therefore needed for early diagnosis and timely management. It mimics a large number of medical and surgical conditions, including malignant neoplasms, inflammatory bowel disease, chronic liver disease, and other gastrointestinal infections (Jadvar 1997).
Index test(s)
Many HIV‐positive people with low CD4 counts have abdominal TB or disseminated TB with abdominal involvement. As sputum smears are frequently negative in HIV‐associated TB, it is common clinical practice, supported by WHO guidelines, to reach TB diagnosis on the basis of imaging results and clinical case definitions (Wilson 2006; WHO 2016a). Ultrasound is such an imaging test that can be used as a diagnostic tool (Heller 2010a; Heller 2010b; Patel 2011; Giordani 2013). Ultrasound uses sound waves to produce images of structures and organs within the body, and is particularly relevant in low‐resource settings as it is more accessible and cheaper than using computed tomography (CT). Ultrasound techniques for TB diagnosis are also easily learned and quick to perform (less than 10 minutes) (Heller 2010a).
Abdominal ultrasound (an ultrasound examination evaluating the abdominal cavity) is useful in HIV‐positive patients with suspected abdominal TB or disseminated TB with abdominal involvement. Suggestive findings include lymphadenopathy, ascites, and focal liver and splenic lesions (Heller 2010a; Heller 2010b; Patel 2011; Giordani 2013). However, the ultrasound findings are non‐specific and various other diseases may present with the same features. For example, intra‐abdominal lymphadenopathy can be due to other infections (for example, cryptococcosis, histoplasmosis); lymphomas (non‐Hodgkin's lymphoma and Hodgkin's lymphoma); and Kaposi's sarcoma (Martin‐Bates 1993).
Clinical pathway
Any structure or organ in the abdominal cavity (for example, gastrointestinal tract, pancreatobiliary system, peritoneum, and lymph nodes) can be affected by TB disease. The presentation varies considerably and depends on the specific organ involved; other diseases are also often mimicked (Sharma 2004a). Common presenting symptoms are abdominal pain, anorexia, bowel disturbances, fever, and weight loss; whereas the clinical examination often reveals abdominal tenderness, ascites, and solid organ enlargement (for example, hepatomegaly, splenomegaly, or hepatosplenomegaly) (Ibrahim 2005; Mandal 2011).
Essential diagnostic tests, as suggested by the WHO for individuals who are suspected of having abdominal TB or disseminated TB with abdominal involvement, include a chest x‐ray, sputum evaluation (if able to produce) for bacteriological confirmation of TB disease (smear or culture or Xpert MTB/RIF), and blood cultures (WHO 2013b). Urine specimens remain a convenient clinical sample for the diagnosis of TB. Although conventional TB diagnostics applied to urine specimens have limited clinical utility, the use of urinary lipoarabinomannan (LAM) has been recommended by the WHO in HIV‐positive adults with advanced immunosuppression (CD4 cell count less than or equal to 100 cells/µL) or in HIV‐positive adults who are seriously ill (respiratory rate > 30/min, temperature > 39°C, heart rate > 120/min and unable to walk unaided) regardless of their CD4 cell count (WHO 2015; Shah 2016). These tests are usually done in the primary care setting and higher.
Abdominal ultrasound has become part of the initial diagnostic work up in adults living with HIV where abdominal TB or disseminated TB with abdominal involvement is suspected (especially in those with a low CD4 count), despite the lack of robust evidence of validity from large studies (NICE 2016). The diagnostic pathway might differ in different settings if there are ultrasound findings suggestive of TB. In low‐resource settings this might be enough evidence to initiate anti‐TB treatment, but in high‐resource settings it would prompt site‐specific investigations which could include CT scan, paracentesis, laparoscopy, fine needle aspiration, or stool examination.
A presumptive diagnosis of abdominal TB or disseminated TB with abdominal involvement can be made in the setting of known active pulmonary TB, although less than half of chest radiographs are compatible with active or healed TB (Chow 2002).
Routine laboratory studies are nonspecific and include a mild normocytic and normochromic anaemia, hypoalbuminaemia, and raised erythrocyte sedimentation rate (Kapoor 1998; Chow 2002; Sharma 2004a). A normal leukocyte count is present in most patients. However, the WHO further recommends immediate initiation of anti‐TB therapy in patients living with HIV who have clinical features of disseminated TB (WHO 2016a). Bacteriological confirmation of TB from any specimen remains important but treatment should not be delayed until results become available (Figure 1). Patients started on anti‐TB therapy without bacteriological confirmation should be assessed after one month to evaluate the clinical response to treatment. Patients should be re‐assessed and an alternative diagnosis sought if there is no clinical improvement.
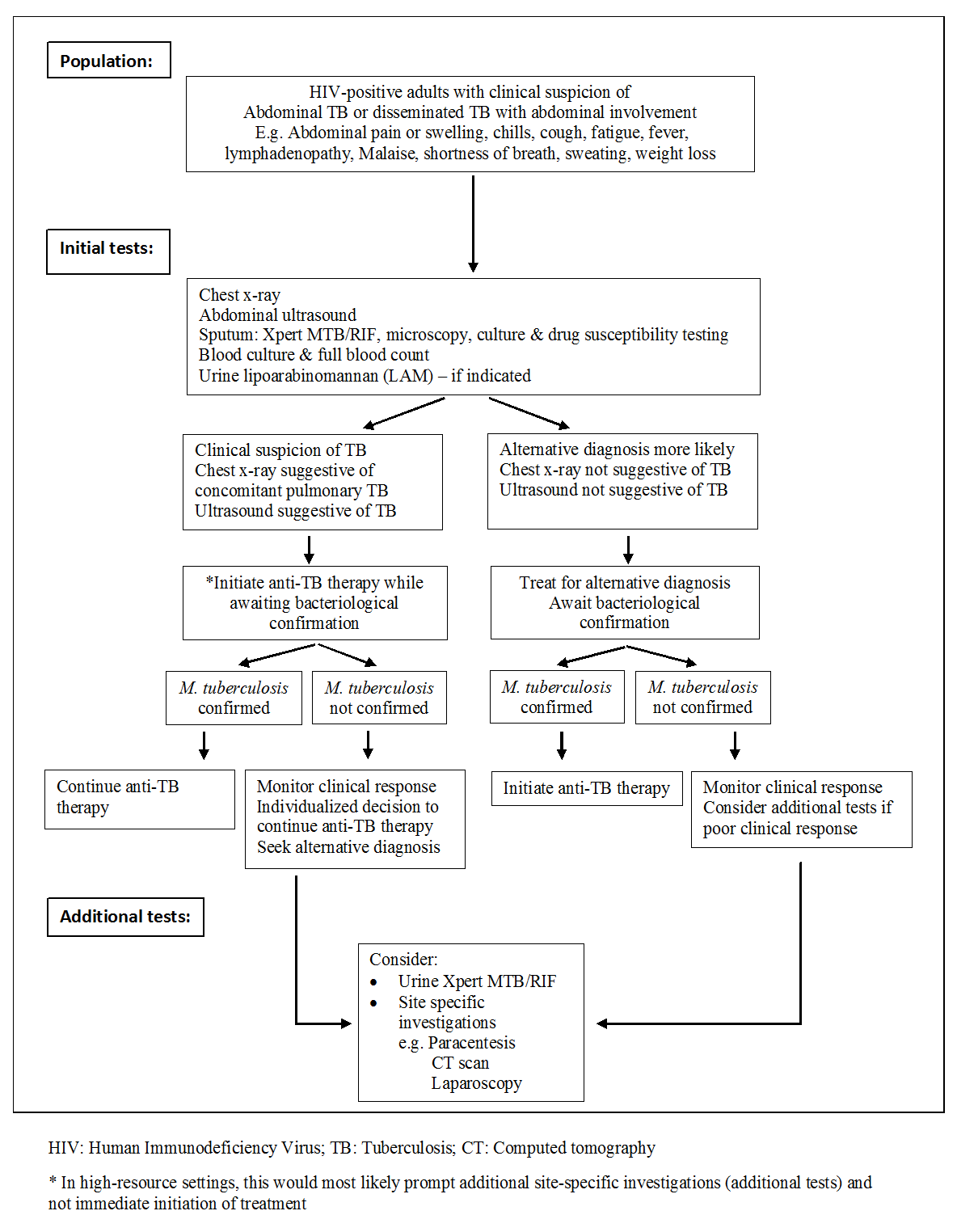
Diagnostic workup of HIV‐positive adults with suspected abdominal tuberculosis or disseminated tuberculosis with abdominal involvement
Alternative test(s)
Ascitic fluid analysis suggestive of abdominal TB or disseminated TB with abdominal involvement includes a leukocyte count of 150 to 4000 cells/mL, which consists predominantly of lymphocytes (Sharma 2004a; Sanai 2005). The ascitic fluid is usually an exudate with the protein content greater than 30 g/L and the serum‐ascites albumin gradient (SAAG) less than 11 g/L (Sharma 2004a; Sanai 2005). Adenosine deaminase activity (ADA) of ascitic fluid (> 39 IU/L) is also suggestive of abdominal TB (Riquelme 2006), while the ascites to blood glucose ratio is usually less than 0.96 (Wilkins 1984). Acid‐Fast Bacilli (AFB) smear and culture of ascitic fluid also have disappointingly low yields (Chow 2003).
Different imaging modalities can be useful to diagnose abdominal TB or disseminated TB with abdominal involvement. Abdominal x‐rays are of very limited value, but can assist with the diagnosis of intestinal obstruction and perforation (Debi 2014). CT features include thickening of the peritoneum, omentum, and bowel wall; lymph nodes (especially if these have hypodense centres due to caseous necrosis); and ascites with strands, debris, and fine septations (Sharma 2004a; Lee 2012). The excellent soft tissue resolution and multiplanar acquisition of Magnetic Resonance Imaging (MRI) resulted in it being used to evaluate solid organs and lymphadenopathy (Joshi 2014). However, MRI is expensive and access is very limited in low‐resource settings. Barium studies may be useful for intrinsic bowel abnormalities such as strictures, fistulae, and erosions (Sharma 2004a; Debi 2014).
Colonoscopy with biopsy is a useful non‐operative diagnostic procedure to obtain material for histology and culture (Kim 1998). Mucosal nodules and transverse ulcers in the bowel are very suggestive of TB, with definitive results obtained from tissue sent for polymerase chain reaction (PCR), Ziehl‐Neelsen stain, and culture (Kim 1998; Sharma 2004a). Laparoscopy is useful in two ways: (i) it allows visual inspection of the peritoneum; and (ii) it permits specimens for histology, AFB stain, and culture to be obtained. However, imaging modalities as described above provide a safer and less expensive alternative, but may be less specific since they are unable to provide a definitive microbiological diagnosis (Sanai 2005).
Expanded clinical case definitions were developed to diagnose smear‐negative TB in HIV‐positive patients living in low‐resource settings (Wilson 2006), including abdominal TB or disseminated TB with abdominal involvement (Wilson 2006; WHO 2016a). For example, a patient presenting with symptoms and signs suggestive of abdominal TB or disseminated TB with abdominal involvement can be started on antituberculous treatment if the ascitic fluid consists of a lymphocytic exudate along with either a fever ≥ 38°C on two occasions or drenching sweats for greater than two weeks (Wilson 2006). In this study, the positive predictive value for abdominal lymph nodes diagnosed by ultrasound was 94% (Wilson 2006). Augmented by the use of objective criteria to monitor response to treatment within the first eight weeks, this approach has reasonable diagnostic accuracy (Wilson 2006).
Rationale
Rapid, simple, inexpensive, and accurate diagnostic tests for TB in general and TB in HIV‐positive patients are needed. The value of diagnostic tests for TB in HIV‐positive patients is that they may be able to reduce both time to TB treatment initiation and inappropriate use of empiric TB treatment. The latter is associated with unnecessary costs, inconvenience, and potential adverse drug reactions of antituberculous treatment (Wilson 2006).
The accurate diagnosis of TB in HIV‐positive patients is difficult as is evident by the poor global case detection rate (WHO 2016b). Many HIV‐positive people with low CD4 counts have abdominal TB or disseminated TB with abdominal involvement. Ultrasound can be a useful diagnostic tool to identify abdominal TB or disseminated TB with abdominal involvement. Ultrasound is non‐invasive, portable, inexpensive, and safe. It is widely available in low‐resource settings that have limited access to CT and MRI scanners (Heller 2010a). Multiple studies of various quality and designs have looked at the use of abdominal ultrasound as a diagnostic tool for abdominal TB or disseminated TB with abdominal involvement; with varying sensitivity, specificity, and predictive values for diagnosing TB (Monill‐Serra 1997; Mugala 2006; Sinkala 2009; Sculier 2010; Patel 2011; Heller 2013). Abdominal ultrasound may be used alone, in combination with existing tests (chest radiograph, full blood count), or as an add‐on following negative results from existing tests (smear microscopy, sputum Xpert MTB/RIF, sputum culture, chest radiograph). The role of abdominal ultrasound as an add‐on test is an important clinical question because it may reflect the way that abdominal ultrasound is used in practice, especially in low‐resource settings. However, after a scoping search, we did not find any studies that have evaluated the accuracy of ultrasound as an add‐on test. Therefore, this Cochrane Review will address the accuracy of ultrasound used alone or in combination with other tests.
Objectives
To determine the diagnostic accuracy of abdominal ultrasound as a standalone test for detecting abdominal TB or disseminated TB with abdominal involvement in HIV‐positive adults.
Secondary objectives
-
To determine the diagnostic accuracy of combinations of abdominal ultrasound and existing tests (chest radiograph, full blood count) for detecting abdominal TB or disseminated TB with abdominal involvement in HIV‐positive adults.
-
To investigate potential sources of heterogeneity in test accuracy, including clinical setting, ultrasound training level, and type of reference standard.
Methods
Criteria for considering studies for this review
Types of studies
We will include cross‐sectional, cohort, or diagnostic case‐control studies (prospective and retrospective) that compare the result of the index tests (abdominal ultrasound and abdominal ultrasound plus existing tests) with one of the reference standards (see Reference standards). We will include conference abstracts if we can obtain the required data (as specified below). Case‐control studies may overestimate sensitivity and specificity, but because we anticipate identifying few relevant studies, we will include them. If there is a sufficient number of studies, we will examine the effect of excluding case‐control studies in sensitivity analyses. We will include studies in which the study authors report the numbers of true positives (TP), true negatives (TN), false positives (FP), and false negatives (FN), or which we can derive from reported statistics, or obtain from the study authors. We will exclude descriptive studies (for example, case series).
Participants
We will include all HIV‐positive adults (≥ 18 years) with a clinical suspicion of abdominal TB or disseminated TB with abdominal involvement (excluding genitourinary TB), who were investigated using an abdominal ultrasound examination. We will also consider studies that include confirmed cases of abdominal TB and controls. We will not place any restrictions on setting. Although abdominal ultrasound can be used to evaluate children, microbiological confirmation of TB is far more difficult than in adults, and so we will exclude children.
Index tests
We will include studies that evaluate the accuracy of abdominal ultrasound. We will not place any restrictions on the type of ultrasound machine used or the qualification of the person performing the ultrasound, but we will record these data. A positive result will be an ultrasound scan with abnormal findings suggestive of abdominal TB or disseminated TB with abdominal involvement including — but not limited to — free abdominal fluid, abdominal lymph nodes, hepatic lesions, and splenic lesions. A negative result will be an ultrasound scan with no abnormal findings.
We will deem test combinations as positive if the abdominal ultrasound is positive and either the chest x‐ray or the full blood count are positive. We will deem the chest x‐ray to be positive if any radiological features are suggestive of TB. A negative result will be a chest x‐ray with no radiological features suggestive of TB. We will consider a full blood count to be positive if a normocytic (mean corpuscular volume 80 to 100 fl) anaemia (haemoglobin less than 13 g/dL in men, or less than 12 g/dL in women) were present (WHO 2008).
Target conditions
Active disease due to M. tuberculosis – either abdominal TB or disseminated TB with abdominal involvement.
Reference standards
We will use a hierarchy of reference standards. The reference standard diagnosis typically relates to microbiological confirmation (microscopy or culture), although histopathological characteristics strongly support a diagnosis of active TB in clinically and epidemiologically appropriate settings. Xpert MTB/RIF assay (an automated nucleic acid amplification test) can also identify M. tuberculosis. A clinical diagnosis of TB is sometimes used in the absence of confirmative tests, for example, probable TB can be defined as the clinical picture of TB without objective diagnostic TB criteria and treated for TB by the attending physician. Although this approach is clinically useful, it is very subjective as it relies on the clinical gestalt of the treating physician. It is therefore seen as a lower quality reference standard.
The primary (higher quality) reference standard will be bacteriological confirmation of any clinical specimen including (i) at least one specimen culture positive for M. tuberculosis, (ii) microscopic identification of AFB on stained sputum smears, lymph node aspirate, or any other specimen; or iii) Xpert MTB/RIF positive (WHO 2013a). We will consider a positive result on any of these tests as a positive result for the microbiological (higher quality) reference standard and a TB case, since not all of the tests might have been performed or might have a positive result. The reference standard will be either solid or liquid culture for M. tuberculosis complex (Lawn 2011). The sensitivity of smear microscopy can be increased by examining more than one sample, using fluorescence microscopy, and using physical and chemical sputum processing techniques including centrifugation, sedimentation, and bleach (Steingart 2006a; Steingart 2006b). We will therefore include studies that use any of these techniques.
The secondary (lower quality) reference standard will be clinical diagnosis of TB without microbiological confirmation. A clinically diagnosed TB case is one that has been diagnosed with active TB by a healthcare practitioner and anti‐TB therapy has subsequently been initiated. This definition lacks bacteriological confirmation but includes cases diagnosed on the basis of suggestive histology (necrotising granulomatous inflammation), x‐ray abnormalities, and extrapulmonary cases without laboratory confirmation (WHO 2013a). Using clinical diagnosis as a reference standard could potentially bias test accuracy because abdominal ultrasound is often used to inform the clinical decision to treat for TB. We will include these studies as incorporation bias had a small effect in diagnostic accuracy estimates (Rutjes 2006). However, we will assess the impact of incorporation bias with a sensitivity analysis and have also adapted the revised tool for the Quality Assessment of Diagnostic Accuracy Studies (QUADAS‐2)
Search methods for identification of studies
Electronic searches
We will search the electronic databases, MEDLINE, Embase, BIOSIS, and MEDION. The MEDLINE search strategy is outlined in Appendix 1. We will further translate the MEDLINE search to Embase and BIOSIS databases to identify additional records. To avoid missing studies, we will not use a diagnostic search filter or restrict searches by date or language.
Searching other resources
We will examine the reference lists of relevant reviews and studies; and search web sites of the WHO, the Stop TB Partnership, and the National Institute of Allergy and Infectious Diseases (NIAID). We will also perform forward citation searching of relevant articles using the PubMed related articles feature, Google Scholar, and ISI citation indices. Furthermore, where necessary we will contact study authors for additional information.
Data collection and analysis
Selection of studies
Two review authors (DJvH and RG) will independently judge study eligibility by examining the title and abstract of each article identified by the literature search and will exclude obviously irrelevant studies. If either review authors considers the abstract to be potentially eligible, we will obtain the full‐text article. The two review authors will independently assess each full‐text article against the predefined inclusion and exclusion criteria as discussed in the ʽCriteria for considering studies for this review' section. The two review authors will resolve any disagreements by discussion. If the review authors cannot reach consensus, a third review author (GrM) will have the final decision. We will list all articles excluded after full‐text assessment and their reasons for exclusion in the ʽCharacteristics of excluded studies' table. We will illustrate the study selection process using a PRISMA diagram.
Data extraction and management
Two review authors (DJvH and RG) will independently extract data using a pre designed data extraction form.
We will extract the following data.
-
Authors, publication year, and journal.
-
Study design.
-
Characteristics study participants (age and gender).
-
Study inclusion/exclusion criteria.
-
Study setting.
-
TB prevalence in study setting.
-
HIV prevalence in study setting.
-
Antiretroviral therapy (ART) status.
-
Reference standard (bacteriological, clinical).
-
Index test ‐ general: abdominal ultrasound normal or abnormal.
-
Index test ‐ specific: individual findings on ultrasound.
-
Additional tests (and their results).
-
Training level of person performing the ultrasound.
-
Number of indeterminate, missing or unavailable test results.
-
Number of TP, TN, FP, and FN.
We will resolve any discrepancies in data extraction by discussion. If needed, we will consult a third review author (GrM). We will contact the authors of primary studies if we cannot resolve any disagreements. In the event that this is unsuccessful, we will report the disagreement in the review.
Assessment of methodological quality
We will use the revised tool for the Quality Assessment of Diagnostic Accuracy Studies (QUADAS‐2) to assess the risk of bias and applicability of included studies (Whiting 2011). We have tailored the tool to the context of the review as shown in Appendix 2. Two review authors (DJvH and RG) will independently assess the methodological quality using the tailored QUADAS‐2 tool. We will resolve any disagreements through consensus or by consulting a third review author (EAO). We will use both graphics and text to present results.
Statistical analysis and data synthesis
Patients are highly likely to have more than one abnormal finding per ultrasound. In our primary analyses, we will use the individual patient as the unit of analysis (that is, any abnormal finding versus none) and not individual ultrasound findings. Clinically, it is also useful to know the accuracy of individual ultrasound findings as it is plausible that some findings are better indicators of TB than others. Therefore, if there is sufficient data, we will consider the accuracy of individual ultrasound findings in secondary analyses. In these analyses, the patient will still be the unit of analysis as in each analysis we will examine a single finding per patient (for example, free abdominal fluid present or not) rather than multiple findings per patient.
Studies are likely to use different criteria or thresholds to determine the positivity of ultrasound. For example, studies may define an ultrasound scan as positive based on the presence of any abnormal abdominal finding including (but not limited to) organ enlargement, the presence or number of hepatic or splenic lesions, or the presence or size of abdominal nodes. We will construct 2 x 2 tables using the criteria specified in the studies. We will perform preliminary analyses by plotting estimates of sensitivity and specificity from the included studies on forest plots and in receiver operating characteristic (ROC) space using the software, Review Manager 5 (RevMan 5) (RevMan 2014).
If there are sufficient data, we will perform meta‐analyses using hierarchical models. To allow for differences in thresholds between studies, we will use the hierarchical summary ROC (HSROC) model to estimate summary ROC (SROC) curves (Rutter 2001). If studies use a common positivity criteria or threshold, we will estimate summary sensitivities and specificities (summary points) using functions of the parameters of the HSROC model. Alternatively, we will use the bivariate model if estimation of summary points is more appropriate given the nature of the data. We will fit the HSROC model using the statistical software SAS version 9.4 (SAS 2016) and the bivariate model using Stata 15 (STATA 2015). If analyses using hierarchical models fail to converge due to sparse data or few studies, we will simplify the models as appropriate based on the focus of inference (summary curves or summary points) (Takwoingi 2015).
Investigations of heterogeneity
Potential sources of heterogeneity include type of reference standard (higher quality versus lower quality), clinical setting (primary care clinic versus hospital), and ultrasound training level (radiologist versus sonographer). We will use these factors as categorical covariates on forest plots and SROC plots for visual assessment of heterogeneity. If sufficient data are available, we will investigate the effect of each covariate by performing subgroup analyses or meta‐regression. We will perform meta‐regression by including a covariate in a hierarchical model.
Sensitivity analyses
If there are sufficient data, we will perform sensitivity analyses to examine the influence of risk of bias and study design on test accuracy. We will examine the impact of risk of bias in each of the four QUADAS‐2 domains (patient selection, index test, reference standard, and flow and timing) by excluding studies at high or unclear risk of bias from the main analyses. To examine the effect of study design, we will exclude case‐control studies. To assess the impact of incorporation bias, we will exclude studies that incorporated the index test on the summary estimates.
Assessment of reporting bias
We will not assess reporting bias.
Assessment of the quality of evidence (certainty of the evidence)
We will use the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach (GRADE 2013) and GRADEpro Guideline Development Tool (GDT) software (GRADEpro GDT 2015) to assess the certainty of evidence (also called the quality of evidence). We will rate the quality of evidence as either high (not downgraded), moderate (downgraded by one level), low (downgraded by two levels), or very low (downgraded by more than two levels) for five domains: risk of bias, indirectness, inconsistency, imprecision, and publication bias.
For each outcome, the quality of evidence will start as high if there are high quality observational studies (cross‐sectional or cohort studies) that enrolled participants with diagnostic uncertainty. We will, if indicated, use our judgement to classify the reason for downgrading as either serious (downgraded by one level) or very serious (downgraded by two levels).
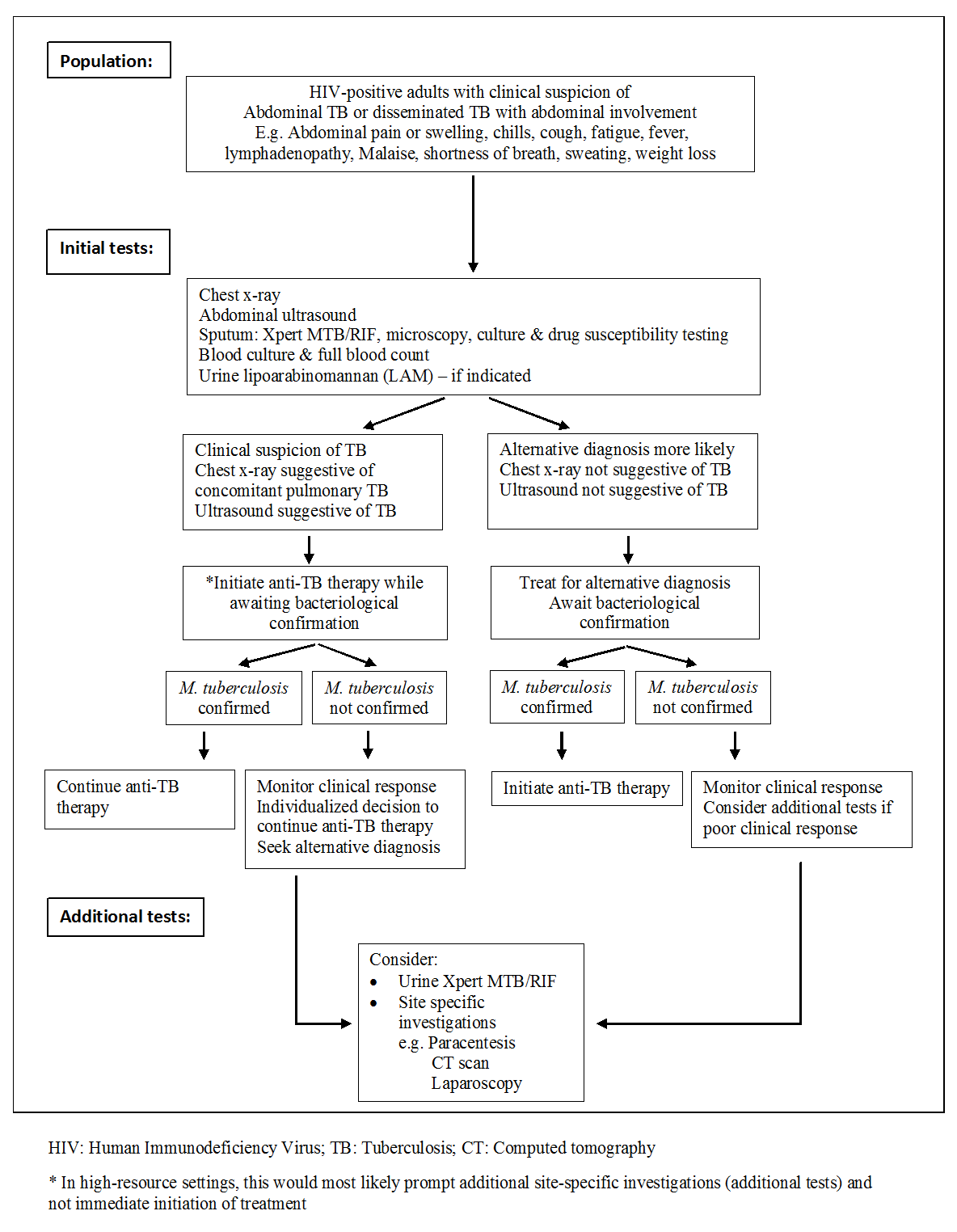
Diagnostic workup of HIV‐positive adults with suspected abdominal tuberculosis or disseminated tuberculosis with abdominal involvement

