Abdominal ultrasound for diagnosing abdominal tuberculosis or disseminated tuberculosis with abdominal involvement in HIV‐positive individuals
Abstract
Background
Accurate diagnosis of tuberculosis in people living with HIV is difficult. HIV‐positive individuals have higher rates of extrapulmonary tuberculosis and the diagnosis of tuberculosis is often limited to imaging results. Ultrasound is such an imaging test that is widely used as a diagnostic tool (including point‐of‐care) in people suspected of having abdominal tuberculosis or disseminated tuberculosis with abdominal involvement.
Objectives
To determine the diagnostic accuracy of abdominal ultrasound for detecting abdominal tuberculosis or disseminated tuberculosis with abdominal involvement in HIV‐positive individuals.
To investigate potential sources of heterogeneity in test accuracy, including clinical setting, ultrasound training level, and type of reference standard.
Search methods
We searched for publications in any language up to 4 April 2019 in the following databases: MEDLINE, Embase, BIOSIS, Science Citation Index Expanded (SCI‐EXPANDED), Social Sciences Citation Index (SSCI), Conference Proceedings Citation Index‐ Science (CPCI‐S), and also ClinicalTrials.gov and the WHO International Clinical Trials Registry Platform to identify ongoing trials.
Selection criteria
We included cross‐sectional, cohort, and diagnostic case‐control studies (prospective and retrospective) that compared the result of the index test (abdominal ultrasound) with one of the reference standards. We only included studies that allowed for extraction of numbers of true positives (TPs), true negatives (TNs), false positives (FPs), and false negatives (FNs). Participants were HIV‐positive individuals aged 15 years and older. A higher‐quality reference standard was the bacteriological confirmation of Mycobacterium tuberculosis from any clinical specimen, and a lower‐quality reference standard was a clinical diagnosis of tuberculosis without microbiological confirmation. We excluded genitourinary tuberculosis.
Data collection and analysis
For each study, two review authors independently extracted data using a standardized form. We assessed the quality of studies using a tailored Quality Assessment of Diagnostic Accuracy Studies‐2 (QUADAS‐2) tool. We used the bivariate model to estimate pooled sensitivity and specificity. When studies were few we simplified the bivariate model to separate univariate random‐effects logistic regression models for sensitivity and specificity. We explored the influence of the type of reference standard on the accuracy estimates by conducting separate analyses for each type of reference standard. We assessed the certainty of the evidence using the GRADE approach.
Main results
We included 11 studies. The risks of bias and concern about applicability were often high or unclear in all domains. We included six studies in the main analyses of any abnormal finding on abdominal ultrasound; five studies reported only individual lesions.
The six studies of any abnormal finding were cross‐sectional or cohort studies. Five of these (83%) were conducted in low‐ or middle‐income countries, and one in a high‐income country. The proportion of participants on antiretroviral therapy was none (1 study), fewer then 50% (4 studies), more than 50% (1 study), and not reported (5 studies). The first main analysis, studies using a higher‐quality reference standard (bacteriological confirmation), had a pooled sensitivity of 63% (95% confidence interval (CI) 43% to 79%; 5 studies, 368 participants; very low‐certainty evidence) and a pooled specificity of 68% (95% CI 42% to 87%; 5 studies, 511 participants; very low‐certainty evidence). If the results were to be applied to a hypothetical cohort of 1000 people with HIV where 200 (20%) have tuberculosis then:
‐ About 382 individuals would have an ultrasound result indicating tuberculosis; of these, 256 (67%) would be incorrectly classified as having tuberculosis (false positives).
‐ Of the 618 individuals with a result indicating that tuberculosis is not present, 74 (12%) would be incorrectly classified as not having tuberculosis (false negatives).
In the second main analysis involving studies using a lower‐quality reference standard (clinical diagnosis), the pooled sensitivity was 68% (95% CI 45% to 85%; 4 studies, 195 participants; very low‐certainty evidence) and the pooled specificity was 73% (95% CI 41% to 91%; 4 studies, 202 participants; very low‐certainty evidence).
Authors' conclusions
In HIV‐positive individuals thought to have abdominal tuberculosis or disseminated tuberculosis with abdominal involvement, abdominal ultrasound appears to have 63% sensitivity and 68% specificity when tuberculosis was bacteriologically confirmed. These estimates are based on data that is limited, varied, and low‐certainty.
The low sensitivity of abdominal ultrasound means clinicians should not use a negative test result to rule out the disease, but rather consider the result in combination with other diagnostic strategies (including clinical signs, chest x‐ray, lateral flow urine lipoarabinomannan assay (LF‐LAM), and Xpert MTB/RIF). Research incorporating the test into tuberculosis diagnostic algorithms will help in delineating more precisely its value in diagnosing abdominal tuberculosis or disseminated tuberculosis with abdominal involvement.
Plain language summary
Abdominal ultrasound for diagnosing abdominal tuberculosis or disseminated tuberculosis with abdominal involvement in people with HIV
Why is improving tuberculosis diagnosis in people with HIV important?
Diagnosing active tuberculosis in people living with HIV is challenging. People with advanced immunosuppression have high rates of extrapulmonary tuberculosis (tuberculosis outside the lungs).
What is the aim of this review?
The aim of this review is to find out how accurate an ultrasound examination of the abdomen (abdominal ultrasound) is for diagnosing tuberculosis in people with HIV suspected of having tuberculosis in the abdomen or widespread tuberculosis (disseminated tuberculosis) involving the abdomen.
What was studied in the review?
Abdominal ultrasound can be done after other tests (e.g. the chest x‐ray did not indicate tuberculosis ) or it can be done before other tests in people suspected of having tuberculosis. This review focuses on situations where other tests are not available.
What are the main results in this review?
We found 11 studies, but only six were relevant for the main analyses. The six studies were divided into two groups. In the first group tuberculosis was diagnosed by identifying the organism causing tuberculosis from any specimen (microbiological confirmation). For the second group, tuberculosis was diagnosed when healthcare personnel suspected tuberculosis and started anti‐tuberculosis treatment, but without identifying the organism (clinical diagnosis). Three studies provided results for both groups.
The review included five studies (a total of 879 participants) with microbiological confirmation. The results showed that if abdominal ultrasound were to be used in a group of 1000 people with HIV where 200 (20%) have tuberculosis then:
‐ About 382 individuals would have an ultrasound result indicating tuberculosis; of these, 256 (67%) would be incorrectly classified as having tuberculosis (false positives).
‐ Of the 618 individuals with a result indicating that tuberculosis is not present, 74 (12%) would be incorrectly classified as not having tuberculosis (false negatives).
How reliable are the results of the studies in this review?
Microbiological confirmation is likely to be a reliable method for deciding whether people really have tuberculosis; clinical diagnosis is likely to be less trustworthy. We found problems in both groups with how studies were conducted. Decreasing the number of false positive results may make abdominal ultrasound appear more accurate than it is. Numbers shown are an average across studies. As estimates from individual studies varied, we cannot be sure that abdominal ultrasound will always produce these results. Not enough people have been studied for us to be confident about the results.
Who do the results of the review apply to?
Studies included in the main analyses were done in Cambodia, India, South Africa, South Sudan, Spain, and Tanzania. Reasons for including people differed between the studies. Four studies used trained radiologists (specialists) or sonographers; two used doctors trained in ultrasound (non‐specialists), and two included people without any suspicion of tuberculosis. Across the studies, the percentage of people with a final diagnosis of tuberculosis ranged from 18% to 64%.
What are the implications of this review?
If the test is used to rule in the disease in the absence of other evidence, then, the chance of diagnosing someone with tuberculosis when they actually do not have it is high. Chances of missing a diagnosis of tuberculosis when the test is positive are lower, but a negative test alone is probably insufficient to rule out the disease. These findings should be considered when deciding whether or not to use abdominal ultrasound to test for tuberculosis involving the abdomen and how to interpret the results in the context of other clinical and diagnostic test information.
How up‐to‐date is this review?
The review authors searched for studies up to 4 April 2019.
Authors' conclusions
Summary of findings
| Review question: Should abdominal ultrasound be used to diagnose abdominal tuberculosis or disseminated tuberculosis with abdominal involvement in HIV‐positive individuals? Patient or population: HIV‐positive individuals Setting: Healthcare facility Index test: Abdominal ultrasound Reference standard: We considered two reference standards. The higher‐quality reference standard was bacteriological confirmation of M tuberculosis (any clinical specimen including (i) at least one specimen culture positive for M tuberculosis, (ii) microscopic identification of acid‐fast bacilli on stained sputum smears, lymph node aspirate, or any other specimen; or iii) Xpert MTB/RIF positive). The lower‐quality reference standard was clinical diagnosis of TB without microbiological confirmation (including cases diagnosed on the basis of: i) suggestive histology (necrotizing granulomatous inflammation), ii) x‐ray abnormalities, iii) extrapulmonary cases without laboratory confirmation, and iv) anti‐tuberculosis therapy initiated by a healthcare practitioner for cases with a high suspicion of tuberculosis). Threshold: Any abnormality found on abdominal ultrasound Study design: Cross‐sectional and cohort Limitations: A small number of studies and participants were included in the analyses. Risks of bias were generally high in the patient selection domain | ||||||
| Test result | Number of results per 1000 HIV‐positive individuals tested (95% CI) | Number of studies | Number of participants | Certainty of the evidence (GRADE) | ||
| Prevalence 10% | Prevalence 20% | Prevalence 40% | ||||
| Bacteriological confirmation as reference standard: pooled sensitivity = 63% (95% CI 43% to 79%) and pooled specificity = 68% (95% CI 42% to 87%) | ||||||
| True positives (participants correctly classified as having tuberculosis) | 63 (43 to 79) | 126 (86 to 158) | 252 (172 to 316) | 5 | 368 | ⊕⊝⊝⊝ |
| False negatives (participants incorrectly classified as not having tuberculosis) | 37 (21 to 57) | 74 (42 to 114) | 148 (84 to 228) | |||
| True negatives (participants correctly classified as not having tuberculosis) | 612 (378 to 783) | 544 (336 to 696) | 408 (252 to 522) | 5 | 511 | ⊕⊝⊝⊝ |
| False positives (participants incorrectly classified as having tuberculosis) | 288 (117 to 522) | 256 (104 to 464) | 192 (78 to 348) | |||
| Abbreviations: CI: confidence interval GRADE certainty of evidence (GRADEpro GDT 2015; Schünemann 2016) The table displays normalized frequencies within a hypothetical cohort of 1000 people at three different tuberculosis prevalences (pre‐test probabilities): 10%, 20% and 40%. We selected prevalence values based on the range of prevalence observed across the included studies. We estimated confidence intervals based on those around the point estimates for pooled sensitivity and specificity. Explanations aRisk of bias: We rated one study at high risk for participant selection since it excluded people unable to produce sputum (Griesel 2019‐h). We downgraded the certainty of the evidence by one level. | ||||||
Background
Target condition being diagnosed
Tuberculosis is caused by the bacillus Mycobacterium tuberculosis. Although it usually affects the lungs (pulmonary tuberculosis), it can also spread to other body sites (extrapulmonary tuberculosis) (WHO 2018).
An estimated 10 million people were diagnosed with tuberculosis in 2017, and 1.6 million people died from tuberculosis (WHO 2018). Resource‐limited countries are the most affected; for example, the African region of the World Health Organization (WHO) had the second highest estimated number of incident cases (2.5 million), but the highest incidence rate (237 versus 133 globally) and mortality rate (HIV‐positive: 24 versus 4.0 globally; HIV‐negative: 39 versus 17 globally) per 100,000 people (WHO 2018).
The probability of developing tuberculosis is higher among people living with HIV. Approximately 920,000 people diagnosed worldwide with tuberculosis in 2017 were HIV‐positive (WHO 2018), with HIV prevalence among incident tuberculosis cases in the African region at 27% (WHO 2018).
The worldwide case detection rate in 2016 was only an estimated 61% (WHO 2017), reflecting a mixture of under‐reporting of detected cases and underdiagnosis of tuberculosis. The low detection rate possibly relates to delays in diagnosis, which could be from problems with tuberculosis diagnostic tests (accuracy and availability), the negative influence of HIV infection on the performance of diagnostic tests, and HIV co‐infection and the opportunistic conditions that complicate it (Palmieri 2002; Dawson 2010; Padmapriyadarsini 2011; Horne 2019; WHO 2017). Other factors might be weaknesses in health systems and broader social and economic influences (for example, undernourishment, poverty) on the tuberculosis epidemic (WHO 2017). The diagnosis of active tuberculosis in HIV‐positive people with advanced immunosuppression is challenging due to more atypical clinical presentations; other opportunistic pulmonary infections with similar presentations; a high proportion of negative sputum smears; and high rates of extrapulmonary tuberculosis (Sharma 2005). This is illustrated by autopsy studies, which indicate a very high proportion of tuberculosis in HIV‐positive adults (32% to 47%); almost half (46%) of adult tuberculosis cases remained undiagnosed before death (Gupta 2015).
An estimated 14% of the 6.4 million incident tuberculosis cases in 2017 were extrapulmonary tuberculosis (WHO 2018). In people with HIV‐associated tuberculosis, extrapulmonary tuberculosis accounts for up to 50% of all tuberculosis cases (Sharma 2004b; Kingkaew 2009; Namme 2013), and is often disseminated (two or more non‐contiguous sites simultaneously infected) (Sharma 2005). Any anatomical site can be involved, but the commonest sites are the lymph nodes, pleura, meninges, and the abdominal cavity (Sharma 2005). Many terms are used in the literature to describe tuberculosis in the abdominal cavity. For the purposes of this Cochrane Review, we use the terms abdominal tuberculosis or disseminated tuberculosis with abdominal involvement, excluding genitourinary tuberculosis. Many abdominal structures can be affected in abdominal tuberculosis or disseminated tuberculosis with abdominal involvement, including involvement of the gastrointestinal tract, peritoneum, omentum, mesentery, intra‐abdominal lymph nodes, and solid organs (liver, spleen, pancreas) (Sharma 2004b). People often present with non‐specific symptoms and signs, and a high index of suspicion is therefore needed for early diagnosis and timely management. It mimics a large number of medical and surgical conditions, including malignant neoplasms, inflammatory bowel disease, chronic liver disease, and other gastrointestinal infections (Jadvar 1997).
Index test(s)
Many HIV‐positive people with low CD4 counts have abdominal tuberculosis or disseminated tuberculosis with abdominal involvement. As sputum smears are frequently negative in HIV‐associated tuberculosis, it is common clinical practice, supported by WHO guidelines, to reach a tuberculosis diagnosis on the basis of imaging results and clinical case definitions (Wilson 2006; WHO 2016). Ultrasound is such an imaging test that can be used as a diagnostic tool (Heller 2010a; Heller 2010b; Patel 2011; Giordani 2013; Sharma 2017), although the only WHO recommendation refers to the use of ultrasound to diagnose pericardial effusions (WHO 2006). Ultrasound uses sound waves to produce images of structures and organs within the body, and has traditionally been performed by trained specialists in dedicated radiology departments. However, the numerous advantages of ultrasound (e.g. rapidly performed, portable, non‐invasive, repeatable, etc.) have led to many physicians in different specialties adopting ultrasound (Adhikari 2014). The use of ultrasound by trained medical professionals (non‐radiologists) is particularly relevant in resource‐limited settings. Computed tomography (CT) or magnetic resonance imaging (MRI) is expensive, mostly only available in tertiary‐level settings, and require specially‐trained personnel to perform and report these examinations. Many low‐income and middle‐income countries have a high tuberculosis burden (WHO 2018), but without widespread access to specialists and tertiary‐level imaging. However, ultrasound machines are mostly accessible and their use by non‐radiologists would be of great value.
Abdominal ultrasound (an ultrasound examination evaluating the abdominal cavity) may be useful in HIV‐positive people with suspected abdominal tuberculosis or disseminated tuberculosis with abdominal involvement. Ultrasound techniques to diagnosis HIV‐associated tuberculosis are easily learned by non‐radiologists and quick to perform (less than 10 minutes) (Heller 2010a). The ultrasound findings are non‐specific, and various other diseases may present with the same features. For example, intra‐abdominal lymphadenopathy can be due to other infections (for example, cryptococcosis, histoplasmosis); lymphomas (non‐Hodgkin's lymphoma and Hodgkin's lymphoma); and Kaposi's sarcoma (Martin‐Bates 1993).
Clinical pathway
Any structure or organ in the abdominal cavity (for example, gastrointestinal tract, pancreatobiliary system, peritoneum, and lymph nodes) can be affected by tuberculosis disease. The presentation varies considerably and depends on the specific organ involved (Sharma 2017); other diseases are also often mimicked (Sharma 2004a). Common presenting symptoms are abdominal pain, anorexia, bowel disturbances, fever, and weight loss. The clinical examination often reveals abdominal tenderness, ascites, and solid organ enlargement (for example, hepatomegaly, splenomegaly, or hepatosplenomegaly) (Ibrahim 2005; Mandal 2011; Sharma 2017).
Essential diagnostic tests for individuals who are suspected of having abdominal tuberculosis or disseminated tuberculosis with abdominal involvement include a chest x‐ray, sputum evaluation (if able to produce) for bacteriological confirmation of tuberculosis disease (smear or culture or Xpert MTB/RIF), and blood cultures (WHO 2013b). Urine specimens remain a convenient clinical sample for the diagnosis of tuberculosis. Although conventional tuberculosis diagnostics applied to urine specimens have limited clinical utility, the use of urinary lipoarabinomannan (LAM) has been recommended by the WHO in HIV‐positive adults with advanced immunosuppression (CD4 cell count of 100 cells/µL or less) or in HIV‐positive adults who are seriously ill (respiratory rate above 30/min, temperature above 39 °C, heart rate above 120/min and unable to walk unaided), regardless of their CD4 cell count (WHO 2015; Shah 2016). These tests are usually done in the primary care setting and higher.
Abdominal ultrasound has become part of the initial diagnostic work‐up in adults living with HIV where abdominal tuberculosis or disseminated tuberculosis with abdominal involvement is suspected (especially in those with a low CD4 count), despite the lack of robust evidence of validity from large studies (NICE 2016). The diagnostic pathway might vary in different settings if there are ultrasound findings suggestive of tuberculosis. In resource‐limited settings this might be enough evidence to initiate anti‐tuberculosis treatment, but in high‐resource settings it would prompt site‐specific investigations which could include CT scan, paracentesis, laparoscopy, fine needle aspiration, or stool examination.
A presumptive diagnosis of abdominal tuberculosis or disseminated tuberculosis with abdominal involvement can be made in the setting of known active pulmonary tuberculosis, although fewer than half of chest radiographs are compatible with active or healed tuberculosis (Chow 2002). However, data are lacking in HIV‐positive individuals.
WHO recommends immediate initiation of anti‐tuberculosis therapy in people living with HIV who have clinical features of disseminated tuberculosis (WHO 2016). Bacteriological confirmation of tuberculosis from any specimen remains important, but treatment should not be delayed until results become available (Figure 1). People started on anti‐tuberculosis therapy without bacteriological confirmation should be assessed after one month to evaluate the clinical response to treatment. They should be re‐assessed and an alternative diagnosis sought if there is no clinical improvement.

Diagnostic workup of HIV‐positive individuals with suspected abdominal tuberculosis or disseminated tuberculosis with abdominal involvement
Role of index test(s)
Abdominal ultrasound is often combined with existing tests such as chest x‐ray, haemoglobin, etc. to reach a diagnosis of abdominal tuberculosis or disseminated tuberculosis with abdominal involvement in clinical practice. However, all the existing tests that could inform a confirmed diagnosis may not always be available.
Alternative test(s)
Ascitic fluid analysis suggestive of abdominal tuberculosis or disseminated tuberculosis with abdominal involvement includes a leukocyte count of 150 to 4000 cells/mL, which consists predominantly of lymphocytes (Sharma 2004a; Sanai 2005). The ascitic fluid is usually an exudate with the protein content greater than 30 g/L and the serum‐ascites albumin gradient (SAAG) less than 11 g/L (Sharma 2004a; Sanai 2005). Adenosine deaminase activity (ADA) of ascitic fluid (> 39 IU/L) is also suggestive of abdominal tuberculosis (Riquelme 2006), while the ascites to blood glucose ratio is usually less than 0.96 (Wilkins 1984). Acid‐fast bacilli (AFB) smear and culture of ascitic fluid also have disappointingly low yields (Chow 2003), while Xpert MTB/RIF for peritoneal tuberculosis using peritoneal fluid has a pooled sensitivity of 59% (credible interval (CrI) 45 to 74) and a pooled specificity of 98% (CrI 96 to 99) (Kohli 2018).
Different imaging modalities can be useful to diagnose abdominal tuberculosis or disseminated tuberculosis with abdominal involvement. Abdominal x‐rays are of very limited value, but can assist with the diagnosis of intestinal obstruction and perforation (Debi 2014). CT features include thickening of the peritoneum, omentum, and bowel wall; lymph nodes (especially if these have hypodense centres due to caseous necrosis); and ascites with strands, debris, and fine septations (Sharma 2004a; Lee 2012). The excellent soft tissue resolution and multiplanar acquisition of MRI have resulted in it being used to evaluate solid organs and lymphadenopathy (Joshi 2014). However, CT and especially MRI are expensive and access is very limited in resource‐limited settings. Barium studies may be useful for intrinsic bowel abnormalities such as strictures, fistulae, and erosions (Sharma 2004a; Debi 2014).
Colonoscopy with biopsy is a useful non‐operative diagnostic procedure to obtain material for histology and culture (Kim 1998). Mucosal nodules and transverse ulcers in the bowel are very suggestive of tuberculosis, with definitive results obtained from tissue sent for polymerase chain reaction (PCR), Ziehl‐Neelsen stain, and culture (Kim 1998; Sharma 2004a). Laparoscopy is useful in two ways: (i) it allows visual inspection of the peritoneum; and (ii) it permits specimens for histology, AFB stain, and culture to be obtained. However, imaging modalities as described above provide a safer, less invasive and less expensive alternative, but may be less specific since they are unable to provide a definitive microbiological diagnosis (Sanai 2005).
Most studies relating to the diagnosis of tuberculosis were done in HIV‐negative people and the true diagnostic accuracy of the above tests in those living with HIV remains uncertain. Expanded clinical case definitions were developed to diagnose smear‐negative tuberculosis in HIV‐positive people living in resource‐limited settings (Wilson 2006), including abdominal tuberculosis or disseminated tuberculosis with abdominal involvement (Wilson 2006; WHO 2016). For example, a person presenting with symptoms and signs suggestive of abdominal tuberculosis or disseminated tuberculosis with abdominal involvement can be started on anti‐tuberculosis treatment if the ascitic fluid consists of a lymphocytic exudate along with either a fever of 38 °C or more on two occasions or drenching sweats for more than two weeks (Wilson 2006). In this study, the positive predictive value for abdominal lymph nodes diagnosed by ultrasound was 94% (Wilson 2006). Augmented by the use of objective criteria to monitor response to treatment within the first eight weeks, this approach has reasonable diagnostic accuracy (Wilson 2006).
Rationale
Multiple studies of various quality and designs have looked at the use of abdominal ultrasound as a diagnostic tool for abdominal tuberculosis or disseminated tuberculosis with abdominal involvement, with varying sensitivity, specificity, and predictive values for diagnosing tuberculosis (Monill‐Serra 1997‐l; Mugala 2006; Sinkala 2009‐l; Sculier 2010‐h; Patel 2011). Abdominal ultrasound may be used alone, in combination with existing tests (chest radiograph, full blood count), or as an add‐on following negative results from existing tests (smear microscopy, sputum Xpert MTB/RIF, sputum culture, chest radiograph). The role of abdominal ultrasound as an add‐on test is an important clinical question because it may reflect the way that abdominal ultrasound is used in practice, especially in resource‐limited settings. However, after a scoping search, we did not find any studies that have evaluated the accuracy of ultrasound as an add‐on test or in combination with other tests.
Objectives
To determine the diagnostic accuracy of abdominal ultrasound for detecting abdominal tuberculosis or disseminated tuberculosis with abdominal involvement in HIV‐positive individuals.
Secondary objectives
To investigate potential sources of heterogeneity in test accuracy, including clinical setting, ultrasound training level, and type of reference standard.
Methods
Criteria for considering studies for this review
Types of studies
We included cross‐sectional, cohort, or diagnostic case‐control studies (prospective and retrospective) that compared the result of the index test (abdominal ultrasound) with one of the reference standards (see Reference standards). Case‐control studies may overestimate sensitivity and specificity, but we include them because we anticipated identifying few relevant studies. We only included studies in which the study authors reported the numbers of true positives (TPs), true negatives (TNs), false positives (FPs), and false negatives (FNs), or where we were able to derive the data from reported statistics. We also wrote to all study authors where data were missing. We excluded descriptive studies (for example, case series).
Participants
We included all HIV‐positive individuals (aged 15 years and older) with a clinical suspicion of abdominal tuberculosis or disseminated tuberculosis with abdominal involvement (excluding genitourinary tuberculosis), who were investigated using an abdominal ultrasound examination. We also considered studies that included confirmed cases of abdominal tuberculosis and controls. We did not place any restrictions on setting. Although abdominal ultrasound can be used to evaluate children, microbiological confirmation of tuberculosis is far more difficult than in adults, and so we excluded children where possible.
Index tests
We included studies that evaluated the accuracy of abdominal ultrasound. We did not place any restrictions on the type of ultrasound machine used or the qualification of the person performing the ultrasound, but recorded these data. A positive result was an ultrasound scan with abnormal findings suggestive of abdominal tuberculosis or disseminated tuberculosis with abdominal involvement, including, but not limited to, free abdominal fluid, abdominal lymph nodes, hepatic lesions, and splenic lesions. A negative result was an ultrasound scan with no abnormal findings.
Target conditions
Active disease due to M tuberculosis – either abdominal tuberculosis or disseminated tuberculosis with abdominal involvement.
Reference standards
We used a hierarchy of reference standards. The reference standard diagnosis typically relates to microbiological confirmation (microscopy or culture), although histopathological characteristics strongly support a diagnosis of active tuberculosis in clinically and epidemiologically appropriate settings. Xpert MTB/RIF assay (an automated nucleic acid amplification test) can also identify M tuberculosis. A clinical diagnosis of tuberculosis is sometimes used in the absence of confirmative tests, for example, probable tuberculosis can be defined as the clinical picture of tuberculosis without objective diagnostic tuberculosis criteria and treated for tuberculosis by the attending physician. Although this approach is clinically useful, it is very subjective as it relies on the clinical gestalt of the treating physician. We therefore viewed it as a lower‐quality reference standard.
The primary (higher‐quality) reference standard was bacteriological confirmation of any clinical specimen including (i) at least one specimen culture positive for M tuberculosis, (ii) microscopic identification of AFB on stained sputum smears, lymph node aspirate, or any other specimen; or iii) Xpert MTB/RIF positive (WHO 2013a). We considered a positive result on any of these tests as a positive result for the microbiological (higher‐quality) reference standard and a tuberculosis case, since not all of the tests might have been performed or might have a positive result. The reference standard for culture was either solid or liquid culture for M tuberculosis complex (Lawn 2011). The sensitivity of smear microscopy can be increased by examining more than one sample, using fluorescence microscopy, and using physical and chemical sputum processing techniques including centrifugation, sedimentation, and bleach (Steingart 2006a; Steingart 2006b). We therefore included studies that used any of these techniques.
The secondary (lower‐quality) reference standard was clinical diagnosis of tuberculosis without microbiological confirmation. A clinically diagnosed tuberculosis case is one that has been diagnosed with active tuberculosis by a healthcare practitioner and where anti‐tuberculosis therapy has subsequently been initiated. This definition lacks bacteriological confirmation but includes cases diagnosed on the basis of suggestive histology (necrotizing granulomatous inflammation), x‐ray abnormalities, and extrapulmonary cases without laboratory confirmation (WHO 2017). Using clinical diagnosis as a reference standard could potentially bias test accuracy because abdominal ultrasound is often used to inform the clinical decision to treat for tuberculosis (incorporation bias). We included these studies, as incorporation bias had a small effect in diagnostic accuracy estimates (Rutjes 2006), and we used an adapted version of the revised tool for the Quality Assessment of Diagnostic Accuracy Studies (QUADAS‐2).
Search methods for identification of studies
Electronic searches
Vittoria Lutje (VL), the Information Specialist for the Cochrane Infectious Diseases Group (CIDG), performed literature searches up to 4 April 2019, without language restrictions. She searched MEDLINE (PubMed, 1946 to 4 April 2019); Embase (Ovid, 1947 to 4 April 2019); Biosis (Web of Science, 1926 to 4 April 2019); Science Citation Index Expanded (SCI‐EXPANDED), Social Sciences Citation Index (SSCI), both 1900 to 4 April 2019, and Conference Proceedings Citation Index‐ Science (CPCI‐S), 1990 to 4 April 2019, (all three in the Web of Science). She also searched ClinicalTrials.gov and the WHO International Clinical Trials Registry Platform (ICTRP; apps.who.int/trialsearch/) for trials in progress. The search terms and strategy are reported in Appendix 1.
Searching other resources
We examined the reference lists of relevant reviews and studies; and searched websites of the WHO, the Stop TB Partnership, and the National Institute of Allergy and Infectious Diseases (NIAID). We also performed forward citation searching of relevant articles using the PubMed ‘related articles' feature, Google Scholar, and ISI citation indices. We also contacted study authors for additional information if we deemed it necessary.
Data collection and analysis
Selection of studies
Two review authors (DJvH and RG) independently judged study eligibility by examining the title and abstract of each article identified by the literature search and excluded obviously irrelevant studies. We obtained the full‐text article if either review author considered the abstract to be potentially eligible. The two review authors independently assessed each full‐text article against the predefined inclusion and exclusion criteria, as stated in the ‘Criteria for considering studies for this review' section. The two review authors resolved any disagreements by discussion. If the review authors could not reach consensus, a third review author (GrM) made the final decision. We maintained a list of all articles excluded after full‐text assessment and their reasons for exclusion in the ‘Characteristics of excluded studies' table. The study selection process is also illustrated using a PRISMA flow diagram.
Data extraction and management
We developed a standardized data extraction form before two review authors (DJvH and RG) independently extracted data. The extracted data were:
-
Details of study: first author, publication year, journal, study design, inclusion/exclusion criteria
-
Characteristics of study population: age, gender, estimated tuberculosis prevalence in study setting; estimated HIV prevalence in study setting, antiretroviral therapy (ART) status
-
Reference standard: bacteriological, clinical
-
Index test: general (abdominal ultrasound normal or abnormal), specific (individual findings on ultrasound), training level of person performing the ultrasound, additional tests (and their results)
-
Details of outcome: number of indeterminate, missing or unavailable test results, number of TP, TN, FP, and FN results
We resolved any discrepancies in data extraction by discussion, and a third review author (GrM) had the final say.
Assessment of methodological quality
We used the revised tool for the Quality Assessment of Diagnostic Accuracy Studies (QUADAS‐2) to assess the risks of bias and applicability of included studies (Whiting 2011). We tailored the tool to the context of the review, as shown in Appendix 2. Two review authors (DJvH and RG) independently assessed methodological quality using the tailored QUADAS‐2 tool. We resolved any disagreements through consensus or by consulting a third review author (EAO). We present the results in graphs, text, and the ‘Characteristics of included studies' table.
Statistical analysis and data synthesis
In our primary meta‐analyses, we used the individual participant as the unit of analysis (that is, any abnormal finding versus none) and not individual ultrasound findings. Clinically, it is also useful to know the accuracy of individual ultrasound findings, as it is plausible that some findings are better indicators of tuberculosis than others. We therefore determined the accuracy of individual ultrasound findings in secondary analyses.
We only included studies that reported test thresholds to enable us to construct 2 x 2 tables and also to select an appropriate method of meta‐analysis. Studies used different criteria to determine the positivity of ultrasound. For example, studies may define an ultrasound scan as positive based on the presence of any abnormal abdominal finding including (but not limited to) organ enlargement, the presence or number of hepatic or splenic lesions, or the presence or size of abdominal nodes. For the primary analysis we thus defined the threshold as the presence or absence of any abnormal lesion. In order to produce clinically meaningful results, we conducted two separate sets of primary meta‐analyses by estimating the pooled sensitivity and specificity for each type of reference standard (higher quality and lower quality).
For the secondary analyses (individual lesion as unit of analysis), we did not estimate the pooled sensitivity and specificity because some studies did not report thresholds and those that did used different thresholds. We only report the range of sensitivity and specificity.
We used the number of TPs, FPs, FNs, and TNs to construct 2 x 2 tables using the criteria specified in the studies. We plotted the estimates of sensitivity and specificity from the included studies on forest plots using Review Manager 5 software (Review Manager 2014).
We used the bivariate model (Chu 2006) to estimate pooled sensitivity and specificity at common thresholds. We fitted the models using the xtmelogit command in Stata version 15.0 (StataCorp, College Station, TX, USA).
Investigations of heterogeneity
Potential sources of heterogeneity included the type of reference standard (higher quality versus lower quality), clinical setting (any setting versus tertiary/referral hospital), and ultrasound training level (radiologist versus non‐radiologist). We stratified the primary analysis by the type of reference standard. Due to the small number of included studies and sample sizes we did not investigate other sources of heterogeneity.
Sensitivity analyses
We did not perform sensitivity analyses because of the small number of included studies.
Assessment of reporting bias
We did not carry out a formal assessment of publication bias.
Assessment of the certainty of the evidence
We used the GRADE approach (Schünemann 2016) and GRADEpro Guideline Development Tool (GDT) software (GRADEpro GDT 2015) to assess the certainty of the evidence (also called the quality of the evidence). We rated the certainty of the evidence as either high (not downgraded), moderate (downgraded by one level), low (downgraded by two levels), or very low (downgraded by more than two levels) for five domains: risk of bias, indirectness, inconsistency, imprecision, and publication bias. For each domain, the certainty of evidence started as high if there were high‐quality observational studies (cross‐sectional or cohort studies) that enrolled participants with diagnostic uncertainty. We used our judgement to classify the reason for downgrading as either serious (downgraded by one level) or very serious (downgraded by two levels).
Two review authors (DJvH and RG) discussed judgements and applied GRADE in the following way.
Risk of bias: we used the tailored QUADAS‐2 to assess risks of bias.
Indirectness: we used the tailored QUADAS‐2 for concerns of applicability and evaluated the studies for important differences between the populations studied (for example, age) and the setting. We made judgements on whether the differences were sufficient to lower our certainty in the results.
Inconsistency: we downgraded the certainty of the evidence for unexplained inconsistency in sensitivity and specificity estimates.
Imprecision: we considered a point estimate to be substantially different if it would alter a clinical decision. We considered the width of the CI, and whether a different clinical decision would be made if the lower or upper boundary of the CI represented the truth. We also made judgements on the imprecision of projected ranges for TP, FN, TN, and FP for a given prevalence of tuberculosis.
Publication bias: as recommended, we did not downgrade the certainty of evidence for publication bias for the following reasons (Schünemann in press). We did not detect studies done for‐profit interest. Included studies had small sample sizes and accuracy estimates were low and imprecise. We did an extensive search in electronic databases and grey literature and did not identify completed studies that were unpublished. We only identified one ongoing study, the results of which are not yet registered in the Pan African Clinical Trials Registry (Trial ID: PACTR201712002829221) (PACTR201712002829221).
Results
Results of the search
Our search yielded 1129 records. We identified two additional studies through contact with experts. After we removed one duplicate, we had 1130 records. We excluded 1089 records based on a review of title, abstract, or both. We retrieved 41 full‐text articles and excluded 30 studies for the following reasons: descriptive study (22 studies); ineligible participant population (2 studies); no reference standard reported (2 studies); ineligible index test evaluated (1 study); only abnormal index test reported (2 studies); and not a diagnostic accuracy study (1 study). We therefore include 11 unique studies in this review (Barreiros 2008‐h; Bobbio 2019‐l; Dominguez‐Castellano 1998‐h; Griesel 2019‐h; Kaneria 2009‐l; Monill‐Serra 1997‐l; Ndege 2019‐h; O'Keefe 1998‐h; Sculier 2010‐h; Sinkala 2009‐l; Weber 2018‐h). We listed the excluded studies and reasons for their exclusion in the Characteristics of excluded studies section. Figure 2 shows the flow of studies through the screening process.

Study flow diagram.
Three studies were conducted in low‐income countries, three in lower‐middle‐income countries, two in upper‐middle‐income countries, and three in high‐income countries. We noted poor reporting on the estimated prevalence of tuberculosis and HIV in study setting, qualification of sonographer and setting in which ultrasound was performed. Studies used different criteria to determine the positivity of ultrasound (see Characteristics of included studies section). Key findings of included studies are presented in Table 1 and Table 2.
| Author (publication year) | Study design | Country | Clinical setting | Target condition definition | Qualification of person performing index test | Sample size | Tuberculosisproportion in study |
| Case‐control | Germany | Not reported | Gastro‐intestinal tuberculosis | Not reported | 25a (7 cases, 18 pulmonary tuberculosis controls) | ‐ | |
| Cross‐sectional | South Sudan | Referral hospital | Extra‐pulmonary tuberculosis | Trained non‐radiologist | 100 | 24% | |
| Cross‐sectional | Spain | Not reported | Extra‐pulmonary tuberculosis | Sonographer | 116 | 55% (higher) 58% (lower) | |
| Cross‐sectional | South Africa | Non‐tertiary hospital | Culture‐positive tuberculosis | Sonographer | 377 | 53% | |
| Case‐control | India | Not reported | Pulmonary tuberculosis, extra‐pulmonary tuberculosis, disseminated tuberculosis | Not reported | 90 (45 cases, 45 HIV‐positive controls without any pathology) | ‐ | |
| Case‐control | Spain | Not reported | Disseminated tuberculosis | Not reported | 152 (76 cases, 76 HIV‐positive controls without any pathology) | ‐ | |
| Cohort | Tanzania | Referral hospital | Pulmonary tuberculosis, extra‐pulmonary tuberculosis, disseminated tuberculosis | Board‐certified sonographers | 100 (191 original study sample) | 46% (higher) 64% (lower) | |
| Cross‐sectional | South Africa | Non‐tertiary hospital | Disseminated tuberculosis | Radiologist | 35 (44 original study sample) | 34% | |
| Cross‐sectional | Cambodia | Referral hospital | Disseminated tuberculosis | Radiologist | 212 | 18% | |
| Cross‐sectional | Zambia | Tertiary hospital | Abdominal tuberculosis | Not reported | 31 | 71% | |
| Cohort | India | Tertiary hospital | Disseminated tuberculosis | Trained non‐radiologist | 81 (425 original study sample) | 30% (higher) 49% (lower) |
aIncludes five HIV‐negative participants.
Suffix (h) indicates higher‐quality reference standard; suffix (l) indicates lower‐quality reference standard.
| Author (publication year) | Index test variable included (threshold) | Reference standard quality and definition |
| Ascites (any) Lymphadenopathy (abdominal and perihepatic nodes with longitudinal diameter > 20 mm) Splenomegaly (> 135 mm) | Lower: Clinical, endoscopic, histologic, radiologic and operative findings including microbiology and polymerase chain reaction of biopsies taken during endoscopy | |
| Any abnormality (Presence of ≥ 1: i) pericardial effusion, ii) periportal/para‐aortic lymph nodes (> 15 mm diameter), iii) focal splenic lesions, iv) pleural effusion or consolidation of the lung, v) ascites without alternative explanation) | Lower: Sputum microscopy OR clinical reasons OR Focused Assessment with Sonography in HIV‐associated tuberculosis (FASH) | |
| Any abnormality (presence of ≥ 1: i) multiple hypoechoic splenic lesions (< 10 mm), ii) any abdominal adenopathy, iii) hypo‐ or hyperechoic liver lesions) | Higher: Microscopy OR culture | |
| Any abnormality (presence of ≥ 1: i) abdominal lymph nodes (any size), ii) splenic hypoechoic lesions, iii) splenomegaly (≥ 110 mm), iv) any one of abdominal, pleural, or pericardial effusions) Ascites (any) Lymphadenopathy (any size) Splenic lesions (hypoechoic) Splenomegaly (≥ 110 mm) | Higher: Positive culture for M tuberculosis from any site | |
| Ascites (any) Hepatomegaly (not defined) Lymphadenopathy (diameter > 15 mm) Splenic lesions (multiple, hypoechoic, 5 mm to 10 mm diameter) Splenomegaly (not defined) | Lower: Lymphocytic predominance and elevated adenosine deaminase (ADA) levels in pleural or ascitic fluid OR granulomatous lymphadenitis and acid‐fast bacilli in lymph node OR sputum microscopy | |
| Ascites (any) Hepatomegaly (not defined) Lymphadenopathy (> 15 mm diameter) Splenic lesions (hypoechoic nodes) Splenomegaly (long axis > 120 mm or subjective impression) | Lower: Blood culture positive for M tuberculosis OR medullary bone or liver biopsy with granulomatous inflammation or culture positive for M tuberculosis OR microbiological or histopathological confirmation in ≥ 2 non‐contiguous extra‐pulmonary sites | |
| Any abnormality (presence of ≥ 1: i) pleural or pericardial effusion, ii) ascites, iii) abdominal lymph nodes > 15 mm, iv) hypoechogenic lesions in the liver or spleen, v) ileum wall thickening > 4 mm or destructed ileum wall architecture) Ascites (any) Hepatomegaly (not defined) Lymphadenopathy (> 15 mm diameter) Splenomegaly (not defined) | Higher: Xpert MTB/RIF assay and/or bacteriologic culture (growth of M tuberculosis) | |
| Ascites (any) Lymphadenopathy (not defined) | Higher: Positive mycobacterial blood or bone marrow cultures OR positive mycobacterial cultures from 2 or more other sites OR post mortem evidence | |
| Any abnormality (presence of ≥ 1: i) any lymph nodes ≥ 12 mm, ii) ascites, iii) hepatomegaly, iv) splenomegaly, v) hepatic or splenic hypoechoic lesions with or without organ enlargement) | Higher: Positive culture for M tuberculosis from any site | |
| Ascites (any) Hepatomegaly (not defined) Lymphadenopathy (not defined) Splenomegaly (not defined) | Lower: Positive bacteriological culture OR granulomatous inflammation with positive Ziehl‐Neelsen (ZN) staining on microscopy OR granulomatous inflammation on microscopy OR visual inspection on laparoscopy consistent with tuberculosis (presence of tubercles, fibro‐adhesive peritonitis, or caseating lymphadenopathy) and favourable response to anti‐tuberculous treatment | |
| Any abnormality (presence of ≥ 1: i) pericardial or pleural effusion, ii) focal liver or splenic lesions, iii) abdominal lymphadenopathy) Ascites (any) Hepatomegaly (not defined) Lymphadenopathy (≥ 15 mm diameter) Splenic lesions (multiple, hypoechoic, 2 mm to 5 mm diameter) | Higher: Positive fluorescent microscopy, polymerase chain reaction, or tuberculosis culture |
Suffix (h) indicates higher quality reference standard; suffix (l) indicates lower quality reference standard
We contacted the authors of all 11 studies, of whom five responded. We received unpublished data from four studies (Weber 2018‐h; Bobbio 2019‐l; Griesel 2019‐h; Ndege 2019‐h), and one study clarified the qualification of the sonographer (O'Keefe 1998‐h).
Methodological quality of included studies
We present the results of the methodological assessment of the 11 studies in Figure 3. The results are reported below separately for studies included in the primary analyses (any abnormal finding) and those included in the secondary analyses (individual lesions). Studies that used a higher‐quality reference standard are indicated with the suffix ‘h' and studies that used a lower‐quality reference standard are indicated with the suffix ‘l'.

Risk of bias and applicability concerns summary: review authors' judgements about each domain for each included study. Suffix (h) indicates higher quality reference standard; suffix (l) indicates lower quality reference standard.
Studies of any abnormal finding included in primary analyses
Six studies with a higher‐level reference standard contributed data (Figure 3). One study was considered to be at high risk of bias in the patient selection domain since it excluded people unable to produce sputum (Griesel 2019‐h). Concerns about applicability (i.e. are there concerns that the included participants do not match the review question?) were deemed high in four studies, since they included asymptomatic people (Sculier 2010‐h; Bobbio 2019‐l) or were conducted in a referral or tertiary setting (Sculier 2010‐h; Weber 2018‐h; Bobbio 2019‐l; Ndege 2019‐h). One study was deemed of unclear concern as the setting in which the ultrasound was done was not reported (Dominguez‐Castellano 1998‐h). In the index test domain, we considered one study to be at unclear risk of bias because, although the study did specify thresholds for positivity, the test was sometimes interpreted with knowledge of the results of the reference standard (Weber 2018‐h). We considered the conduct and interpretation of the index test to be of high concern for applicability in one study where the ultrasound was performed by a trained radiologist (Sculier 2010‐h). In the reference standard domain, all studies used a higher‐quality reference standard (microbiological confirmation). We regarded two studies as being of high concern for applicability, as neither study speciated mycobacteria isolated in culture (Sculier 2010‐h; Weber 2018‐h). For the flow and timing domain, we considered one study to be at unclear risk of bias because the study did not report the interval between the index test and the reference standard, and it was unclear if all participants received the same reference standard (Dominguez‐Castellano 1998‐h).
For the main analyses (abnormal versus normal ultrasound examination), four studies with a lower‐level reference standard contributed data (Figure 3). We considered one study to be at high risk of bias in the patient selection domain because it did not enrol participants consecutively or randomly (Bobbio 2019‐l). Concerns about applicability (i.e. are there concerns that the included participants do not match the review question?) were deemed high in three studies since they included asymptomatic participants (Bobbio 2019‐l), or the study was conducted in a referral or tertiary setting (Weber 2018‐l; Bobbio 2019‐l; Ndege 2019‐l). We rated one study at unclear concern as the setting in which the ultrasound was done was not reported (Dominguez‐Castellano 1998‐l). In the index test domain, we considered one study to be at unclear risk of bias because the index test was sometimes interpreted with knowledge of the results of the reference standard (Weber 2018‐l). In the reference standard domain, we considered all studies to be at high risk of bias because the studies included a lower‐quality reference standard (clinical diagnosis) ( Dominguez‐Castellano 1998‐l; Weber 2018‐l; Bobbio 2019‐l; Ndege 2019‐l). We rated one study at unclear concern for applicability since it is unclear whether all clinically diagnosed participants improved on anti‐tuberculosis treatment (Weber 2018‐l). In terms of the flow and timing domain, we considered one study to be at unclear risk of bias because the study did not report the interval between the index test and the reference standard, and it was unclear if all participants received the same reference standard (Dominguez‐Castellano 1998‐l). We judged one study to be at high risk of bias because not all participants received a reference standard and not all participants received the same reference standard (Bobbio 2019‐l).
Studies of individual lesions included in secondary analyses
Nine studies contributed data (Figure 3). In the patient selection domain, we deemed five studies (56%) to be at high risk of bias because: i) three studies used a case‐control design (Monill‐Serra 1997‐l; Barreiros 2008‐hKaneria 2009‐l); ii) one study excluded patients with a CD4 cell count of 200 or more (O'Keefe 1998‐h); and iii) one study excluded patients unable to produce sputum (Griesel 2019‐h). For applicability, we judged four studies (44%) to be at high concern since one study included HIV‐negative participants (Barreiros 2008‐h), and the ultrasound examination was performed in a tertiary or referral centre in three studies (Sinkala 2009‐l; Weber 2018‐h; Ndege 2019‐h). We rated three studies at unclear concern as the setting in which the ultrasound was done was not reported (Monill‐Serra 1997‐l; Dominguez‐Castellano 1998‐l; Kaneria 2009‐l). In the index test domain we judged five studies (56%) to be at unclear risk of bias because four studies did not specify (or it was unclear) whether index test results were interpreted without knowledge of the results of the reference standard (Monill‐Serra 1997‐l; O'Keefe 1998‐h; Kaneria 2009‐l; Weber 2018‐h), and three studies did not report prespecified thresholds (O'Keefe 1998‐h; Kaneria 2009‐l; Sinkala 2009‐l). We considered the conduct and interpretation of the index test to be of high concern for applicability in one study where the ultrasound was performed by a trained radiologist (O'Keefe 1998‐h); we rated four studies at unclear concern since we were not able to make a decision on the qualification of the person performing the index tests (Monill‐Serra 1997‐l; Barreiros 2008‐h; Kaneria 2009‐l; Sinkala 2009‐l). Five studies (56%) used a lower‐quality reference standard and were deemed at high risk of bias in the reference standard domain (Monill‐Serra 1997‐l; Dominguez‐Castellano 1998‐l; Barreiros 2008‐h; Kaneria 2009‐l; Sinkala 2009‐l). We rated five studies at high concern for applicability for the reference standard since mycobacteria isolated in culture were not speciated (Monill‐Serra 1997‐l; Barreiros 2008‐h; Kaneria 2009‐l; Sinkala 2009‐l; Weber 2018‐h). For the flow and timing domain, we considered one study to be at high risk of bias because not all participants received a reference standard and not all participants received the same reference standard (Kaneria 2009‐l). Four studies were deemed to be at unclear risk of bias since: i) three studies did not report the interval between the index test and the reference standard, and it was unclear if all participants received the same reference standard (Monill‐Serra 1997‐l; Dominguez‐Castellano 1998‐l; Barreiros 2008‐h); and ii) one study did not report the interval between the index test and the reference standard, and not all participants received the same reference standard (O'Keefe 1998‐h).
Findings
For the diagnostic accuracy of abdominal ultrasound (main and secondary analyses), the 11 studies included 1319 participants. The median number of participants in the studies was 100 (interquartile range (IQR) 58 to 134). The proportion of tuberculosis cases in the non‐case‐control studies ranged from 17.5% (Sculier 2010‐h) to 71.0% (Sinkala 2009‐l), median 40.6% (IQR 27.5 to 53.7). Table 1 present key characteristics for each of the 11 studies. Three studies used a case‐control design (Monill‐Serra 1997‐l; Barreiros 2008‐h; Kaneria 2009‐l) and eight studies used cross‐sectional or cohort design (Dominguez‐Castellano 1998‐h; O'Keefe 1998‐h; Sinkala 2009‐l; Sculier 2010‐h; Weber 2018‐h; Bobbio 2019‐l; Griesel 2019‐h; Ndege 2019‐h). Eight studies (73%) were conducted in low‐income or middle‐income countries, while the remaining three studies were conducted in high‐income countries. Results of the primary and secondary analyses are summarized in Table 3.
| Abdominal ultrasound finding | Number of studies | Number of participants (tuberculosiscases) | Pooled sensitivity (95% CI) % | Pooled specificity (95% CI) % | Range of sensitivity % | Range of specificity % |
| Any abnormality (higher‐quality reference standard) | 5 | 879 (368) | 63 (43 to 79) | 68 (72 to 87) | 35 to 82 | 20 to 92 |
| Any abnormality (lower‐quality reference standard) | 4 | 397 (149) | 68 (45 to 85) | 73 (41 to 91) | 37 to 88 | 22 to 92 |
| Splenic lesions | 6 | 916 (477) | Not calculated | Not calculated | 13 to 62 | 86 to 100 |
| Intra‐abdominal lymph nodes | 8 | 917 (455) | Not calculated | Not calculated | 22 to 86 | 56 to 100 |
| Ascites | 8 | 891 (433) | Not calculated | Not calculated | 4 to 73 | 33 to 100 |
| Splenomegaly | 6 | 775 (397 | Not calculated | Not calculated | 5 to 62 | 45 to 89 |
| Hepatomegaly | 4 | 373 (189) | Not calculated | Not calculated | 24 to 76 | 20 to 78 |
I. Any abnormal abdominal ultrasound finding for tuberculosis detection
We included six of the 11 studies in the primary analyses (Dominguez‐Castellano 1998‐h; Dominguez‐Castellano 1998‐l; Sculier 2010‐h; Weber 2018‐h; Weber 2018‐l; Bobbio 2019‐l; Griesel 2019‐h; Ndege 2019‐h; Ndege 2019‐l); three studies provided data for each type of reference standard.
Five studies (879 participants) used a higher‐quality reference standard (Dominguez‐Castellano 1998‐h; Sculier 2010‐h; Weber 2018‐h; Griesel 2019‐h; Ndege 2019‐h). Study estimates of sensitivity and specificity ranged from 35% to 82% and from 20% to 92%. The pooled sensitivity and specificity were 63% (95% CI 43% to 79%) and 68% (95% CI 42% to 87%), respectively (Figure 4).

Forest plot of abdominal ultrasound for detecting abdominal TB or disseminated TB with abdominal involvement. TP = true positive; FP = false positive; FN = false negative; TN = true negative. Suffix (h) indicates higher quality reference standard; suffix (l) indicates lower quality reference standard.
Four studies (397 participants) used a lower‐quality reference standard (Dominguez‐Castellano 1998‐l; Weber 2018‐l; Bobbio 2019‐l; Ndege 2019‐l). Sensitivity estimates ranged from 37% to 88% and specificity estimates ranged from 22% to 92% (Figure 4). The pooled sensitivity and specificity were 68% (95% CI 45% to 85%) and 73% (95% CI 41% to 91%), respectively.
II. Splenic lesions on abdominal ultrasound for tuberculosis detection
We included six studies involving 916 participants, of whom 477 had tuberculosis (Monill‐Serra 1997‐l; Dominguez‐Castellano 1998‐l; Kaneria 2009‐l; Weber 2018‐h; Griesel 2019‐h; Ndege 2019‐h). Sensitivity estimates were very heterogeneous and ranged from 13% to 62%. Specificity estimates were less heterogeneous and ranged from 86% to 100% (Figure 5).

Forest plot of individual findings on ultrasound for detecting abdominal TB or disseminated TB with abdominal involvement. TP = true positive; FP = false positive; FN = false negative; TN = true negative. Suffix (h) indicates higher quality reference standard; suffix (l) indicates lower quality reference standard.
III. Intra‐abdominal lymph nodes on abdominal ultrasound for tuberculosis detection
Eight studies involving 917 participants (included 455 tuberculosis cases) reported on intra‐abdominal lymph nodes on abdominal ultrasound (Monill‐Serra 1997‐l; Dominguez‐Castellano 1998‐l; O'Keefe 1998‐h; Barreiros 2008‐h; Sinkala 2009‐l; Weber 2018‐h; Griesel 2019‐h; Ndege 2019‐h). The sensitivities ranged from 22% to 86% and specificities from 56% to 100% (Figure 5).
IV. Ascites on abdominal ultrasound for tuberculosis detection
We included eight studies involving 891 participants, of whom 433 had tuberculosis (Monill‐Serra 1997‐l; O'Keefe 1998‐h; Barreiros 2008‐h; Kaneria 2009‐l; Sinkala 2009‐l; Weber 2018‐h; Griesel 2019‐h; Ndege 2019‐h). Sensitivity and specificity estimates were very heterogeneous and ranged from 4% to 73% and from 33% to 100% respectively (Figure 5).
V. Splenomegaly
Six studies (775 participants, 397 tuberculosis cases) reported splenomegaly (Monill‐Serra 1997‐l; Barreiros 2008‐h; Kaneria 2009‐l; Sinkala 2009‐l; Griesel 2019‐h; Ndege 2019‐h). Estimates were very heterogeneous and ranged from 5% to 62% for sensitivity and 45% to 89% for specificity (Figure 5).
VI. Hepatomegaly
Four studies (373 participants, of whom 189 had tuberculosis) were included for hepatomegaly. The sensitivity ranged from 24% to 76% and specificity from 20% to 78% (Figure 5).
Investigations of heterogeneity
We did not investigate heterogeneity, due to limited data.
Discussion
This systematic review of the diagnostic accuracy of abdominal ultrasound for diagnosing abdominal tuberculosis or disseminated tuberculosis with abdominal involvement in HIV‐positive individuals summarizes the current literature and includes 11 studies. Six studies reported on abdominal ultrasound with any abnormal finding, and nine studies reported on individual ultrasound findings. Studies were conducted in low‐, middle‐ and high‐income countries. Five studies were performed in referral or tertiary‐level healthcare facilities, and in four studies the ultrasound examinations were performed by radiologists.
Summary of main results
We have summarized the main results in summary of findings Table. An abdominal ultrasound with any abnormal finding had a pooled sensitivity of 63% (95% CI 43% to 79%) and a pooled specificity of 68% (95% CI 42% to 87%) when bacteriological confirmation was used as the (higher‐quality) reference standard. The pooled sensitivity was 68% (95% CI 45% to 85%) and the pooled specificity was 73% (95% CI 41% to 91%) when the reference standard was clinical diagnosis without microbiological confirmation (lower‐quality reference standard).
The sensitivity of abdominal ultrasound is of concern, due to the high chance of missing tuberculosis cases (high false negative rate). This means that HIV‐positive individuals who have tuberculosis may be wrongly classified as not having tuberculosis, with a delay in initiating appropriate treatment. Ultrasound examination is operator‐dependent and subjective, with the possibility of missing subtle signs. Ultrasound also evaluates anatomical changes, and abnormalities might not occur in individuals with advanced immunosuppression.
The effect of the type of reference standard used is reflected in the improvement in both the sensitivity and specificity in the lower‐quality reference standard group. The primary concern with a lower‐quality reference standard (clinical diagnosis) is that clinicians may overdiagnose tuberculosis for fear of missing or delaying a diagnosis that could result in excess morbidity and mortality, particularly among HIV‐postive adults. This would result in an overestimation of the diagnostic accuracy of abdominal ultrasound, as fewer false positive and negative results would occur. In addition, in studies where abdominal ultrasound is part of the reference standard, incorporation bias would further result in an overestimation of diagnostic accuracy.
The estimates of sensitivity for the primary and secondary analyses were low and very heterogeneous. This means that a negative abdominal ultrasound should not be used to rule out abdominal tuberculosis or disseminated tuberculosis with abdominal involvement.
Specificity estimates were very heterogeneous, especially for hepatomegaly and splenomegaly.
Application of the main meta‐analytic findings to a hypothetical cohort
The main findings of the review were illustrated by applying the results to a hypothetical cohort of 1000 HIV‐positive individuals thought to have tuberculosis. We presented different scenarios where the tuberculosis prevalence varies from 10% to 20% to 40%. The consequences of false positive results are probably unnecessary initiation of treatment, additional testing with subsequent morbidity, patient anxiety, and possible delay in further diagnostic evaluation. The consequences of false negative results are the continued risk of community transmission of tuberculosis and an increased risk of patient morbidity and mortality.
If the pooled estimates (from using a higher‐quality reference standard) for an abdominal ultrasound with any abnormal finding are applied to a hypothetical cohort of 1000 HIV‐positive individuals where 100 (10%) of them actually have tuberculosis, abdominal ultrasound would be expected to miss 37 tuberculosis cases and falsely diagnose 288 people as tuberculosis cases (summary of findings Table). For a prevalence of 20% (200 tuberculosis cases), 74 tuberculosis cases will be missed and 256 people will be falsely diagnosed as having tuberculosis (Figure 6) while for a prevalence of 40% (400 tuberculosis cases), 148 tuberculosis cases will be missed and 192 people will be falsely diagnosed as having tuberculosis (summary of findings Table).

Flow diagram summarizing the main results in hypothetical cohort with TB prevalence 20%
Strengths and weaknesses of the review
The findings in this review are based on comprehensive literature searches, strict inclusion criteria, and standardized data extraction. The search included studies published in all languages and we corresponded with study authors to obtain additional and unpublished data. However, as diagnostic accuracy studies are poorly indexed, we acknowledge that we may have missed some studies despite the comprehensive search.
The main limitations of the review were the small number of studies and participants included in the analyses. The results were very heterogeneous with a high false negative rate, and should therefore be interpreted with caution. The high risks of bias in the patient selection domain and the reference standard domain further weaken our confidence in the results. A further limitation in the reference standard was the use of microscopic identification of acid‐fast bacilli on stained sputum smears. Although smear positivity has high specificity in high tuberculosis prevalence settings, it is not a perfect reference standard as smear will also detect non‐tuberculous mycobacteria, which are found in a higher proportion in low‐prevalence tuberculosis settings.
Applicability of findings to the review question
We had high concern about the applicability of the included studies to our review question. We foresee that in clinical practice abdominal ultrasound to diagnose abdominal tuberculosis or disseminated tuberculosis with abdominal involvement in HIV‐positive individuals would be most beneficial when performed by non‐radiologists in non‐tertiary endemic settings. Most studies were performed in tertiary settings with trained radiologists or sonographers performing the ultrasound examination, and it is possible that the accuracy of abdominal ultrasound may be lower when performed in a different setting or by less experienced users. The predictive values of any diagnostic test are influenced by disease prevalence, so the inclusion of studies performed in low tuberculosis‐burden countries would have decreased the positive predictive value of abdominal ultrasound. Two studies included HIV‐positive participants without a clinical suspicion of tuberculosis. In these studies, abdominal ultrasound has been used as a screening test and not a diagnostic test. This will further affect the diagnostic accuracy of abdominal ultrasound and increase the risk of inappropriate additional testing and initiation of anti‐tuberculous treatment. Studies were carried out under research conditions, and it is possible that the diagnostic accuracy of abdominal ultrasound might be lower in routine practice.

Diagnostic workup of HIV‐positive individuals with suspected abdominal tuberculosis or disseminated tuberculosis with abdominal involvement

Study flow diagram.

Risk of bias and applicability concerns summary: review authors' judgements about each domain for each included study. Suffix (h) indicates higher quality reference standard; suffix (l) indicates lower quality reference standard.

Forest plot of abdominal ultrasound for detecting abdominal TB or disseminated TB with abdominal involvement. TP = true positive; FP = false positive; FN = false negative; TN = true negative. Suffix (h) indicates higher quality reference standard; suffix (l) indicates lower quality reference standard.
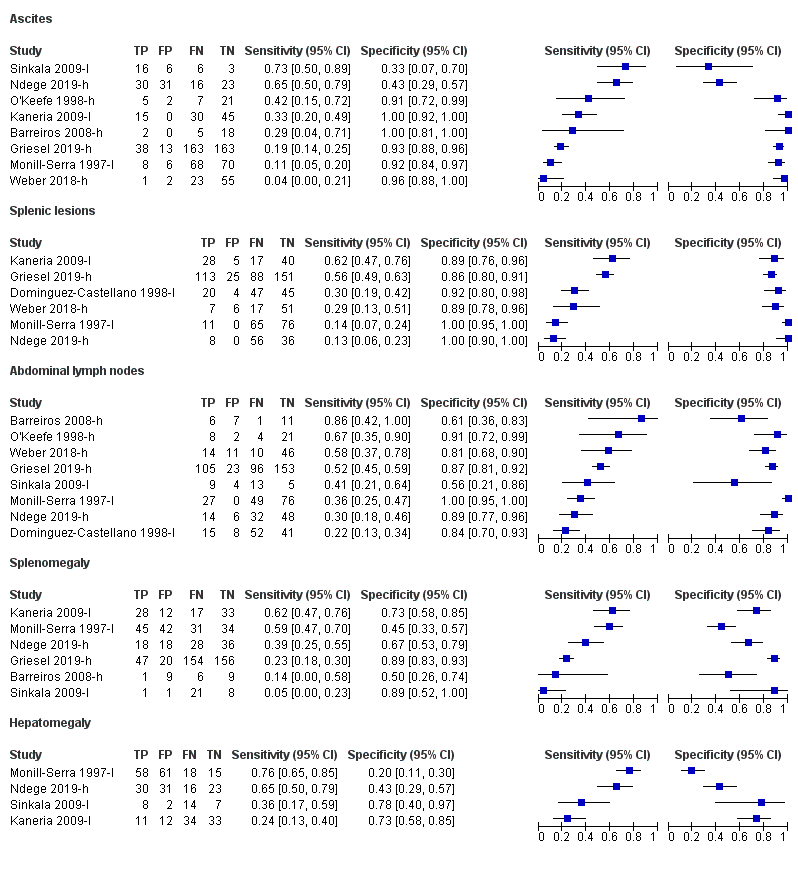
Forest plot of individual findings on ultrasound for detecting abdominal TB or disseminated TB with abdominal involvement. TP = true positive; FP = false positive; FN = false negative; TN = true negative. Suffix (h) indicates higher quality reference standard; suffix (l) indicates lower quality reference standard.

Flow diagram summarizing the main results in hypothetical cohort with TB prevalence 20%

Abnormal abdominal ultrasound (higher quality).

Abnormal abdominal ultrasound (lower quality).

Abdominal lymph nodes.
| Review question: Should abdominal ultrasound be used to diagnose abdominal tuberculosis or disseminated tuberculosis with abdominal involvement in HIV‐positive individuals? Patient or population: HIV‐positive individuals Setting: Healthcare facility Index test: Abdominal ultrasound Reference standard: We considered two reference standards. The higher‐quality reference standard was bacteriological confirmation of M tuberculosis (any clinical specimen including (i) at least one specimen culture positive for M tuberculosis, (ii) microscopic identification of acid‐fast bacilli on stained sputum smears, lymph node aspirate, or any other specimen; or iii) Xpert MTB/RIF positive). The lower‐quality reference standard was clinical diagnosis of TB without microbiological confirmation (including cases diagnosed on the basis of: i) suggestive histology (necrotizing granulomatous inflammation), ii) x‐ray abnormalities, iii) extrapulmonary cases without laboratory confirmation, and iv) anti‐tuberculosis therapy initiated by a healthcare practitioner for cases with a high suspicion of tuberculosis). Threshold: Any abnormality found on abdominal ultrasound Study design: Cross‐sectional and cohort Limitations: A small number of studies and participants were included in the analyses. Risks of bias were generally high in the patient selection domain | ||||||
| Test result | Number of results per 1000 HIV‐positive individuals tested (95% CI) | Number of studies | Number of participants | Certainty of the evidence (GRADE) | ||
| Prevalence 10% | Prevalence 20% | Prevalence 40% | ||||
| Bacteriological confirmation as reference standard: pooled sensitivity = 63% (95% CI 43% to 79%) and pooled specificity = 68% (95% CI 42% to 87%) | ||||||
| True positives (participants correctly classified as having tuberculosis) | 63 (43 to 79) | 126 (86 to 158) | 252 (172 to 316) | 5 | 368 | ⊕⊝⊝⊝ |
| False negatives (participants incorrectly classified as not having tuberculosis) | 37 (21 to 57) | 74 (42 to 114) | 148 (84 to 228) | |||
| True negatives (participants correctly classified as not having tuberculosis) | 612 (378 to 783) | 544 (336 to 696) | 408 (252 to 522) | 5 | 511 | ⊕⊝⊝⊝ |
| False positives (participants incorrectly classified as having tuberculosis) | 288 (117 to 522) | 256 (104 to 464) | 192 (78 to 348) | |||
| Abbreviations: CI: confidence interval GRADE certainty of evidence (GRADEpro GDT 2015; Schünemann 2016) The table displays normalized frequencies within a hypothetical cohort of 1000 people at three different tuberculosis prevalences (pre‐test probabilities): 10%, 20% and 40%. We selected prevalence values based on the range of prevalence observed across the included studies. We estimated confidence intervals based on those around the point estimates for pooled sensitivity and specificity. Explanations aRisk of bias: We rated one study at high risk for participant selection since it excluded people unable to produce sputum (Griesel 2019‐h). We downgraded the certainty of the evidence by one level. | ||||||
| Author (publication year) | Study design | Country | Clinical setting | Target condition definition | Qualification of person performing index test | Sample size | Tuberculosisproportion in study |
| Case‐control | Germany | Not reported | Gastro‐intestinal tuberculosis | Not reported | 25a (7 cases, 18 pulmonary tuberculosis controls) | ‐ | |
| Cross‐sectional | South Sudan | Referral hospital | Extra‐pulmonary tuberculosis | Trained non‐radiologist | 100 | 24% | |
| Cross‐sectional | Spain | Not reported | Extra‐pulmonary tuberculosis | Sonographer | 116 | 55% (higher) 58% (lower) | |
| Cross‐sectional | South Africa | Non‐tertiary hospital | Culture‐positive tuberculosis | Sonographer | 377 | 53% | |
| Case‐control | India | Not reported | Pulmonary tuberculosis, extra‐pulmonary tuberculosis, disseminated tuberculosis | Not reported | 90 (45 cases, 45 HIV‐positive controls without any pathology) | ‐ | |
| Case‐control | Spain | Not reported | Disseminated tuberculosis | Not reported | 152 (76 cases, 76 HIV‐positive controls without any pathology) | ‐ | |
| Cohort | Tanzania | Referral hospital | Pulmonary tuberculosis, extra‐pulmonary tuberculosis, disseminated tuberculosis | Board‐certified sonographers | 100 (191 original study sample) | 46% (higher) 64% (lower) | |
| Cross‐sectional | South Africa | Non‐tertiary hospital | Disseminated tuberculosis | Radiologist | 35 (44 original study sample) | 34% | |
| Cross‐sectional | Cambodia | Referral hospital | Disseminated tuberculosis | Radiologist | 212 | 18% | |
| Cross‐sectional | Zambia | Tertiary hospital | Abdominal tuberculosis | Not reported | 31 | 71% | |
| Cohort | India | Tertiary hospital | Disseminated tuberculosis | Trained non‐radiologist | 81 (425 original study sample) | 30% (higher) 49% (lower) | |
| aIncludes five HIV‐negative participants. Suffix (h) indicates higher‐quality reference standard; suffix (l) indicates lower‐quality reference standard. | |||||||
| Author (publication year) | Index test variable included (threshold) | Reference standard quality and definition |
| Ascites (any) Lymphadenopathy (abdominal and perihepatic nodes with longitudinal diameter > 20 mm) Splenomegaly (> 135 mm) | Lower: Clinical, endoscopic, histologic, radiologic and operative findings including microbiology and polymerase chain reaction of biopsies taken during endoscopy | |
| Any abnormality (Presence of ≥ 1: i) pericardial effusion, ii) periportal/para‐aortic lymph nodes (> 15 mm diameter), iii) focal splenic lesions, iv) pleural effusion or consolidation of the lung, v) ascites without alternative explanation) | Lower: Sputum microscopy OR clinical reasons OR Focused Assessment with Sonography in HIV‐associated tuberculosis (FASH) | |
| Any abnormality (presence of ≥ 1: i) multiple hypoechoic splenic lesions (< 10 mm), ii) any abdominal adenopathy, iii) hypo‐ or hyperechoic liver lesions) | Higher: Microscopy OR culture | |
| Any abnormality (presence of ≥ 1: i) abdominal lymph nodes (any size), ii) splenic hypoechoic lesions, iii) splenomegaly (≥ 110 mm), iv) any one of abdominal, pleural, or pericardial effusions) Ascites (any) Lymphadenopathy (any size) Splenic lesions (hypoechoic) Splenomegaly (≥ 110 mm) | Higher: Positive culture for M tuberculosis from any site | |
| Ascites (any) Hepatomegaly (not defined) Lymphadenopathy (diameter > 15 mm) Splenic lesions (multiple, hypoechoic, 5 mm to 10 mm diameter) Splenomegaly (not defined) | Lower: Lymphocytic predominance and elevated adenosine deaminase (ADA) levels in pleural or ascitic fluid OR granulomatous lymphadenitis and acid‐fast bacilli in lymph node OR sputum microscopy | |
| Ascites (any) Hepatomegaly (not defined) Lymphadenopathy (> 15 mm diameter) Splenic lesions (hypoechoic nodes) Splenomegaly (long axis > 120 mm or subjective impression) | Lower: Blood culture positive for M tuberculosis OR medullary bone or liver biopsy with granulomatous inflammation or culture positive for M tuberculosis OR microbiological or histopathological confirmation in ≥ 2 non‐contiguous extra‐pulmonary sites | |
| Any abnormality (presence of ≥ 1: i) pleural or pericardial effusion, ii) ascites, iii) abdominal lymph nodes > 15 mm, iv) hypoechogenic lesions in the liver or spleen, v) ileum wall thickening > 4 mm or destructed ileum wall architecture) Ascites (any) Hepatomegaly (not defined) Lymphadenopathy (> 15 mm diameter) Splenomegaly (not defined) | Higher: Xpert MTB/RIF assay and/or bacteriologic culture (growth of M tuberculosis) | |
| Ascites (any) Lymphadenopathy (not defined) | Higher: Positive mycobacterial blood or bone marrow cultures OR positive mycobacterial cultures from 2 or more other sites OR post mortem evidence | |
| Any abnormality (presence of ≥ 1: i) any lymph nodes ≥ 12 mm, ii) ascites, iii) hepatomegaly, iv) splenomegaly, v) hepatic or splenic hypoechoic lesions with or without organ enlargement) | Higher: Positive culture for M tuberculosis from any site | |
| Ascites (any) Hepatomegaly (not defined) Lymphadenopathy (not defined) Splenomegaly (not defined) | Lower: Positive bacteriological culture OR granulomatous inflammation with positive Ziehl‐Neelsen (ZN) staining on microscopy OR granulomatous inflammation on microscopy OR visual inspection on laparoscopy consistent with tuberculosis (presence of tubercles, fibro‐adhesive peritonitis, or caseating lymphadenopathy) and favourable response to anti‐tuberculous treatment | |
| Any abnormality (presence of ≥ 1: i) pericardial or pleural effusion, ii) focal liver or splenic lesions, iii) abdominal lymphadenopathy) Ascites (any) Hepatomegaly (not defined) Lymphadenopathy (≥ 15 mm diameter) Splenic lesions (multiple, hypoechoic, 2 mm to 5 mm diameter) | Higher: Positive fluorescent microscopy, polymerase chain reaction, or tuberculosis culture | |
| Suffix (h) indicates higher quality reference standard; suffix (l) indicates lower quality reference standard | ||
| Abdominal ultrasound finding | Number of studies | Number of participants (tuberculosiscases) | Pooled sensitivity (95% CI) % | Pooled specificity (95% CI) % | Range of sensitivity % | Range of specificity % |
| Any abnormality (higher‐quality reference standard) | 5 | 879 (368) | 63 (43 to 79) | 68 (72 to 87) | 35 to 82 | 20 to 92 |
| Any abnormality (lower‐quality reference standard) | 4 | 397 (149) | 68 (45 to 85) | 73 (41 to 91) | 37 to 88 | 22 to 92 |
| Splenic lesions | 6 | 916 (477) | Not calculated | Not calculated | 13 to 62 | 86 to 100 |
| Intra‐abdominal lymph nodes | 8 | 917 (455) | Not calculated | Not calculated | 22 to 86 | 56 to 100 |
| Ascites | 8 | 891 (433) | Not calculated | Not calculated | 4 to 73 | 33 to 100 |
| Splenomegaly | 6 | 775 (397 | Not calculated | Not calculated | 5 to 62 | 45 to 89 |
| Hepatomegaly | 4 | 373 (189) | Not calculated | Not calculated | 24 to 76 | 20 to 78 |
| Test | No. of studies | No. of participants |
| 1 Abnormal abdominal ultrasound (higher quality) Show forest plot | 5 | 879 |
| 2 Abnormal abdominal ultrasound (lower quality) Show forest plot | 4 | 397 |
| 3 Ascites Show forest plot | 8 | 891 |
| 4 Splenic lesions Show forest plot | 6 | 916 |
| 5 Abdominal lymph nodes Show forest plot | 8 | 917 |
| 6 Splenomegaly Show forest plot | 6 | 775 |
| 7 Hepatomegaly Show forest plot | 4 | 373 |