Ecografía abdominal para el diagnóstico de la tuberculosis abdominal o la tuberculosis diseminada con compromiso abdominal en pacientes con pruebas positivas para VIH
Referencias
References to studies included in this review
References to studies excluded from this review
References to ongoing studies
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Study characteristics | |||
| Patient sampling | Case‐control design | ||
| Patient characteristics and setting | Country: Germany Setting: Not reported High tuberculosis burden country: No High HIV‐associated tuberculosis burden country: No Sample size: 7 cases (of these 3 HIV‐negative); 18 controls (of these 9 HIV‐negative) Median age (range): Cases 41 (27 ‐ 66); Controls 36 (21 ‐ 69) Gender proportion (M:F): Cases 3:4; Controls 11:7 Proportion on antiretroviral therapy (ART): Not reported | ||
| Index tests | Sonographer qualification: Not reported Threshold(s):
| ||
| Target condition and reference standard(s) | Target condition: Intestinal tuberculosis Confirmation of active tuberculosis: “…based on clinical, endoscopic, histologic, radiologic and operative findings including microbiology (in all) and polymerase chain reaction (PCR) (in 5 patients) of biopsies taken during endoscopy.” | ||
| Flow and timing | |||
| Comparative | |||
| Notes | Second control group of healthy persons not included 4 cases and 9 controls were HIV‐positive Cases had pulmonary tuberculosis only (randomly selected) Reference standard results not delineated | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| High | High | ||
| DOMAIN 2: Index Test Ascites | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Unclear | ||
| DOMAIN 2: Index Test Abdominal lymph nodes | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Unclear | ||
| DOMAIN 2: Index Test Splenomegaly | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Unclear | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Was incorporation bias avoided? | Yes | ||
| High | High | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Unclear | ||
| Were all patients included in the analysis? | Yes | ||
| Did all patients received a reference standard? | Yes | ||
| Unclear | |||
| Study characteristics | |||
| Patient sampling | Cross‐sectional design | ||
| Patient characteristics and setting | Country: South Sudan Setting: Referral hospital High tuberculosis burden country: No High HIV‐associated tuberculosis burden country: No Sample size: 100 Median age (range): Not available (only categories available) Gender proportion (M:F): 48:52 Proportion on antiretroviral therapy (ART): 3% | ||
| Index tests | Sonographer qualification: Clinician trained in ultrasound Threshold(s): At least one of
| ||
| Target condition and reference standard(s) | Target condition: Disseminated tuberculosis Confirmation of active tuberculosis: Acid‐fast bacilli sputum smears, ultrasound, clinical diagnosis | ||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| High | High | ||
| DOMAIN 2: Index Test Abnormal abdominal ultrasound (lower quality) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | No | ||
| Was incorporation bias avoided? | No | ||
| High | High | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | Yes | ||
| Did all patients received a reference standard? | No | ||
| High | |||
| Study characteristics | |||
| Patient sampling | Prospective cross‐sectional | ||
| Patient characteristics and setting | Country: Spain Setting: Not reported High tuberculosis burden country: No High HIV‐associated tuberculosis burden country: No Sample size:116 Age: 31.56 ± 4.68 years (mean ± SD) Gender proportion: Not reported Proportion on antiretroviral therapy (ART): Not reported | ||
| Index tests | Sonographer qualification: “Medical sonographer” Threshold(s):
| ||
| Target condition and reference standard(s) | Target condition: Pulmonary tuberculosis, Extra‐pulmonary tuberculosis and disseminated tuberculosis (with or without abdominal involvement) Confirmation of active tuberculosis: Smear microscopy, Lowenstein culture | ||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Unclear | ||
| DOMAIN 2: Index Test Abnormal abdominal ultrasound (higher quality) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Was incorporation bias avoided? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Unclear | ||
| Were all patients included in the analysis? | Yes | ||
| Did all patients received a reference standard? | Yes | ||
| Unclear | |||
| Study characteristics | |||
| Patient sampling | Prospective cross‐sectional | ||
| Patient characteristics and setting | Country: Spain Setting: Not reported High tuberculosis burden country: No High HIV‐associated tuberculosis burden country: No Sample size:116 Age: 31.56 ± 4.68 years (mean ± SD) Gender proportion: Not reported Proportion on antiretroviral therapy (ART): Not reported | ||
| Index tests | Sonographer qualification: “Medical sonographer” Threshold(s):
| ||
| Target condition and reference standard(s) | Target condition: Pulmonary tuberculosis, extra‐pulmonary tuberculosis and disseminated tuberculosis (with or without abdominal involvement) Confirmation of active tuberculosis: Compatible with clinical and radiography findings with improvement to anti‐tuberculosis treatment | ||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Unclear | ||
| DOMAIN 2: Index Test Abnormal abdominal ultrasound (lower quality) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test Splenic lesions | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test Abdominal lymph nodes | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | No | ||
| Was incorporation bias avoided? | Unclear | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Unclear | ||
| Were all patients included in the analysis? | Yes | ||
| Did all patients received a reference standard? | Yes | ||
| Unclear | |||
| Study characteristics | |||
| Patient sampling | Prospective cross‐sectional | ||
| Patient characteristics and setting | Country: South Africa Setting: Secondary‐level hospitals High tuberculosis burden country: Yes High HIV‐associated tuberculosis burden country: Yes Sample size: 377 Age: Median (IQR) tuberculosis cases: 35 (30 ‐ 41); Non‐tuberculosis controls: 36 (30 ‐ 42) Gender proportion (M:F) tuberculosis cases: 64:137; Non‐tuberculosis controls: 64:112 Proportion on antiretroviral therapy (ART): tuberculosis cases: 59/201 (29%); Non‐tuberculosis controls: 61/176 (35%) | ||
| Index tests | Sonographer qualification: Trained sonographers Threshold(s):
| ||
| Target condition and reference standard(s) | Target condition: Tuberculosis Confirmation of active tuberculosis: Positive culture for M tuberculosis | ||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | No | ||
| High | Low | ||
| DOMAIN 2: Index Test Abnormal abdominal ultrasound (higher quality) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test Ascites | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test Splenic lesions | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test Abdominal lymph nodes | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test Splenomegaly | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Was incorporation bias avoided? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Did all patients received a reference standard? | Yes | ||
| Low | |||
| Study characteristics | |||
| Patient sampling | Case‐control | ||
| Patient characteristics and setting | Country: India Setting: Not reported High tuberculosis burden country: Yes High HIV‐associated tuberculosis burden country: Yes Sample size: 90 Age: Mean (range) Cases: Male 36.4 (24 ‐ 60), Female 33.41 (25 ‐ 60); Controls: Male 39.46 (24 ‐ 60), Female 38.71 (25 ‐ 61) Gender proportion: M:F Cases: 31:14; Controls: 30:15 Proportion on antiretroviral therapy (ART): Cases: 7/45 (15.6%); Controls: 15/30 (50%) | ||
| Index tests | Sonographer qualification: Not reported Threshold(s): Not reported | ||
| Target condition and reference standard(s) | Target condition: Pulmonary tuberculosis, extra‐pulmonary tuberculosis and disseminated tuberculosis (with or without abdominal involvement) Confirmation of active tuberculosis: Microscopic identification of AFB and compatible clinical findings | ||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| High | Unclear | ||
| DOMAIN 2: Index Test Ascites | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Unclear | Unclear | ||
| DOMAIN 2: Index Test Splenic lesions | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Unclear | Unclear | ||
| DOMAIN 2: Index Test Splenomegaly | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Unclear | Unclear | ||
| DOMAIN 2: Index Test Hepatomegaly | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Unclear | Unclear | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Was incorporation bias avoided? | Unclear | ||
| High | High | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | Yes | ||
| Did all patients received a reference standard? | No | ||
| High | |||
| Study characteristics | |||
| Patient sampling | Case‐control | ||
| Patient characteristics and setting | Country: Spain Setting: Not reported High tuberculosis burden country: No High HIV‐associated tuberculosis burden country: No Sample size: 152 Age: Cases: Mean 30; Range 20 ‐ 49; Controls: Not reported Gender proportion: M:F Cases: 56:20; Controls: Not reported Proportion on antiretroviral therapy (ART): Not reported | ||
| Index tests | Sonographer qualification: Not reported Threshold(s):
| ||
| Target condition and reference standard(s) | Target condition: Disseminated tuberculosis (with or without abdominal involvement) Confirmation of active tuberculosis: Microbiological (culture) or histopathological examination | ||
| Flow and timing | |||
| Comparative | |||
| Notes | Controls were HIV‐positive with no associated neoplastic illness or opportunistic infection | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | No | ||
| High | Unclear | ||
| DOMAIN 2: Index Test Ascites | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Unclear | Unclear | ||
| DOMAIN 2: Index Test Splenic lesions | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Unclear | Unclear | ||
| DOMAIN 2: Index Test Abdominal lymph nodes | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Unclear | Unclear | ||
| DOMAIN 2: Index Test Splenomegaly | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Unclear | Unclear | ||
| DOMAIN 2: Index Test Hepatomegaly | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Unclear | Unclear | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Was incorporation bias avoided? | Yes | ||
| High | High | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Unclear | ||
| Were all patients included in the analysis? | Yes | ||
| Did all patients received a reference standard? | Yes | ||
| Unclear | |||
| Study characteristics | |||
| Patient sampling | Prospective cohort | ||
| Patient characteristics and setting | Country: Tanzania Setting: Referral hospital High tuberculosis burden country: Yes High HIV‐associated tuberculosis burden country: Yes Sample size: 100 (original study size including HIV‐negative n = 191) Age: Median 38 years; IQR 32 ‐ 44 years Gender proportion: M:F 47:53 Proportion on antiretroviral therapy (ART): 56% | ||
| Index tests | Sonographer qualification: Board‐certified sonographers Threshold(s):
| ||
| Target condition and reference standard(s) | Confirmed tuberculosis was defined as ≥ 1 positive microbiological result from any site confirmed by Xpert MTB/RIF assay and/or bacteriologic culture (growth of M tuberculosis) in sputum, pleural fluid, ascites, cerebrospinal fluid, urine or lymph node aspirate | ||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | High | ||
| DOMAIN 2: Index Test Abnormal abdominal ultrasound (higher quality) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test Ascites | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test Splenic lesions | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test Abdominal lymph nodes | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test Splenomegaly | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test Hepatomegaly | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Was incorporation bias avoided? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Did all patients received a reference standard? | Yes | ||
| Low | |||
| Study characteristics | |||
| Patient sampling | Prospective cohort | ||
| Patient characteristics and setting | Country: Tanzania Setting: Referral hospital High tuberculosis burden country: Yes High HIV‐associated tuberculosis burden country: Yes Sample size: 100 (original study size including HIV‐negative n = 191) Age: Median 38 years; IQR 32 ‐ 44 years Gender proportion: M:F 47:53 Proportion on antiretroviral therapy (ART): 56% | ||
| Index tests | Sonographer qualification: Board‐certified sonographers Threshold(s):
| ||
| Target condition and reference standard(s) | Confirmed tuberculosis was defined as ≥ 1 positive microbiological result from any site confirmed by Xpert MTB/RIF assay and/or bacteriologic culture (growth of M tuberculosis) in sputum, pleural fluid, ascites, cerebrospinal fluid, urine or lymph node aspirate. In addition, the identification of acid‐fast bacilli in sputum by another health centre, or adenosine deaminase (ADA) ≥ 40 U/ml in pleural fluid, ≥ 35 U/ml in pericardial fluid and ≥ 30 U/ml in ascitic fluid were accepted as microbiological confirmation. Probable tuberculosis was defined as negative microbiological tests in a participant in whom anti‐tuberculosis therapy (prescribed based on clinical suspicion or on chest x‐ray) in the absence of an alternative diagnosis led to a resolution of clinical signs and symptoms, radiographic and sonographic signs, and to an increase in body weight documented 2 months after start of anti‐tuberculosis treatment | ||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | High | ||
| DOMAIN 2: Index Test Abnormal abdominal ultrasound (lower quality) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Was incorporation bias avoided? | Unclear | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | Yes | ||
| Did all patients received a reference standard? | Yes | ||
| Unclear | |||
| Study characteristics | |||
| Patient sampling | Prospective cross‐sectional | ||
| Patient characteristics and setting | Country: South Africa Setting: Non‐tertiary setting High tuberculosis burden country: Yes High HIV‐associated tuberculosis burden country: Yes Sample size: 35 (original study size n = 44) Age: Mean 32.9; Range 18.4 ‐ 53.3 Gender proportion: M:F 26:18 Proportion on antiretroviral therapy (ART): 0/44 (0%) | ||
| Index tests | Sonographer qualification: Radiologist Threshold(s): Not reported | ||
| Target condition and reference standard(s) | Target condition: Disseminated tuberculosis with abdominal involvement) Confirmation of active tuberculosis: Microbiological (culture) or postmortem evidence | ||
| Flow and timing | |||
| Comparative | |||
| Notes | Only 35/44 had ultrasound examination | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | No | ||
| High | Low | ||
| DOMAIN 2: Index Test Ascites | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Unclear | High | ||
| DOMAIN 2: Index Test Abdominal lymph nodes | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Unclear | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Was incorporation bias avoided? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | Yes | ||
| Did all patients received a reference standard? | Yes | ||
| Unclear | |||
| Study characteristics | |||
| Patient sampling | Prospective cross‐sectional | ||
| Patient characteristics and setting | Country: Cambodia Setting: “not‐for‐profit referral hospital” High tuberculosis burden country: Yes High HIV‐associated tuberculosis burden country: No Sample size: 212 Age: Median (IQR) 34 (29 ‐ 41.5) years (included participants < 18 years) Gender proportion: M 40%, F 60% Proportion on antiretroviral therapy (ART): Not reported | ||
| Index tests | Sonographer qualification: “Trained radiologist” Threshold(s):
| ||
| Target condition and reference standard(s) | Target condition: Disseminated tuberculosis (with or without abdominal involvement) Confirmation of active tuberculosis: Culture | ||
| Flow and timing | |||
| Comparative | |||
| Notes | Substudy | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | High | ||
| DOMAIN 2: Index Test Abnormal abdominal ultrasound (higher quality) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Was incorporation bias avoided? | Yes | ||
| Low | High | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Did all patients received a reference standard? | Yes | ||
| Low | |||
| Study characteristics | |||
| Patient sampling | Prospective cross‐sectional | ||
| Patient characteristics and setting | Country: Zambia Setting: “secondary and tertiary care hospital” High tuberculosis burden country: Yes High HIV‐associated tuberculosis burden country: Yes Sample size: 31 Age: Mean (SD) All: 33.4 (8.3) years (in text: mean 33.1 range 18 ‐ 54); tuberculosis: 30.7 (6.9); No tuberculosis: 39.8 (8) Gender proportion: M:F All: 8:23; tuberculosis: 7:15; No tuberculosis: 1:8 Proportion on antiretroviral therapy (ART): Not reported | ||
| Index tests | Sonographer qualification: Not reported Threshold(s): Not reported | ||
| Target condition and reference standard(s) | Target condition: Abdominal tuberculosis Confirmation of active tuberculosis: “…definitive diagnosis of tuberculosis was made by demonstration of M tuberculosis infection via positive bacteriological culture and/or granulomatous inflammation on histopathological examination with positive Ziehl‐Neelsen (ZN) staining on microscopy. A presumptive diagnosis of tuberculosis was made when granulomatous inflammation was seen on microscopy, or when visual inspection on laparoscopy was consistent with tuberculosis and the patient's clinical response to anti‐tuberculous treatment was good. Laparoscopic features felt to be consistent with tuberculosis for the purpose of making a presumptive diagnosis were the presence of tubercles, fibro adhesive peritonitis, or caseating lymphadenopathy.” | ||
| Flow and timing | |||
| Comparative | |||
| Notes | Ultrasound used as part of inclusion and exclusion criteria (selection bias) | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | High | ||
| DOMAIN 2: Index Test Ascites | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Low | Unclear | ||
| DOMAIN 2: Index Test Abdominal lymph nodes | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Unclear | Unclear | ||
| DOMAIN 2: Index Test Splenomegaly | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Unclear | Unclear | ||
| DOMAIN 2: Index Test Hepatomegaly | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Unclear | Unclear | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | No | ||
| Was incorporation bias avoided? | Yes | ||
| High | High | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Did all patients received a reference standard? | Yes | ||
| Low | |||
| Study characteristics | |||
| Patient sampling | Prospective controlled cohort | ||
| Patient characteristics and setting | Country: India Setting: Tertiary setting High tuberculosis burden country: Yes High HIV‐associated tuberculosis burden country: Yes Sample size: 81 (original study size including HIV‐negative n = 425) Age: Overall median (IQR) 43 (31.5 ‐ 55); HIV only 43 (38 ‐ 48) (included participants < 18 years) Gender proportion: Overall: M 328/425 (77%); HIV‐positive M 56/81 (69%) Proportion on antiretroviral therapy (ART): 29/81 (35.8%) | ||
| Index tests | Sonographer qualification: Clinician trained in the study’s ultrasound protocol but without formal ultrasound training Threshold(s):
| ||
| Target condition and reference standard(s) | Target condition: Pulmonary tuberculosis and extra‐pulmonary tuberculosis Confirmation of active tuberculosis: “…’confirmed tuberculosis' (i.e., positive fluorescent microscopy, polymerase chain reaction, or tuberculosis culture)…” | ||
| Flow and timing | |||
| Comparative | |||
| Notes | Includes patients ≥ 16 years “…therapeutic and diagnostic management was fully the responsibility of the attending hospital doctor.” Additional info received from authors | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | High | ||
| DOMAIN 2: Index Test Abnormal abdominal ultrasound (higher quality) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | No | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test Ascites | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | No | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test Splenic lesions | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | No | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test Abdominal lymph nodes | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | No | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Was incorporation bias avoided? | Yes | ||
| Low | High | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | Yes | ||
| Did all patients received a reference standard? | Yes | ||
| Low | |||
| Study characteristics | |||
| Patient sampling | Prospective controlled cohort | ||
| Patient characteristics and setting | Country: India Setting: Tertiary setting High tuberculosis burden country: Yes High HIV‐associated tuberculosis burden country: Yes Sample size: 81 (original study size including HIV‐negative n = 425) Age: Overall median (IQR) 43 (31.5 ‐ 55); HIV only 43 (38 ‐ 48) (included participants < 18 years) Gender proportion: Overall: M 328/425 (77%); HIV‐positive M 56/81 (69%) Proportion on antiretroviral therapy (ART): 29/81 (35.8%) | ||
| Index tests | Sonographer qualification: Clinician trained in the study’s ultrasound protocol but without formal ultrasound training Threshold(s):
| ||
| Target condition and reference standard(s) | Target condition: Pulmonary tuberculosis and extra‐pulmonary tuberculosis Confirmation of active tuberculosis: “…’clinical tuberculosis’ (no microbiological confirmation, but clinical tuberculosis diagnosis and tuberculosis treatment initiated)…” | ||
| Flow and timing | |||
| Comparative | |||
| Notes | Includes patients ≥ 16 years “…therapeutic and diagnostic management was fully the responsibility of the attending hospital doctor.” Additional info received from authors | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | High | ||
| DOMAIN 2: Index Test Abnormal abdominal ultrasound (lower quality) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | No | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Unclear | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | No | ||
| Was incorporation bias avoided? | No | ||
| High | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | Yes | ||
| Did all patients received a reference standard? | Yes | ||
| Low | |||
Suffix (h) indicates higher‐quality reference standard; suffix (l) indicates lower‐quality reference standard
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Descriptive study | |
| No reference standard | |
| Descriptive study | |
| Descriptive study | |
| Descriptive study | |
| Descriptive study | |
| Descriptive study | |
| Descriptive study | |
| Descriptive study | |
| Ineligible index test | |
| Descriptive study | |
| Descriptive study | |
| Descriptive study | |
| Descriptive study | |
| Descriptive study | |
| Ineligible patient population | |
| Descriptive study | |
| Descriptive study | |
| Only abnormal index test reported | |
| Only abnormal index test reported | |
| Descriptive study | |
| Descriptive study | |
| Not a diagnostic accuracy study | |
| Descriptive study | |
| Descriptive study | |
| No reference standard | |
| Descriptive study | |
| Descriptive study | |
| Ineligible patient population | |
| Descriptive study |
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Ultrasound in managing tuberculosis: A randomized controlled two‐center study |
| Target condition and reference standard(s) | Target condition: Extrapulmonary tuberculosis Reference standard: Not stipulated |
| Index and comparator tests | Index test: eFASH (extended focused assessment with sonography for HIV and tuberculosis) and a management algorithm Comparator group: Standard of care (Management according to the decision of the treating physician) |
| Starting date | September 2018 |
| Contact information | |
| Notes |
Data
Presented below are all the data for all of the tests entered into the review.
| Test | No. of studies | No. of participants |
| 1 Abnormal abdominal ultrasound (higher quality) Show forest plot | 5 | 879 |
| Test 1  Abnormal abdominal ultrasound (higher quality). | ||
| 2 Abnormal abdominal ultrasound (lower quality) Show forest plot | 4 | 397 |
| Test 2  Abnormal abdominal ultrasound (lower quality). | ||
| 3 Ascites Show forest plot | 8 | 891 |
| Test 3  Ascites. | ||
| 4 Splenic lesions Show forest plot | 6 | 916 |
| Test 4  Splenic lesions. | ||
| 5 Abdominal lymph nodes Show forest plot | 8 | 917 |
| Test 5  Abdominal lymph nodes. | ||
| 6 Splenomegaly Show forest plot | 6 | 775 |
| Test 6  Splenomegaly. | ||
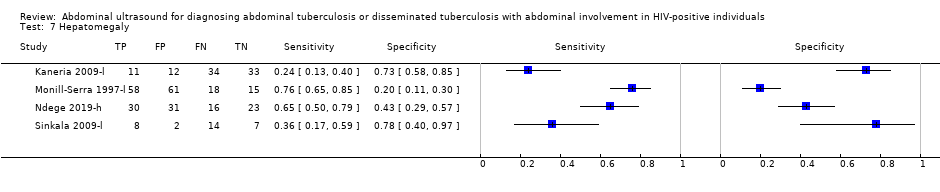
| 7 Hepatomegaly Show forest plot | 4 | 373 |
| Test 7  Hepatomegaly. | ||

Diagnostic workup of HIV‐positive individuals with suspected abdominal tuberculosis or disseminated tuberculosis with abdominal involvement

Study flow diagram.

Risk of bias and applicability concerns summary: review authors' judgements about each domain for each included study. Suffix (h) indicates higher quality reference standard; suffix (l) indicates lower quality reference standard.

Forest plot of abdominal ultrasound for detecting abdominal TB or disseminated TB with abdominal involvement. TP = true positive; FP = false positive; FN = false negative; TN = true negative. Suffix (h) indicates higher quality reference standard; suffix (l) indicates lower quality reference standard.
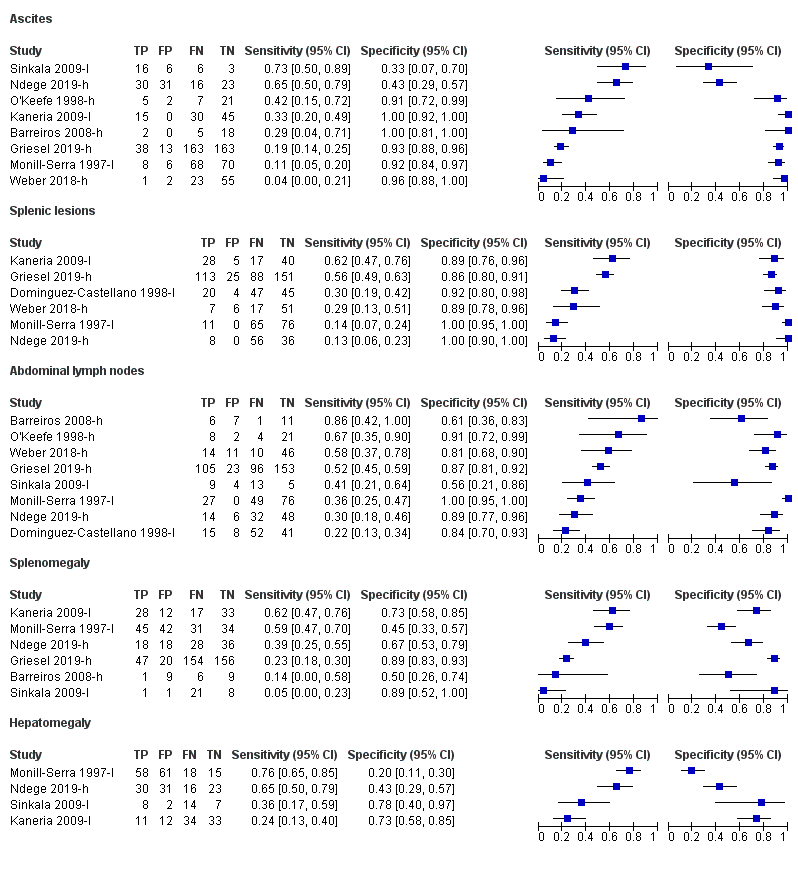
Forest plot of individual findings on ultrasound for detecting abdominal TB or disseminated TB with abdominal involvement. TP = true positive; FP = false positive; FN = false negative; TN = true negative. Suffix (h) indicates higher quality reference standard; suffix (l) indicates lower quality reference standard.

Flow diagram summarizing the main results in hypothetical cohort with TB prevalence 20%

Abnormal abdominal ultrasound (higher quality).

Abnormal abdominal ultrasound (lower quality).

Abdominal lymph nodes.
| Review question: Should abdominal ultrasound be used to diagnose abdominal tuberculosis or disseminated tuberculosis with abdominal involvement in HIV‐positive individuals? Patient or population: HIV‐positive individuals Setting: Healthcare facility Index test: Abdominal ultrasound Reference standard: We considered two reference standards. The higher‐quality reference standard was bacteriological confirmation of M tuberculosis (any clinical specimen including (i) at least one specimen culture positive for M tuberculosis, (ii) microscopic identification of acid‐fast bacilli on stained sputum smears, lymph node aspirate, or any other specimen; or iii) Xpert MTB/RIF positive). The lower‐quality reference standard was clinical diagnosis of TB without microbiological confirmation (including cases diagnosed on the basis of: i) suggestive histology (necrotizing granulomatous inflammation), ii) x‐ray abnormalities, iii) extrapulmonary cases without laboratory confirmation, and iv) anti‐tuberculosis therapy initiated by a healthcare practitioner for cases with a high suspicion of tuberculosis). Threshold: Any abnormality found on abdominal ultrasound Study design: Cross‐sectional and cohort Limitations: A small number of studies and participants were included in the analyses. Risks of bias were generally high in the patient selection domain | ||||||
| Test result | Number of results per 1000 HIV‐positive individuals tested (95% CI) | Number of studies | Number of participants | Certainty of the evidence (GRADE) | ||
| Prevalence 10% | Prevalence 20% | Prevalence 40% | ||||
| Bacteriological confirmation as reference standard: pooled sensitivity = 63% (95% CI 43% to 79%) and pooled specificity = 68% (95% CI 42% to 87%) | ||||||
| True positives (participants correctly classified as having tuberculosis) | 63 (43 to 79) | 126 (86 to 158) | 252 (172 to 316) | 5 | 368 | ⊕⊝⊝⊝ |
| False negatives (participants incorrectly classified as not having tuberculosis) | 37 (21 to 57) | 74 (42 to 114) | 148 (84 to 228) | |||
| True negatives (participants correctly classified as not having tuberculosis) | 612 (378 to 783) | 544 (336 to 696) | 408 (252 to 522) | 5 | 511 | ⊕⊝⊝⊝ |
| False positives (participants incorrectly classified as having tuberculosis) | 288 (117 to 522) | 256 (104 to 464) | 192 (78 to 348) | |||
| Abbreviations: CI: confidence interval GRADE certainty of evidence (GRADEpro GDT 2015; Schünemann 2016) The table displays normalized frequencies within a hypothetical cohort of 1000 people at three different tuberculosis prevalences (pre‐test probabilities): 10%, 20% and 40%. We selected prevalence values based on the range of prevalence observed across the included studies. We estimated confidence intervals based on those around the point estimates for pooled sensitivity and specificity. Explanations aRisk of bias: We rated one study at high risk for participant selection since it excluded people unable to produce sputum (Griesel 2019‐h). We downgraded the certainty of the evidence by one level. | ||||||
| Author (publication year) | Study design | Country | Clinical setting | Target condition definition | Qualification of person performing index test | Sample size | Tuberculosisproportion in study |
| Case‐control | Germany | Not reported | Gastro‐intestinal tuberculosis | Not reported | 25a (7 cases, 18 pulmonary tuberculosis controls) | ‐ | |
| Cross‐sectional | South Sudan | Referral hospital | Extra‐pulmonary tuberculosis | Trained non‐radiologist | 100 | 24% | |
| Cross‐sectional | Spain | Not reported | Extra‐pulmonary tuberculosis | Sonographer | 116 | 55% (higher) 58% (lower) | |
| Cross‐sectional | South Africa | Non‐tertiary hospital | Culture‐positive tuberculosis | Sonographer | 377 | 53% | |
| Case‐control | India | Not reported | Pulmonary tuberculosis, extra‐pulmonary tuberculosis, disseminated tuberculosis | Not reported | 90 (45 cases, 45 HIV‐positive controls without any pathology) | ‐ | |
| Case‐control | Spain | Not reported | Disseminated tuberculosis | Not reported | 152 (76 cases, 76 HIV‐positive controls without any pathology) | ‐ | |
| Cohort | Tanzania | Referral hospital | Pulmonary tuberculosis, extra‐pulmonary tuberculosis, disseminated tuberculosis | Board‐certified sonographers | 100 (191 original study sample) | 46% (higher) 64% (lower) | |
| Cross‐sectional | South Africa | Non‐tertiary hospital | Disseminated tuberculosis | Radiologist | 35 (44 original study sample) | 34% | |
| Cross‐sectional | Cambodia | Referral hospital | Disseminated tuberculosis | Radiologist | 212 | 18% | |
| Cross‐sectional | Zambia | Tertiary hospital | Abdominal tuberculosis | Not reported | 31 | 71% | |
| Cohort | India | Tertiary hospital | Disseminated tuberculosis | Trained non‐radiologist | 81 (425 original study sample) | 30% (higher) 49% (lower) | |
| aIncludes five HIV‐negative participants. Suffix (h) indicates higher‐quality reference standard; suffix (l) indicates lower‐quality reference standard. | |||||||
| Author (publication year) | Index test variable included (threshold) | Reference standard quality and definition |
| Ascites (any) Lymphadenopathy (abdominal and perihepatic nodes with longitudinal diameter > 20 mm) Splenomegaly (> 135 mm) | Lower: Clinical, endoscopic, histologic, radiologic and operative findings including microbiology and polymerase chain reaction of biopsies taken during endoscopy | |
| Any abnormality (Presence of ≥ 1: i) pericardial effusion, ii) periportal/para‐aortic lymph nodes (> 15 mm diameter), iii) focal splenic lesions, iv) pleural effusion or consolidation of the lung, v) ascites without alternative explanation) | Lower: Sputum microscopy OR clinical reasons OR Focused Assessment with Sonography in HIV‐associated tuberculosis (FASH) | |
| Any abnormality (presence of ≥ 1: i) multiple hypoechoic splenic lesions (< 10 mm), ii) any abdominal adenopathy, iii) hypo‐ or hyperechoic liver lesions) | Higher: Microscopy OR culture | |
| Any abnormality (presence of ≥ 1: i) abdominal lymph nodes (any size), ii) splenic hypoechoic lesions, iii) splenomegaly (≥ 110 mm), iv) any one of abdominal, pleural, or pericardial effusions) Ascites (any) Lymphadenopathy (any size) Splenic lesions (hypoechoic) Splenomegaly (≥ 110 mm) | Higher: Positive culture for M tuberculosis from any site | |
| Ascites (any) Hepatomegaly (not defined) Lymphadenopathy (diameter > 15 mm) Splenic lesions (multiple, hypoechoic, 5 mm to 10 mm diameter) Splenomegaly (not defined) | Lower: Lymphocytic predominance and elevated adenosine deaminase (ADA) levels in pleural or ascitic fluid OR granulomatous lymphadenitis and acid‐fast bacilli in lymph node OR sputum microscopy | |
| Ascites (any) Hepatomegaly (not defined) Lymphadenopathy (> 15 mm diameter) Splenic lesions (hypoechoic nodes) Splenomegaly (long axis > 120 mm or subjective impression) | Lower: Blood culture positive for M tuberculosis OR medullary bone or liver biopsy with granulomatous inflammation or culture positive for M tuberculosis OR microbiological or histopathological confirmation in ≥ 2 non‐contiguous extra‐pulmonary sites | |
| Any abnormality (presence of ≥ 1: i) pleural or pericardial effusion, ii) ascites, iii) abdominal lymph nodes > 15 mm, iv) hypoechogenic lesions in the liver or spleen, v) ileum wall thickening > 4 mm or destructed ileum wall architecture) Ascites (any) Hepatomegaly (not defined) Lymphadenopathy (> 15 mm diameter) Splenomegaly (not defined) | Higher: Xpert MTB/RIF assay and/or bacteriologic culture (growth of M tuberculosis) | |
| Ascites (any) Lymphadenopathy (not defined) | Higher: Positive mycobacterial blood or bone marrow cultures OR positive mycobacterial cultures from 2 or more other sites OR post mortem evidence | |
| Any abnormality (presence of ≥ 1: i) any lymph nodes ≥ 12 mm, ii) ascites, iii) hepatomegaly, iv) splenomegaly, v) hepatic or splenic hypoechoic lesions with or without organ enlargement) | Higher: Positive culture for M tuberculosis from any site | |
| Ascites (any) Hepatomegaly (not defined) Lymphadenopathy (not defined) Splenomegaly (not defined) | Lower: Positive bacteriological culture OR granulomatous inflammation with positive Ziehl‐Neelsen (ZN) staining on microscopy OR granulomatous inflammation on microscopy OR visual inspection on laparoscopy consistent with tuberculosis (presence of tubercles, fibro‐adhesive peritonitis, or caseating lymphadenopathy) and favourable response to anti‐tuberculous treatment | |
| Any abnormality (presence of ≥ 1: i) pericardial or pleural effusion, ii) focal liver or splenic lesions, iii) abdominal lymphadenopathy) Ascites (any) Hepatomegaly (not defined) Lymphadenopathy (≥ 15 mm diameter) Splenic lesions (multiple, hypoechoic, 2 mm to 5 mm diameter) | Higher: Positive fluorescent microscopy, polymerase chain reaction, or tuberculosis culture | |
| Suffix (h) indicates higher quality reference standard; suffix (l) indicates lower quality reference standard | ||
| Abdominal ultrasound finding | Number of studies | Number of participants (tuberculosiscases) | Pooled sensitivity (95% CI) % | Pooled specificity (95% CI) % | Range of sensitivity % | Range of specificity % |
| Any abnormality (higher‐quality reference standard) | 5 | 879 (368) | 63 (43 to 79) | 68 (72 to 87) | 35 to 82 | 20 to 92 |
| Any abnormality (lower‐quality reference standard) | 4 | 397 (149) | 68 (45 to 85) | 73 (41 to 91) | 37 to 88 | 22 to 92 |
| Splenic lesions | 6 | 916 (477) | Not calculated | Not calculated | 13 to 62 | 86 to 100 |
| Intra‐abdominal lymph nodes | 8 | 917 (455) | Not calculated | Not calculated | 22 to 86 | 56 to 100 |
| Ascites | 8 | 891 (433) | Not calculated | Not calculated | 4 to 73 | 33 to 100 |
| Splenomegaly | 6 | 775 (397 | Not calculated | Not calculated | 5 to 62 | 45 to 89 |
| Hepatomegaly | 4 | 373 (189) | Not calculated | Not calculated | 24 to 76 | 20 to 78 |
| Test | No. of studies | No. of participants |
| 1 Abnormal abdominal ultrasound (higher quality) Show forest plot | 5 | 879 |
| 2 Abnormal abdominal ultrasound (lower quality) Show forest plot | 4 | 397 |
| 3 Ascites Show forest plot | 8 | 891 |
| 4 Splenic lesions Show forest plot | 6 | 916 |
| 5 Abdominal lymph nodes Show forest plot | 8 | 917 |
| 6 Splenomegaly Show forest plot | 6 | 775 |
| 7 Hepatomegaly Show forest plot | 4 | 373 |