Abdominal ultrasound for diagnosing abdominal tuberculosis or disseminated tuberculosis with abdominal involvement in HIV‐positive adults
Appendices
Appendix 1. MEDLINE search strategy (OVID)
1 extrapulmonary tuberculosis.mp.
2 Peritonitis, Tuberculous/ or Tuberculosis, Gastrointestinal/ or Tuberculosis, Hepatic/
3 abdominal tuberculosis.mp.
4 Tuberculosis, Hepatic/ or liver tuberculosis.mp. or gastric tuberculosis.mp or intestinal tuberculosis.mp
5 Tuberculosis, Miliary/
6 disseminated tuberculosis.mp.
7 1 or 2 or 3 or 4 or 5 or 6
8 HIV infection.mp. or HIV Infections/
9 exp HIV/
10 human immunodeficiency virus.mp.
11 Acquired Immunodeficiency Syndrome/ or acquired immunodeficiency syndrome.mp.
12 (acquired immun* and deficiency syndrome).mp.
13 (HIV* adj2 (people or person* or patient*)).mp. or PLHIV.mp
14 8 or 9 or 10 or 11 or 12 or 13
15 7 and 14
16 Abdomen/us [Ultrasonography]
17 Radiography/ or (X‐ray*).mp
18 ultrasound.mp. or barium.mp
19 Tomography, X‐Ray Computed/
20 (comput* adj2 tomograph*).mp.
21 Magnetic Resonance Imaging/
22 (MRI or CAT).mp.
23 Ultrasonography/ or ultrasonograph*.mp.
24 Bacteriological Techniques/ or Sputum/ or sputum specimen.mp.
25 liquid culture system.mp.
26 Xpert MTB*.mp.
27 Genotype MTBDR*.mp.
28 lipoarabinomannan.mp. or LAM.mp or LF‐LAM.mp
29 (QuantiFERON‐TB‐Gold). mp or Tuberculin Test/ or tuberculin.mp
30 Diagnostic Imaging/ or Point‐of‐Care Systems/
31 CD4 Lymphocyte Count/ or (CD4 adj2 (cells or lymphocytes).mp
32 Laparotomy.mp or laparoscopy.mp or (fine needle aspiration).mp
33 Ascites/diagnosis or Ascites/microbiology or Paracentesis/ or Laparoscopy/
34 colonoscopy.mp. or Colonoscopy/
35 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 or
31 or 32 or 33 or 34
36 15 and 35
This is the preliminary search strategy for MEDLINE (OVID), which we will adapt to search other electronic databases. We will report all search strategies in full in the final version of the review.
Appendix 2. QUADAS‐2 tool tailored to the context of the review
| Domain | Patient selection | Index test | Reference standard | Flow and timing |
| Description | Methods of patient selection | How index test was conducted and reported | How reference standard was conducted and reported | Describe patients that did not receive and time interval between index test or reference standard |
| Signalling questions (yes, no, or unclear) | Consecutive or random sample of patients?
| Index test results interpreted without knowledge of the results of reference standard?
| Reference standard likely to correctly classify the target condition?
| Was there an appropriate interval between index test and reference standard?
|
| Was a case‐control design avoided?
| Pre‐specified threshold used?
| Reference standard results interpreted without knowledge of the results of index test?
| Did all patients receive a reference standard?
| |
| Did the study avoid inappropriate exclusions?
| Was incorporation bias avoided (inclusion of index test as part of the reference standard)?
| Did all patients receive the same reference standard?
| ||
| Were all patients included in the analysis?
| ||||
| Risk of bias¹(high, low, or unclear) | Could the selection of patients have introduced bias? | Could the conduct or interpretation of the index test have introduced bias? | Could the reference standard, its conduct, or its interpretation has introduced bias? | Could the patient flow have introduced bias? |
| Applicability concerns (high, low, or unclear) | Are there concerns that the included patients do not match the review question?
| Are there concerns that the index test, its conduct, or interpretation differs from the review question?
| Are there concerns that the target condition as defined by the reference standard does not match the review question?
| Not applicable |
Abbreviations: TB: tuberculosis
¹Grading criteria for ʽRisk of bias' assessment
-
If all signalling questions for a domain are answered ʽyes' then we will judge the risk of bias to be ʽlow'.
-
If any signalling question is answered ʽno' this will flag the potential for bias and we will judge risk of bias with a senior review author.
-
If all signalling questions or most of them were answered ʽno', then we will judge the risk of bias as ʽhigh'.
-
We will assign the ʽunclear' category when the study authors report insufficient data to permit a judgment.
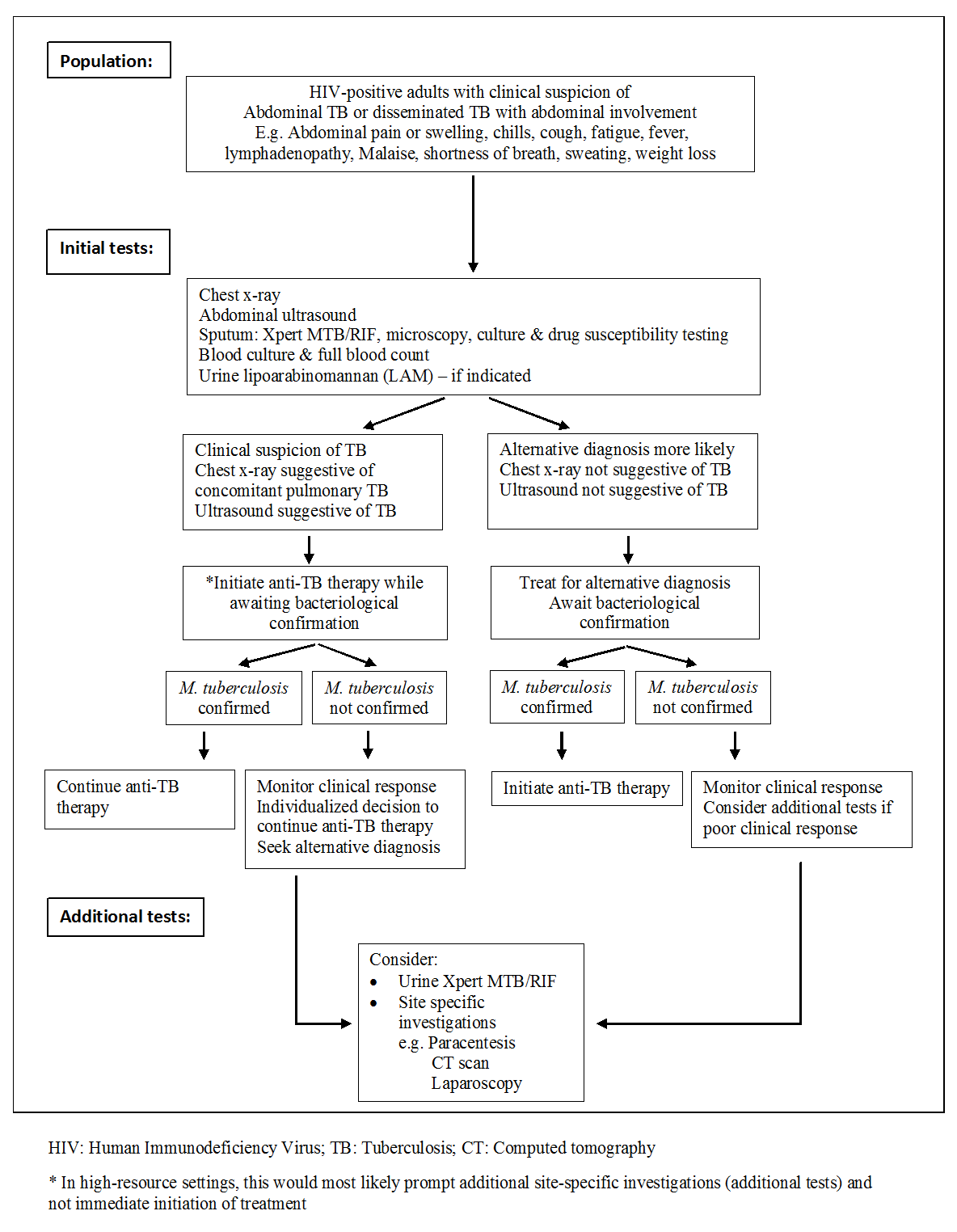
Diagnostic workup of HIV‐positive adults with suspected abdominal tuberculosis or disseminated tuberculosis with abdominal involvement

