Prebiotics for the prevention of hyperbilirubinaemia in neonates
Abstract
This is a protocol for a Cochrane Review (Intervention). The objectives are as follows:
To determine whether administration of prebiotics reduces the incidence of hyperbilirubinaemia among term and preterm infants compared with enteral supplementation of milk with distilled water or no supplementation.
Background
Description of the condition
Hyperbilirubinaemia occurs in approximately two‐thirds of all newborns during the first days of life (Lauer 2011). Mild elevations in bilirubin levels usually do not have serious side effects apart from recognisable jaundice (Maisels 2008).
The pathogenesis of jaundice in preterm infants is the same as in term infants, but because of liver and gastrointestinal tract prematurity, hyperbilirubinaemia can be more dangerous during the late preterm period (Maisels 2012). Furthermore, sometimes because of complications related to prematurity, enteral feeding in late preterm infants is delayed, which, in turn, may limit intestinal flow and bacterial colonisation, resulting in more effective enterohepatic circulation, hence high serum bilirubin (Gartner 2001). The major complication of hyperbilirubinaemia ‐ kernicterus, or bilirubin‐induced encephalopathy ‐ can occur at high bilirubin levels. Severe haemolysis and certain diseases (e.g. isoimmune haemolytic disease, glucose‐6‐phosphate deficiency (G6PD), asphyxia, sepsis, acidosis, hypoalbuminaemia) are risk factors for kernicterus (Maisels 2009). This devastating neurological dysfunction can cause permanent neurodevelopmental handicaps (Xiong 2011). Although, nowadays, kernicterus is rarely seen to result from early aggressive phototherapy, it still occurs (Kaplan 2011). Neonatal jaundice can cause parental concern and can increase hospital costs due to hospital re‐admissions (Burke 2009). Therefore, proper and timely treatment of hyperbilirubinaemia is of critical importance.
Current recommendations for management of neonatal hyperbilirubinaemia focus on determining age‐specific bilirubin levels before initiating phototherapy (Maisels 2012). Phototherapy does not seem to cause serious side effects, but recently some clinical trials have raised concerns based on animal or cell culture studies regarding its potential to damage DNA (Cetinkursun 2005; Ramy 2016; Roll 2005; Rosenstein 1984; Tatli 2008; Yahia 2014). Investigators have observed that hyperbilirubinaemia did not influence DNA damage and apoptosis, whereas phototherapy (both conventional and intensive types) was associated with DNA damage in full‐term infants (Ramy 2016; Tatli 2008; Yahia 2014) and induced apoptosis in peripheral blood lymphocytes (Yahia 2014). Therefore, other types of treatment for hyperbilirubinaemia are needed.
The increased enterohepatic circulation of bilirubin has a known role in the incidence of neonatal jaundice (Sato 2013), and interventions that reduce the enterohepatic circulation of bilirubin can be beneficial, as they reduce the production of bilirubin.
Prebiotics are observed to have favourable effects on the enterohepatic cycle including better gastrointestinal motility and improved frequency and viscosity of stool (Indrio 2009; Westerbeek 2011). Thus, it has been hypothesised that with enteral feeding supplementation of prebiotics during enterohepatic circulation (when bilirubin circulates within the intestine), less conjugated bilirubin is converted to unconjugated bilirubin, leading to improvement in hyperbilirubinaemia status and reduction of jaundice among neonates. It is worth noting that the exact mechanism of action of prebiotics for hyperbilirubinaemia and how long it takes for prebiotics to delay onset of neonatal jaundice remain unclear.
Description of the intervention
Prebiotics are “non‐digestible food components that affect the host beneficially by selectively stimulating the growth and/or activity of one or a limited number of bacteria in the colon, thereby improving host health” (Gibson 1995). Oligosaccharides are the most common archetype of prebiotics. Lactulose and inulin are other types of prebiotics. Prebiotics are found in human breast milk as oligosaccharides (Armanian 2014; Srinivasjois 2013).
Our selected intervention consists of "enteral supplementation with any type of prebiotics". Prebiotics can take the form of galacto‐oligosaccharides (GOSs), fructo‐oligosaccharides (FOSs), acidic oligosaccharides (AOSs), lactulose, or inulin. The most frequently used prebiotics are oligosaccharides (Srinivasjois 2013), which are found in human breast milk (Armanian 2014; Srinivasjois 2013). They usually consist of a mixture of short‐chain carbohydrates (with a degree of polymerisation between 2 and 60) with long‐chain carbohydrates (9:1) that are non‐digestible by neonatal digestive systems (Cummings 2002). Oligosaccharides usually are given at a dosage of 0.5 to 1.5 gr/kg/d initiated at day 2 to 10 after birth, added to neonatal enteral milk.
Distilled water rarely has an effect on neonatal jaundice. Therefore, to eliminate the confounding effects of different types of placebo, our review will be conducted to compare enteral supplementation of prebiotics added to milk versus distilled water or no supplementation. Trials included in this review must have continued for at least seven days. For management of neonatal jaundice, all infants in both groups will be treated according to the protocol for phototherapy described in guidelines of the American Academy of Pediatrics 2004.
How the intervention might work
Literature suggests positive effects of prebiotics on neonatal outcomes, such as gastrointestinal (GI) motility, frequency and viscosity of stool, enteral tolerance, and necrotising enterocolitis (NEC) (Armanian 2014; Indrio 2009; Modi 2010; Srinivasjois 2013; Westerbeek 2011). Prebiotics mainly stimulate rapid growth of beneficial bacteria within the colon (Armanian 2016; Cummings 2002; Oozeer 2013; Sherman 2009). Oligosaccharides represent one types of prebiotic in the gut that is decomposed by microbial flora into short‐chain fatty acids, hydrogen, and carbon dioxide. Short‐chain fatty acids have a mild laxative effect because they reduce the pH level of the stool, making it more acidic (Rao 2009) and thus changing stool consistency and frequency of defecation. This could be beneficial in preventing production of bilirubin by enterohepatic circulation. As prebiotics enter the GI system, the enterohepatic circulation of bilirubin rotates slowly with better and faster GI evacuation. When bilirubin is evacuated faster from the GI system, the opportunity for conversion of conjugated bilirubin to unconjugated bilirubin is lessened, hence less jaundice occurs. Therefore, supplementation with prebiotics can reduce the production of unconjugated bilirubin. On the other hand, distilled water rarely has an effect on neonatal jaundice and can act as a preferred placebo.
Why it is important to do this review
Concerns about the use of phototherapy surround its potentially damaging effects on DNA (Ramy 2016; Tatli 2008; Yahia 2014) and increased mortality among term and particularly preterm infants (Tyson 2012) with intensive use. Investigators have evaluated other methods, such as administration of prebiotics, for management of hyperbilirubinaemia in neonates. However, to the best of our knowledge, no review has evaluated the impact of prebiotics on neonatal hyperbilirubinaemia.
To date, nearly three studies have examined effects of prebiotics on neonatal hyperbilirubinaemia (Armanian 2015; Bisceglia 2009; Riskin 2010). In two of these studies, lower bilirubin levels detected by transcutaneous bilirubinometry were associated with enteral feeding supplementation by oligosaccharides (Armanian 2015; Bisceglia 2009). On the other hand, it has been found that colonies of lactobacilli and bifidobacteria within the gut cannot convert bilirubin into excretory products (Bisceglia 2009), and some researchers have observed that Clostridium (a pathogenic microorganism that can be reduced by prebiotics) can convert bilirubin to non‐toxic excretory derivatives (Petit 1999; Konickova 2012; Vitek 2006). Hence, enteral feeding supplementation with prebiotics may lead to an unexpected increase in conversion of bilirubin into recyclable products. Therefore, researchers are interested in discerning the effects of prebiotics on neonatal hyperbilirubinaemia.
In summary, literature is inconsistent in terms of effects of feeding supplementation with prebiotics on neonatal hyperbilirubinaemia. This review can shed light on clinical trials investigating effects of prebiotics on neonatal hyperbilirubinaemia.
Objectives
To determine whether administration of prebiotics reduces the incidence of hyperbilirubinaemia among term and preterm infants compared with enteral supplementation of milk with distilled water or no supplementation.
Methods
Criteria for considering studies for this review
Types of studies
We will include in the review all randomised controlled trials (RCTs) comparing enteral feeding supplementation with prebiotics versus distilled water or no supplementation.
Types of participants
We will include all studies that enrolled neonates and will categorise neonates into three groups: term neonates (gestational age ≥ 37 weeks); late preterm neonates (35 to < 37 weeks' gestation); and premature neonates (< 35 weeks' gestation). We will exclude infants with the following conditions: asphyxia, major congenital anomalies, Rhesus isoimmunisation, G6PD deficiency, inborn errors, proven sepsis or other infection, cephalohaematoma, and subgaleal haemorrhage. We will also exclude infants with enteral feeding supplementation with specific formulas, probiotics, or zinc.
Types of interventions
Enteral feeding supplementation given with prebiotics during the first ten days of life is the intervention under study. Prebiotics can be provided in the form of galacto‐oligosaccharides (GOSs), fructo‐oligosaccharides (FOSs), acidic oligosaccharides (AOSs), lactulose, or inulin. We will include studies that initiated supplementation with prebiotics between days 2 and 10 of life at a dosage of 0.5 to 1.5 gr/kg/d, with concentrations of 1% and 0.8 gr/dL for oligosaccharides, lactulose, and inulin. To eliminate confounding effects of prebiotics in human breast milk and in formula, we will include studies that added these compounds to neonatal enteral milk. We will include studies of three types: trials that add prebiotics to breast milk in breast‐fed only infants; trials that add prebiotics to formula in bottle‐fed only infants; and trials providing mixed forms (i.e. adding prebiotics to both breast milk and formula). Investigators must compare interventions versus enteral supplementation of milk with distilled water, no supplementation, or both. We will include in this review only trials that are continued for at least seven days. For management of neonatal jaundice, all infants in both groups will be treated according to the protocol for phototherapy described in guidelines of the American Academy of Pediatrics 2004.
Types of outcome measures
Primary outcomes
-
Neonatal hyperbilirubinaemia: incidence of hyperbilirubinaemia at any time during the first ten days of life, considered as the primary outcome. Hyperbilirubinemia is defined:
-
-
for term and late preterm neonates (gestational age ≥ 35 weeks): as total bilirubin (TB) level eligible for phototherapy, as described in guidelines of the American Academy of Pediatrics 2004 (Figure 1), or as absolute TB level ≥ 15 mg/dL; and
-
for premature neonates (< 35 weeks’ gestation): as TB level that is eligible for phototherapy, as described in guidelines of the American Academy of Pediatrics 2004 (Figure 2), or as absolute TB level > 1% of body weight.
-

Guidelines for phototherapy in infants at ≥ 35 weeks' gestation.
(American Academy of Pediatrics Subcommittee on Hyperbilirubinemia. Management of hyperbilirubinemia in the newborn infant 35 or more weeks of gestation. Pediatrics 2004;114:297.)
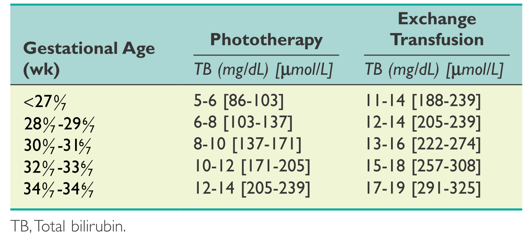
Suggested guidelines for initiating phototherapy or exchange transfusion in premature infants.
(Maisels MJ, Watchko JF, Bhutani VK, et al. An approach to the management of hyperbilirubinemia in the preterm infant less than 35 weeks of gestation. Journal of Perinatology 2012;32:660‐664.)
-
Acute bilirubin encephalopathy: defined as a clinical syndrome, in the presence of severe hyperbilirubinaemia, that ranges from the initial phase of lethargy, hypotonia, reduced movement, and poor sucking and suckling to the advanced phase of deep stupor, hypertonia, inability to feed, and shrill cry or seizures (Canadian Pediatric Society 2007).
Secondary outcomes
-
Maximum plasma unconjugated bilirubin levels (at any time during study period)
-
Treatment with phototherapy
-
Duration of phototherapy (days)
-
Exchange transfusion: receipt of any exchange transfusion (single or double volume)
-
Chronic bilirubin encephalopathy or kernicterus: defined by a tetrad of choreoathetoid cerebral palsy, high‐frequency sensorineural hearing loss, and palsy of vertical gaze and dental enamel hypoplasia assessed at 6 months' corrected age (Okumura 2009)
-
Stool frequency: total number of defecations recorded per day during intervention
-
Length of hospital stay (days)
-
Neonatal mortality
-
Major neurodevelopmental disability
-
Major neurodevelopmental disabilities considered
-
Cerebral palsy
-
Developmental delay or intellectual impairment
-
-
Bayley or Griffith assessment more than two standard deviations (SDs) below the mean, or intellectual impairment (IQ) > 2 SDs below the mean
-
Neuromotor development (Bayley Scales of Infant Development ‐ Psychomotor Development Index (BSID PDI)) assessed among survivors
-
Mental development (Bayley Scales of Infant Development ‐ Mental Development Index (BSID MDI)) assessed among survivors
-
Blindness/vision (< 6/60 in both eyes)
-
Sensorineural deafness requiring amplification
-
-
The review will report these components of long‐term outcomes for all studies that have evaluated children after 18 months' chronological age. Review authors will perform separate analyses for children 18 to 24 months of age and 3 to 5 years of age. We will consider all categories
-
-
Side effects: diarrhoea (stool > 40 gr/kg/d) and dehydration (weight loss > 10% birth weight) recorded during the intervention
Search methods for identification of studies
We will follow the search strategy used by the Cochrane Neonatal Review Group (CNRG) to identify studies. We will apply no language restrictions.
Electronic searches
We will use criteria and standard methods of the Cochrane Collaboration and the Cochrane Neonatal Review Group and will undertake a comprehensive search including:
-
the Cochrane Central Register of Controlled Trials (CENTRAL) in the Cochrane Library (April 2015) (Appendix 1);
-
MEDLINE (January 1996 to current) (Appendix 2);
-
Embase (January 1980 to current) (Appendix 3); and
-
the Cumulative Index to Nursing and Allied Health Literature (CINAHL; 1982 to current).
We will check Proceedings of the Prenatal Society of Australia and New Zealand (PSANZ ‐ 2005 to current) and of the Pediatric Academic Societies (PAS) ‐ American Pediatric Society, Society for Pediatric Research, and European Society for Pediatric Research (2000 to current).
We will combine search terms with the highly sensitive search strategy presented in Boluyt 2008 to identify RCTs on neonates in the light of sensitive key terms in our search strategy (Appendix 4). The proposed search strategy will use a combination of controlled vocabulary; we will use subject terms and free text terms for MEDLINE and will adapt these for use in the other databases mentioned.
Searching other resources
We will apply no language and publication restrictions; therefore, we will search reference lists of identified clinical trials and review articles and will contact authors of published articles to ask about possible unpublished trials. We will search all ongoing and recently completed clinical trials available through www.ClinicalTrials.gov and www.Controlled‐trials.com.
Data collection and analysis
We will employ standard methods of the Cochrane Collaboration, as described in the Cochrane Handbook for Systematic Reviews of Interventions, and of the Cochrane Neonatal Review Group (Higgins 2011).
Selection of studies
Two review authors will independently check the titles and abstracts of all studies retrieved through the literature search to identify those that meet the inclusion criteria, will independently confirm their eligibility by reviewing the full text of retrieved articles, and will resolve disagreements through discussion. We will consult the Cochrane Neonatal Review Group for advice when necessary.
Data extraction and management
We will design a data extraction form and will evaluate its validity and reliability before use. At least two review authors will independently extract data and will resolve differences in data interpretation with assistance provided by trial authors. We will resolve uncertainties regarding trial information and incomplete or missing data by contacting trial authors. We will enter data into Review Manager (RevMan 5.3) and will check them for accuracy.
Assessment of risk of bias in included studies
Two review authors will independently assess the risk of bias (low, high, or unclear) of all included trials using the Cochrane ‘Risk of bias’ tool (Higgins 2011) for the following domains.
-
Selection bias.
-
Performance bias.
-
Attrition bias.
-
Reporting bias.
-
Any other bias.
We will resolve disagreements by discussion or by consultation with a third assessor. See Appendix 5 for a detailed description of risk of bias for each domain.
Measures of treatment effect
We will present pooled estimates of dichotomous outcomes as risk ratios (RRs) or risk differences (RDs) with corresponding 95% confidence intervals (CIs). For continuous outcomes, we will use mean differences (MDs) along with 95% CIs to compare intervention and control groups. When outcomes are measured by different methods or by different scales, we will use standardised mean differences (SMDs). If data are missing or incomplete and the RD statistic is statistically significant, we will present the number needed to treat for an additional beneficial or harmful outcome (NNTB/NNTH).
Unit of analysis issues
We will consider the participating infant as the unit of analysis in trials of cross‐over design and in parallel clinical trials, and the participating neonate unit as the unit of analysis in cluster‐randomised trials. We may consider appropriate corrections on reported estimates from included cluster‐randomised trials. We will deal with an approximate analysis as suggested by the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We will multiply the standard error of the reported effect estimate by the square root of the design effect and will calculate the design effect from the average cluster size and the intracluster correlation coefficient (ICC). If such a correction is not possible, we will include the proposed cluster trial in the review but not in the meta‐analysis.
Dealing with missing data
If clarification of information or missing data is needed, we will contact the relevant study authors. We will exclude from the meta‐analysis studies with a high drop‐out rate for which we cannot obtain appropriate details of missing data and will perform a sensitivity analysis to evaluate the impact of exclusion on results of analysis.
Assessment of heterogeneity
We will use the I2 statistic and the following categories to assess for heterogeneity among studies.
-
Less than 25%: no heterogeneity.
-
25% to 49%: low heterogeneity.
-
50% to 74%: moderate heterogeneity.
-
75% or greater: high heterogeneity.
We will use Cochran's Chi2 Q test and the associated P value in evaluating heterogeneity. We will consider P < 0.1 as an indication of the presence of heterogeneity. We will present a Galbraith plot for heterogeneity and will perform visual assessment to identify obvious overlaps and outliers. In cases of moderate or high heterogeneity, we will explore potential sources of heterogeneity by performing sensitivity analysis and subgroup analyses.
Assessment of reporting biases
We plan to use funnel plots to assess possible reporting or publication bias. For funnel plots to be meaningful, ten or more trials must be included. We will conduct Begg and Egger linear tests in evaluating publication bias.
Data synthesis
We will apply standard methods of the Cochrane Neonatal Review Group and RevMan V.5.3 for meta‐analyses. We will use a fixed‐effect method in combining the effects of studies that are sufficiently similar. When heterogeneity between studies is evident, we will perform random‐effects subgroup analysis or meta‐regression, if appropriate. For estimates of combined risk ratio and risk difference, we will use the Mantel‐Haenszel method. We will use mean differences (MDs) or standardised mean difference (SMDs) to combine estimates of quantitative data as reported in clinical trials measuring the same outcome using the same scale and in those using different scales or measurement methods, respectively. We will report risk ratios (RRs) and MDs and SMDs along with 95% CIs and will base analysis on numbers needed to treat for additional beneficial and harmful outcomes.
Quality of evidence
We will use the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) approach, as outlined in the GRADE Handbook (Schünemann 2013), to assess the quality of evidence for the following (clinically relevant) outcomes: neonatal hyperbilirubinaemia, need for phototherapy, exchange transfusion, acute bilirubin encephalopathy/kernicterus, and stool frequency.
Two review authors will independently assess the quality of evidence for each of the outcomes above. We will consider evidence from RCTs as high quality but will downgrade the evidence one level for serious (or two levels for very serious) limitations on the basis of the following: design (risk of bias), consistency across studies, directness of evidence, precision of estimates, and presence of publication bias. We will use the GRADEpro GDT Guideline Development Tool to create a ‘Summary of findings’ table to report the quality of evidence.
The GRADE approach yields an assessment of the quality of a body of evidence according to one of four grades.
-
High: We are very confident that the true effect lies close to that of the estimate of the effect.
-
Moderate: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different.
-
Low: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect.
-
Very low: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect.
Subgroup analysis and investigation of heterogeneity
We will run a subgroup analysis based on the following.
-
Gestational age (term neonates (gestational age ≥ 37 weeks), late preterm neonates (35 to 37 weeks' gestation), premature neonates (< 35 weeks' gestation)).
-
Birth weight (≥ 2500 grams, < 2500 grams, < 1500 grams).
-
Type of prebiotic (oligosaccharide, lactulose, inulin).
-
Type of feeding (only breast milk‐fed infants, only formula‐fed infants, infants given a mixed form of feeding).
-
Duration of supplementation.
Sensitivity analysis
We will perform sensitivity analyses based on both outcomes and baseline characteristics if the chance of bias in study findings is high, to assess the impact of bias on results of the meta‐analysis.
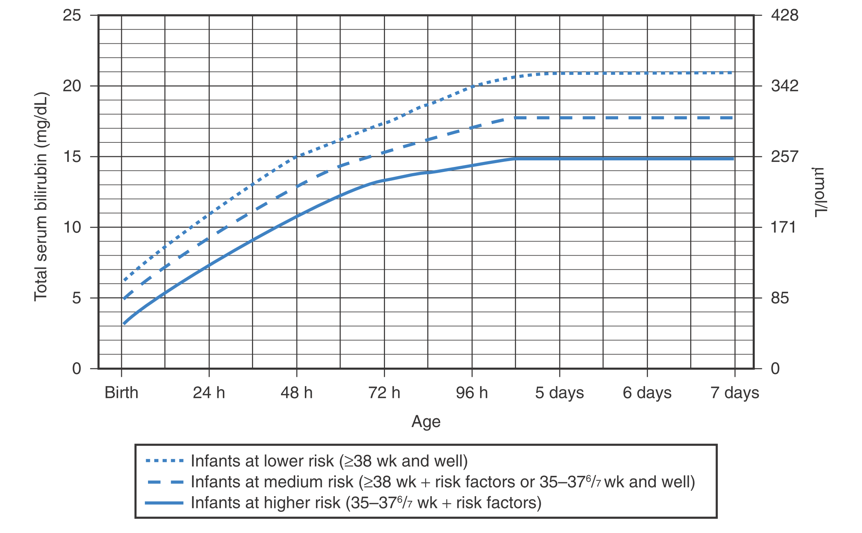
Guidelines for phototherapy in infants at ≥ 35 weeks' gestation.
(American Academy of Pediatrics Subcommittee on Hyperbilirubinemia. Management of hyperbilirubinemia in the newborn infant 35 or more weeks of gestation. Pediatrics 2004;114:297.)

Suggested guidelines for initiating phototherapy or exchange transfusion in premature infants.
(Maisels MJ, Watchko JF, Bhutani VK, et al. An approach to the management of hyperbilirubinemia in the preterm infant less than 35 weeks of gestation. Journal of Perinatology 2012;32:660‐664.)

