Betabloqueantes e inhibidores del sistema de renina‐angiotensina‐aldosterona para la insuficiencia cardíaca crónica con fracción de expulsión conservada
Resumen
Antecedentes
Los betabloqueantes y los inhibidores del sistema de renina‐angiotensina‐aldosterona mejoran la supervivencia y reducen la morbilidad en los pacientes con insuficiencia cardíaca con fracción de expulsión reducida del ventrículo izquierdo . Hay incertidumbre con respecto a si estos tratamientos son beneficiosos para los pacientes con insuficiencia cardíaca con fracción de expulsión conservada y se requiere una revisión integral de la evidencia.
Objetivos
Evaluar los efectos de los betabloqueantes, los inhibidores de la enzima convertidora de angiotensina, los bloqueadores de los receptores de angiotensina, los inhibidores de la neprilesina y del receptor de angiotensina y los antagonistas de los receptores mineralocorticoides en los pacientes con insuficiencia cardíaca con fracción de expulsión conservada.
Métodos de búsqueda
Se hicieron búsquedas en CENTRAL, MEDLINE, Embase y en dos registros de ensayos clínicos el 25 julio 2017 para identificar estudios elegibles. Se revisaron las listas de referencias de los estudios primarios y de los artículos de revisión para obtener estudios adicionales. No hubo ninguna restricción de idioma o de fecha.
Criterios de selección
Se incluyeron los ensayos controlados aleatorios con un diseño de grupos paralelos que reclutaron a participantes adultos con insuficiencia cardíaca con fracción de expulsión conservada, definida por una fracción de expulsión del ventrículo izquierdo mayor del 40%.
Obtención y análisis de los datos
Dos autores de la revisión seleccionaron de forma independiente los estudios para la inclusión y extrajeron los datos. Los resultados incluidos evaluaron la mortalidad cardiovascular, la hospitalización por insuficiencia cardíaca, la hiperpotasemia, la mortalidad por todas las causas y la calidad de vida. Se calcularon los cocientes de riesgos (CR) y, cuando fue posible, los cocientes de riesgos instantáneos (CRI) para los resultados dicotómicos. Para los datos continuos se calcularon la diferencia de medias (DM) o la diferencia de medias estandarizada (DME). Cuando fue necesario, se estableció contacto con los autores de los ensayos para obtener los datos faltantes.
Resultados principales
En todas las comparaciones se incluyeron 37 ensayos controlados aleatorios (207 informes) con un total de 18 311 participantes.
Se incluyeron diez estudios (3087 participantes) que investigaron los betabloqueantes (BB). Un análisis agrupado indicó una reducción de la mortalidad cardiovascular (15% de los participantes del brazo de intervención versus 19% del brazo control; CR 0,78; intervalo de confianza del 95% [IC] 0,62 a 0,99; número necesario a tratar para lograr un beneficio [NNTB] 25; 1046 participantes; tres estudios). Sin embargo, la calidad de la evidencia fue baja y no se observaron efectos sobre la mortalidad cardiovascular cuando el análisis se limitó a los estudios con un bajo riesgo de sesgo (CR 0,81; IC del 95%: 0,50 a 1,29; 643 participantes; un estudio). No hubo efectos sobre la mortalidad por todas las causas, la hospitalización por insuficiencia cardíaca ni las medidas de calidad de vida; sin embargo, hay incertidumbre acerca de estos efectos debido a la evidencia limitada disponible.
Se incluyeron 12 estudios (4408 participantes) que investigaron a los antagonistas de los receptores mineralocorticoides (ARM) y la calidad de la evidencia se consideró moderada. El tratamiento con ARM redujo la hospitalización por insuficiencia cardíaca (11% de los participantes del brazo de intervención versus 14% del brazo control; CR 0,82; IC del 95%: 0,69 a 0,98; NNTB 41; 3714 participantes; tres estudios; evidencia de calidad moderada); sin embargo, se observó poco o ningún efecto sobre la mortalidad cardiovascular y por todas las causas, así como sobre las medidas de calidad de vida. El tratamiento con ARM se asoció con un riesgo mayor de hiperpotasemia (16% de los participantes del grupo de intervención versus 8% del grupo control; CR 2,11; IC del 95%: 1,77 a 2,51; 4291 participantes; seis estudios; evidencia de alta calidad).
Se incluyeron ocho estudios (2061 participantes) que investigaron los inhibidores de la enzima convertidora de angiotensina (IECA) y la calidad general de la evidencia se consideró moderada. La evidencia indicó que es probable que el tratamiento con IECA tenga poco o ningún efecto sobre la mortalidad cardiovascular, la mortalidad por todas las causas, la hospitalización por insuficiencia cardíaca o la calidad de vida. Los datos del efecto de los IECA sobre la hiperpotasemia solo se obtuvieron de uno de los estudios incluidos.
Se incluyeron ocho estudios (8755 participantes) que investigaron los bloqueadores de los receptores de angiotensina (BRA) y la calidad general de la evidencia se consideró alta. La evidencia indicó que el tratamiento con BRA tiene poco o ningún efecto sobre la mortalidad cardiovascular, la mortalidad por todas las causas, la hospitalización por insuficiencia cardíaca o la calidad de vida. Los BRA se asociaron con un mayor riesgo de hiperpotasemia (0,9% de los participantes del grupo de intervención versus 0,5% del grupo control; CR 1,88; IC del 95%: 1,07 a 3,33; 7148 participantes; dos estudios; evidencia de alta calidad).
Se identificó un único estudio controlado con placebo en curso que investiga el efecto de los inhibidores de la neprilesina y del receptor de angiotensina (INRA) en pacientes con insuficiencia cardíaca con fracción de expulsión conservada.
Conclusiones de los autores
Hay evidencia de que el tratamiento con ARM reduce la hospitalización por insuficiencia cardíaca en la insuficiencia cardíaca con fracción de expulsión conservada; sin embargo, los efectos sobre los resultados relacionados con la mortalidad y sobre la calidad de vida todavía no están claros. La evidencia disponible de los betabloqueantes, los IECA, los BRA y los INRA es limitada y todavía no está claro si estos tratamientos tienen una función en el tratamiento de la ICFEc a falta de una indicación alternativa para su uso. Esta revisión integral destaca la brecha que persiste en la evidencia, que actualmente se analiza en varios ensayos clínicos grandes en curso.
PICO
Resumen en términos sencillos
Betabloqueantes e inhibidores del sistema de renina‐angiotensina‐aldosterona para la insuficiencia cardíaca crónica con fracción de expulsión conservada
Pregunta de la revisión
Se investigaron los efectos de los betabloqueantes (BB), los antagonistas de los receptores mineralocorticoides (ARM), los inhibidores de la enzima convertidora de angiotensina (IECA), los bloqueadores de los receptores de angiotensina (BRA) y los inhibidores de la neprilesina y del receptor de angiotensina (INRA) sobre la supervivencia, los ingresos hospitalarios para la insuficiencia cardíaca, la calidad de vida y los niveles de potasio en los pacientes con insuficiencia cardíaca con fracción de expulsión conservada.
Antecedentes
La insuficiencia cardíaca es una afección frecuente que se presenta cuando la función del músculo cardíaco está deteriorada y se asocia con síntomas de disnea y fatiga, así como con una reducción de la supervivencia. En cerca de la mitad de los casos donde hay una reducción de la contracción (insuficiencia cardíaca con fracción de expulsión reducida, ICFEr), se sabe que varios tratamientos son efectivos para mejorar la supervivencia y reducir la hospitalización. En los casos restantes donde hay un deterioro de la relajación (insuficiencia cardíaca con fracción de expulsión conservada, ICFEc), no está claro si los mismos tratamientos farmacológicos también son efectivos para mejorar los resultados.
Criterios de selección
Se procuró investigar si los tratamientos de la ICFEr también son efectivos para la ICFEc. Se realizó una búsqueda exhaustiva de todos los ensayos que investigaron los BB, los ARM, los IECA, los BRA o los INRA (evidencia actualizada hasta el 25 de julio de 2017).
Resultados y conclusiones
Se incluyeron diez estudios con 3087 participantes asignados al azar a BB, 12 estudios con 4408 participantes asignados al azar a ARM, ocho estudios con 2061 participantes asignados al azar a IECA y ocho estudios con 8755 participantes asignados al azar a BRA. La evidencia se combinó en un análisis agrupado para cada clase de fármaco y para cada uno de los resultados evaluados. No todos los estudios incluidos formaron parte de cada análisis.
Se encontró que los betabloqueantes pueden mejorar la mortalidad cardiovascular; sin embargo, la calidad de la evidencia fue baja debido al tamaño pequeño de los ensayos y a la incertidumbre acerca de los métodos utilizados. Para los ARM, los resultados indican una reducción de la hospitalización por insuficiencia cardíaca y que tienen poco o ningún efecto en la mortalidad cardiovascular y por todas las causas; sin embargo, la calidad de la evidencia fue solamente moderada. Para los IECA, el tratamiento probablemente tiene poco o ningún efecto sobre los resultados de mortalidad cardiovascular, mortalidad por todas las causas y hospitalización por insuficiencia cardíaca; sin embargo, la calidad de la evidencia fue solamente moderada. Se encontró evidencia de alta calidad para el tratamiento con BRA y los resultados indican poco o ningún efecto de este tratamiento. No hubo estudios finalizados disponibles para los INRA. Se encontró que el tratamiento con ARM y BRA aumentó el riesgo de niveles elevados de potasio en sangre.
En conclusión, los BB pueden mejorar los resultados en los pacientes con ICFEc; sin embargo, este resultado todavía no está claro. Se encontró que los ARM dieron lugar a una reducción leve en el riesgo de hospitalización debido a insuficiencia cardíaca. El tratamiento con IECA probablemente no tiene efectos; sin embargo, este resultado todavía no está claro. La evidencia indica que el tratamiento con BRA tiene poco o ningún efecto beneficioso en los pacientes con ICFEc.
Calidad de la evidencia
La calidad de la evidencia varió de alta a baja entre los resultados y las clases de fármacos estudiadas. Con la excepción de los BRA, faltaron ensayos a gran escala en la ICFEc para las intervenciones y los resultados probados.
Conclusiones de los autores
Summary of findings
| Beta‐blockers compared to placebo or no treatment for chronic heart failure with preserved ejection fraction | ||||||
| Patient or population: chronic heart failure with preserved ejection fraction | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
| Risk with placebo/no treatment | Risk with Beta‐blockers | |||||
| Cardiovascular mortality (RR) | Study population | RR 0.78 | 1046 | ⊕⊕⊝⊝ | Three additional studies (ELANDD; SWEDIC; Takeda 2004) reported that no deaths occurred | |
| 173 per 1000 | 135 per 1000 | |||||
| Heart failure hospitalisation (RR) | Study population | RR 0.73 | 449 | ⊕⊝⊝⊝ | Follow‐up unclear for SWEDIC. ELANDD reported that no hospitalisation due to heart failure occurred | |
| 117 per 1000 | 86 per 1000 | |||||
| Hyperkalaemia | 245 (1 RCT) | ⊕⊝⊝⊝ | J‐DHF reported one participant in the intervention group (N = 120) experienced hyperkalaemia but did not report on this outcome for the control group. No further data were available from any of the other studies. | |||
| All‐cause mortality (RR) | Study population | RR 0.82 | 1105 | ⊕⊕⊝⊝ | Follow‐up unclear for Adamyan 2010. ELANDD, SWEDIC and Takeda 2004 reported that no deaths occurred | |
| 243 per 1000 | 199 per 1000 | |||||
| Quality of life (Minnesota) | Mean quality of life (Minnesota) was 24 | MD 1 lower | ‐ | 93 | ⊕⊝⊝⊝ | Lower = better, 5 point difference considered to be clinically meaningful |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded by one level due to unclear selection bias in most studies. 2 Downgraded by one level due to concerns about the smaller study being more precise than the larger study. 3 Downgraded by one level due to large variation in size of effect. 4 Downgraded by two levels due to few events and wide CI. 5 Downgraded by two levels due to very small sample size. 6 Suspected publication bias; this is a patient‐relevant outcome that is not reported in most studies. 7 Downgraded by two levels due to incomplete reporting. | ||||||
| MRA compared to placebo or no treatment for chronic heart failure with preserved ejection fraction | ||||||
| Patient or population: chronic heart failure with preserved ejection fraction | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
| Risk with placebo/no treatment | Risk with MRA | |||||
| Cardiovascular mortality (RR) | Study population | RR 0.90 | 4070 | ⊕⊕⊕⊝ | Two additional trials (RAAM‐PEF, Kurrelmeyer 2014) reported that no deaths occurred | |
| 88 per 1000 | 79 per 1000 | |||||
| Heart failure hospitalisation (RR) | Study population | RR 0.82 | 3714 | ⊕⊕⊕⊝ | Three additional trials (ALDO‐DHF ,Kurrelmeyer 2014, Upadhya 2017) reported that no hospitalisation due to heart failure occurred | |
| 136 per 1000 | 112 per 1000 | |||||
| Hyperkalaemia | Study population | RR 2.11 | 4291 | ⊕⊕⊕⊕ | Two trials defined hyperkalaemia ≥ 5.5 mEg/L | |
| 83 per 1000 | 175 per 1000 | |||||
| All‐cause mortality | Study population | RR 0.91 | 4207 | ⊕⊕⊕⊝ | Two additional trials (RAAM‐PEF, Kurrelmeyer 2014) reported that no deaths occurred | |
| 133 per 1000 | 121 per 1000 | |||||
| Quality of life (Minnesota) | Mean quality of life (Minnesota) ranged from 20 to 25 | MD 0.84 higher | ‐ | 511 | ⊕⊕⊝⊝ | Lower = better, 5 points are considered a clinically significant difference We did not pre‐specify which QoL scale was to be reported in the 'Summary of findings' table. To aid comparisons among 'Summary of findings' tables we chose to include the Minnesota Living with Heart Failure questionnaire and not the SMD across two scales |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded by one level due to imprecision. 2 Downgraded by one level because one trial was open label. 3 Downgraded by one level due to small sample size. | ||||||
| ACEI compared to placebo or no treatment for chronic heart failure with preserved ejection fraction | ||||||
| Patient or population: chronic heart failure with preserved ejection fraction | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
| Risk with placebo/no treatment | Risk with ACEI | |||||
| Cardiovascular mortality (RR) | Study population | RR 0.93 | 945 | ⊕⊕⊕⊝ | One additional trial (Kitzman 2010) reported that no deaths occurred | |
| 86 per 1000 | 81 per 1000 | |||||
| Heart failure hospitalisation (RR) | Study population | RR 0.86 | 1019 | ⊕⊕⊕⊝ | ||
| 13 per 1000 | 11 per 1000 | |||||
| Hyperkalaemia | 74 (1 RCTs) | ⊕⊝⊝⊝ | One trial (Zi 2003) reported 2 events in the intervention group (N = 36), 0 events in the control group (N = 38) (RR 5.27, 95% CI 0.26 to 106.16) | |||
| All‐cause mortality (RR) | Study population | RR 0.99 | 1079 | ⊕⊕⊕⊝ | One additional trial (Kitzman 2010) reported that no deaths occurred | |
| 119 per 1000 | 119 per 1000 | |||||
| Quality of life (Minnesota) | Mean quality of life (Minnesota) ranged from 10.9 to 29 | MD 0.09 lower | ‐ | 154 | ⊕⊕⊝⊝ | Scale: 0 to 105, lower = better, 5 point difference considered clinically relevant One trial (SNEGOVIK) reported mean change from baseline of ‐19.8 for intervention and ‐10.7 for control |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded by one level due to wide CI. 2 Downgraded by one level due to risk of bias (open label). 3 Downgraded by one level due to low sample size. 4 Downgraded by one level due to unclear selection bias. | ||||||
| ARB compared to placebo or no treatment for chronic heart failure with preserved ejection fraction | ||||||
| Patient or population: chronic heart failure with preserved ejection fraction | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
| Risk with placebo/no treatment | Risk with ARB | |||||
| Cardiovascular mortality (RR) | Study population | RR 1.02 | 7254 | ⊕⊕⊕⊕ | One additional trial (Parthasarathy 2009) reported that no deaths occurred | |
| 131 per 1000 | 133 per 1000 | |||||
| Heart failure hospitalisation (RR) | Study population | RR 0.92 | 7254 | ⊕⊕⊕⊕ | ||
| 171 per 1,‐000 | 157 per 1,‐000 | |||||
| Hyperkalaemia | Study population | RR 1.88 | 7148 | ⊕⊕⊕⊕ | ||
| 3 per 1,000 | 5 per 1,000 | |||||
| All‐cause mortality (RR) | Study population | RR 1.01 | 7964 | ⊕⊕⊕⊕ | One additional trial (Parthasarathy 2009) reported that no deaths occurred | |
| 72 per 1000 | 73 per 1,‐000 | |||||
| Quality of life (Minnesota) | Mean quality of life (Minnesota) ranged from 10.9 to 31.6 | MD 0.41 higher | ‐ | 3117 | ⊕⊕⊕⊕ | Scale: 0 to 105, lower = better, 5 point difference considered clinically relevant |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
Antecedentes
Descripción de la afección
La insuficiencia cardíaca es un síndrome clínico que se caracteriza por disnea y fatiga debido a anomalías de la estructura y la función cardíaca por un gasto cardíaco inadecuado o por presiones elevadas en el llenado ventricular, o ambos (Ponikowski 2016). Según los datos disponibles de los Estados Unidos y Europa, se calcula que la prevalencia de la insuficiencia cardíaca es del 1% al 12% en la población adulta y se proyecta que aumente con el envejecimiento poblacional y la mejoría de la supervivencia de las enfermedades cardiovasculares (Roger 2013). La insuficiencia cardíaca es un problema significativo de salud pública que representa el 5% de los ingresos hospitalarios de urgencia en el Reino Unido y se asocia con mortalidad significativa, con una estimación de la supervivencia a los cinco años del 50% (NICE 2010). La insuficiencia cardíaca se clasifica según la fracción de eyección del ventrículo izquierdo (FEVI) en insuficiencia cardíaca con fracción de expulsión reducida (ICFEr, habitualmente considerada como una FEVI < 40%), e insuficiencia cardíaca con fracción de expulsión conservada (ICFEc, habitualmente una FEVI > 40%). Recientemente, la European Society of Cardiology definió un subgrupo intermedio como insuficiencia cardíaca con fracción de expulsión intermedia (ICFEm), definida como una FEVI del 40% al 49% (Ponikowski 2016). El American College of Cardiology la definió como ICFEc "borderline", definida como una FEVI del 41% al 49% (Yancy 2013). En esta revisión, la ICFEc se definió como FEVI > 40% porque los ensayos de ICFEc completados y en curso han utilizado un rango de valores de corte de FEVI entre el 40% y el 50%. La ICFEc representa aproximadamente la mitad de todos los casos de insuficiencia cardíaca; los resultados de mortalidad son similares a los de la ICFEr (Gerber 2015).
Descripción de la intervención
La inhibición neurohumoral con betabloqueantes (BB), inhibidores de la enzima convertidora de angiotensina (IECA) y antagonistas de los receptores mineralocorticoides (ARM) da lugar a una mejoría en la supervivencia y una reducción de las hospitalizaciones por insuficiencia cardíaca en los pacientes con ICFEr (CIBIS Investigators 1999; Consensus Trial Study Group 1987; Flather 2005; Hjalmarson 2000; Kotecha 2014; MERIT‐HF Study Group 1999; Packer 1999; Packer 2002; Packer 2001; Pitt 1999; Ponikowski 2016; SOLVD Investigators 1991; SOLVD Investigators 1992; Zannad 2011). Cuando los IECA o los ARM están contraindicados o no se toleran, se recomiendan los antagonistas de los receptores de angiotensina (BRA) como una opción para ambos, aunque la evidencia es limitada (Granger 2003). Los inhibidores de la neprilesina y del receptor de angiotensina (INRA) se recomiendan como una opción para los IECA, con eficacia superior en los pacientes con ICFEr que permanecen sintomáticos a pesar del tratamiento óptimo (McMurray 2014). Aunque en la ICFEc se observa activación neurohumoral (Hogg 2005), en esta población se han realizado relativamente menos ensayos clínicos de los tratamientos con inhibidores neurohumorales. La evidencia existente a partir de ensayos individuales de BB, ARM, IECA, BRA o ARM en pacientes con ICFEc no apoya una reducción de la mortalidad con estos tratamientos (Ponikowski 2016). Sin embargo, evidencia limitada indica que el candesartán (un BRA) (Yusuf 2003) y la espironolactona (un ARM) (Pitt 2014) pueden ser efectivos para reducir el número de pacientes hospitalizados con insuficiencia cardíaca.
Esta revisión quiso determinar si la inhibición neurohumoral con tratamientos que mejoren la mortalidad y la morbilidad en los pacientes con ICFEr (BB, ARM, IECA, BRA e INRA) tiene efectos beneficiosos similares en los pacientes con ICFEc.
De qué manera podría funcionar la intervención
En los pacientes con ICFEc, la función cardíaca inadecuada desencadena respuestas neurohumorales compensatorias similares a las observadas en la ICFEr (Hogg 2005). La activación del sistema renina‐angiotensina‐aldosterona (SRAA) y el aumento del tono del sistema nervioso simpático pueden ser mecanismos de adaptación a corto plazo; sin embargo, es probable que la activación crónica sea perjudicial. Modelos de enfermedades preclínicas de ICFEc indican que la activación del SRAA provoca hipertrofia por adaptación deficiente y fibrosis (Sharma 2014). Los IECA, los BRA o los ARM inhiben los componentes del SRAA para contrarrestar la hiperactivación que se presenta en los pacientes con insuficiencia cardíaca. Los INRA combinan la inhibición del SRAA con un BRA (valsartán) con un aumento del sistema del péptido natriurético mediante la inhibición de la neprilesina (sacubitril). La neprilesina es una endopeptidasa neutra que degrada varios péptidos vasoactivos endógenos que sirven para contrarrestar algunos de los efectos de la activación del SRAA (McMurray 2014). Es probable que los efectos beneficiosos del tratamiento con betabloqueantes en los pacientes con ICFEr estén mediados por una reducción de los efectos perjudiciales de un aumento del tono simpático que pueden incluir el aumento de la frecuencia cardíaca, los efectos energéticos miocárdicos adversos y la estimulación del SRAA (Sackner‐Bernstein 1995). Estos mecanismos también pueden ser importantes en la ICFEc y el efecto de los betabloqueantes para aumentar el tiempo de llenado diastólico puede ser particularmente importante (Sharma 2014). La población de pacientes con ICFEc es heterogénea con respecto a la etiología de la enfermedad y las comorbilidades. Sin embargo, es posible que la activación neurohumoral represente un mecanismo fisiopatológico común que se podría dirigir de forma exitosa para mejorar los resultados clínicos en los pacientes con insuficiencia cardíaca a través del espectro de la FEVI.
Por qué es importante realizar esta revisión
Hay incertidumbre con respecto a si los betabloqueantes o los inhibidores del SRAA son efectivos en la reducción de la mortalidad y hospitalización por insuficiencia cardíaca y para mejorar la calidad de vida en los pacientes con ICFEc. Las guías no ofrecen recomendaciones específicas de tratamiento con respecto a su uso más allá del control de las comorbilidades, aparte de una recomendación para la administración de los BRA para reducir las hospitalizaciones (recomendación IIb, Yancy 2013) y para la administración de los ARM (recomendación débil; evidencia de calidad moderada, Ezekowitz 2017). El National Institute for Health and Care Excellence (NICE) del Reino Unido señaló la revisión de la evidencia como una prioridad de investigación (NICE 2010).
Una revisión sistemática y metanálisis recientes de la farmacoterapia en la ICFEc incluyó los betabloqueantes y los inhibidores del SRAA (IECA, BRA y ARM), e indicó una reducción en la mortalidad cardiovascular y por todas las causas con el tratamiento con los betabloqueantes (Zheng 2017). Se necesita una revisión actualizada con una estrategia de búsqueda más integral para informar las recomendaciones de nuevas guías y la realización de ensayos clínicos adicionales.
Objetivos
Evaluar los efectos de los betabloqueantes, los inhibidores de la enzima convertidora de angiotensina, los bloqueadores de los receptores de angiotensina, los inhibidores de la neprilesina y del receptor de angiotensina y los antagonistas de los receptores mineralocorticoides en los pacientes con insuficiencia cardíaca con fracción de expulsión conservada.
Métodos
Criterios de inclusión de estudios para esta revisión
Tipos de estudios
Se incluyeron los ensayos controlados aleatorios (ECA) con diseño de grupos paralelos. Se excluyeron los ensayos cruzados porque se consideraron inapropiados para la pregunta de revisión debido a la naturaleza progresiva de la insuficiencia cardíaca.
Fueron elegibles para inclusión los estudios publicados como texto completo o como resúmenes, o solamente disponibles como datos no publicados.
Tipos de participantes
Se incluyeron los estudios con participantes adultos (edad ≥ 18 años) con ICFEc definida por una fracción de expulsión del ventrículo izquierdo mayor del 40% (FEVI > 40%). Se reconoció que era probable que hubiera heterogeneidad significativa entre las poblaciones de estudio con respecto a la definición de la enfermedad, y en la Discusión se incluye un resumen narrativo. Se estableció contacto con los autores de los estudios para obtener datos sobre el subgrupo de interés en los estudios con poblaciones mixtas con respecto a la fracción de expulsión.
Tipos de intervenciones
Se realizaron metanálisis separados de los estudios que compararon BB, ARM, IECA o BRA, además de atención habitual, con placebo o control ningún tratamiento. No se realizó un metanálisis de los INRA porque no se identificaron ensayos.
Tipos de medida de resultado
Resultados primarios
-
Mortalidad cardiovascular.
-
Hospitalización por insuficiencia cardíaca.
-
Hiperpotasemia.
Resultados secundarios
-
Mortalidad por todas las causas.
-
Calidad de vida (medida con el Minnesota Living With Heart Failure Questionnaire o el Kansas City Cardiomyopathy Questionnaire).
-
Retiro debido a eventos adversos (hipotensión, hiperpotasemia o deterioro renal).
El informe en el ensayo de algún otro de los resultados enumerados no fue un criterio de inclusión para la revisión. Los resultados se evaluaron al seguimiento más largo informado.
Métodos de búsqueda para la identificación de los estudios
Búsquedas electrónicas
We identified trials through systematic searches of the following bibliographic databases on 25 July 2017:
-
Cochrane Central Register of Controlled Trials (CENTRAL) in the Cochrane Library (Wiley, Issue 6, June 2017);
-
MEDLINE (Ovid, 1946 to July Week 2 2017);
-
MEDLINE In‐Process & Other Non‐Indexed Citations, Epub Ahead of Print (Ovid, 24 July 2017); and
-
Embase and Embase Classic (embase.com, 1974 to 25 July 2017).
The search strategies used are included in Appendix 1. We applied the Cochrane sensitivity‐maximising RCT filter (Lefebvre 2011) to the main segment of MEDLINE (Ovid) (point 2 above) but did not use the filter when searching the MEDLINE Epub and In‐Process database segments. We used the multi‐term Embase filter with the best optimisation of sensitivity and specificity (Wong 2006) translated from Ovid to embase.com syntax.
We did not impose any restriction on language of publication.
Búsqueda de otros recursos
We searched ClinicalTrials.gov (https://clinicaltrials.gov/) and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) Search Portal (http://apps.who.int/trialsearch/) on 25 July 2017 to identify ongoing and unpublished trials. Search terms for the trials registers are also listed in Appendix 1.
We checked all primary references of included studies and systematic reviews for additional references. For any studies identified as eligible from clinical trial register records, we searched for the trials registry number on PubMed for publications about this study.
We contacted study authors to clarify details or obtain additional information not included in the published reports.
Obtención y análisis de los datos
Selección de los estudios
Two review authors (KM and NM) independently screened titles and abstracts of all records identified in our search and coded them as 'retrieve' (eligible or potentially eligible/unclear) or 'do not retrieve'. In the event of disagreement, a third review author was asked to arbitrate (TL). We then retrieved the full‐text study reports for records identified as eligible, potentially eligible or unclear. Two review authors (KM and NM) independently screened the full‐text and identified studies for inclusion. We recorded reasons for exclusion of ineligible studies. We resolved any disagreement by consensus or consulted a third review author (TL). We identified and excluded duplicates and collated multiple reports of the same study so that each study rather than each report is the unit of interest in the review. We recorded the selection process and completed a PRISMA flow diagram (Figure 1) and Characteristics of excluded studies table.
Extracción y manejo de los datos
We used a data collection form to record study characteristics and outcome data from included studies, which had been piloted on two studies in the review (PEP‐CHF; TOPCAT). Some modifications were made after the pilot phase. Two review authors (NM and TL) extracted study characteristics from included studies as follows:
-
Methods: study design, duration of follow‐up, details of any 'run in' period, number of study centres and location, study setting, withdrawals, and start/end date of enrolment.
-
Participants: N randomised/withdrawn/lost to follow‐up/analysed, mean age/age range, % male, inclusion criteria, exclusion criteria, systolic blood pressure, heart rate, body mass index, serum creatinine, B‐type natriuretic peptide, NT pro B‐type natriuretic peptide, LVEF, New York Heart Association (NYHA) class, comorbidity (hypertension, diabetes, atrial fibrillation, hospitalisation for heart failure, coronary heart disease, stroke, medications at baseline).
-
Interventions: intervention, comparison, concomitant medications (diuretic, digoxin, BB, ACEI, ARB, MRA).
-
Outcomes: planned and reported.
-
Notes: sources of funding, and notable conflicts of interest of trial authors.
Two review authors (NM and TL) independently extracted outcome data from included studies. Disagreements were resolved by consensus. One review author (NM) transferred data into the Review Manager (RevMan 2014) file. One review author (TL) double‐checked that data were entered correctly by comparing the data presented in the systematic review with the data extraction sheet.
Evaluación del riesgo de sesgo de los estudios incluidos
Two review authors (NM and TL) independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Disagreements were resolved by consensus. We assessed the risk of bias according to the following domains.
-
Random sequence generation.
-
Allocation concealment.
-
Blinding of participants and personnel.
-
Blinding of outcome assessment.
-
Incomplete outcome data.
-
Selective outcome reporting.
-
Other bias.
We judged each potential source of bias as high, low or unclear and provided quotes from study reports together with justification for our judgment in the 'Risk of bias' table. We summarised the 'Risk of bias' judgements across different studies for each of the domains listed. Where information on risk of bias related to unpublished data or correspondence with a trialist, we noted this in the 'Risk of bias' table.
When considering treatment effects in the pooled analysis, we accounted for risk of bias for the studies that contributed to each outcome tested.
Assessment of bias in conducting the systematic review
We conducted the review according to the published protocol and reported protocol deviations in the Differences between protocol and review section.
Medidas del efecto del tratamiento
We analysed dichotomous data as risk ratios (RR) with 95% confidence intervals (CI) and continuous data as mean difference (MD) or standardised mean difference (SMD) with 95% CIs. We analysed mortality data as hazard ratios (HR). We used SMD for one analysis when combining quality of life data reported for two different scales. We entered data presented as a scale with a consistent direction of effect.
Cuestiones relativas a la unidad de análisis
We included one three‐arm trial (Hong Kong DHF); because two intervention arms contributed to two separate comparisons, no unit of analysis issue arose.
Manejo de los datos faltantes
We contacted investigators or study sponsors to verify key study characteristics and obtain missing numerical outcome data where possible.
Evaluación de la heterogeneidad
We used the I² statistic to measure heterogeneity among the trials in each analysis. We considered possible causes in cases of substantial heterogeneity (I² ≥ 50%).
Evaluación de los sesgos de notificación
We pooled fewer than 10 trials for each comparison. Therefore, we did not examine funnel plots to explore possible small‐study biases for the primary outcomes.
Síntesis de los datos
We undertook meta‐analyses only where this was meaningful, that is, if the treatments, participants and the underlying clinical question were similar enough for pooling to make sense. We used a fixed‐effect model in the absence of substantial heterogeneity (I² < 50%) and a random‐effects model when unexplained substantial heterogeneity was present (I² ≥ 50%). We applied a random‐effects model for quality of life analyses for MRA to account the high heterogeneity observed for the Kansas City Cardiomyopathy Questionnaire (KCCQ) (Analysis 2.7; I² = 86%) and to permit a combined analysis with outcome data from the Minnesota Living With Heart Failure Questionnaire (MLHFQ) (Analysis 2.6; I² = 50%).
We considered two relevant quality of life scales: the MLHFQ or KCCQ. The MLHFQ score has a range from 0 to 105, lower scores indicate better quality of life. The KCCQ score has a range from 0 to 100, higher scores indicate better quality of life. To account for the difference in the direction of the scale of the KCCQ, the mean values were multiplied by ‐1 (Cochrane Handbookfor Systematic Reviews of Interventions, section 9.2.3.2, Deeks 2011). For the purpose of interpretation, we considered a five point difference in score as clinically significant for the MLHFQ (Rector 1995) and KCCQ (Spertus 2005).
'Summary of findings' table
We created 'Summary of findings' tables for each of our four interventions and included the following outcomes: cardiovascular mortality, heart failure hospitalisation, all‐cause mortality, quality of life (Minnesota Living with Heart Failure Questionnaire) and hyperkalaemia. We used the five GRADE considerations (study limitations, inconsistencies, imprecision, indirectness and publication bias) to assess the quality of the body of evidence as it related to the studies which contributed data to the meta‐analyses for the prespecified outcomes. We used methods and recommendations described in Section 8.5 and Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) using GRADEpro software (GRADEpro GDT). Four review authors assessed the quality of evidence (TL, NM, KM, CD). We documented our justification for decisions to downgrade the quality of evidence using footnotes.
Análisis de subgrupos e investigación de la heterogeneidad
We planned to carry out the following subgroup analyses.
-
Age.
-
Sex.
-
Heart failure with mid‐range ejection fraction (HFmrEF) LVEF 40% to 49% and preserved LVEF ≥ 50%.
-
Length of follow‐up < 12 months and ≥ 12 months.
We were only able to perform a subgroup analysis based on length of follow‐up because data for the prespecified subgroups were unavailable.
We used the outcomes cardiovascular mortality and hospitalisation for heart failure in subgroup analyses.
We used the formal test for subgroup interactions in Review Manager (RevMan 2014).
Análisis de sensibilidad
We performed a sensitivity analysis for risk of bias by performing a pooled analysis including only studies with a low risk of bias (where at least four of the six domains for bias assessment were judged to be low risk and no domain was at high risk of bias).
Results
Description of studies
Results of the search
The database searches retrieved 7364 records and the search of clinical trial registries retrieved 467 records. After de‐duplication, 5789 records were screened by title and abstract. Of these, 5241 records did not meet the inclusion criteria and were excluded. The remaining 548 records were assessed for eligibility in full‐text and 303 studies (324 references) were excluded.
Included studies
We included 37 studies (207 reports) that involved a total of 18,311 participants.
Beta‐blockers
We included 10 studies (3087 participants) that investigated beta‐blockers for HFpEF. Of these, five studies compared beta‐blockers versus placebo (ELANDD; Mittal 2017; Sahoo 2016; SENIORS; SWEDIC) and five versus usual care (Adamyan 2010; Aronow 1997; J‐DHF; Shu 2005; Takeda 2004). Four studies investigated carvedilol: Adamyan 2010 (up to 50 mg daily), J‐DHF (up to 10 mg twice daily), SWEDIC (up to 25 mg twice daily or 50 mg twice daily in people weighing over 85 kg), Takeda 2004 (up to 20 mg daily). Two studies used nebivolol: ELANDD (up to 10 mg daily) and SENIORS (up to 10 mg daily). One study used propranolol: Aronow 1997 (30 mg, 3 times daily); and two studies investigated metoprolol succinate: Mittal 2017; Sahoo 2016 (up to 100 mg daily). Shu 2005 investigated bisoprolol (up to 10 mg daily).
Numbers of participants randomised ranged from 40 (Mittal 2017; Takeda 2004) to 643 (SENIORS).
Four were multicentre studies. ELANDD was conducted across 12 centres in eight countries in Europe; J‐DHF was assumed to have taken place in Japan; SENIORS took place in 11 countries (Czech Republic, France, Germany, Hungary, Italy, Netherlands, Romania, Spain, Switzerland, UK and Ukraine), and SWEDIC took place in 12 centres in Sweden. Mittal 2017 and Sahoo 2016 were each conducted in one centre in India. Adamyan 2010, Aronow 1997 and Shu 2005 did not report numbers of centres or countries, but we assumed that Adamyan 2010 likely took place in Armenia. Takeda 2004 was a single centre trial in Japan.
Three studies did not report LVEF of the included participants at baseline (Adamyan 2010; Shu 2005; SWEDIC). Six studies reported LVEF at baseline with a mean ranging from 56% to 63% (Aronow 1997; ELANDD; J‐DHF; Mittal 2017; Sahoo 2016; Takeda 2004). SENIORS included participants with a "clinical history of chronic HF with at least 1 of the following features: documented hospital admission within the previous 12 months with a discharge diagnosis of congestive HF or documented LVEF ≤ 35% within the previous 6 months". The SENIORS study reported a subgroup of participants with LVEF > 40% and these outcome data were used in our analysis (643 participants).
Most participants were NYHA class II (51% to 78%). Shu 2005 did not report participants' NYHA class at baseline. Participants' mean age ranged from 30 years to 81 years; six studies reported mean age less than 70 years (Adamyan 2010; ELANDD; Mittal 2017; Sahoo 2016; Shu 2005; SWEDIC) and four reported mean age above 70 years (Aronow 1997; J‐DHF; SENIORS; Takeda 2004).
Three studies were funded by industry (ELANDD; SENIORS; SWEDIC); two studies were funded by not‐for‐profit organisations (J‐DHF; Mittal 2017); and five did not report sources of funding (Adamyan 2010; Aronow 1997; Sahoo 2016; Shu 2005; Takeda 2004).
Mineralocorticoid receptor antagonists (MRA)
We included 12 studies that investigated MRAs for HFpEF. Of these, eight compared MRA versus placebo (ALDO‐DHF; AREA IN‐CHF; Kurrelmeyer 2014; Mottram 2004; RAAM‐PEF; STRUCTURE; TOPCAT; Upadhya 2017) and four versus usual care (Karapysh 2015; Mak 2009; Orea‐Tejeda 2007; Wang 2010). Nine studies investigated spironolactone (ALDO‐DHF; Kurrelmeyer 2014; Mottram 2004; STRUCTURE; Upadhya 2017 (25 mg/d); Karapysh 2015; Orea‐Tejeda 2007 (25 mg/d up‐titrated if tolerated to 50 mg/d); TOPCAT (15 mg/d, increased to a maximum of 45 mg/d); Wang 2010 (50 mg/d)). Two studies used eplerenone (Mak 2009; RAAM‐PEF (25 mg/d to a maximum of 50 mg/d)). AREA IN‐CHF investigated canrenone at a maximum dose of 50 mg/d.
Numbers of participants randomised ranged from 28 (Orea‐Tejeda 2007) to 3445 (TOPCAT). Four were multicentre trials; ALDO‐DHF included 10 centres in Germany and Austria; AREA IN‐CHF was conducted in 46 centres in Italy; STRUCTURE had centres in Poland (the number is unclear but publication states "of each centre"); and TOPCAT was conducted across 233 sites in six countries (Argentina, Brazil, Canada, Georgia, Russia, USA). Two studies were single‐centre trials in USA (Kurrelmeyer 2014; RAAM‐PEF). Mottram 2004 was a single centre trial in Australia. Wang 2010 was a single centre trial in Taiwan. Mak 2009 was a single‐centre trial but the country was unspecified. Three trials did not report on numbers of centres or countries (Karapysh 2015; Orea‐Tejeda 2007; Upadhya 2017).
Two studies (Karapysh 2015; Mottram 2004) did not report participants' LVEF at baseline. AREA IN‐CHF had a mean LVEF at baseline of 39.9% (intervention) and 39.7% (control) for the overall included participants (N = 467). However, we obtained outcome data for the subgroup of participants with LVEF > 40% (N = 225). The LVEF in the remaining seven studies ranged from 62% to 72%.
Most participants in five studies were NYHA class II (52% to 88%; ALDO‐DHF; Mak 2009; RAAM‐PEF; STRUCTURE; TOPCAT). Most participants in two studies were NYHA class III (58% to 64%; Kurrelmeyer 2014; Upadhya 2017). Three studies did not report NYHA class for participants eligible for inclusion in our review (AREA IN‐CHF; Karapysh 2015; Mottram 2004). Orea‐Tejeda 2007 reported that most participants in the intervention arm were NYHA class III (57.1%) and NYHA class I (75%) in the control arm.
Participants' mean age ranged from 54.5 years to 80 years; seven studies included participants whose mean age was less than 70 years (ALDO‐DHF; AREA IN‐CHF; Karapysh 2015; Mottram 2004; Orea‐Tejeda 2007; STRUCTURE; TOPCAT). In four studies, participants' mean age was over 70 years (Kurrelmeyer 2014; Mak 2009; RAAM‐PEF; Upadhya 2017).
AREA IN‐CHF was industry funded; six studies were funded by not‐for‐profit organisations (ALDO‐DHF; Kurrelmeyer 2014; RAAM‐PEF; STRUCTURE; TOPCAT; Upadhya 2017). Five studies did not report sources of funding (Karapysh 2015; Mak 2009; Mottram 2004; Orea‐Tejeda 2007; Wang 2010).
Angiotensin converting enzyme inhibitors (ACEI)
We included eight studies that investigated ACEIs for HFpEF. Of these, three compared ACEI with placebo (Kitzman 2010; PEP‐CHF; Zi 2003), and five versus usual care (Aronow 1993; Aronow 1998; Hong Kong DHF; SNEGOVIK; Yuksek 2012). Two studies investigated enalapril (Aronow 1993, up to 20 mg daily; Kitzman 2010, up to 10 mg daily). Aronow 1998 investigated benazepril (up to 40 mg/d). Two studies investigated perindopril (PEP‐CHF, up to 4 mg daily; Yuksek 2012, up to 10 mg). Hong Kong DHF investigated ramipril in one of two active arms (maximum of 10 mg daily). Two studies investigated quinapril (SNEGOVIK, dose not reported; Zi 2003, up to 40 mg daily).
Numbers of participants randomised ranged from 21 (Aronow 1993) to 850 (PEP‐CHF). Two studies were reportedly multicentre trials (Hong Kong DHF; PEP‐CHF). Hong Kong DHF did not report details on the number of centres. PEP‐CHF was conducted at 53 centres in Bulgaria (3), Czech Republic (5), Hungary (10), Ireland (1), Poland (26), Russia (1), Slovakia (2), and the UK (5). Zi 2003 took place at one hospital in the UK and Yuksek 2012 was conducted in Turkey. The countries or number of centres were not reported in four studies (Aronow 1993; Aronow 1998; Kitzman 2010; SNEGOVIK).
The mean LVEF of the included participants at baseline was not reported by two studies (SNEGOVIK; Zi 2003). LVEF ranged from 61% to 69% in five studies (Aronow 1993; Aronow 1998; Hong Kong DHF; Kitzman 2010; PEP‐CHF). Most participants were classified in NYHA class II in four studies (Hong Kong DHF; Kitzman 2010; PEP‐CHF; Zi 2003) and in NYHA class III in one study (Aronow 1993). Two studies did not report participants' NYHA class at baseline (Aronow 1998; SNEGOVIK).
Participants' mean age ranged from 70 years to 82 years with all studies equal to or over a mean age of 70 years.
Four studies did not report funding sources (Aronow 1993; Aronow 1998; SNEGOVIK; Yuksek 2012). Three studies were industry funded (Hong Kong DHF; PEP‐CHF; Zi 2003) and one study was funded by a not‐for profit organisation (Kitzman 2010).
Angiotensin receptor blockers (ARB)
We included eight studies that investigated ARBs for HFpEF. Of these, five compared ARB versus placebo (CAN‐DHF; CHARM‐Preserved; I‐PRESERVE; Kasama 2005; Parthasarathy 2009) and three compared ARB versus usual care (CandHeart; Hong Kong DHF; SUPPORT). Four studies investigated candesartan (CAN‐DHF; CandHeart; CHARM‐Preserved (up to 32 mg daily), Kasama 2005 (8 mg to 12 mg daily)). Two studies investigated irbesartan (one of the two active treatment arms in Hong Kong DHF (up to 75 mg daily), I‐PRESERVE (up to 300 mg)). Parthasarathy 2009 investigated valsartan (80 mg daily). SUPPORT investigated olmesartan (up to 40 mg daily).
Numbers of participants randomised ranged from 22 (CAN‐DHF) to 4128 (I‐PRESERVE). Seven were multicentre trials: CAN‐DHF was conducted at eight centres in Germany; CandHeart at 70 centres in Italy; CHARM‐Preserved was conducted at 618 centres in 26 countries; I‐PRESERVE involved 293 centres in 25 countries; Parthasarathy 2009 was conducted at five centres each in Germany and the UK; and SUPPORT was conducted at 17 centres in Japan. Hong Kong DHF was reported to be a multicentre trial but no details were provided on numbers of centres or countries. Kasama 2005 was reported to be a single‐centre trial in Japan.
The mean LVEF of the included participants at baseline was not reported by CAN‐DHF and ranged from 49% to 72% in seven studies (CandHeart; CHARM‐Preserved; Hong Kong DHF; I‐PRESERVE; Kasama 2005; Parthasarathy 2009; SUPPORT). Most participants were assessed as NYHA class II at baseline in five studies (CandHeart; CHARM‐Preserved; Hong Kong DHF; Kasama 2005; SUPPORT); NYHA class III in I‐PRESERVE; and was not reported by two studies (CAN‐DHF; Parthasarathy 2009).
Participants' mean age ranged from 61 years to 75 years. Mean age was below 70 years in six studies (CAN‐DHF; CandHeart; CHARM‐Preserved; Kasama 2005; Parthasarathy 2009; SUPPORT) and over 70 years in two studies (Hong Kong DHF; I‐PRESERVE).
Six studies were funded by industry (CAN‐DHF; CandHeart; CHARM‐Preserved; Hong Kong DHF; I‐PRESERVE; Parthasarathy 2009). SUPPORT was funded by a not‐for‐profit organisation. Kasama 2005 did not report the source of funding.
Angiotensin receptor neprilysin inhibitors (ARNI)
We did not identify any completed trials that compared ARNI to placebo or no treatment control. However, one completed and two ongoing active controlled studies were identified investigating ARNI for HFpEF. Although people with HFpEF and co‐existing hypertension are often treated with the active comparators used in these studies (ARB or ACEI), these therapies are not considered as usual care for HFpEF.
The PARAMOUNT study, which randomised participants with HFpEF defined as heart failure with LVEF ≥ 45%, investigated ARNI (sacubitril/valsartan), (N = 149) or matching ARB (valsartan), (N = 152) (Zile 2016). The primary outcome measure was change in N‐terminal pro b‐type natriuretic peptide (NT‐proBNP), and secondary outcomes included echocardiographic parameters, NYHA class, and quality of life (KCCQ). During the 36‐week follow‐up, one death occurred in the ARNI group and two deaths in the ARB group. Hyperkalaemia and quality of life outcomes did not differ between groups (Solomon 2012). PERSPECTIVE (NCT02884206) is an ongoing RCT to examine the effect of LCZ696 compared to valsartan on cognitive outcomes in participants with HFpEF, defined as LVEF > 40%. NCT03066804 is a four‐arm, parallel group study to compare the effects of sacubitril/valsartan, enalapril, valsartan and placebo in participants with HFpEF (LVEF ≥ 45%), with estimated enrolment of 2200 participants.
PARAGON‐HF (NCT01920711) is a large active comparator RCT comparing sacubitril/valsartan with valsartan for a composite primary outcome of cardiovascular death and heart failure hospitalisation. The study is ongoing with 4822 participants enrolled, and an estimated study completion date of March 2019.
Excluded studies
We excluded 303 studies (324 references) based on full‐text assessment. Details for the reasons for exclusion are provided in the Characteristics of excluded studies table. In summary we made exclusions based on:
-
wrong population: n = 116;
-
wrong intervention: n = 8;
-
wrong comparator: n = 20;
-
wrong study design: n = 118;
-
subgroup of interest but no response to our enquiry for data: n = 8;
-
unclear eligibility and no response to our enquiry for details: n = 10;
-
unclear eligibility and no current contact details: n = 13;
-
completed status in trial registry record but no published results and no response to our enquiry for data: n = 1;
-
missing data and response that no details can be provided: n = 6;
-
retraction: n = 1; and
-
did not take place as planned: n = 2.
Studies awaiting classification
We identified eight studies that await classification (Anonymous 2003d; Botoni 2010; Dielievska 2015; Gao 2010; Liu 2006; Metra 1999; Rapezzi 1999; Zheng 2009; Characteristics of studies awaiting classification). We are waiting to retrieve the full‐text (n = 4), responses from translators (n = 3) and response from the trialists to clarify eligibility (n = 1).
Ongoing studies
We identified five ongoing studies (EudraCT 2013‐000867‐10; IMPRESS‐AF; NCT02901184; NCT03066804; Zhou 2010; Characteristics of ongoing studies). Three studies are investigating MRAs: spironolactone versus placebo (EudraCT 2013‐000867‐10; IMPRESS‐AF); spironolactone versus usual care (NCT02901184). NCT03066804 is a four‐arm trial comparing ARNI (sacubitril/valsartan), ACEI (enalapril), ARB (valsartan) and matching placebo. Zhou 2010 is testing a beta‐blocker (metoprolol succinate) versus usual care.
Risk of bias in included studies
The risk of bias assessments are detailed in the Characteristics of included studies tables, and summarised in the text below and in Figure 2 and Figure 3.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
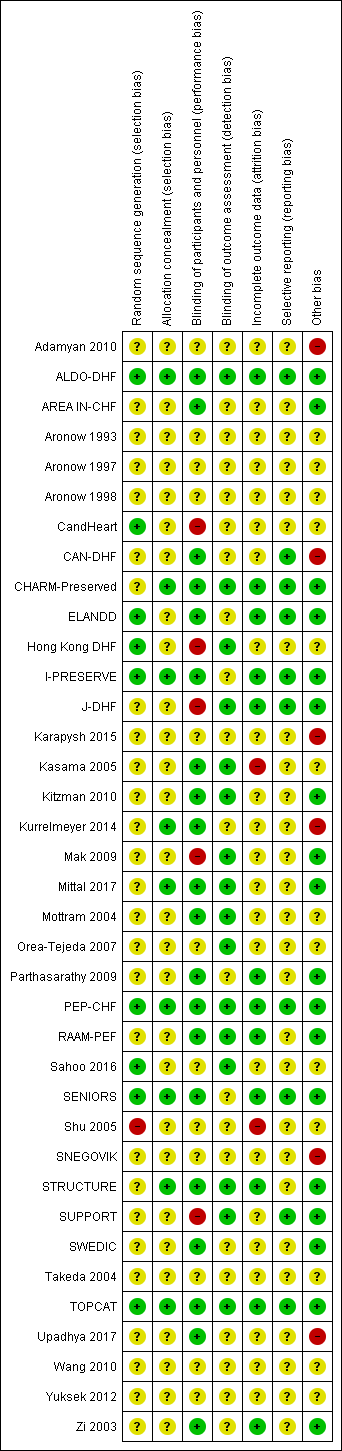
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Nine studies reported random sequence methods and were rated as low risk of bias (ALDO‐DHF; CandHeart; ELANDD; Hong Kong DHF; I‐PRESERVE; PEP‐CHF; Sahoo 2016; SENIORS; TOPCAT). We assessed 28 studies at unclear risk of bias for this domain because no information was provided in study reports.
Nine studies used a method for allocation concealment that was judged to be of low risk of bias (ALDO‐DHF; CHARM‐Preserved; I‐PRESERVE; Kurrelmeyer 2014; Mittal 2017; PEP‐CHF; SENIORS; STRUCTURE; TOPCAT). We assessed 28 studies at unclear risk of bias for this domain because no information was provided in study reports.
Blinding
We assessed 20 studies as low risk of bias regarding blinding of participants and personnel (ALDO‐DHF; AREA IN‐CHF; CAN‐DHF; CHARM‐Preserved; ELANDD; I‐PRESERVE; Kasama 2005; Kitzman 2010; Kurrelmeyer 2014; Mittal 2017; Mottram 2004; Parthasarathy 2009; PEP‐CHF; RAAM‐PEF; SENIORS; STRUCTURE; SWEDIC; TOPCAT; Upadhya 2017; Zi 2003). Five studies were open‐label designs and therefore judged to be at high risk of bias for this domain (CandHeart; Hong Kong DHF; J‐DHF; Mak 2009; SUPPORT). The remaining 12 studies were assessed at unclear risk of bias because no information was provided.
Detection bias was judged to be at low risk in 16 studies (ALDO‐DHF; CHARM‐Preserved; Hong Kong DHF; J‐DHF; Kasama 2005; Kitzman 2010; Mak 2009; Mittal 2017; Mottram 2004; Orea‐Tejeda 2007; PEP‐CHF; RAAM‐PEF; Sahoo 2016; STRUCTURE; SUPPORT; TOPCAT). The remaining 21 studies did not provide information and were judged to be at unclear risk of detection bias.
Incomplete outcome data
Attrition bias was judged to be at low risk in 12 studies (ALDO‐DHF; CHARM‐Preserved; ELANDD; I‐PRESERVE; J‐DHF; Parthasarathy 2009; PEP‐CHF; RAAM‐PEF; SENIORS; STRUCTURE; TOPCAT; Zi 2003). We judged Kasama 2005 to be at high risk of bias for this domain because the study report did not indicate if losses to follow‐up or withdrawals occurred. All other 25 studies were assessed as unclear risk of bias for attrition bias as no information was reported to allow judgement.
Selective reporting
We assessed 10 studies to be at low risk of reporting bias (ALDO‐DHF; CAN‐DHF; CHARM‐Preserved; ELANDD; I‐PRESERVE; J‐DHF; PEP‐CHF; SENIORS; SUPPORT; TOPCAT). These 10 studies reported planned outcomes in either published protocols or clinical trial registers before enrolment started. We were unable to assess reporting bias in 27 studies because either no information was available in the form of protocols or clinical trial registry entries, or they were published/entered after enrolment was completed.
Other potential sources of bias
We judged 18 studies to be at low risk of other biases (mainly based on providing details on funding and declaring any conflict of interest by the authors) (ALDO‐DHF; AREA IN‐CHF; CHARM‐Preserved; ELANDD; I‐PRESERVE; J‐DHF; Kitzman 2010; Mak 2009; Mittal 2017; Parthasarathy 2009; PEP‐CHF; RAAM‐PEF; SENIORS; STRUCTURE; SUPPORT; SWEDIC; TOPCAT; Zi 2003).
We judged six studies to be at high risk of other bias. Kurrelmeyer 2014 was originally registered as an observational study and this detail was changed after completion of the trial but before the results were published. Five studies (Adamyan 2010; CAN‐DHF; Karapysh 2015; SNEGOVIK; Upadhya 2017) were published as conference abstracts only; withholding the full results from publication may present a form of bias. The remaining 26 studies were judged to be at unclear risk of bias.
Effects of interventions
See: Summary of findings for the main comparison Beta‐blockers compared to placebo or no treatment for chronic heart failure with preserved ejection fraction; Summary of findings 2 MRA compared to placebo or no treatment for chronic heart failure with preserved ejection fraction; Summary of findings 3 ACEI compared to placebo or no treatment for chronic heart failure with preserved ejection fraction; Summary of findings 4 ARB compared to placebo or no treatment for chronic heart failure with preserved ejection fraction
Beta‐blockers versus placebo or no treatment
We included 10 studies that involved a total of 3087 participants that assessed beta‐blockers versus placebo or no treatment. The main outcomes for this comparison are included in summary of findings Table for the main comparison.
Cardiovascular mortality
Six studies reported cardiovascular mortality (Aronow 1997; ELANDD; J‐DHF; SENIORS; SWEDIC; Takeda 2004). Three studies reported that no deaths occurred (ELANDD; SWEDIC; Takeda 2004). We included three studies in the meta‐analysis (Aronow 1997; J‐DHF; SENIORS) (15% of participants in the intervention arm versus 19% in the control arm; RR 0.78; 95% CI 0.62 to 0.99; NNTB 25; 1046 participants; I² = 0%; low‐quality evidence; Analysis 1.1).
J‐DHF reported cardiovascular mortality but with different numbers for events within the same table (Table 2 in the primary reference). We contacted the study authors to seek clarification but are yet to receive a response; we used the higher numbers in the analysis.
SENIORS reported a hazard ratio (HR 0.80; 95% CI 0.49 to 1.32; 643 participants).
Heart failure hospitalisation
We included five studies that reported heart failure hospitalisation (ELANDD; J‐DHF; Shu 2005; SWEDIC; Takeda 2004). ELANDD reported that no hospitalisation occurred due to heart failure. Data from four studies (J‐DHF; Shu 2005; SWEDIC; Takeda 2004) contributed to the meta‐analysis (RR 0.73; 95% CI 0.47 to 1.13; 449 participants; I² = 22%; very low‐quality evidence; Analysis 1.2).
Hyperkalaemia
J‐DHF reported that one participant in the intervention group (N = 120) experienced hyperkalaemia but did not report on this outcome for the control group (very low‐quality evidence). No further data were available from any other studies.
All‐cause mortality
We included seven studies that reported all‐cause mortality (Adamyan 2010; Aronow 1997; ELANDD; J‐DHF; SENIORS; SWEDIC; Takeda 2004). Of these, three studies reported that no deaths occurred (ELANDD; SWEDIC; Takeda 2004). We included data from four studies in the meta‐analysis (Adamyan 2010; Aronow 1997; J‐DHF; SENIORS) (RR 0.82; 95% CI 0.67 to 1.00; 1105 participants; I² = 0%; low‐quality evidence; Analysis 1.3).
J‐DHF reported all‐cause mortality but with different numbers for events within the same table (Table 2 in the primary reference). We contacted the study authors to seek clarification but are yet to receive a response. We used the higher number of deaths in the analysis.
SENIORS reported a hazard ratio (HR 0.92; 95% CI 0.61 to 1.36; 643 participants).
Quality of life
We included two studies that reported quality of life (ELANDD; Mittal 2017). Mittal 2017 reported quality of life using SF‐36, which was not a scale we considered for our analysis. ELANDD reported end scores for the Minnesota Living with Heart Failure Questionnaire (MLHFQ) total score and showed MD ‐1.00 between the treatment arms, favouring the intervention (95% CI ‐9.05 to 7.05; 93 participants; very low‐quality evidence).
Withdrawal due to adverse event
We included five studies that reported withdrawals due to adverse events (Aronow 1997; ELANDD; J‐DHF; Mittal 2017; Sahoo 2016). Mittal 2017 and Sahoo 2016 reported no withdrawals due to adverse events. Aronow 1997 reported 11 withdrawals due to "worsening CHF [chronic heart failure] in 7 patients and hypotension in 4 patients" but did not provide this information by intervention arm. Only two studies (ELANDD; J‐DHF) contributed data for meta‐analysis (9% of participants in the intervention arm versus 0% in the control arm, RR 18.07; 95% CI 2.45 to 133.04; 338 participants; I² = 0%; Analysis 1.5; number needed to harm (NNTH) 11).
Mineralocorticoid receptor antagonists (MRA) versus placebo or no treatment
We included 12 studies (4408 participants) that assessed MRA versus placebo or no treatment. The main outcomes for this comparison are included in summary of findings Table 2. The findings for this comparison were driven by one trial (TOPCAT). Four trials (Karapysh 2015; Mottram 2004; Orea‐Tejeda 2007; Wang 2010) did not contribute any outcome data of interest for this review.
Cardiovascular mortality
We included five studies that reported cardiovascular mortality (ALDO‐DHF; AREA IN‐CHF; Kurrelmeyer 2014; RAAM‐PEF; TOPCAT). Of these, two studies reported that no deaths occurred (Kurrelmeyer 2014; RAAM‐PEF). We included data from three studies in the meta‐analysis (ALDO‐DHF; AREA IN‐CHF; TOPCAT) (RR 0.90; 95% CI 0.74 to 1.11; 4070 participants; I² = 0%; moderate‐quality evidence; Analysis 2.1).
TOPCAT also reported a hazard ratio (HR 0.90; 95% CI 0.73 to 1.12; 3445 participants).
Heart failure hospitalisation
We included six studies that reported heart failure hospitalisation (ALDO‐DHF; AREA IN‐CHF; Kurrelmeyer 2014; RAAM‐PEF; TOPCAT; Upadhya 2017). Of these, three studies reported no hospitalisations due to heart failure (ALDO‐DHF; Kurrelmeyer 2014; Upadhya 2017). We included data from three studies in the meta‐analysis (AREA IN‐CHF; RAAM‐PEF; TOPCAT) (11% of participants in the intervention arm versus 14% in the control arm, RR 0.82; 95% CI 0.69 to 0.98; 3714 participants; NNTB 41; I² = 22%; moderate‐quality evidence; Analysis 2.2).
Hazard ratios for time to first heart failure hospitalisation were reported for two studies (AREA IN‐CHF; TOPCAT) (HR 0.82; 95% CI 0.69 to 0.98; 3670 participants; I² = 59%; Analysis 2.3). The substantial heterogeneity was explained by differences in population characteristics (TOPCAT, LVEF ≥ 45%; AREA IN‐CHF subgroup, LVEF 40% to 45%).
Hyperkalaemia
We included six studies that reported hyperkalaemia (ALDO‐DHF; AREA IN‐CHF; Kurrelmeyer 2014; RAAM‐PEF; STRUCTURE; TOPCAT) (16% of participants in the intervention arm versus 8% in the control arm, RR 2.11; 95% CI 1.77 to 2.51; 4291 participants; I² = 0%; high‐quality evidence; Analysis 2.4).
All‐cause mortality
We included eight studies that reported all‐cause mortality (ALDO‐DHF; AREA IN‐CHF; Kurrelmeyer 2014; Mak 2009; RAAM‐PEF; STRUCTURE; TOPCAT; Upadhya 2017). Of these, three studies reported that no deaths occurred (Kurrelmeyer 2014; RAAM‐PEF; STRUCTURE). The meta‐analysis included data from five studies (ALDO‐DHF; AREA IN‐CHF; Mak 2009; TOPCAT; Upadhya 2017) (RR 0.91; 95% CI 0.78 to 1.06; 4207 participants; I² = 0%; moderate‐quality evidence; Analysis 2.5).
TOPCAT also reported a hazard ratio (HR 0.91; 95% CI 0.77 to 1.08; 3445 participants).
Quality of life
We included six studies that reported quality of life (ALDO‐DHF; Kurrelmeyer 2014; Mak 2009; RAAM‐PEF; TOPCAT; Upadhya 2017). TOPCAT reported quality of life in a report by Lewis 2016, but the end scores per treatment arm were not provided. We contacted the investigators and await details.
Three studies (ALDO‐DHF; Mak 2009; Upadhya 2017) reported total MLFHQ scores and were pooled for analysis (MD 0.84; 95% CI ‐2.30 to 3.98; 511 participants; I² = 0%; low‐quality evidence; Analysis 2.8). Kurrelmeyer 2014 and RAAM‐PEF reported Kansas City Cardiomyopathy Questionnaire (KCCQ) results and were pooled (MD ‐0.78; 95% CI ‐28.02 to 26.46; 92 participants; I² = 86%; Analysis 2.7). The substantial heterogeneity could not be explained.
All five studies that used MLHFQ and KCCQ were pooled (SMD 0.05; 95% CI ‐0.23 to 0.34; 603 participants; I² = 50%; Analysis 2.6). The substantial heterogeneity could not be explained.
Withdrawal due to adverse event
Four studies reported this outcome (ALDO‐DHF; Kurrelmeyer 2014; TOPCAT; Upadhya 2017) and contributed to the meta‐analysis (RR 1.10; 95% CI 1.00 to 1.21; 3986 participants; I² = 0%; Analysis 2.9).
Angiotensin converting enzyme inhibitors (ACEI) versus placebo or no treatment
We included eight studies that involved a total of 2061 participants that assessed ACEI versus placebo or no treatment. The main outcomes for this comparison are presented in summary of findings Table 3. The findings for this comparison were driven by PEP‐CHF. Two studies (Aronow 1993; Yuksek 2012) did not contribute any outcome data of interest for this review.
Cardiovascular mortality
Three studies reported cardiovascular mortality (Hong Kong DHF; Kitzman 2010; PEP‐CHF). Kitzman 2010 reported that no deaths occurred. Hong Kong DHF and PEP‐CHF contributed data to the meta‐analysis (RR 0.93; 95% CI 0.61 to 1.42; 945 participants; I² = 0%; moderate‐quality evidence; Analysis 3.1).
PEP‐CHF also reported a hazard ratio (HR 0.98; 95% CI 0.63 to 1.52; 850 participants).
Heart failure hospitalisation
Three studies (Hong Kong DHF; PEP‐CHF; Zi 2003) reported heart failure hospitalisation and were pooled for analysis (RR 0.86, 95% CI 0.64 to 1.15; 1019 participants; I² = 0%; moderate‐quality evidence; Analysis 3.2).
PEP‐CHF also reported a hazard ratio (HR 0.86, 95% CI 0.61 to 1.20; 850 participants).
Hyperkalaemia
Zi 2003 reported hyperkalaemia (RR 5.27; 95% CI 0.26 to 106.16; 74 participants; very low‐quality evidence; Analysis 3.3).
All‐cause mortality
We included five studies that reported all‐cause mortality (Aronow 1998; Hong Kong DHF; Kitzman 2010; PEP‐CHF; Zi 2003). Kitzman 2010 reported that no deaths occurred. Four studies (Aronow 1998; Hong Kong DHF; PEP‐CHF; Zi 2003) contributed to the meta‐analysis (RR 0.99; 95% CI 0.71 to 1.38; 1079 participants; I² = 0%; moderate‐quality evidence; Analysis 3.4).
PEP‐CHF also reported a hazard ratio (HR 1.09; 95% CI 0.75 to 1.58; 850 participants).
Quality of life
Three studies reported quality of life assessed using the MLHFQ scale (Hong Kong DHF; Kitzman 2010; SNEGOVIK). SNEGOVIK reported quality of life assessment based on the MLHFQ scale as change from baseline per treatment arm (‐18.9 for intervention, ‐10.7 for control). We were unsuccessful in our attempts to contact study authors to obtain scores at the end of follow‐up. Two studies (Hong Kong DHF; Kitzman 2010) contributed to the meta‐analysis (MD ‐0.09; 95% CI ‐3.66 to 3.48; 154 participants; I² = 4%; low‐quality evidence; Analysis 3.5).
Zi 2003 assessed quality of life using the McMaster quality of life questionnaire and reported end scores at six months and reported 12.9 ± 3.1 for the intervention and 13.1 ± 4.7 for the control arm.
Withdrawal due to adverse event
Three studies (Hong Kong DHF; PEP‐CHF; Zi 2003) reported this outcome and were pooled for analysis (RR 1.53; 95% CI 0.26 to 9.00; 1019 participants; I² = 59%; Analysis 3.6).
Angiotensin receptor blockers (ARB) versus placebo or no treatment
We included eight studies that involved a total of 8755 participants that assessed ARB versus placebo or no treatment. The main outcomes for this comparison are included in summary of findings Table 4. The findings for this comparison were driven by two studies (CHARM‐Preserved; I‐PRESERVE). Three trials (CAN‐DHF; CandHeart; Kasama 2005) did not contribute any outcome data of interest for this review.
Cardiovascular mortality
Four studies reported this outcome (CHARM‐Preserved; Hong Kong DHF; I‐PRESERVE; Parthasarathy 2009). Parthasarathy 2009 reported that no deaths occurred. Three studies (CHARM‐Preserved; Hong Kong DHF; I‐PRESERVE) contributed to the meta‐analysis (RR 1.02; 95% CI 0.90 to 1.14; 7254 participants; I² = 0%; high‐quality evidence; Analysis 4.1).
Two studies (CHARM‐Preserved; I‐PRESERVE) were also pooled for analysis (HR 1.00; 95% CI 0.89 to 1.13; 5087 participants; Analysis 4.2).
Heart failure hospitalisation
Three studies (CHARM‐Preserved; Hong Kong DHF; I‐PRESERVE) reported this outcome and were pooled for analysis (RR 0.92; 95% CI 0.83 to 1.02; 7254 participants; I² = 0%; high‐quality evidence; Analysis 4.3).
Two studies (CHARM‐Preserved; I‐PRESERVE) were also pooled for analysis (HR 0.90; 95% CI 0.80 to 1.01; 7148 participants; Analysis 4.4).
Hyperkalaemia
Two studies reported this outcome and were pooled for analysis (CHARM‐Preserved; I‐PRESERVE) (0.9% of participants in the intervention group and 0.5% in the control group; RR 1.88; 95% CI 1.07 to 3.33; participants = 7148; high‐quality evidence; Analysis 4.5).
All‐cause mortality
Five studies reported this outcome (CHARM‐Preserved; Hong Kong DHF; I‐PRESERVE; Parthasarathy 2009; SUPPORT). Parthasarathy 2009 reported that no deaths occurred. Four studies (CHARM‐Preserved; Hong Kong DHF; I‐PRESERVE; SUPPORT) contributed to the meta‐analysis (RR 1.01; 95% CI 0.92 to 1.11; 7964 participants; I² = 0%; high‐quality evidence; Analysis 4.6). For the SUPPORT trial, data for participants with LVEF ≥ 50% were analysed according to the definition of HFpEF used in this trial.
Two studies (I‐PRESERVE; SUPPORT) were also pooled for analysis (HR 0.99; 95% CI 0.88 to 1.12; 4838 participants; Analysis 4.7).
Quality of life
Four studies reported this outcome (CHARM‐Preserved; Hong Kong DHF; I‐PRESERVE; Parthasarathy 2009). CHARM‐Preserved reported quality of life (MLHF) in a study report (Lewis 2007): however, end scores per treatment arm were not provided. Three studies (Hong Kong DHF; I‐PRESERVE; Parthasarathy 2009) contributed to the meta‐analysis for MLHF (MD 0.41; 95% CI ‐0.86 to 1.67; 3117 participants; I² = 19%; high‐quality evidence; Analysis 4.8).
Withdrawal due to adverse event
Four studies (CHARM‐Preserved; Hong Kong DHF; I‐PRESERVE; Parthasarathy 2009) reported this outcome and contributed to the meta‐analysis (16% of participants in the intervention arm versus 13% in the control arm; RR 1.22; 95% CI 1.09 to 1.36; 7406 participants; I² = 0%; Analysis 4.9; NNTH 33).
Angiotensin receptor neprilysin inhibitors (ARNI) versus placebo or no treatment
We did not identify any completed trials that assessed ARNI.
Subgroup analyses
We were unable to perform a subgroup analysis for length of follow up for cardiovascular mortality as all included studies fell into one category of follow‐up (≥ 12 months: Analysis 1.1; Analysis 2.1; Analysis 3.1; Analysis 4.1). Similarly, we were unable to perform a subgroup analysis for ARB and heart failure hospitalisation (Analysis 4.3).
Heart failure hospitalisation for the comparison of beta‐blockers versus control (Analysis 1.2) showed no difference among the subgroups (< 12 months: RR 0.31; 95% CI 0.09 to 1.02; 67 participants; 1 study; versus ≥ 12 months: RR 0.79; 95% CI 0.48 to 1.31; 285 participants; studies = 2). SWEDIC did not report length of follow‐up.
Heart failure hospitalisation for the comparison of MRA versus control (Analysis 2.2) showed a confirmation of the overall effect estimate (RR 0.82; 95% CI 0.69 to 0.98; 3714 participants; 3 studies) only in the subgroup for follow‐up ≥ 12 months (RR 0.82; 95% CI 0.69 to 0.98; 3670 participants; 2 studies) while the much smaller study of shorter duration (< 12 months) showed RR 0.55; 95% CI 0.05 to 5.61; 44 participants.
Heart failure hospitalisation for the comparison of ACEI versus control (Analysis 3.2) showed no difference between subgroups (< 12 months: RR 0.42; 95% CI 0.09 to 2.04; 74 participants; 1 study; versus ≥ 12 months: RR 0.88; 95% CI 0.66 to 1.19; 945 participants; 2 studies).
Sensitivity analyses
We conducted a sensitivity analysis by only including studies assessed at low risk of bias. Across comparisons, the estimates were not significantly changed with the exception of beta‐blockers where no effect on cardiovascular mortality was observed (1 low risk of bias study (SENIORS): RR 0.81; 95% CI 0.50 to 1.29 versus overall analysis of 3 studies: RR 0.78; 95% CI 0.62 to 0.99).
Discusión
Resumen de los resultados principales
Se examinó la evidencia de los efectos de los BB y los inhibidores del SRAA en el tratamiento de la ICFEc. Se incluyeron 37 ensayos, informados en 207 publicaciones con un total de 18 311 participantes. Se identificaron cinco ensayos en curso con brazos de tratamiento que incluyeron las intervenciones evaluadas en esta revisión. Otros ocho estudios esperan la evaluación.
Se realizó un análisis agrupado de los resultados mortalidad cardiovascular y por todas las causas, hospitalización por insuficiencia cardíaca, calidad de vida e hiperpotasemia. Se utilizaron los datos del cuestionario Minnesota Living with Heart Failure (MLHF) para los resultados calidad de vida porque este fue el instrumento que se informó con mayor frecuencia.
Los retiros debidos a eventos adversos se informaron de manera inconsistente; estos datos no se pudieron incluir en las tablas de "Resumen de hallazgos".
Se realizó un análisis de sensibilidad con la inclusión solamente de los estudios considerados con bajo riesgo general de sesgo. Las estimaciones del efecto no cambiaron de forma significativa, excepto para los betabloqueantes.
Betabloqueantes
Un total de diez estudios incluidos (3087 participantes) evaluaron los betabloqueantes en comparación con placebo o ninguna intervención. Se realizaron metanálisis que incluyeron hasta cuatro estudios y 1105 participantes. Los resultados indicaron que el tratamiento puede mejorar la mortalidad cardiovascular; sin embargo, la calidad de la evidencia fue baja debido a la imprecisión y al riesgo de sesgo. Cuando se realizó un análisis de sensibilidad en el que se incluyeron solamente los estudios con bajo riesgo general de sesgo, no persistieron los efectos sobre la mortalidad cardiovascular. Los dos estudios más grandes (J‐DHF; SENIORS) informaron tasas altas de interrupción del fármaco de estudio debido a intolerancia, en lugar de a eventos adversos, lo que puede haber atenuado cualquier efecto verdadero del tratamiento. No hay seguridad acerca de los efectos del tratamiento farmacológico con betabloqueantes en los pacientes con ICFEc. Está en curso un gran ensayo controlado aleatorio abierto, de resultados clínicos, del metoprolol para la ICFEc (Zhou 2010, beta‐PRESERVE).
Antagonistas de los receptores mineralocorticoides (ARM)
Un total de 12 estudios (4408 participantes) evaluaron los ARM en comparación con placebo o ninguna intervención. En los metanálisis se combinó la evidencia de hasta seis ensayos y 4291 participantes. Se encontró que el tratamiento con ARM reduce el riesgo de hospitalización por insuficiencia cardíaca, pero se encontró poco o ningún efecto sobre la mortalidad cardiovascular y por todas las causas; sin embargo, la calidad de la evidencia fue moderada y todavía existe incertidumbre sobre estos efectos del tratamiento. Como se esperaba, el tratamiento con ARM se asoció con un mayor riesgo de hiperpotasemia; por lo tanto, en los pacientes tratados con ARM se requiere la monitorización del potasio. Está en curso un gran ensayo de resultados clínicos con asignación al azar por registro de espironolactona para la ICFEc, que se debe completar en 2022 (NCT02901184: Spironolactone Initiation Registry Randomized Interventional Trial in Heart Failure with Preserved Ejection Fraction, SPIRRIT).
Inhibidores de la enzima convertidora de angiotensina (IECA)
Un total de ocho estudios incluidos (2062 participantes) evaluaron IECA versus placebo o ninguna intervención. Se realizó un metanálisis de los datos de cuatro ensayos y 1079 participantes. Se encontró que probablemente hubo poco o ningún efecto sobre la mortalidad cardiovascular, la mortalidad por todas las causas, la hospitalización por insuficiencia cardíaca o la calidad de vida, y hubo datos limitados sobre la hiperpotasemia. No hubo ensayos clínicos grandes (> 1000 participantes) disponibles y la calidad de la evidencia se consideró moderada debido a la imprecisión. La efectividad del tratamiento con IECA en el tratamiento de los pacientes con ICFEc todavía no está clara.
Antagonistas de los receptores de angiotensina (BRA)
Un total de ocho estudios incluidos (8755 participantes) evaluaron el tratamiento con BRA en pacientes con ICFEc y la calidad de la evidencia se consideró alta. En el metanálisis se combinó la evidencia de hasta cuatro ensayos y 7964 participantes y se encontró poca o ninguna diferencia general en los resultados mortalidad cardiovascular, mortalidad por todas las causas, hospitalización por insuficiencia cardíaca o calidad de vida. El estudio CHARM encontró un efecto sobre la hospitalización por insuficiencia cardíaca sobre la base del análisis del tiempo hasta el evento y la fortaleza de esta asociación aumentó en un análisis posterior basado en los eventos recurrentes (Rogers 2014). Como era de esperar, el tratamiento con BRA se asoció con un mayor riesgo de hiperpotasemia; por lo tanto, es necesario monitorizar el potasio.
Compleción y aplicabilidad general de las pruebas
Esta revisión proporciona la evaluación más integral de la evidencia hasta la fecha. Se incluyeron 37 estudios (207 informes) que incorporaron a 18 311 participantes. Los ensayos incluidos evaluaron los betabloqueantes (diez estudios, 3087 participantes), los ARM (12 estudios, 4408 participantes), los IECA (ocho estudios, 2061 participantes) y los BRA (ocho estudios, 8755 participantes).
Al buscar en los registros de ensayos clínicos se identificaron cinco ensayos clínicos en curso y es posible que varios de ellos influyan en los resultados de la revisión. También se identificaron ocho estudios que se clasificaron como "Estudios en espera de clasificación", porque no había información suficiente para determinar si estos estudios cumplían con los criterios de inclusión de esta revisión. Estos estudios fueron en su mayoría pequeños y, por lo tanto, es poco probable que influyeran en los resultados de esta revisión. Se identificó un total de 207 informes de 37 ensayos, ocho estudios en espera de clasificación y cinco ensayos en curso, en comparación con el total de 22 identificados por Zheng 2017 para las mismas comparaciones.
El umbral de FEVI para definir a las poblaciones de los ensayos de ICFEc varió entre los estudios incluidos y puede contribuir a la indireccionalidad, con implicaciones para la aplicabilidad de la evidencia. Nueve estudios incluyeron a participantes con un valor de corte de la fracción de expulsión del 40%, diez utilizaron el 45%, 14 utilizaron el 50% y uno utilizó el 55%. Adamyan 2010 incluyó a participantes con fracción de expulsión preservada, pero no especificó el valor de corte. SENIORS informó un subgrupo de participantes con FEVI > 40% y en esta revisión se obtuvieron los resultados de un subgrupo con FEVI > 40% de AREA IN‐CHF. Ningún ensayo informó resultados del subgrupo en el rango intermedio del 40% al 49%, por lo que no fue posible investigar este subgrupo.
Los estudios incluidos tenían fechas de comienzo del reclutamiento desde 1997 a 2011. En los estudios más recientes, los péptidos natriuréticos tipo B se han utilizado como un criterio de inclusión clave para mejorar la especificidad de la población con ICFEc y ampliar las poblaciones de los ensayos con pacientes en mayor riesgo de resultados clínicos (p.ej. CAN‐DHF; Mak 2009; RAAM‐PEF). De igual manera, en los estudios más recientes se han incluido nuevas medidas de función diastólica para aumentar la especificidad (p.ej. ELANDD; J‐DHF; PEP‐CHF). Se observó una heterogeneidad clínica considerable entre las poblaciones de estudio con respecto a las comorbilidades y a las terapias cardiovasculares al inicio, lo que puede influir en la aplicabilidad de la evidencia.
Calidad de la evidencia
Se utilizó el método GRADE para evaluar la calidad de la evidencia para los resultados de mortalidad cardiovascular, mortalidad por todas las causas, hospitalización por insuficiencia cardíaca, calidad de vida (evaluada mediante el cuestionario Minnesota Living with Heart Failure) e hiperpotasemia. Para los betabloqueantes, la calidad de la evidencia para los resultados clínicos (mortalidad cardiovascular, mortalidad por todas las causas y hospitalización por insuficiencia cardíaca) varió de baja a muy baja. En el análisis combinado la mayoría de los participantes provinieron de un subgrupo de un gran ensayo único; los otros estudios incluidos fueron pequeños, con riesgo alto o incierto de sesgo.
Para los ARM, el estudio TOPCAT contribuyó con la mayoría de los participantes al metanálisis, por lo que la calidad general de la evidencia para los resultados clínicos se consideró moderada. Se observaron diferencias en las poblaciones de participantes entre los estudios incluidos (TOPCAT, FEVI > 40%; RAAM‐PEF, FEVI ≥ 50%; AREA IN‐CHF FEVI 40% al 45%) y se informó que la fracción de expulsión es un modificador del efecto del tratamiento con los ARM (Solomon 2016). En particular, un análisis post hoc del estudio TOPCAT informó diferencias importantes en las tasas de eventos placebo entre los participantes reclutados en América (Argentina, Brasil, Canadá, EE.UU.) y los participantes reclutados en Rusia y Georgia (Pfeffer 2015). Además, un subestudio de farmacología de los participantes a los 12 meses (206 participantes de EE.UU. y Canadá; 160 participantes de Rusia) encontró que los metabolitos del fármaco no fueron detectables en una proporción más grande de los participantes de Rusia en comparación con los participantes de los EE.UU. y Canadá (30% versus 3%, P < 0,001) (de Denus 2017). Un análisis de subgrupos geográfico indicó el posible beneficio clínico de la espironolactona en la ICFEc en los participantes reclutados en América (Estados Unidos, Canadá, Brasil, Argentina; mortalidad cardiovascular CRI 0,74; IC del 95%: 0,57 a 0,97; mortalidad por todas las causas CRI 0,83; IC del 95%: 0,68 a 1,02; hospitalización por insuficiencia cardíaca CRI 0,82; IC del 95%: 0,67 a 0,99). Estos resultados se investigarán más a fondo en el estudio SPIRRIT en curso (NCT02901184).
Para los BRA, varios ensayos grandes contribuyeron con un gran número de eventos al metanálisis de los resultados clínicos de mortalidad y hospitalización por insuficiencia cardíaca y la calidad de la evidencia fue alta. Para los IECA, en el análisis combinado se incluyeron menos ensayos, los números de eventos fueron bajos y la calidad de la evidencia se consideró moderada. Para los betabloqueantes, la calidad de la evidencia fue baja debido a los tamaños pequeños de los estudios y al riesgo de sesgo.
Sesgos potenciales en el proceso de revisión
Aunque se realizó una búsqueda exhaustiva en las principales bases de datos, es posible que los estudios en los registros de ensayos clínicos se perdieran, no se hayan informado o ambos. Cuando no se proporcionó información sobre subgrupos ni resultados relevantes se intentó establecer contacto con los autores de los estudios, pero solamente se recibió un número limitado de respuestas. Debido al escaso número de estudios incluidos por análisis no fue posible evaluar formalmente la existencia de sesgo de publicación.
Acuerdos y desacuerdos con otros estudios o revisiones
Los resultados de la presente revisión en gran parte fueron consistentes con los de la revisión integral más reciente de la evidencia de los betabloqueantes y los inhibidores del SRAA en los pacientes con ICFEc (Zheng 2017). Sin embargo, se observaron diferencias importantes. El análisis de la presente revisión incluyó más estudios en cada comparación, pero los estudios adicionales fueron pequeños y no modificaron de forma significativa las estimaciones generales del efecto. Una distinción adicional es que aquí se utilizó para el metanálisis un modelo de efectos fijos en lugar de un modelo de efectos aleatorios debido a la heterogeneidad baja entre los estudios para todas las comparaciones. Se encontró una reducción en la mortalidad cardiovascular con el tratamiento con betabloqueantes, pero este resultado no fue consistente con un análisis de sensibilidad que incluyó solamente los estudios considerados con bajo riesgo de sesgo. A diferencia de Zheng 2017, no se encontró un efecto del tratamiento con betabloqueantes sobre la mortalidad por todas las causas que lograra significación estadística.

Study flow diagram

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
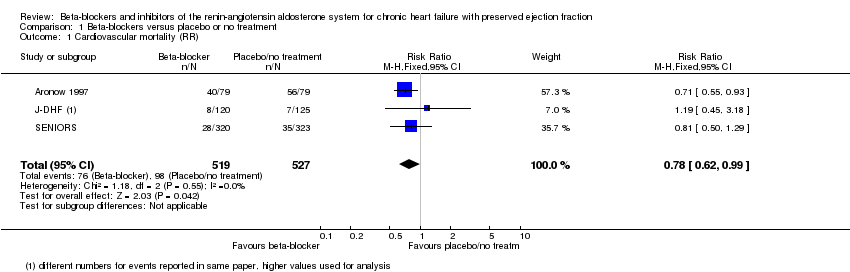
Comparison 1 Beta‐blockers versus placebo or no treatment, Outcome 1 Cardiovascular mortality (RR).

Comparison 1 Beta‐blockers versus placebo or no treatment, Outcome 2 Heart failure hospitalisation (RR).
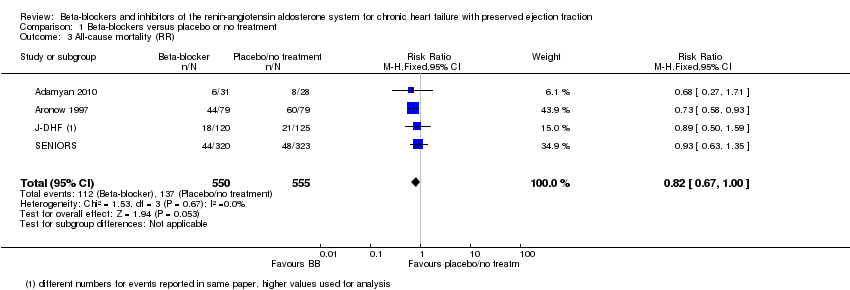
Comparison 1 Beta‐blockers versus placebo or no treatment, Outcome 3 All‐cause mortality (RR).
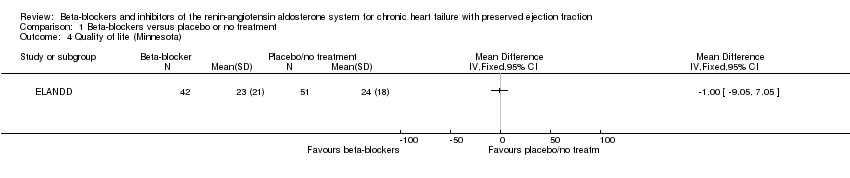
Comparison 1 Beta‐blockers versus placebo or no treatment, Outcome 4 Quality of life (Minnesota).
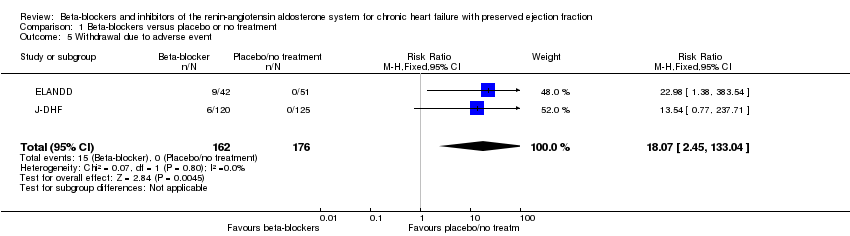
Comparison 1 Beta‐blockers versus placebo or no treatment, Outcome 5 Withdrawal due to adverse event.

Comparison 2 Mineralocorticoid receptor antagonists versus placebo or no treatment, Outcome 1 Cardiovascular mortality (RR).
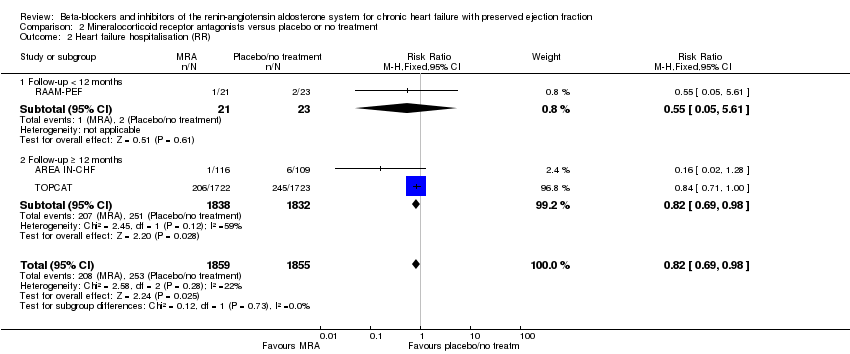
Comparison 2 Mineralocorticoid receptor antagonists versus placebo or no treatment, Outcome 2 Heart failure hospitalisation (RR).
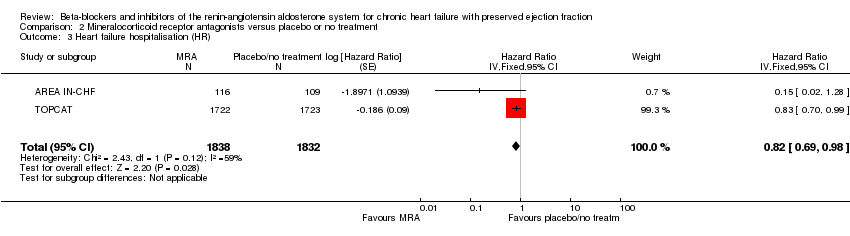
Comparison 2 Mineralocorticoid receptor antagonists versus placebo or no treatment, Outcome 3 Heart failure hospitalisation (HR).

Comparison 2 Mineralocorticoid receptor antagonists versus placebo or no treatment, Outcome 4 Hyperkalaemia.
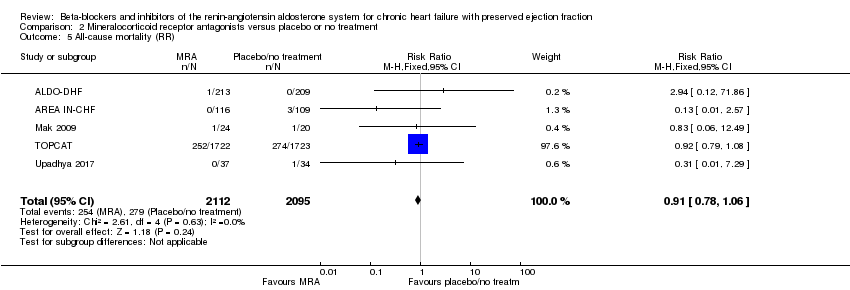
Comparison 2 Mineralocorticoid receptor antagonists versus placebo or no treatment, Outcome 5 All‐cause mortality (RR).

Comparison 2 Mineralocorticoid receptor antagonists versus placebo or no treatment, Outcome 6 Quality of life.

Comparison 2 Mineralocorticoid receptor antagonists versus placebo or no treatment, Outcome 7 Quality of life (KCCQ).

Comparison 2 Mineralocorticoid receptor antagonists versus placebo or no treatment, Outcome 8 Quality of life (Minnesota).

Comparison 2 Mineralocorticoid receptor antagonists versus placebo or no treatment, Outcome 9 Withdrawal due to adverse event.

Comparison 3 Angiotensin converting enzyme inhibitors versus placebo or no treatment, Outcome 1 Cardiovascular mortality (RR).
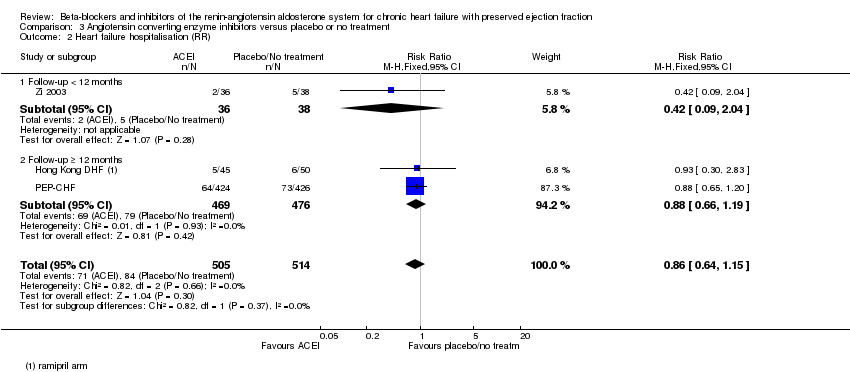
Comparison 3 Angiotensin converting enzyme inhibitors versus placebo or no treatment, Outcome 2 Heart failure hospitalisation (RR).

Comparison 3 Angiotensin converting enzyme inhibitors versus placebo or no treatment, Outcome 3 Hyperkalaemia.
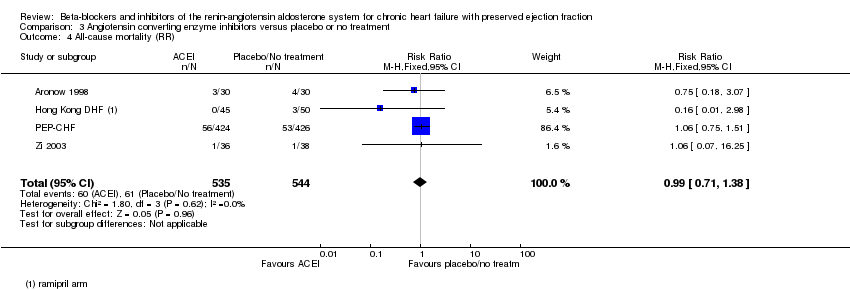
Comparison 3 Angiotensin converting enzyme inhibitors versus placebo or no treatment, Outcome 4 All‐cause mortality (RR).

Comparison 3 Angiotensin converting enzyme inhibitors versus placebo or no treatment, Outcome 5 Quality of life (Minnesota).

Comparison 3 Angiotensin converting enzyme inhibitors versus placebo or no treatment, Outcome 6 Withdrawal due to adverse event.

Comparison 4 Angiotensin receptor blockers versus placebo or no treatment, Outcome 1 Cardiovascular mortality (RR).

Comparison 4 Angiotensin receptor blockers versus placebo or no treatment, Outcome 2 Cardiovascular mortality (HR).

Comparison 4 Angiotensin receptor blockers versus placebo or no treatment, Outcome 3 Heart failure hospitalisation (RR).

Comparison 4 Angiotensin receptor blockers versus placebo or no treatment, Outcome 4 Heart failure hospitalisation (HR).
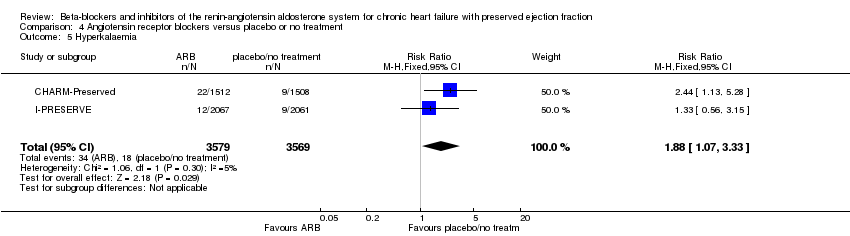
Comparison 4 Angiotensin receptor blockers versus placebo or no treatment, Outcome 5 Hyperkalaemia.
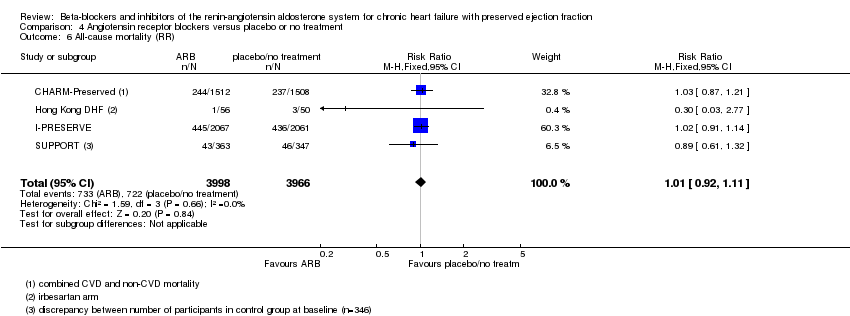
Comparison 4 Angiotensin receptor blockers versus placebo or no treatment, Outcome 6 All‐cause mortality (RR).
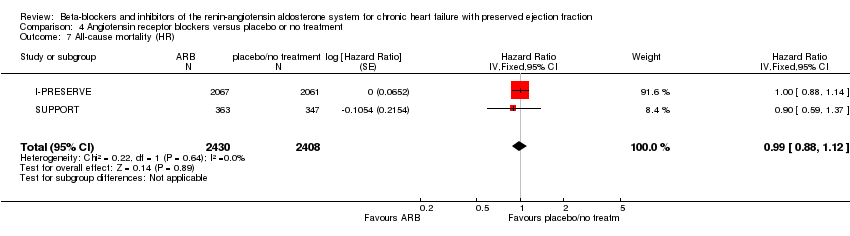
Comparison 4 Angiotensin receptor blockers versus placebo or no treatment, Outcome 7 All‐cause mortality (HR).

Comparison 4 Angiotensin receptor blockers versus placebo or no treatment, Outcome 8 Quality of life (Minnesota).
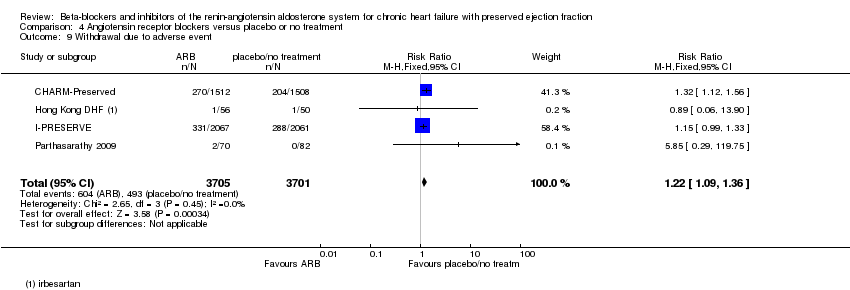
Comparison 4 Angiotensin receptor blockers versus placebo or no treatment, Outcome 9 Withdrawal due to adverse event.
| Beta‐blockers compared to placebo or no treatment for chronic heart failure with preserved ejection fraction | ||||||
| Patient or population: chronic heart failure with preserved ejection fraction | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
| Risk with placebo/no treatment | Risk with Beta‐blockers | |||||
| Cardiovascular mortality (RR) | Study population | RR 0.78 | 1046 | ⊕⊕⊝⊝ | Three additional studies (ELANDD; SWEDIC; Takeda 2004) reported that no deaths occurred | |
| 173 per 1000 | 135 per 1000 | |||||
| Heart failure hospitalisation (RR) | Study population | RR 0.73 | 449 | ⊕⊝⊝⊝ | Follow‐up unclear for SWEDIC. ELANDD reported that no hospitalisation due to heart failure occurred | |
| 117 per 1000 | 86 per 1000 | |||||
| Hyperkalaemia | 245 (1 RCT) | ⊕⊝⊝⊝ | J‐DHF reported one participant in the intervention group (N = 120) experienced hyperkalaemia but did not report on this outcome for the control group. No further data were available from any of the other studies. | |||
| All‐cause mortality (RR) | Study population | RR 0.82 | 1105 | ⊕⊕⊝⊝ | Follow‐up unclear for Adamyan 2010. ELANDD, SWEDIC and Takeda 2004 reported that no deaths occurred | |
| 243 per 1000 | 199 per 1000 | |||||
| Quality of life (Minnesota) | Mean quality of life (Minnesota) was 24 | MD 1 lower | ‐ | 93 | ⊕⊝⊝⊝ | Lower = better, 5 point difference considered to be clinically meaningful |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded by one level due to unclear selection bias in most studies. 2 Downgraded by one level due to concerns about the smaller study being more precise than the larger study. 3 Downgraded by one level due to large variation in size of effect. 4 Downgraded by two levels due to few events and wide CI. 5 Downgraded by two levels due to very small sample size. 6 Suspected publication bias; this is a patient‐relevant outcome that is not reported in most studies. 7 Downgraded by two levels due to incomplete reporting. | ||||||
| MRA compared to placebo or no treatment for chronic heart failure with preserved ejection fraction | ||||||
| Patient or population: chronic heart failure with preserved ejection fraction | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
| Risk with placebo/no treatment | Risk with MRA | |||||
| Cardiovascular mortality (RR) | Study population | RR 0.90 | 4070 | ⊕⊕⊕⊝ | Two additional trials (RAAM‐PEF, Kurrelmeyer 2014) reported that no deaths occurred | |
| 88 per 1000 | 79 per 1000 | |||||
| Heart failure hospitalisation (RR) | Study population | RR 0.82 | 3714 | ⊕⊕⊕⊝ | Three additional trials (ALDO‐DHF ,Kurrelmeyer 2014, Upadhya 2017) reported that no hospitalisation due to heart failure occurred | |
| 136 per 1000 | 112 per 1000 | |||||
| Hyperkalaemia | Study population | RR 2.11 | 4291 | ⊕⊕⊕⊕ | Two trials defined hyperkalaemia ≥ 5.5 mEg/L | |
| 83 per 1000 | 175 per 1000 | |||||
| All‐cause mortality | Study population | RR 0.91 | 4207 | ⊕⊕⊕⊝ | Two additional trials (RAAM‐PEF, Kurrelmeyer 2014) reported that no deaths occurred | |
| 133 per 1000 | 121 per 1000 | |||||
| Quality of life (Minnesota) | Mean quality of life (Minnesota) ranged from 20 to 25 | MD 0.84 higher | ‐ | 511 | ⊕⊕⊝⊝ | Lower = better, 5 points are considered a clinically significant difference We did not pre‐specify which QoL scale was to be reported in the 'Summary of findings' table. To aid comparisons among 'Summary of findings' tables we chose to include the Minnesota Living with Heart Failure questionnaire and not the SMD across two scales |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded by one level due to imprecision. 2 Downgraded by one level because one trial was open label. 3 Downgraded by one level due to small sample size. | ||||||
| ACEI compared to placebo or no treatment for chronic heart failure with preserved ejection fraction | ||||||
| Patient or population: chronic heart failure with preserved ejection fraction | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
| Risk with placebo/no treatment | Risk with ACEI | |||||
| Cardiovascular mortality (RR) | Study population | RR 0.93 | 945 | ⊕⊕⊕⊝ | One additional trial (Kitzman 2010) reported that no deaths occurred | |
| 86 per 1000 | 81 per 1000 | |||||
| Heart failure hospitalisation (RR) | Study population | RR 0.86 | 1019 | ⊕⊕⊕⊝ | ||
| 13 per 1000 | 11 per 1000 | |||||
| Hyperkalaemia | 74 (1 RCTs) | ⊕⊝⊝⊝ | One trial (Zi 2003) reported 2 events in the intervention group (N = 36), 0 events in the control group (N = 38) (RR 5.27, 95% CI 0.26 to 106.16) | |||
| All‐cause mortality (RR) | Study population | RR 0.99 | 1079 | ⊕⊕⊕⊝ | One additional trial (Kitzman 2010) reported that no deaths occurred | |
| 119 per 1000 | 119 per 1000 | |||||
| Quality of life (Minnesota) | Mean quality of life (Minnesota) ranged from 10.9 to 29 | MD 0.09 lower | ‐ | 154 | ⊕⊕⊝⊝ | Scale: 0 to 105, lower = better, 5 point difference considered clinically relevant One trial (SNEGOVIK) reported mean change from baseline of ‐19.8 for intervention and ‐10.7 for control |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded by one level due to wide CI. 2 Downgraded by one level due to risk of bias (open label). 3 Downgraded by one level due to low sample size. 4 Downgraded by one level due to unclear selection bias. | ||||||
| ARB compared to placebo or no treatment for chronic heart failure with preserved ejection fraction | ||||||
| Patient or population: chronic heart failure with preserved ejection fraction | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
| Risk with placebo/no treatment | Risk with ARB | |||||
| Cardiovascular mortality (RR) | Study population | RR 1.02 | 7254 | ⊕⊕⊕⊕ | One additional trial (Parthasarathy 2009) reported that no deaths occurred | |
| 131 per 1000 | 133 per 1000 | |||||
| Heart failure hospitalisation (RR) | Study population | RR 0.92 | 7254 | ⊕⊕⊕⊕ | ||
| 171 per 1,‐000 | 157 per 1,‐000 | |||||
| Hyperkalaemia | Study population | RR 1.88 | 7148 | ⊕⊕⊕⊕ | ||
| 3 per 1,000 | 5 per 1,000 | |||||
| All‐cause mortality (RR) | Study population | RR 1.01 | 7964 | ⊕⊕⊕⊕ | One additional trial (Parthasarathy 2009) reported that no deaths occurred | |
| 72 per 1000 | 73 per 1,‐000 | |||||
| Quality of life (Minnesota) | Mean quality of life (Minnesota) ranged from 10.9 to 31.6 | MD 0.41 higher | ‐ | 3117 | ⊕⊕⊕⊕ | Scale: 0 to 105, lower = better, 5 point difference considered clinically relevant |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Cardiovascular mortality (RR) Show forest plot | 3 | 1046 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.62, 0.99] |
| 2 Heart failure hospitalisation (RR) Show forest plot | 4 | 449 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.47, 1.13] |
| 2.1 Follow‐up < 12 months | 1 | 67 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.31 [0.09, 1.02] |
| 2.2 Follow‐up ≥ 12 months | 2 | 285 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.48, 1.31] |
| 2.3 Follow‐up unknown | 1 | 97 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.31 [0.26, 107.85] |
| 3 All‐cause mortality (RR) Show forest plot | 4 | 1105 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.67, 1.00] |
| 4 Quality of life (Minnesota) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5 Withdrawal due to adverse event Show forest plot | 2 | 338 | Risk Ratio (M‐H, Fixed, 95% CI) | 18.07 [2.45, 133.04] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Cardiovascular mortality (RR) Show forest plot | 3 | 4070 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.74, 1.11] |
| 2 Heart failure hospitalisation (RR) Show forest plot | 3 | 3714 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.69, 0.98] |
| 2.1 Follow‐up < 12 months | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.05, 5.61] |
| 2.2 Follow‐up ≥ 12 months | 2 | 3670 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.69, 0.98] |
| 3 Heart failure hospitalisation (HR) Show forest plot | 2 | 3670 | Hazard Ratio (Fixed, 95% CI) | 0.82 [0.69, 0.98] |
| 4 Hyperkalaemia Show forest plot | 6 | 4291 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.11 [1.77, 2.51] |
| 5 All‐cause mortality (RR) Show forest plot | 5 | 4207 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.78, 1.06] |
| 6 Quality of life Show forest plot | 5 | 603 | Std. Mean Difference (IV, Random, 95% CI) | 0.05 [‐0.23, 0.34] |
| 7 Quality of life (KCCQ) Show forest plot | 2 | 92 | Mean Difference (IV, Random, 95% CI) | ‐0.78 [‐28.02, 26.46] |
| 8 Quality of life (Minnesota) Show forest plot | 3 | 511 | Mean Difference (IV, Random, 95% CI) | 0.84 [‐2.30, 3.98] |
| 9 Withdrawal due to adverse event Show forest plot | 4 | 3986 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [1.00, 1.21] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Cardiovascular mortality (RR) Show forest plot | 2 | 945 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.61, 1.42] |
| 2 Heart failure hospitalisation (RR) Show forest plot | 3 | 1019 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.64, 1.15] |
| 2.1 Follow‐up < 12 months | 1 | 74 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.09, 2.04] |
| 2.2 Follow‐up ≥ 12 months | 2 | 945 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.66, 1.19] |
| 3 Hyperkalaemia Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 All‐cause mortality (RR) Show forest plot | 4 | 1079 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.71, 1.38] |
| 5 Quality of life (Minnesota) Show forest plot | 2 | 154 | Mean Difference (IV, Fixed, 95% CI) | ‐0.09 [‐3.66, 3.48] |
| 6 Withdrawal due to adverse event Show forest plot | 3 | 1019 | Risk Ratio (M‐H, Random, 95% CI) | 1.53 [0.26, 9.00] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Cardiovascular mortality (RR) Show forest plot | 3 | 7254 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.90, 1.14] |
| 2 Cardiovascular mortality (HR) Show forest plot | 2 | 5087 | Hazard Ratio (Fixed, 95% CI) | 1.00 [0.89, 1.13] |
| 3 Heart failure hospitalisation (RR) Show forest plot | 3 | 7254 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.83, 1.02] |
| 4 Heart failure hospitalisation (HR) Show forest plot | 2 | 7148 | Hazard Ratio (Fixed, 95% CI) | 0.90 [0.80, 1.01] |
| 5 Hyperkalaemia Show forest plot | 2 | 7148 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.88 [1.07, 3.33] |
| 6 All‐cause mortality (RR) Show forest plot | 4 | 7964 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.92, 1.11] |
| 7 All‐cause mortality (HR) Show forest plot | 2 | 4838 | Hazard Ratio (Fixed, 95% CI) | 0.99 [0.88, 1.12] |
| 8 Quality of life (Minnesota) Show forest plot | 3 | 3117 | Mean Difference (IV, Fixed, 95% CI) | 0.41 [‐0.86, 1.67] |
| 9 Withdrawal due to adverse event Show forest plot | 4 | 7406 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [1.09, 1.36] |

