Betabloqueantes e inhibidores del sistema de renina‐angiotensina‐aldosterona para la insuficiencia cardíaca crónica con fracción de expulsión conservada
Appendices
Appendix 1. Search strategies
CENTRAL
#1 MeSH descriptor: [Heart Failure] explode all trees
#2 ((heart or cardia* or myocardial) near/3 (failure or insufficienc* or decompensat*)):ab,ti,kw
#3 #1 or #2
#4 MeSH descriptor: [Ventricular Dysfunction] explode all trees
#5 MeSH descriptor: [Ventricular Function] explode all trees
#6 ((preserved or normal or greater) near/5 ("ejection fraction" or "EF" or "LVEF")):ab,ti,kw
#7 ("preserved systolic function" or "normal systolic function" or "HFpEF" or "HF‐pEF" or "HFnEF" or "HF‐nEF" or "DHF" or diastolic*):ab,ti,kw
#8 #4 or #5 or #6 or #7
#9 #3 and #8
#10 MeSH descriptor: [Adrenergic beta‐Antagonists] explode all trees
#11 (beta near/2 (antagonist* or block* or receptor*)):ab,ti,kw
#12 (acebutolol or adimolol or afurolol or alprenolol or amosulalol or arotinolol or atenolol or befunolol or betaxolol or bevantolol or bisoprolol or bopindolol or bornaprolol or brefonalol or bucindolol or bucumolol or bufetolol or bufuralol or bunitrolol or bunolol or bupranolol or butofilolol or butoxamine or carazolol or carteolol or carvedilol or celiprolol or cetamolol or chlortalidone or cloranolol or cyanoiodopindolol or cyanopindolol or deacetylmetipranolol or diacetolol or dihydroalprenolol or dilevalol or epanolol or esmolol or exaprolol or falintolol or flestolol or flusoxolol or hydroxybenzylpinodolol or hydroxycarteolol or hydroxy benazepril or indenolol or iodocyanopindolol or iodopindolol or iprocrolol or isoxaprolol or labetalol or landiolol or levobunolol or levomoprolol or medroxalol or mepindolol or methylthiopropranolol or metipranolol or metoprolol or moprolol or nadolol or oxprenolol or penbutolol or pindolol or nadolol or nebivolol or nifenalol or nipradilol or oxprenolol or pafenolol or pamatolol or penbutolol or pindolol or practolol or primidolol or prizidilol or procinolol or pronetalol or propranolol or proxodolol or ridazolol or salcardolol or soquinolol or sotalol or spirendolol or talinolol or tertatolol or tienoxolol or tilisolol or timolol or tolamolol or toliprolol or tribendilol or xibenolol or Betapace or Blocadren or Bystolic or Cartrol or Coreg or Corgard or Inderal or Kerlone or Levatol or Lopressor or Normodyne or Sectral or Tenormin or Toprol or Trandate or Visken or Zebeta):ab,ti,kw
#13 MeSH descriptor: [Angiotensin‐Converting Enzyme Inhibitors] explode all trees
#14 ((angiotensin* or dipeptidyl* or 'kininase ii') near/3 (convert* or enzyme or inhibit* or recept* or block*)):ab,ti,kw
#15 (ace near/1 inhibit*):ab,ti,kw
#16 acei:ab,ti,kw
#17 (alacepril or altiopril or ancovenin or benazepril* or captopril or ceranapril or ceronapril or cilazapril or deacetylalacepril or delapril or derapril or enalapril* or epicaptopril or fasidotril* or fosinopril or foroxymithine or gemopatrilat or idrapril or ilepatril or imidapril* or indolapril or libenzapril or lisinopril or moexipril* or omapatrilat or pentopril* or perindopril* or pivopril or quinapril* or ramipril* or rentiapril or sampatrilat or saralasin or "s nitrosocaptopril" or spirapril* or temocapril* or teprotide or trandolapril* or utibapril* or zabicipril* or zofenopril* or Aceon or Accupril or Altace or Capoten or Lotensin or Mavik or Monopril or Prinivil or Univas or Vasotec or Zestril):ab,ti,kw
#18 MeSH descriptor: [Angiotensin Receptor Antagonists] explode all trees
#19 (angiotensin near/3 ('receptor antagonist*' or "receptor block*")):ab,ti,kw
#20 (arb or arbs):ab,ti,kw
#21 (abitesartan or azilsartan or candesartan or elisartan or embusartan or eprosartan or fimasartan or fonsartan or forasartan or irbesartan or "KT3‐671" or losartan or milfasartan or olmesartan or pomisartan or pratosartan or ripisartan or saprisartan or sparsentan or tasosartan or telmisartan or Losartan or zolasartan or Edarbi or Blopress or Atacand or Amias or Ratacand or Eprozar or Aprovel or Karvea or Avapro or Cozaar or Benicar or Olmecip or Micardis or Diovan):ab,ti,kw
#22 MeSH descriptor: [Neprilysin] this term only and with qualifier(s): [Antagonists & inhibitors ‐ AI]
#23 (neprilysin near/1 (inhibit* or antagonist*)):ab,ti,kw
#24 arni:ab,ti,kw
#25 (sacubitril or sacubitrilat or lbq657 or "lbq 657" or ahu377 or "ahu 377" or entresto or lcz696 or "lcz 696")
#26 MeSH descriptor: [Mineralocorticoid Receptor Antagonists] explode all trees
#27 ((mineralocorticoid or aldosterone) near/3 (antagonist* or block* or inhibit*)):ab,ti,kw
#28 ("canrenoic acid" or canrenone or eplerenone or finerenone or "oxprenoate potassium" or spironolactone or aldactone or contaren or inspra or luvion or phanurane or spiroletan):ab,ti,kw
#29 {or #10‐#28}
#30 #9 and #29
MEDLINE (Ovid)
1. exp Heart Failure/
2. ((heart or cardia* or myocardial) adj3 (failure or insufficienc* or decompensat*)).tw.
3. 1 or 2
4. exp Ventricular Dysfunction/
5. exp Ventricular Function/
6. ((preserved or normal or greater) adj5 (ejection fraction or EF or LVEF)).tw.
7. (preserved systolic function or normal systolic function or HFpEF or HF‐pEF or HFnEF or HF‐nEF or DHF or diastolic*).tw.
8. 4 or 5 or 6 or 7
9. 3 and 8
10. exp Adrenergic beta‐Antagonists/
11. (beta adj2 (antagonist* or block* or receptor*)).tw.
12. (acebutolol or adimolol or afurolol or alprenolol or amosulalol or arotinolol or atenolol or befunolol or betaxolol or bevantolol or bisoprolol or bopindolol or bornaprolol or brefonalol or bucindolol or bucumolol or bufetolol or bufuralol or bunitrolol or bunolol or bupranolol or butofilolol or butoxamine or carazolol or carteolol or carvedilol or celiprolol or cetamolol or chlortalidone or cloranolol or cyanoiodopindolol or cyanopindolol or deacetylmetipranolol or diacetolol or dihydroalprenolol or dilevalol or epanolol or esmolol or exaprolol or falintolol or flestolol or flusoxolol or hydroxybenzylpinodolol or hydroxycarteolol or hydroxymetoprolol or indenolol or iodocyanopindolol or iodopindolol or iprocrolol or isoxaprolol or labetalol or landiolol or levobunolol or levomoprolol or medroxalol or mepindolol or methylthiopropranolol or metipranolol or metoprolol or moprolol or nadolol or oxprenolol or penbutolol or pindolol or nadolol or nebivolol or nifenalol or nipradilol or oxprenolol or pafenolol or pamatolol or penbutolol or pindolol or practolol or primidolol or prizidilol or procinolol or pronetalol or propranolol or proxodolol or ridazolol or salcardolol or soquinolol or sotalol or spirendolol or talinolol or tertatolol or tienoxolol or tilisolol or timolol or tolamolol or toliprolol or tribendilol or xibenolol or Betapace or Blocadren or Bystolic or Cartrol or Coreg or Corgard or Inderal or Kerlone or Levatol or Lopressor or Normodyne or Sectral or Tenormin or Toprol or Trandate or Visken or Zebeta).mp.
13. exp Angiotensin‐Converting Enzyme Inhibitors/
14. ((angiotensin* or dipeptidyl* or kininase ii) adj3 (convert* or enzyme or inhibit* or recept* or block*)).tw.
15. (ace adj inhibit*).tw.
16. acei.tw.
17. (alacepril or altiopril or ancovenin or benazepril* or captopril or ceranapril or ceronapril or cilazapril or deacetylalacepril or delapril or derapril or enalapril* or epicaptopril or fasidotril* or fosinopril or foroxymithine or gemopatrilat or idrapril or ilepatril or imidapril* or indolapril or libenzapril or lisinopril or moexipril* or omapatrilat or pentopril* or perindopril* or pivopril or quinapril* or ramipril* or rentiapril or sampatrilat or saralasin or s nitrosocaptopril or spirapril* or temocapril* or teprotide or trandolapril* or utibapril* or zabicipril* or zofenopril* or Aceon or Accupril or Altace or Capoten or Lotensin or Mavik or Monopril or Prinivil or Univas or Vasotec or Zestril).mp.
18. exp Angiotensin Receptor Antagonists/
19. (angiotensin adj3 (receptor antagonist* or receptor block*)).tw.
20. (arb or arbs).tw.
21. (abitesartan or azilsartan or candesartan or elisartan or embusartan or eprosartan or fimasartan or fonsartan or forasartan or irbesartan or KT3‐671 or losartan or milfasartan or olmesartan or pomisartan or pratosartan or ripisartan or saprisartan or sparsentan or tasosartan or telmisartan or valsartan or zolasartan or Edarbi or Blopress or Atacand or Amias or Ratacand or Eprozar or Aprovel or Karvea or Avapro or Cozaar or Benicar or Olmecip or Micardis or Diovan).mp.
22. Neprilysin/ai [Antagonists & Inhibitors]
23. (neprilysin adj (inhibit* or antagonist*)).tw.
24. arni.tw.
25. (Sacubitril or "ahu 377" or ahu377 or Sacubitrilat or lbq657 or "lbq 657" or ahu377 or "ahu 377" or Entresto or lcz696 or "lcz 696").mp.
26. exp Mineralocorticoid Receptor Antagonists/
27. ((mineralocorticoid or aldosterone) adj3 (antagonist* or block* or inhibit*)).tw.
28. (canrenoic acid or canrenone or eplerenone or finerenone or oxprenoate potassium or spironolactone or Aldactone or Contaren or Inspra or Luvion or Phanurane or Spiroletan).mp.
29. or/10‐28
30. 9 and 29
31. randomized controlled trial.pt.
32. controlled clinical trial.pt.
33. randomized.ab.
34. placebo.ab.
35. drug therapy.fs.
36. randomly.ab.
37. trial.ab.
38. groups.ab.
39. 31 or 32 or 33 or 34 or 35 or 36 or 37 or 38
40. exp animals/ not humans.sh.
41. 39 not 40
42. 30 and 41
Embase
#33 #31 AND #32
#32 random*:ab,ti OR placebo* OR ((double NEXT/1 blind*):ab,ti)
#31 #10 AND #30
#30 #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28OR #29
#29 'canrenoic acid' OR canrenone OR eplerenone OR finerenone OR 'oxprenoate potassium' OR spironolactone OR aldactone OR contaren OR inspra OR luvion OR phanurane OR spiroletan
#28 ((mineralocorticoid OR aldosterone) NEAR/3 (antagonist* OR block* OR inhibit*)):ab,ti
#27 'mineralocorticoid antagonist'/exp
#26 sacubitril OR sacubitrilat OR lbq657 OR 'lbq 657' OR 'ahu377' OR 'ahu 377' OR entresto OR lcz696 OR 'lcz 696'
#25 arni:ab,ti
#24 (neprilysin NEAR/1 (inhibit* OR antagonist*)):ab,ti
#23 'enkephalinase inhibitor'/exp
#22 abitesartan OR azilsartan OR candesartan OR elisartan OR embusartan OR eprosartan OR fimasartan OR fonsartan OR forasartanOR irbesartan OR 'kt3‐671' OR losartan OR milfasartan OR olmesartan OR pomisartan OR pratosartan OR ripisartan OR saprisartanOR sparsentan OR tasosartan OR telmisartan OR valsartan OR zolasartan OR edarbi OR blopress OR atacand OR amias OR ratacandOR eprozar OR aprovel OR karvea OR avapro OR cozaar OR benicar OR olmecip OR micardis OR diovan
#21 arb:ab,ti OR arbs:ab,ti
#20 (angiotensin NEAR/3 ('receptor antagonist*' OR 'receptor block*')):ab,ti
#19 'angiotensin receptor antagonist'/exp
#18 alacepril OR altiopril OR ancovenin OR benazepril* OR captopril OR ceranapril OR ceronapril OR cilazapril OR deacetylalacepril OR delapril OR derapril OR enalapril* OR epicaptopril OR fasidotril* OR fosinopril OR foroxymithine OR gemopatrilat OR idrapril OR ilepatril OR imidapril* OR indolapril OR libenzapril OR lisinopril OR moexipril* OR omapatrilat OR pentopril* OR perindopril* OR pivopril OR quinapril* OR ramipril* OR rentiapril OR sampatrilat OR saralasin OR 's nitrosocaptopril' OR spirapril* OR temocapril*OR teprotide OR trandolapril* OR utibapril* OR zabicipril* OR zofenopril* OR aceon OR accupril OR altace OR capoten OR lotensinOR mavik OR monopril OR prinivil OR univas OR vasotec OR zestril
#17 acei:ab,ti
#16 (ace NEAR/1 inhibit*):ab,ti
#15 ((angiotensin* OR dipeptidyl* OR 'kininase ii') NEAR/3 (convert* OR enzyme OR inhibit* OR recept* OR block*)):ab,ti
#14 'dipeptidyl carboxypeptidase inhibitor'/exp
#13 acebutolol OR adimolol OR afurolol OR alprenolol OR amosulalol OR arotinolol OR atenolol OR befunolol OR betaxolol OR bevantololOR bisoprolol OR bopindolol OR bornaprolol OR brefonalol OR bucindolol OR bucumolol OR bufetolol OR bufuralol OR bunitrolol OR bunolol OR bupranolol OR butofilolol OR butoxamine OR carazolol OR carteolol OR carvedilol OR celiprolol OR cetamolol OR chlortalidone OR cloranolol OR cyanoiodopindolol OR cyanopindolol OR deacetylmetipranolol OR diacetolol OR dihydroalprenololOR dilevalol OR epanolol OR esmolol OR exaprolol OR falintolol OR flestolol OR flusoxolol OR hydroxybenzylpinodolol OR hydroxycarteolol OR hydroxymetoprolol OR indenolol OR iodocyanopindolol OR iodopindolol OR iprocrolol OR isoxaprolol OR labetalol OR landiolol OR levobunolol OR levomoprolol OR medroxalol OR mepindolol OR methylthiopropranolol OR metipranololOR metoprolol OR moprolol OR nadolol OR nebivolol OR nifenalol OR nipradilol OR oxprenolol OR pafenolol OR pamatolol OR penbutolol OR pindolol OR practolol OR primidolol OR prizidilol OR procinolol OR pronetalol OR propranolol OR proxodolol OR ridazolol OR salcardolol OR soquinolol OR sotalol OR spirendolol OR talinolol OR tertatolol OR tienoxolol OR tilisolol OR timolol OR tolamolol OR toliprolol OR tribendilol OR xibenolol OR betapace OR blocadren OR bystolic OR cartrol OR coreg OR corgard OR inderal OR kerlone OR levatol OR lopressor OR normodyne OR sectral OR tenormin OR toprol OR trandate OR visken OR zebeta
#12 (beta NEAR/2 (antagonist* OR block* OR receptor*)):ab,ti
#11 'beta adrenergic receptor blocking agent'/exp
#10 #3 AND #9
#9 #4 OR #5 OR #6 OR #7 OR #8
#8 'preserved systolic function':ab,ti OR 'normal systolic function':ab,ti OR hfpef:ab,ti OR 'hf‐pef':ab,ti OR hfnef:ab,ti OR 'hf‐nef':ab,ti OR dhf:ab,ti OR diastolic*:ab,ti
#7 ((preserved OR normal OR greater) NEAR/5 ('ejection fraction' OR ef OR lvef)):ab,ti
#6 'systolic dysfunction'/exp
#5 'diastolic dysfunction'/exp
#4 'heart ventricle function'/de
#3 #1 OR #2
#2 ((heart OR cardia* OR myocardial) NEAR/3 (failure OR insufficienc* OR decompensat*)):ab,ti
#1 'heart failure'/exp
ClinicalTrials.gov
Advanced Search‐‐Limited to study type: interventional studies
("heart failure" AND ("preserved ejection fraction" OR "normal ejection fraction" OR "preserved systolic function" OR "normal systolic function")) OR "diastolic heart failure" OR "HFpEF" OR "HF‐pEF" OR "HFnEF" OR "HF‐nEF" OR "DHF"
WHO International Clinical Trials Registry Platform (ICTRP) Search Portal
Standard Search
heart failure AND preserved ejection fraction OR heart failure AND normal ejection fraction OR
heart failure AND preserved systolic function OR heart failure AND normal systolic function OR
diastolic heart failure OR HFpEF OR HF‐pEF OR HFnEF OR HF‐nEF OR DHF

Study flow diagram

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
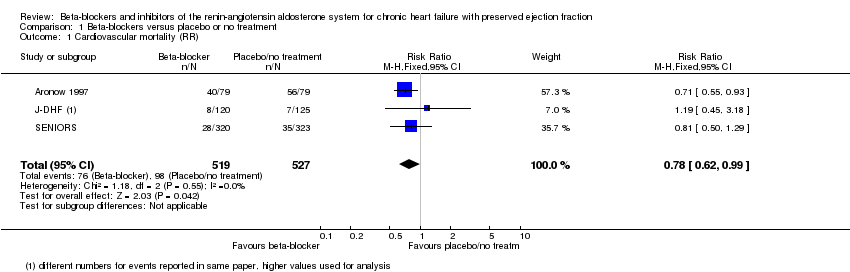
Comparison 1 Beta‐blockers versus placebo or no treatment, Outcome 1 Cardiovascular mortality (RR).

Comparison 1 Beta‐blockers versus placebo or no treatment, Outcome 2 Heart failure hospitalisation (RR).
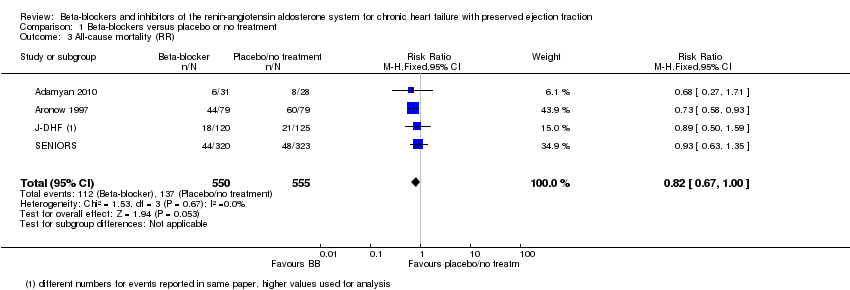
Comparison 1 Beta‐blockers versus placebo or no treatment, Outcome 3 All‐cause mortality (RR).
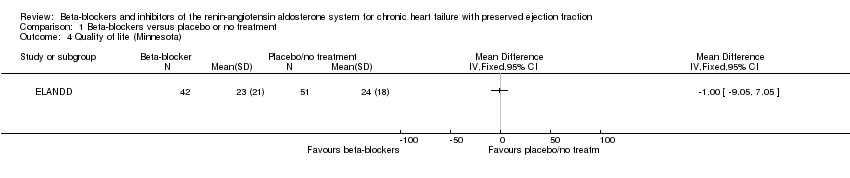
Comparison 1 Beta‐blockers versus placebo or no treatment, Outcome 4 Quality of life (Minnesota).
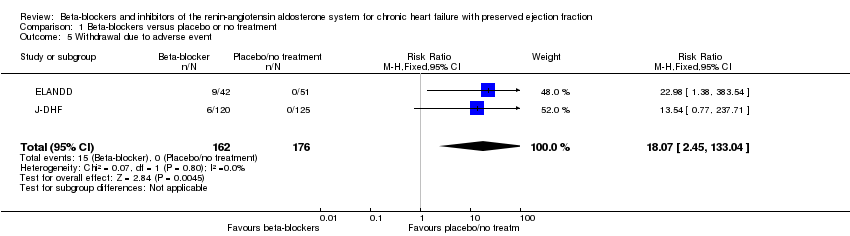
Comparison 1 Beta‐blockers versus placebo or no treatment, Outcome 5 Withdrawal due to adverse event.

Comparison 2 Mineralocorticoid receptor antagonists versus placebo or no treatment, Outcome 1 Cardiovascular mortality (RR).
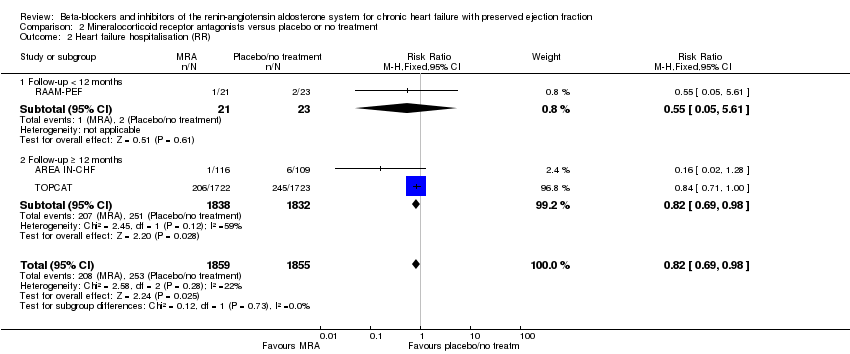
Comparison 2 Mineralocorticoid receptor antagonists versus placebo or no treatment, Outcome 2 Heart failure hospitalisation (RR).
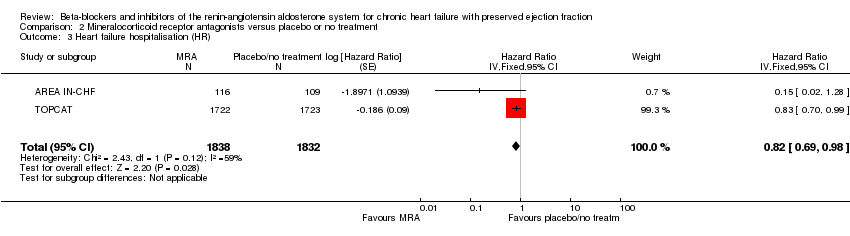
Comparison 2 Mineralocorticoid receptor antagonists versus placebo or no treatment, Outcome 3 Heart failure hospitalisation (HR).

Comparison 2 Mineralocorticoid receptor antagonists versus placebo or no treatment, Outcome 4 Hyperkalaemia.
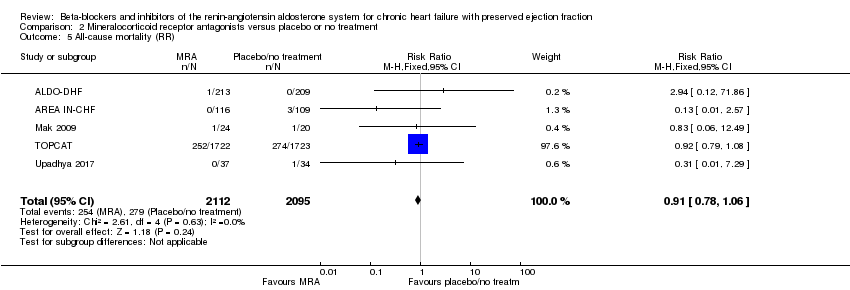
Comparison 2 Mineralocorticoid receptor antagonists versus placebo or no treatment, Outcome 5 All‐cause mortality (RR).

Comparison 2 Mineralocorticoid receptor antagonists versus placebo or no treatment, Outcome 6 Quality of life.

Comparison 2 Mineralocorticoid receptor antagonists versus placebo or no treatment, Outcome 7 Quality of life (KCCQ).

Comparison 2 Mineralocorticoid receptor antagonists versus placebo or no treatment, Outcome 8 Quality of life (Minnesota).

Comparison 2 Mineralocorticoid receptor antagonists versus placebo or no treatment, Outcome 9 Withdrawal due to adverse event.

Comparison 3 Angiotensin converting enzyme inhibitors versus placebo or no treatment, Outcome 1 Cardiovascular mortality (RR).
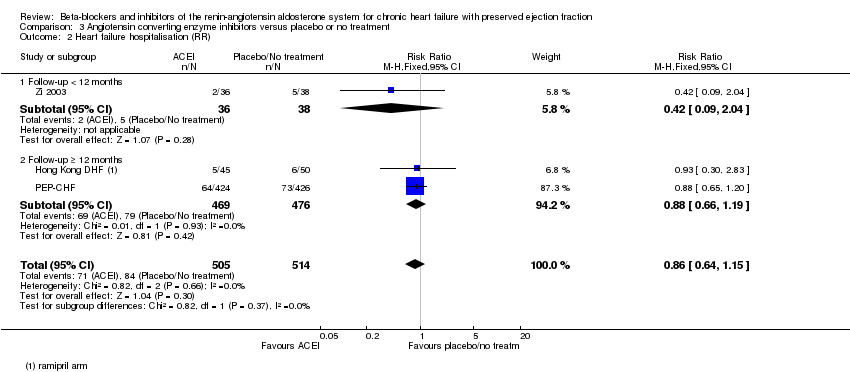
Comparison 3 Angiotensin converting enzyme inhibitors versus placebo or no treatment, Outcome 2 Heart failure hospitalisation (RR).

Comparison 3 Angiotensin converting enzyme inhibitors versus placebo or no treatment, Outcome 3 Hyperkalaemia.
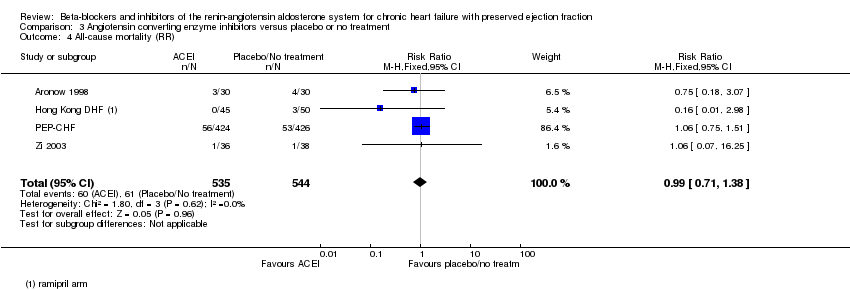
Comparison 3 Angiotensin converting enzyme inhibitors versus placebo or no treatment, Outcome 4 All‐cause mortality (RR).

Comparison 3 Angiotensin converting enzyme inhibitors versus placebo or no treatment, Outcome 5 Quality of life (Minnesota).

Comparison 3 Angiotensin converting enzyme inhibitors versus placebo or no treatment, Outcome 6 Withdrawal due to adverse event.

Comparison 4 Angiotensin receptor blockers versus placebo or no treatment, Outcome 1 Cardiovascular mortality (RR).

Comparison 4 Angiotensin receptor blockers versus placebo or no treatment, Outcome 2 Cardiovascular mortality (HR).

Comparison 4 Angiotensin receptor blockers versus placebo or no treatment, Outcome 3 Heart failure hospitalisation (RR).

Comparison 4 Angiotensin receptor blockers versus placebo or no treatment, Outcome 4 Heart failure hospitalisation (HR).
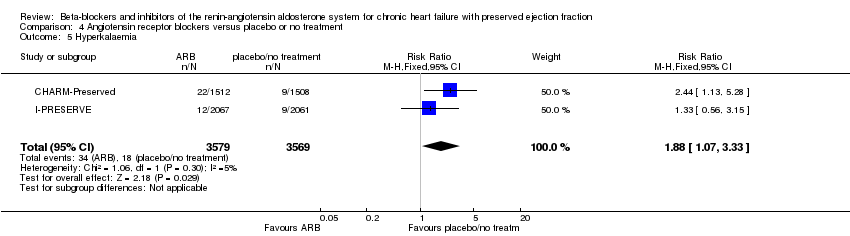
Comparison 4 Angiotensin receptor blockers versus placebo or no treatment, Outcome 5 Hyperkalaemia.
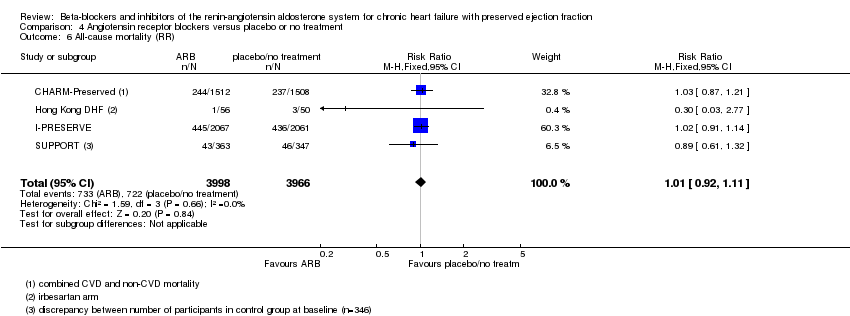
Comparison 4 Angiotensin receptor blockers versus placebo or no treatment, Outcome 6 All‐cause mortality (RR).
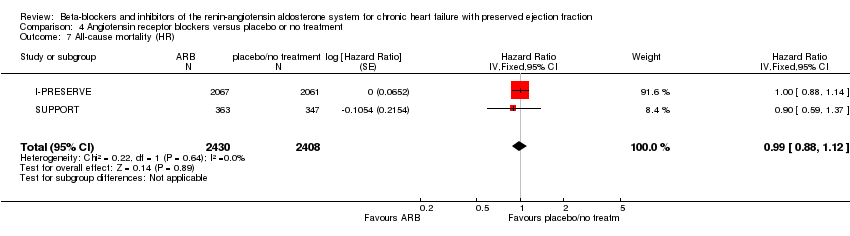
Comparison 4 Angiotensin receptor blockers versus placebo or no treatment, Outcome 7 All‐cause mortality (HR).

Comparison 4 Angiotensin receptor blockers versus placebo or no treatment, Outcome 8 Quality of life (Minnesota).
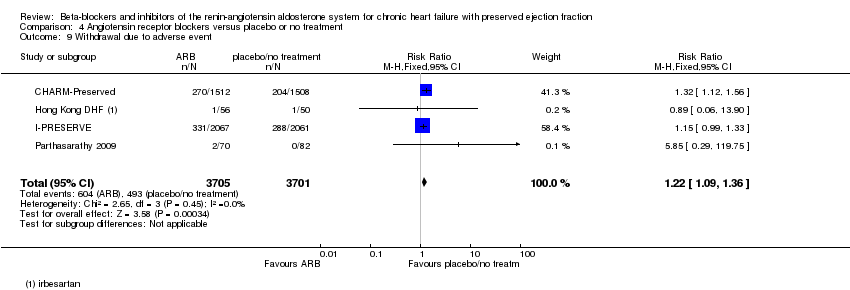
Comparison 4 Angiotensin receptor blockers versus placebo or no treatment, Outcome 9 Withdrawal due to adverse event.
| Beta‐blockers compared to placebo or no treatment for chronic heart failure with preserved ejection fraction | ||||||
| Patient or population: chronic heart failure with preserved ejection fraction | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
| Risk with placebo/no treatment | Risk with Beta‐blockers | |||||
| Cardiovascular mortality (RR) | Study population | RR 0.78 | 1046 | ⊕⊕⊝⊝ | Three additional studies (ELANDD; SWEDIC; Takeda 2004) reported that no deaths occurred | |
| 173 per 1000 | 135 per 1000 | |||||
| Heart failure hospitalisation (RR) | Study population | RR 0.73 | 449 | ⊕⊝⊝⊝ | Follow‐up unclear for SWEDIC. ELANDD reported that no hospitalisation due to heart failure occurred | |
| 117 per 1000 | 86 per 1000 | |||||
| Hyperkalaemia | 245 (1 RCT) | ⊕⊝⊝⊝ | J‐DHF reported one participant in the intervention group (N = 120) experienced hyperkalaemia but did not report on this outcome for the control group. No further data were available from any of the other studies. | |||
| All‐cause mortality (RR) | Study population | RR 0.82 | 1105 | ⊕⊕⊝⊝ | Follow‐up unclear for Adamyan 2010. ELANDD, SWEDIC and Takeda 2004 reported that no deaths occurred | |
| 243 per 1000 | 199 per 1000 | |||||
| Quality of life (Minnesota) | Mean quality of life (Minnesota) was 24 | MD 1 lower | ‐ | 93 | ⊕⊝⊝⊝ | Lower = better, 5 point difference considered to be clinically meaningful |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded by one level due to unclear selection bias in most studies. 2 Downgraded by one level due to concerns about the smaller study being more precise than the larger study. 3 Downgraded by one level due to large variation in size of effect. 4 Downgraded by two levels due to few events and wide CI. 5 Downgraded by two levels due to very small sample size. 6 Suspected publication bias; this is a patient‐relevant outcome that is not reported in most studies. 7 Downgraded by two levels due to incomplete reporting. | ||||||
| MRA compared to placebo or no treatment for chronic heart failure with preserved ejection fraction | ||||||
| Patient or population: chronic heart failure with preserved ejection fraction | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
| Risk with placebo/no treatment | Risk with MRA | |||||
| Cardiovascular mortality (RR) | Study population | RR 0.90 | 4070 | ⊕⊕⊕⊝ | Two additional trials (RAAM‐PEF, Kurrelmeyer 2014) reported that no deaths occurred | |
| 88 per 1000 | 79 per 1000 | |||||
| Heart failure hospitalisation (RR) | Study population | RR 0.82 | 3714 | ⊕⊕⊕⊝ | Three additional trials (ALDO‐DHF ,Kurrelmeyer 2014, Upadhya 2017) reported that no hospitalisation due to heart failure occurred | |
| 136 per 1000 | 112 per 1000 | |||||
| Hyperkalaemia | Study population | RR 2.11 | 4291 | ⊕⊕⊕⊕ | Two trials defined hyperkalaemia ≥ 5.5 mEg/L | |
| 83 per 1000 | 175 per 1000 | |||||
| All‐cause mortality | Study population | RR 0.91 | 4207 | ⊕⊕⊕⊝ | Two additional trials (RAAM‐PEF, Kurrelmeyer 2014) reported that no deaths occurred | |
| 133 per 1000 | 121 per 1000 | |||||
| Quality of life (Minnesota) | Mean quality of life (Minnesota) ranged from 20 to 25 | MD 0.84 higher | ‐ | 511 | ⊕⊕⊝⊝ | Lower = better, 5 points are considered a clinically significant difference We did not pre‐specify which QoL scale was to be reported in the 'Summary of findings' table. To aid comparisons among 'Summary of findings' tables we chose to include the Minnesota Living with Heart Failure questionnaire and not the SMD across two scales |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded by one level due to imprecision. 2 Downgraded by one level because one trial was open label. 3 Downgraded by one level due to small sample size. | ||||||
| ACEI compared to placebo or no treatment for chronic heart failure with preserved ejection fraction | ||||||
| Patient or population: chronic heart failure with preserved ejection fraction | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
| Risk with placebo/no treatment | Risk with ACEI | |||||
| Cardiovascular mortality (RR) | Study population | RR 0.93 | 945 | ⊕⊕⊕⊝ | One additional trial (Kitzman 2010) reported that no deaths occurred | |
| 86 per 1000 | 81 per 1000 | |||||
| Heart failure hospitalisation (RR) | Study population | RR 0.86 | 1019 | ⊕⊕⊕⊝ | ||
| 13 per 1000 | 11 per 1000 | |||||
| Hyperkalaemia | 74 (1 RCTs) | ⊕⊝⊝⊝ | One trial (Zi 2003) reported 2 events in the intervention group (N = 36), 0 events in the control group (N = 38) (RR 5.27, 95% CI 0.26 to 106.16) | |||
| All‐cause mortality (RR) | Study population | RR 0.99 | 1079 | ⊕⊕⊕⊝ | One additional trial (Kitzman 2010) reported that no deaths occurred | |
| 119 per 1000 | 119 per 1000 | |||||
| Quality of life (Minnesota) | Mean quality of life (Minnesota) ranged from 10.9 to 29 | MD 0.09 lower | ‐ | 154 | ⊕⊕⊝⊝ | Scale: 0 to 105, lower = better, 5 point difference considered clinically relevant One trial (SNEGOVIK) reported mean change from baseline of ‐19.8 for intervention and ‐10.7 for control |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded by one level due to wide CI. 2 Downgraded by one level due to risk of bias (open label). 3 Downgraded by one level due to low sample size. 4 Downgraded by one level due to unclear selection bias. | ||||||
| ARB compared to placebo or no treatment for chronic heart failure with preserved ejection fraction | ||||||
| Patient or population: chronic heart failure with preserved ejection fraction | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
| Risk with placebo/no treatment | Risk with ARB | |||||
| Cardiovascular mortality (RR) | Study population | RR 1.02 | 7254 | ⊕⊕⊕⊕ | One additional trial (Parthasarathy 2009) reported that no deaths occurred | |
| 131 per 1000 | 133 per 1000 | |||||
| Heart failure hospitalisation (RR) | Study population | RR 0.92 | 7254 | ⊕⊕⊕⊕ | ||
| 171 per 1,‐000 | 157 per 1,‐000 | |||||
| Hyperkalaemia | Study population | RR 1.88 | 7148 | ⊕⊕⊕⊕ | ||
| 3 per 1,000 | 5 per 1,000 | |||||
| All‐cause mortality (RR) | Study population | RR 1.01 | 7964 | ⊕⊕⊕⊕ | One additional trial (Parthasarathy 2009) reported that no deaths occurred | |
| 72 per 1000 | 73 per 1,‐000 | |||||
| Quality of life (Minnesota) | Mean quality of life (Minnesota) ranged from 10.9 to 31.6 | MD 0.41 higher | ‐ | 3117 | ⊕⊕⊕⊕ | Scale: 0 to 105, lower = better, 5 point difference considered clinically relevant |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Cardiovascular mortality (RR) Show forest plot | 3 | 1046 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.62, 0.99] |
| 2 Heart failure hospitalisation (RR) Show forest plot | 4 | 449 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.47, 1.13] |
| 2.1 Follow‐up < 12 months | 1 | 67 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.31 [0.09, 1.02] |
| 2.2 Follow‐up ≥ 12 months | 2 | 285 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.48, 1.31] |
| 2.3 Follow‐up unknown | 1 | 97 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.31 [0.26, 107.85] |
| 3 All‐cause mortality (RR) Show forest plot | 4 | 1105 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.67, 1.00] |
| 4 Quality of life (Minnesota) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5 Withdrawal due to adverse event Show forest plot | 2 | 338 | Risk Ratio (M‐H, Fixed, 95% CI) | 18.07 [2.45, 133.04] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Cardiovascular mortality (RR) Show forest plot | 3 | 4070 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.74, 1.11] |
| 2 Heart failure hospitalisation (RR) Show forest plot | 3 | 3714 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.69, 0.98] |
| 2.1 Follow‐up < 12 months | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.05, 5.61] |
| 2.2 Follow‐up ≥ 12 months | 2 | 3670 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.69, 0.98] |
| 3 Heart failure hospitalisation (HR) Show forest plot | 2 | 3670 | Hazard Ratio (Fixed, 95% CI) | 0.82 [0.69, 0.98] |
| 4 Hyperkalaemia Show forest plot | 6 | 4291 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.11 [1.77, 2.51] |
| 5 All‐cause mortality (RR) Show forest plot | 5 | 4207 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.78, 1.06] |
| 6 Quality of life Show forest plot | 5 | 603 | Std. Mean Difference (IV, Random, 95% CI) | 0.05 [‐0.23, 0.34] |
| 7 Quality of life (KCCQ) Show forest plot | 2 | 92 | Mean Difference (IV, Random, 95% CI) | ‐0.78 [‐28.02, 26.46] |
| 8 Quality of life (Minnesota) Show forest plot | 3 | 511 | Mean Difference (IV, Random, 95% CI) | 0.84 [‐2.30, 3.98] |
| 9 Withdrawal due to adverse event Show forest plot | 4 | 3986 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [1.00, 1.21] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Cardiovascular mortality (RR) Show forest plot | 2 | 945 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.61, 1.42] |
| 2 Heart failure hospitalisation (RR) Show forest plot | 3 | 1019 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.64, 1.15] |
| 2.1 Follow‐up < 12 months | 1 | 74 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.09, 2.04] |
| 2.2 Follow‐up ≥ 12 months | 2 | 945 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.66, 1.19] |
| 3 Hyperkalaemia Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 All‐cause mortality (RR) Show forest plot | 4 | 1079 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.71, 1.38] |
| 5 Quality of life (Minnesota) Show forest plot | 2 | 154 | Mean Difference (IV, Fixed, 95% CI) | ‐0.09 [‐3.66, 3.48] |
| 6 Withdrawal due to adverse event Show forest plot | 3 | 1019 | Risk Ratio (M‐H, Random, 95% CI) | 1.53 [0.26, 9.00] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Cardiovascular mortality (RR) Show forest plot | 3 | 7254 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.90, 1.14] |
| 2 Cardiovascular mortality (HR) Show forest plot | 2 | 5087 | Hazard Ratio (Fixed, 95% CI) | 1.00 [0.89, 1.13] |
| 3 Heart failure hospitalisation (RR) Show forest plot | 3 | 7254 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.83, 1.02] |
| 4 Heart failure hospitalisation (HR) Show forest plot | 2 | 7148 | Hazard Ratio (Fixed, 95% CI) | 0.90 [0.80, 1.01] |
| 5 Hyperkalaemia Show forest plot | 2 | 7148 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.88 [1.07, 3.33] |
| 6 All‐cause mortality (RR) Show forest plot | 4 | 7964 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.92, 1.11] |
| 7 All‐cause mortality (HR) Show forest plot | 2 | 4838 | Hazard Ratio (Fixed, 95% CI) | 0.99 [0.88, 1.12] |
| 8 Quality of life (Minnesota) Show forest plot | 3 | 3117 | Mean Difference (IV, Fixed, 95% CI) | 0.41 [‐0.86, 1.67] |
| 9 Withdrawal due to adverse event Show forest plot | 4 | 7406 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [1.09, 1.36] |

