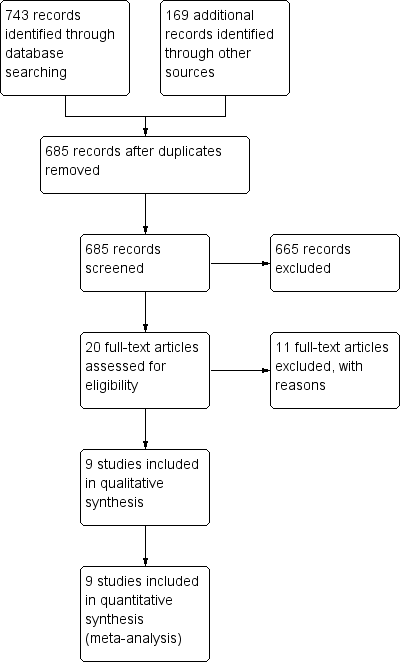
| 1 Relapse Show forest plot | 9 | 804 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.72, 1.24] |
|
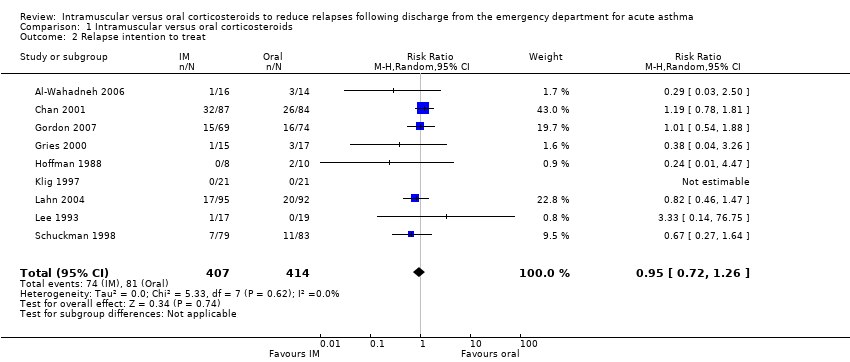
| 2 Relapse intention to treat Show forest plot | 9 | 821 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.72, 1.26] |
|
| 3 Subgroup analysis: children versus adults Show forest plot | 9 | 804 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.72, 1.24] |
|
| 3.1 Children | 4 | 245 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.48, 1.53] |
| 3.2 Adults | 5 | 559 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.71, 1.33] |
| 4 Subgroup analysis: relapse within 10 days and over 10 days post‐discharge Show forest plot | 9 | | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only |
|
| 4.1 Within 10 days post‐discharge | 7 | 742 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.51, 1.07] |
| 4.2 Greater than 10 days post‐discharge | 5 | 556 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.74, 1.33] |
| 5 Subgroup analysis: mild/moderate versus severe exacerbations Show forest plot | 5 | 557 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.70, 1.32] |
|
| 5.1 Mild/moderate exacerbations | 4 | 539 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.71, 1.34] |
| 5.2 Severe exacerbations | 1 | 18 | Risk Ratio (M‐H, Random, 95% CI) | 0.24 [0.01, 4.47] |
| 6 Sensitivity analysis: risk of bias Show forest plot | 5 | 559 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.71, 1.33] |
|
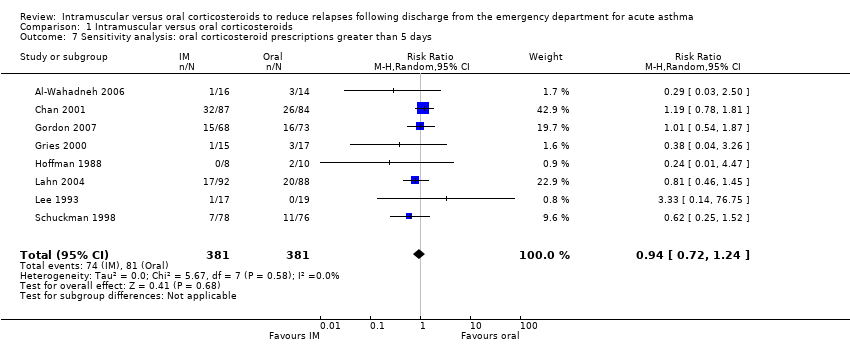
| 7 Sensitivity analysis: oral corticosteroid prescriptions greater than 5 days Show forest plot | 8 | 762 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.72, 1.24] |
|
| 8 Sensitivity analysis: fixed effects Show forest plot | 9 | 804 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.69, 1.19] |
|
| 9 Sensitivity analysis: corticosteroids in ED Show forest plot | 5 | 320 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.45, 1.29] |
|
| 10 Serious adverse events; hospitalization following discharge Show forest plot | 1 | | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
|
| 11 Adverse events Show forest plot | 5 | 404 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.64, 1.07] |
|
| 12 Adverse events: nausea/vomiting/GI distress Show forest plot | 3 | 320 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.09, 3.59] |
|
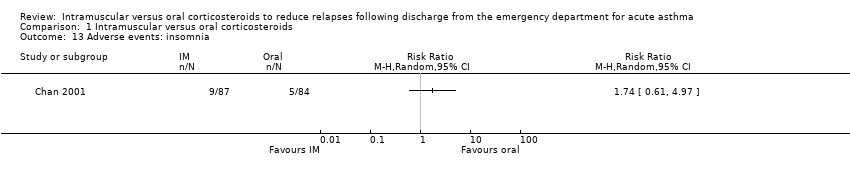
| 13 Adverse events: insomnia Show forest plot | 1 | | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
|
| 14 Adverse events: personality changes Show forest plot | 1 | | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
|
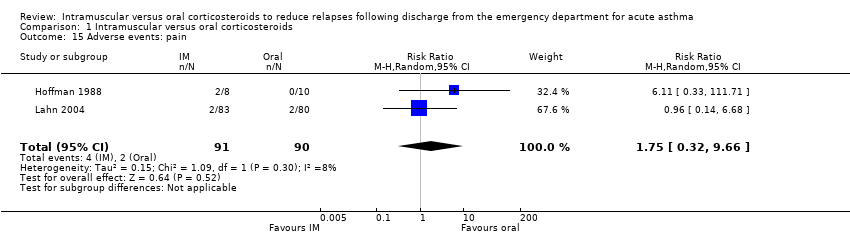
| 15 Adverse events: pain Show forest plot | 2 | 181 | Risk Ratio (M‐H, Random, 95% CI) | 1.75 [0.32, 9.66] |
|
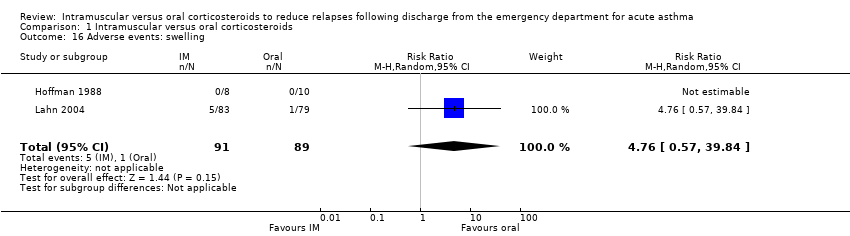
| 16 Adverse events: swelling Show forest plot | 2 | 180 | Risk Ratio (M‐H, Random, 95% CI) | 4.76 [0.57, 39.84] |
|
| 17 Adverse events: redness Show forest plot | 2 | 175 | Risk Ratio (M‐H, Random, 95% CI) | 13.5 [0.77, 235.63] |
|
| 18 Pulmonary function: peak expiratory flow (L/min) Show forest plot | 4 | 272 | Mean Difference (IV, Random, 95% CI) | ‐7.78 [‐38.83, 23.28] |
|
| 19 Pulmonary function: FEV₁/FVC (%) Show forest plot | 1 | | Mean Difference (IV, Random, 95% CI) | Totals not selected |
|
| 20 Symptom persistence Show forest plot | 3 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 0.41 [0.14, 1.20] |
|
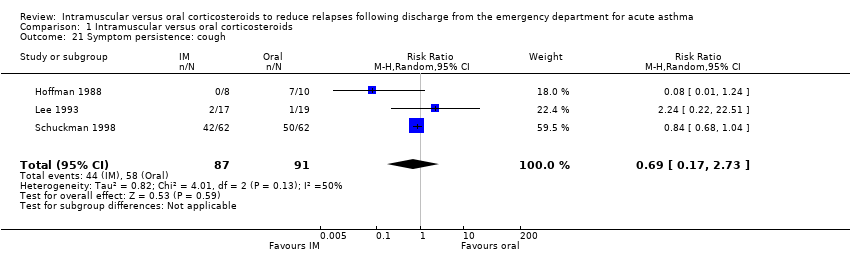
| 21 Symptom persistence: cough Show forest plot | 3 | 178 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.17, 2.73] |
|
| 22 Symptom persistence: wheezing Show forest plot | 3 | 177 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.14, 2.52] |
|
| 23 24‐hour beta agonist use Show forest plot | 2 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.21, 1.37] |
|