Diferentes regímenes de corticosteroides orales para el asma aguda
Resumen
Antecedentes
El asma es una afección respiratoria frecuente a largo plazo que afecta a aproximadamente 300 000 000 de personas en todo el mundo. Los pacientes con asma pueden presentar un empeoramiento a corto plazo de los síntomas del asma; estos episodios a menudo se conocen como "exacerbaciones", "brotes", "ataques" o "asma agudo". Los esteroides orales, que tienen un efecto antiinflamatorio potente, se recomiendan para todas las exacerbaciones del asma, excepto las más leves, y se deben iniciar con prontitud. Los esteroides orales prescritos con mayor frecuencia son la prednisolona y la dexametasona, pero las guías actuales para las dosis varían entre los países y a menudo entre diferentes elaboradores de guías dentro del mismo país. A pesar de la eficacia comprobada, la administración de esteroides se debe equilibrar contra la posibilidad de provocar eventos adversos importantes. Las pruebas con respecto a la dosis óptima de esteroides orales para las exacerbaciones del asma son algo limitadas y tienen como objetivo maximizar la recuperación a la vez que disminuyen los posibles efectos secundarios, que es el tema de esta revisión.
Objetivos
Evaluar la eficacia y la seguridad de cualquier dosis o duración de los esteroides orales versus otra dosis o duración de los esteroides orales en adultos y niños con una exacerbación del asma.
Métodos de búsqueda
Se identificaron ensayos en el registro especializado del Grupo Cochrane de Vías Respiratorias (Cochrane Airways Group Specialised Register, CAGR), ClinicalTrials.gov (www.ClinicalTrials.gov), en el World Health Organization (WHO) trials portal (www.who.int/ictrp/en/) y en las listas de referencias de todos los estudios primarios y artículos de revisión. Esta búsqueda estaba actualizada hasta abril 2016.
Criterios de selección
Se incluyeron los ensayos controlados aleatorios paralelos (ECA), independientemente del cegamiento o la duración, que evaluaron una dosis o duración del esteroide oral versus otra dosis o duración, para el tratamiento de las exacerbaciones del asma. Se incluyeron los estudios que incluyeron adultos y niños con asma de cualquier gravedad, en los que los investigadores analizaron a los adultos y los niños por separado. Se permitieron otras cointervenciones en el tratamiento de una exacerbación del asma, a condición de que no fuera parte del tratamiento asignado al azar. Se incluyeron los estudios informados como texto completo, los publicados como resumen solamente y los datos no publicados.
Obtención y análisis de los datos
Dos revisores de forma independiente examinaron los resultados de la búsqueda de los ensayos incluidos, extrajeron los datos numéricos y evaluaron el riesgo de sesgo; todos los datos se verificaron de forma cruzada para la exactitud. Los desacuerdos se resolvieron mediante discusión con el tercer revisor o con un asesor externo.
Los datos dicotómicos se analizaron como odds ratios (OR) o diferencias de riesgos (DR) y se utilizaron los participantes del estudio como la unidad del análisis; los datos continuos se analizaron como diferencias de medias (DM). Se utilizó un modelo de efectos aleatorios y se realizó un análisis de efectos fijos si se detectó heterogeneidad estadística. Todos los resultados se calificaron mediante GRADE (Grades of Recommendation, Assessment, Development and Evaluation) y los resultados se presentaron en la tabla "Resumen de los hallazgos".
Resultados principales
Se incluyeron 18 estudios que asignaron al azar a 2438 participantes (adultos y niños) y realizaron las comparaciones de interés. Los estudios incluidos evaluaron dosis mayores versus inferiores de prednisolona (n = 4); ciclos más largos versus más cortos de prednisolona (n = 3) o dexametasona (n = 1); ciclos decrecientes versus ciclos no decrecientes de prednisolona (n = 4); y prednisolona versus dexametasona (n = 6). La duración del seguimiento varió de siete días a seis meses. El estudio más pequeño asignó al azar a solamente 15 participantes y el más grande 638 (mediana 93). Las intervenciones y los resultados informados fueron variados y limitaron el número de metanálisis significativos que fue posible realizar.
Para dos de los resultados primarios (ingreso hospitalario y eventos adversos graves) los eventos fueron demasiado poco frecuentes para poder establecer conclusiones acerca de la superioridad de un tratamiento sobre el otro, o su equivalencia. Los investigadores de los estudios incluidos informaron los síntomas de asma de diferentes maneras y pocas veces utilizaron escalas validadas, lo que también limitó las conclusiones. De manera similar, el metanálisis de los resultados secundarios fue obstaculizado por la heterogeneidad entre las intervenciones y las medidas de resultado utilizadas. En general, no se encontraron pruebas convincentes de diferencias en los resultados entre una dosis mayor o un ciclo más largo y una dosis inferior o un ciclo más corto de prednisolona o dexametasona, o entre la prednisolona y la dexametasona.
En general los estudios incluidos tuvieron una calidad metodológica razonable. Los revisores calificaron la mayoría de los resultados de la revisión como de calidad baja o muy baja, lo que significa que no hay seguridad en las estimaciones del efecto. El motivo principal para la disminución fue la imprecisión, pero la falta de direccionalidad y el riesgo de sesgo también redujeron la confianza en algunas estimaciones.
Conclusiones de los autores
Las pruebas no son suficientemente consistentes para mostrar si los regímenes más cortos o de dosis inferior son en general menos eficaces que los regímenes más largos o de dosis mayor, o que realmente los últimos se asocian con más eventos adversos. Cualquier cambio recomendado para la práctica actual debe estar apoyado por los datos de ensayos más grandes, bien diseñados. El diseño de los estudios y las medidas de resultado fueron variados y limitaron el número de metanálisis que fue posible realizar. Un mayor énfasis en la aceptabilidad y en determinar si algunos regímenes pudieran ser más fáciles de cumplir que otros, podría mejorar el informe de las decisiones clínicas en los pacientes individuales.
PICO
Resumen en términos sencillos
Diferentes dosis y duraciones de los esteroides orales para las crisis asmáticas
Antecedentes: Los pacientes con asma a veces presentan crisis asmáticas en las que empeoran síntomas como la tos, la opresión torácica y la dificultad para respirar. A muchos pacientes con crisis asmáticas se les trata con esteroides que se administran generalmente como un tratamiento de corta duración de fármacos en comprimidos o líquidos. Los esteroides funcionan al reducir la inflamación en las vías respiratorias en los pulmones, pero pueden tener efectos secundarios (p.ej. reducción del crecimiento en los niños, hiperactividad, náuseas).
Pregunta de la revisión: Se planificó comparar diferentes dosis o duraciones de esteroides orales administrados a los pacientes con crisis asmáticas. Este es un tema importante porque en diferentes países se utilizan diferentes dosis y duraciones de los esteroides orales para las crisis asmáticas y no se sabe qué régimen tiene mayores probabilidades de mejorar los síntomas a la vez que disminuye los efectos secundarios desagradables.
Características de los estudios: Se incluyeron 18 estudios con 2438 adultos y niños. Los estudios compararon dos tipos de esteroides (prednisolona y dexametasona) o dos dosis o duraciones diferentes del fármaco. El estudio más pequeño incluyó sólo a 15 personas, y el más grande 638. Los estudios siguieron a los pacientes entre siete días y seis meses para ver qué les sucedió. Las pruebas presentadas aquí están actualizadas hasta abril 2016.
Resultados clave: Fue difícil combinar los resultados de los estudios de forma provechosa porque los investigadores utilizaron diversas dosis y duraciones de los esteroides y midieron los resultados de maneras diferentes. Además, eventos como los ingresos hospitalarios y los efectos secundarios graves sucedieron muy pocas veces en estos estudios, lo que dificultó determinar si los ciclos más largos o más cortos o las dosis mayores o inferiores son mejores o más seguros, o si la prednisolona en general es mejor o peor que la dexametasona. Algunos estudios fueron antiguos y no utilizaron las dosis o las duraciones de esteroides que utilizan los médicos hoy en día.
Cualquier cambio en la forma de tratar actualmente los ataques de asma con esteroides orales necesitaría estar apoyado por estudios más grandes que los realizados hasta el presente.
Calidad de la evidencia: Las pruebas presentadas en esta revisión se consideraron en general de calidad baja o muy baja, lo que significa que no hay mucha seguridad con respecto a si los resultados son exactos, principalmente porque muchos estudios no se pudieron combinar. Algunos estudios no explicaron claramente cómo los organizadores del ensayo decidieron qué pacientes recibirían qué dosis de esteroides, y en algunos estudios los participantes y los organizadores del ensayo conocían la dosis administrada. Esto puede haber afectado los resultados de los estudios.
Conclusiones de los autores
Summary of findings
| Adults: higher dose/longer course compared with lower dose/shorter course for acute asthma | ||||||
| Patient or population: adults with an acute exacerbation of asthma Duration range: 3 to 26 weeks | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Risk with lower dose/shorter course | Risk with higher dose/longer course | |||||
| Re‐admission in follow‐up period | Longer vs shorter course prednisolone | OR 1.35 | 142 | ⊕⊕⊝⊝ | ||
| 74 per 1000 | 97 per 1000 | |||||
| Asthma symptoms Asthma severity score | Longer vs shorter course prednisolone | ‐ | 44 | ⊕⊕⊝⊝ | Higher score = Worse symptoms | |
| Mean asthma severity score was 2.6 | Mean asthma severity score in the longer course group was 0.7 lower (1.28 lower to 0.12 lower) | |||||
| Asthma symptoms Complete resolution by day 28 | Longer vs shorter course prednisolone | OR 0.55 | 35 | ⊕⊕⊝⊝ | ||
| 412 per 1000 | 278 per 1000 | |||||
| New exacerbation in follow‐up period Requiring visit to healthcare provider | Longer vs shorter course prednisolone | OR 0.98 | 55 | ⊕⊝⊝⊝ | ||
| 111 per 1000 | 109 per 1000 | |||||
| Stable (same daily dose for 7 days) vs tapered (tapering daily dose over 7 days) prednisolone | OR 3.56 | 41 | ⊕⊝⊝⊝ | No events were reported in the tapered arm and only 2 events in the stable arm, so we were unable to calculate a baseline risk | ||
| No events | Risk difference in the stable (higher total dose) group was 9% (0 to 26%) | |||||
| New exacerbation in follow‐up period Oral corticosteroids prescribed | Longer vs shorter course prednisolone | OR 0.62 | 122 | ⊕⊕⊝⊝ | Lederle 1987 dominates this analysis, as the event rate was much higher than in the other 2 studies, possibly reflecting co‐morbid COPD in the study population. Result should be interpreted with caution | |
| 241 per 1000 | 165 per 1000 (68 to 348) | |||||
| Lung function tests FEV1% predicted | Stable (same daily dose for 7 days) vs tapered (tapering daily dose over 7 days) prednisolone | ‐ | 41 | ⊕⊝⊝⊝ | Higher percentage = Better lung function | |
| Mean FEV1% predicted was 70.6 | Mean FEV1% predicted in the stable dose (higher total dose) was 1.02 lower (4.62 lower to 2.58 higher) | |||||
| All adverse events | Longer vs shorter course prednisolone | OR 4.15 | 43 | ⊕⊝⊝⊝ | ||
| 143 per 1000 | 409 per 1000 | |||||
| *Risk in the intervention group (and its 95% confidence interval) is based on assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) | ||||||
| GRADE Working Group grades of evidence | ||||||
| aLederle 1987 carried a large proportion of the analysis weight for this outcome because event rates were higher in both groups. This may reflect co‐morbid COPD (participants were older and most had an extensive smoking history). Downgraded once for indirectness bConfidence intervals include no difference and an important benefit of a longer or shorter course. Downgraded once for imprecision cConfidence intervals excluded possible benefit of a shorter course, but the effect was based on only 1 study of 44 people. Downgraded once for imprecision dA 1‐7 scale of symptom severity averaged over days 6‐21 was used, making clinical benefit difficult to interpret. Downgraded once for indirectness eNeither treatment regimen used in the one study in this analysis is consistent with current international guidance. Downgraded once for indirectness fThe study contributing most of the analysis weight was unblinded and uncertainties surrounded the selection procedure. Downgraded once for risk of bias gBoth trials contributing to the analysis used a treatment regimen that was inconsistent with current international guidance. Downgraded once for indirectness hThe effect was derived from 2 very similar studies including 41 people in total. Studies had smaller standard deviations than would be expected given the sample sizes. Downgraded once for imprecision iThe result is based on 1 small study and has wide confidence intervals, which do not exclude the possibility of no difference or an important increase in adverse events in the longer course arm, Downgraded twice for imprecision | ||||||
| Adults: prednisolone compared with dexamethasone for acute asthma | ||||||
| Patient or population: adults with an acute exacerbation of asthma Duration: 2 weeks | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Risk with dexamethasone | Risk with prednisolone | |||||
| Re‐admission during follow‐up period | 29 per 1000 | 10 per 1000 | OR 0.35 | 200 | ⊕⊝⊝⊝ | |
| Asthma symptoms Returned to normal activities within 3 days | 901 per 1000 | 800 per 1000 | OR 0.44 | 191 | ⊕⊕⊝⊝ | |
| New exacerbation during follow‐up period Any ED visit after discharge | 48 per 1000 | 63 per 1000 | OR 1.32 | 200 | ⊕⊝⊝⊝ | |
| New exacerbation during follow‐up period Unscheduled visit to primary healthcare provider | 29 per 1000 | 52 per 1000 | OR 1.85 | 200 | ⊕⊝⊝⊝ | |
| *Risk in the intervention group (and its 95% confidence interval) is based on assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) | ||||||
| GRADE Working Group grades of evidence | ||||||
| aOnly 1 study contributed to this outcome with very few events reported in total, resulting in an imprecise estimate with confidence intervals including both important harms and benefits of either regimen. Downgraded twice for imprecision bOnly contributing study judged to be at high risk of attrition bias because of post‐randomisation exclusions and large numbers lost to follow‐up. Downgraded once for risk of bias cOnly 1 study contributed to this outcome with imprecise estimate and confidence intervals not completely excluding the possibility of no differences. Downgraded once for imprecision | ||||||
| Children: higher dose/longer course compared with lower dose/shorter course for acute asthma | ||||||
| Patient or population: children with an acute exacerbation of asthma Duration range: 1 to 4 weeks | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Risk with lower dose/shorter course | Risk with higher dose/longer course | |||||
| Re‐admission during follow‐up period | Higher‐ vs lower‐dose prednisolone | Not estimable | 98 | ⊕⊝⊝⊝ | Only one 3‐arm study (Langton Hewer 1998) contributed events to this analysis. Two lower‐dose arms pooled for this outcome. OR 1.55 (0.24 to 9.78) favouring lower dose | |
| Not pooled | Not pooled | |||||
| Longer vs shorter course prednisolone | OR 0.33 | 201 | ⊕⊕⊝⊝ | |||
| 10 per 1000 | 3 per 1000 | |||||
| Longer vs shorter course dexamethasone | OR 2.22 | 100 | ⊕⊝⊝⊝ | |||
| 19 per 1000 | 42 per 1000 | |||||
| Asthma symptoms Symptom free by 7 days | Longer vs shorter course prednisolone | OR 1.22 | 201 | ⊕⊕⊕⊝ | One other study (Langton Hewer 1998) randomising 98 children to high‐ vs medium‐ vs low‐dose prednisolone reported clinical asthma score at discharge. Small differences in scores were reported with uncertain clinical importance and no consistent dose‐response effect | |
| 307 per 1000 | 351 per 1000 | |||||
| Serious adverse events | Longer vs shorter course prednisolone | Not estimable | 201 | No events occurred in either trial arm | ||
| 0 per 1000 | 0 per 1000 | |||||
| New exacerbation during follow‐up period Oral corticosteroids prescribed | Higher‐ vs lower‐dose prednisolone | OR 1.38 | 231 | ⊕⊕⊝⊝ | ||
| 17 per 1000 | 24 per 1000 | |||||
| Longer vs shorter course prednisolone | OR 0.61 | 201 | ⊕⊕⊕⊝ | |||
| 79 per 1000 | 50 per 1000 | |||||
| Longer vs shorter course dexamethasone | OR 0.24 | 100 | ⊕⊕⊝⊝ | |||
| 154 per 1000 | 42 per 1000 | |||||
| New exacerbation during follow‐up period Unscheduled visit to healthcare provider | Longer vs shorter course dexamethasone | OR 2.17 | 100 | ⊕⊝⊝⊝ | ||
| 96 per 1000 | 188 per 1000 | |||||
| Lung function tests FEV1% predicted at discharge | High vs medium vs low dose | ‐ | 34 | This outcome includes only 1 small study (Langton Hewer 1998) in which a subset of participants were able to perform PFTs. Reported between‐group differences were small and of uncertain clinical importance with no consistent dose‐response effect. | ||
| ‐ | ‐ | |||||
| All adverse events | Longer vs short course prednisolone | OR 0.67 | 201 | ⊕⊕⊕⊝ | ||
| 30 per 1000 | 20 per 1000 | |||||
| *Risk in the intervention group (and its 95% confidence interval) is based on assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) | ||||||
| GRADE Working Group grades of evidence | ||||||
| aOnly 1 study contributed events to this outcome and was assessed to be at high risk of attrition bias because of unbalanced drop‐out from intervention arms. Downgraded once for risk of bias bThe study contributing events had 3 different dose arms, 1 of which is outside the current dosing guidelines. Two other studies reported no events, but intervention involved much higher doses of prednisolone. Downgraded once for indirectness cOnly 1 study contributed to this analysis. Imprecise estimate with confidence intervals including possibility of important harms or benefits. Downgraded twice for imprecision dOnly contributing study considered at high risk of bias in multiple domains. Downgraded once for risk of bias eOnly 1 study contributed to this outcome, resulting in imprecise estimate and confidence intervals including the possibility of important harms or benefits. Downgraded once for imprecision fOnly 2 studies contributed to this outcome with few events, resulting in imprecise estimate and wide confidence intervals including the possibility of important harms or benefits. Downgraded twice for imprecision gOnly 1 study contributed to this outcome, resulting in imprecise estimate, which does not exclude the possibility of no difference. Downgraded once for imprecision | ||||||
| Children: prednisolone compared with dexamethasone for acute asthma | ||||||
| Patient or population: children with acute exacerbation of asthma Duration range: 1.5 to 3 weeks | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Risk with dexamethasone | Risk with prednisolone | |||||
| Admission at initial presentation | 116 per 1000 | 124 per 1000 | OR 1.08 (0.74 to 1.58) | 1007 | ⊕⊕⊝⊝ | |
| Re‐admission during follow‐up period | 22 per 1000 | 10 per 1000 | OR 0.44 (0.15 to 1.33) | 985 | ⊕⊕⊝⊝ | |
| Asthma symptoms scores Pulmonary Index Score (PIS); Patient Self Assessment Score (PSAS); Paediatric Respiratory Assessment Measure (PRAM) | Not pooled | Not pooled | ‐ | 328 (2 RCTs) | ⊕⊝⊝⊝ | Altamimi 2006 reported PIS and PSAS Cronin 2015 reported PRAM (we extracted the result, which excluded re‐enrolments) No between‐group differences were detected |
| Asthma symptoms Persistent cough, wheeze, chest tightness, night‐time wakening and difficulty maintaining normal activities | Not pooled | Not pooled | ‐ | 533 | The number of people experiencing these symptoms at day 10 was not found to be significantly different between the 2 intervention arms | |
| Serious adverse events | Not pooled | Not pooled | Not estimable | 255 | No events were reported in either study | |
| New exacerbation during follow‐up period Unscheduled visit to healthcare provider | 97 per 1000 | 83 per 1000 | OR 0.85 (0.54 to 1.34) | 981 | ⊕⊕⊝⊝ | |
| *Risk in the intervention group (and its 95% confidence interval) is based on assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) | ||||||
| GRADE Working Group grades of evidence | ||||||
| aThe 2 studies contributing most events to this outcome were considered to be at high or unclear risk of selection (Qureshi 2001) and performance and detection bias (Cronin 2015; Qureshi 2001). In addition, Cronin 2015 allowed 19 participants to enrol more than once in the study. Downgraded once for risk of bias bConfidence intervals include possible harms or benefits of either intervention. Downgraded once for imprecision cThe pulmonary index score may lack rigorous evaluation, so clinical interpretation of this score is limited. Downgraded once for indirectness dConfidence intervals for PIS and PSAS include no difference, but we are unsure whether either end of the confidence intervals includes a clinically important effect. Downgraded once for imprecision eThe PSAS score has been adapted from National Institute of Health guidelines and may lack rigorous evaluation, so clinical interpretation is limited. Downgraded once for indirectness fWe were unable to combine the results of these different scales. Downgraded once for inconsistency | ||||||
Antecedentes
Descripción de la afección
El asma es una afección respiratoria frecuente a largo plazo que afecta a aproximadamente 300 000 000 de personas en todo el mundo y causa unas 250 000 muertes cada año (WHO 2007). Entre el 1% y el 18% de las personas en diferentes países son afectados por el asma (GINA 2015), que se caracteriza por inflamación crónica de las vías respiratorias e hiperrespuesta de las vías respiratorias, lo que provoca disnea, sibilancia, opresión torácica y tos. Habitualmente los síntomas son peores de noche y temprano en la mañana y pueden variar con el transcurso del tiempo (CDC 2012; GINA 2015). En gran parte los tratamientos tienen como objetivo reducir la constricción de los músculos lisos de las vías respiratorias mediante la administración de broncodilatadores inhalados (p.ej. agonistas beta2 de acción corta y prolongada) y reducir la inflamación de las vías respiratorias mediante la administración de corticosteroides, que generalmente también se inhalan (BTS/SIGN 2014).
Los pacientes con asma pueden presentar un empeoramiento a corto plazo de los síntomas del asma; estos episodios se conocen como "exacerbaciones", "brotes", "ataques" o "asma agudo". Las exacerbaciones se caracterizan por episodios de "aumento progresivo de la disnea, la tos, la sibilancia o la opresión torácica, o alguna combinación de estos síntomas" (NAEPP 2007). No se ha alcanzado un consenso internacional sobre la definición de un ataque o exacerbación, pero un grupo de trabajo en los EE.UU. propuso recientemente la definición de "un empeoramiento del asma que requiere la administración de corticosteroides sistémicos para prevenir un resultado grave" (Fuhlbrigge 2012).
En los EE.UU., en 2008 más de la mitad de los adultos y niños con asma presentaron al menos una exacerbación del asma(CDC 2011). Los factores desencadenantes de la exacerbación del asma varían de paciente a paciente pero habitualmente incluyen el humo del tabaco, la infección de las vías respiratorias, los ácaros del polvo doméstico, la contaminación del aire, los animales domésticos y el moho (CDC 2006). Según la gravedad, las exacerbaciones del asma suelen requerir un cambio temporal en el régimen de medicación del paciente con asma, por ejemplo, aumento en el uso de broncodilatadores de acción corta como el salbutamol y un ciclo de esteroides sistémicos. Las exacerbaciones más graves pueden requerir tratamiento en un departamento de urgencias o ingreso hospitalario (BTS/SIGN 2014).
Descripción de la intervención
Los esteroides orales se recomiendan para todas las exacerbaciones del asma, excepto las más leves (BTS/SIGN 2014); y se deben iniciar con prontitud (Rowe 2001). Se piensa que las vías intravenosa o intramuscular no ofrecen ventajas sobre la vía oral a menos que el cumplimiento del tratamiento o la absorción intestinal sean motivos de preocupación (Krishnan 2009; Lahn 2004). Se recomienda que los esteroides orales se tomen como dosis única después del desayuno.
Las guías actuales para la dosis varían levemente entre los países, y a menudo entre diferentes elaboradores de guías dentro del mismo país. En el Reino Unido, las guías más recientes(BTS/SIGN 2014) recomiendan para los adultos 40 a 50 mg de prednisolona oral diaria durante al menos cinco días, o hasta la recuperación. Las mismas guías recomiendan una dosis de 20 mg de prednisolona para los niños de dos a cinco años de edad y de 30 a 40 mg para los niños mayores de cinco años. Las recomendaciones GINA 2015 son similares e indican una dosis de 1 mg/kg para los pacientes adultos hasta una dosis máxima diaria de 50 mg, y de 1 a 2 mg/kg para los niños de seis a 11 años de edad hasta una dosis máxima diaria de 40 mg. La guía GINA 2015 recomienda que generalmente es suficiente un ciclo de cinco a siete días en los adultos y de tres a cinco días en los niños.
Actualmente no hay pruebas suficientes para indicar que los esteroides alternativos, como la dexametasona, ofrezcan ninguna ventaja sobre la prednisolona (BTS/SIGN 2014). La prednisolona tienen un gran uso internacional y tiene un costo relativamente bajo; un paquete de 28 comprimidos de 5 mg cuesta solamente £1,29 en el Reino Unido (BNF). No es necesario disminuir gradualmente la dosis cuando se interrumpe, siempre que el paciente ya reciba corticosteroides inhalados, no reciba esteroides orales a largo plazo o haya requerido un ciclo agudo de más de tres semanas de duración (BTS/SIGN 2014; GINA 2015).
De qué manera podría funcionar la intervención
Los glucocorticoides, que incluyen la prednisolona, son inhibidores potentes de la inflamación y se utilizan para tratar una variedad amplia de afecciones inflamatorias y autoinmunes, incluido el asma (Barnes 2003; van der Velden 1998). Se piensa que los glucocorticoides funcionan al unirse a un receptor glucocorticoide celular, lo que da lugar a la disminución de la expresión de diversos genes involucrados en mantener el proceso inflamatorio. A su vez provoca la disminución del reclutamiento y la activación de las células inflamatorias, el aumento de los receptores beta2, la disminución de la permeabilidad microvascular y la producción de moco (Barnes 1992). Los resultados de los estudios de investigación indican una resolución más rápida de los síntomas y una reducción de las tasas de recurrencia entre los pacientes tratados con esteroides orales (Alangari 2014; Krishnan 2009; Rowe 2007).
Por qué es importante realizar esta revisión
A pesar de la eficacia comprobada, la administración de esteroides se debe equilibrar contra la posibilidad de provocar eventos adversos importantes. Los problemas asociados con el tratamiento con esteroides a más largo plazo están bien establecidos e incluyen diabetes, osteoporosis, emaciación muscular, síndrome de Cushing y restricción del crecimiento lineal en los niños (BNF). De hecho, la administración regular de incluso dosis bajas a moderadas diarias de corticosteroides inhalados se asocia con una reducción media de la velocidad de crecimiento lineal de 0,48 cm/año entre los niños(Zhang 2014). Sin embargo, muchos eventos adversos importantes se asocian con el uso a corto plazo, que se recomienda con frecuencia para las exacerbaciones del asma. Estos efectos secundarios incluyen insomnio, náuseas, distensión abdominal, dispepsia, malestar general, vértigo, cefalea y (especialmente en niños) cambios conductuales (BNF; Kayani 2002).
Las pruebas actuales con respecto a la dosis óptima de los esteroides orales para las exacerbaciones del asma son algo limitadas. Bowler 1992 asignó al azar a 76 participantes a recibir hidrocortisona intravenosa a dosis baja, media o alta en un contexto hospitalario durante 48 horas, seguida de dosis bajas, medias o altas de esteroides orales administrados por 12 días. Los autores del estudio concluyeron que la hidrocortisona a dosis baja (50 mg, cuatro veces al día durante 48 horas), seguida de prednisolona a dosis baja (20 mg diarios, reducidos a 5 mg por 12 días), fue tan eficaz como las dosis mayores. En un estudio similar con 20 participantes en el año 2000, los investigadores concluyeron que un ciclo de una semana de esteroides orales después de un ciclo de tres días de esteroides intravenosos fue tan eficaz como un ciclo de dos semanas (Hasegawa 2000). Un estudio con 86 niños de dos a 16 años de edad concluyó que una dosis de prednisolona oral de 1 mg/kg fue igualmente eficaz que 2 mg/kg, pero se asoció con menos eventos adversos conductuales (Kayani 2002). De manera similar, Hewer 1998 no identificó una ventaja de una dosis de 1 ó 2 mg/kg sobre una dosis de 0,5 mg/kg en un estudio de 98 niños ingresados en el hospital con asma agudo.
Un resumen o "revisión general" de la administración de corticosteroides en el asma agudo también analizó esta pregunta e indicó que no hubo pruebas que mostraran que las dosis diarias por encima de 50 a 100 mg son beneficiosas y que una duración del ciclo de cinco a diez días es suficiente en los pacientes a los que se les da el alta(Krishnan 2009). Resultados similares se informaron en Manser 2001. Sin embargo, las conclusiones presentadas en ambas revisiones se basan en estudios en pacientes hospitalizados donde los participantes de al menos uno de los brazos del ensayo recibían esteroides parenterales.
Objetivos
Evaluar la eficacia y la seguridad de cualquier dosis o duración de los esteroides orales versus otra dosis o duración de los esteroides orales en adultos y niños con una exacerbación del asma.
Métodos
Criterios de inclusión de estudios para esta revisión
Tipos de estudios
Se incluyeron ensayos controlados aleatorios (ECA) paralelos, con cegamiento y sin cegamiento, que evaluaron cualquier dosis o duración de los esteroides orales versus otra dosis o duración de los esteroides orales para el tratamiento de una exacerbación del asma. Se excluyeron los ensayos cruzados (crossover) debido a los efectos a largo plazo del tratamiento con esteroides orales y a lo impredecible de una segunda exacerbación. Se incluyeron los estudios informados como texto completo, los publicados como resumen solamente y los datos no publicados.
Tipos de participantes
Se incluyeron estudios en adultos y niños con asma, diagnosticados por el médico o según las guías nacionales o internacionales, que presentaban una exacerbación. Se registró la gravedad de la exacerbación y los criterios utilizados para definirla. Se excluyeron los estudios que reclutaron participantes con otra comorbilidad respiratoria y los que tomaban los esteroides orales a largo plazo.
Tipos de intervenciones
Se incluyeron estudios que compararon cualquier dosis o duración de los esteroides orales con otra dosis o duración de los esteroides orales. Se incluyeron estudios que permitieron otras cointervenciones para el tratamiento de una exacerbación del asma, como agonistas beta2 de acción corta inhalados o nebulizados, a condición de que no formaran parte del tratamiento asignado al azar.
Se incluyeron los participantes que habían acudido a una institución de asistencia sanitaria primaria o a un departamento de urgencias y los que habían sido ingresados al hospital. Se incluyeron participantes que habían recibido tratamiento con esteroides intravenosos o intramusculares antes de comenzar a recibir esteroides orales, a condición de que no formara parte del tratamiento asignado al azar y de que esta vía de administración hubiera finalizado antes de la asignación al azar a brazos de diferentes dosis orales o duración.
Las comparaciones de los estudios elegibles incluyeron, pero no se limitaron a, los siguientes ejemplos.
-
Duración corta versus larga de la misma dosis, p.ej. 40 mg de prednisolona oral diaria durante cinco días versus 40 mg de prednisolona oral diaria durante diez días.
-
Dosis alta versus baja con la misma duración, p.ej. 20 mg de prednisolona oral diaria durante cinco días versus 40 mg de prednisolona oral diaria durante cinco días.
-
Duración corta y dosis alta versus duración larga y dosis baja, p.ej. 50 mg de prednisolona oral durante tres días versus 20 mg de prednisolona oral diaria durante diez días.
Tipos de medida de resultado
Resultados primarios
-
Ingreso / reingreso al hospital.
-
Síntomas de asma al final del ciclo con esteroides.
-
Eventos adversos graves.
Resultados secundarios
-
Nueva exacerbación durante el período de seguimiento después del tratamiento.
-
Prueba de función pulmonar al final del tratamiento / período de seguimiento (volumen espiratorio forzado mínimo en un segundo [FEV1], de preferencia si estaba disponible).
-
Todos los eventos adversos/efectos secundarios.
El informe realizado por los investigadores de uno o más de los resultados enumerados en la presente no fue un criterio de inclusión para la revisión. Los resultados se eligieron como los más importantes para los pacientes después de la consulta con un representante de los pacientes.
Si dentro de un estudio se informó más de una escala que midió la misma construcción, o si se utilizaron diferentes escalas entre los estudios, se analizaron juntas mediante las diferencias de medias estandarizadas, a condición de que la heterogeneidad clínica fuera suficientemente baja para poder realizar un análisis agrupado significativo (p.ej. se evitó la combinación de diferentes escalas de síntomas no validadas).
Cuando fue posible se extrajeron los tipos de eventos adversos presentados; la investigación en el grupo de usuarios indica que los efectos secundarios psicológicos / emocionales / conductuales pueden ser particularmente molestos durante los ciclos con esteroides a corto plazo. Lo anterior se ha informado de manera narrativa cuando no fue posible realizar el metanálisis.
Results
Description of studies
Full details of the conduct and characteristics of each included study can be found in the Characteristics of included studies tables and reasons for exclusion when full texts had to be viewed are given in the Characteristics of excluded studies table.
Results of the search
We identified 1297 references through electronic database searches and an additional 109 records through searches of clinicaltrials.gov and the World Health Organization (WHO) trials portal (http://apps.who.int/trialsearch/). We excluded most (n = 1335) of these references on the basis of title and abstract. We retrieved 71 full texts for more detailed assessment and at this stage excluded 47 additional references (related to 39 individual studies). Reasons for exclusion included wrong comparator, wrong intervention and not a randomised controlled trial. We also excluded three studies that were ongoing, and one study (reported as an abstract only) is still awaiting classification, despite attempts to contact the study author to confirm whether it met out inclusion criteria. We present trial flow in Figure 1.
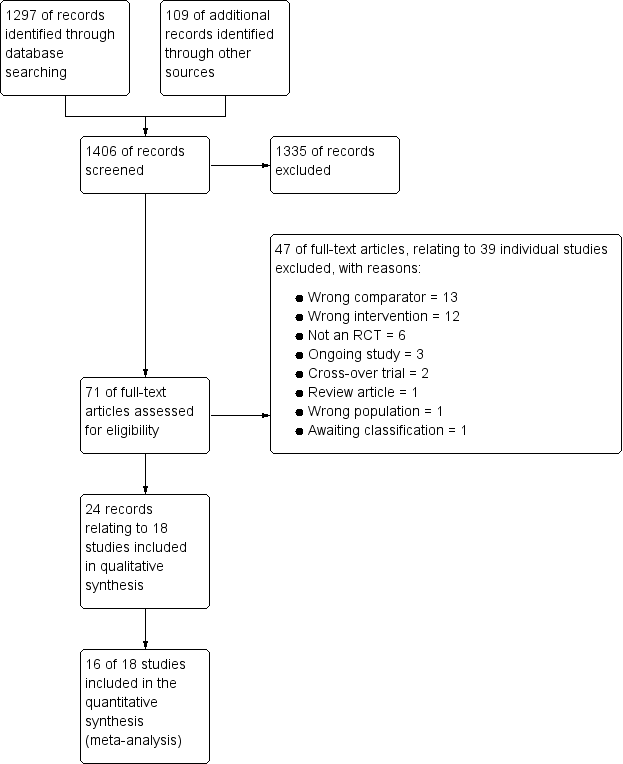
Study flow diagram.
Included studies
Eighteen studies met our inclusion criteria, 16 of which contributed data to at least one meta‐analysis. These studies included a total of 2438 participants who were randomly assigned to comparisons of interest in this review. The largest study included 628 participants, and the smallest just 15. The mean total number of participants was 135, and the median 93. Investigators reported 14 trials as full peer‐reviewed articles, three as abstracts only (Aboeed 2014; Ghafouri 2010; Viska 2008) and one on the clinicaltrials.gov website (NCT00257933), for which we obtained additional unpublished data directly from the trial contact person. We present a summary of the characteristics of included studies in Table 1.
Methods
As per our protocol, all included trials were RCTs with parallel design that compared one dose or duration of oral steroids versus another dose or duration. One study included three relevant arms: high‐, medium‐ and low‐dose oral prednisolone. Trial duration varied, with oral steroid treatment courses ranging from just a single dose to seven weeks of treatment. All studies included a post‐treatment follow‐up period, which ranged in duration from seven days to six months. No studies reported a run‐in period, as recruitment was triggered by an unscheduled presentation with an acute exacerbation of asthma. Outcomes data were extracted at the end of steroid treatment or at the last time point reported, or at both times if available. Trials were conducted in a variety of countries worldwide, but most were carried out in the USA (Aboeed 2014; Cydulka 1998; Ghafouri 2010; Greenberg 2008; Kayani 2002; Kravitz 2011; Lederle 1987; NCT00257933; Qureshi 2001) and the UK (Jones 2002; Langton Hewer 1998; O’Driscoll 1993). The remainder were carried out in Australia (Chang 2008), Canada (Altamimi 2006), Japan (Hasegawa 2000), Indonesia (Viska 2008), India (Karan 2002) and Ireland (Cronin 2015).
Participants
We included studies involving both children and adults. Nine studies (Altamimi 2006; Chang 2008; Cronin 2015; Ghafouri 2010; Greenberg 2008; Kayani 2002; Langton Hewer 1998; NCT00257933; Qureshi 2001) recruited only children (age range one to 18 years depending on the individual study), and seven studies (Cydulka 1998; Jones 2002; Karan 2002; Kravitz 2011; Lederle 1987; O’Driscoll 1993; Viska 2008) recruited only adults (age range 16 to 78 years depending on the individual study). Two studies (Aboeed 2014; Hasegawa 2000) did not report the age range of participants, but the steroid doses administered in Aboeed 2014 would be consistent with adult participants. Most studies did not specify the ethnicity of participants.
All studies included participants with acute exacerbations of asthma. Although reported as having asthma, most of the participants in Lederle 1987 were older men who were current smokers or ex‐smokers, and many may in fact have had chronic obstructive pulmonary disease (COPD) with a degree of reversibility. In most cases, researchers did not report baseline asthma severity and severity of the asthma attack. However, in the majority of studies (Aboeed 2014; Altamimi 2006; Chang 2008; Cronin 2015; Cydulka 1998; Ghafouri 2010; Greenberg 2008; Karan 2002; Kayani 2002; Kravitz 2011; Qureshi 2001), researchers recruited participants in the emergency department (ED) or at an outpatient clinic, and the inclusion criteria in most of these studies required that they must be well enough to be discharged home. Four studies (Jones 2002; Langton Hewer 1998; Lederle 1987; O’Driscoll 1993) recruited participants and commenced randomised treatment on an inpatient basis but completed treatment at home. In one study (NCT00257933), randomised steroid treatment was continued for 48 hours or until discharge, whichever came sooner, followed by five to 10 days of standard oral steroid treatment at the discretion of the treating physician. One study did not report the specific setting in which treatment was commenced (Viska 2008), and in Hasegawa 2000, treatment was initiated in hospital, but it is not clear whether participants remained as inpatients for the duration of their steroid treatment.
Interventions
Studies included a variety of comparisons: longer versus shorter course of prednisolone (Chang 2008; Hasegawa 2000; Jones 2002); higher versus lower dose of prednisolone (Kayani 2002; Langton Hewer 1998; NCT00257933; Viska 2008); longer course of prednisolone versus shorter course of dexamethasone (Aboeed 2014; Altamimi 2006; Cronin 2015; Greenberg 2008; Kravitz 2011; Qureshi 2001); tapering versus non‐tapering course of prednisolone (Cydulka 1998; Karan 2002; O’Driscoll 1993); long‐tapering versus short‐tapering course of prednisolone (Lederle 1987); and finally long versus short course of dexamethasone (Ghafouri 2010). Dosing also varied across studies; we have extracted this information and presented it in the Characteristics of included studies tables, along with the 'prednisolone‐equivalent' total dose received. All participants in Hasegawa 2000 received three days of intravenous methylprednisolone before commencing randomised oral steroid treatment.
Although we did not set out to compare different types of oral steroids, we included the dexamethasone versus prednisolone comparison because these agents were given over different durations, and this was part of our scope. We meta‐analysed these trials separately because, unlike studies that compared a different dose or duration of the same drug, most of these studies gave almost equivalent total doses of steroid in each intervention arm, so any between‐group differences may be related to drug‐specific factors including adherence or palatability. We recognise that in a clinical setting, drug‐specific factors, such as convenience for the patient, may affect an individual practitioner’s choice of drug or regimen.
Most studies stated that participants were allowed to continue use of specified rescue and preventer medication for asthma throughout the study, and in some trials, frequency of use of rescue medication, such as a short‐acting beta2‐agonist, was an efficacy outcome.
Outcomes
Outcomes reported were not consistent across reviews, and validated scales were not always used. Most studies (n = 13) reported some measure of asthma symptoms, at the end of treatment or follow‐up, or time taken for resolution of symptoms. Most (n = 13) also reported relapse rates, defined usually as an unscheduled visit to the ED or another healthcare provider during the follow‐up period. Three studies specifically reported hospitalisation during the follow‐up period, and seven studies reported new exacerbations or another course of oral steroids prescribed during the follow‐up period. Various measures of lung function were also frequently reported (n = 10), as was compliance with prescribed steroid therapy (n = 6). Adverse events were explicitly stated as an outcome measure in only six studies. Four studies recorded rescue medication use, four reported vital signs and three reported asthma severity scores. Two studies assessed adrenal suppression. One study reported Paediatric Asthma Caregiver's Quality of Life Questionnaire (PACQLQ), two reported school days or workdays missed and another used the asthma control test.
Excluded studies
We excluded 46 references (related to 38 individual studies) after assessment of full‐text articles. We excluded 13 studies, as they used a comparator not of interest in this review, for example, intravenous or inhaled steroids were compared with oral steroids. We excluded 12 studies because the intervention was not of interest in this review, for example, studies comparing different doses of intravenous steroids in the acute setting, or interventions including additional randomised treatments not of interest in this review. We excluded six studies as they were not randomised controlled trials and another two because they used a cross‐over trial design. One study was in fact a review article, and another study recruited a mixed population of patients with COPD and asthma. We excluded two studies that were ongoing (NCT01241006; NCT02192827), and one study (Tanifuji 2001; reported as an abstract only) is still awaiting classification, despite attempts to contact the study author to confirm whether it met out inclusion criteria.
Risk of bias in included studies
For details of the risk of bias rating for each study and the supporting evidence for each rating, see the Characteristics of included studies table. A summary of risk of bias judgements by study and domain (sequence generation, allocation concealment, blinding, incomplete data and selective reporting) can be found in Figure 2.
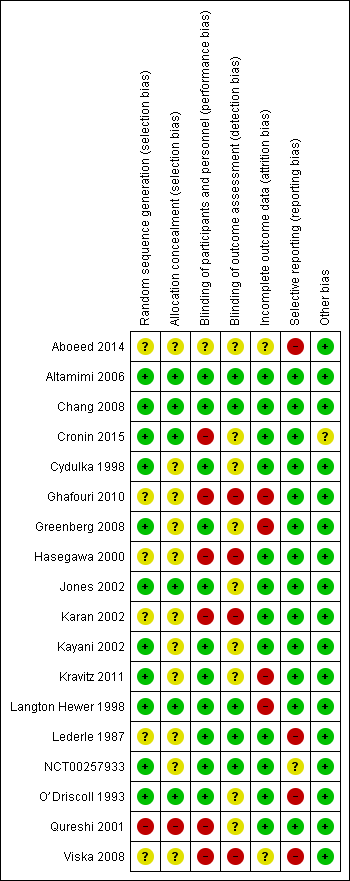
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Six studies (Altamimi 2006; Chang 2008; Cronin 2015; Jones 2002; Langton Hewer 1998; O’Driscoll 1993) described the generation of a random sequence and concealment of allocation of participants in sufficient detail for review authors to assess them as having low risk of selection bias. We considered five other studies (Cydulka 1998; Greenberg 2008; Kayani 2002; Kravitz 2011; NCT00257933) to be at low risk of bias for random sequence generation but at unclear risk of bias for allocation concealment, which was not described in sufficient detail to allow a judgement.
Six studies (Aboeed 2014; Ghafouri 2010; Hasegawa 2000; Karan 2002; Lederle 1987; Viska 2008) did not provide sufficient details of random sequence generation or allocation concealment for review authors to make a judgement, and so we considered these studies to be at unclear risk of bias in both domains. We assessed Qureshi 2001 as having high risk of bias for random sequence generation and allocation concealment, as participants were allocated to the two intervention arms on the basis of the day of the month they presented to the ED.
Blinding
We judged most studies (n = 11; Altamimi 2006; Chang 2008; Cydulka 1998; Greenberg 2008; Jones 2002; Kayani 2002; Kravitz 2011; Langton Hewer 1998; Lederle 1987; NCT00257933; O’Driscoll 1993) to be at low risk of performance bias, as participants and trial personnel were adequately blinded. Five studies (Altamimi 2006; Chang 2008; Langton Hewer 1998; Lederle 1987; NCT00257933) clearly described blinding of outcome assessors, and we judged these studies to be at low risk of detection bias. We assessed the remaining six studies as having unclear risk of detection bias, as blinding of outcome assessors was not clearly described.
We considered Aboeed 2014 to be at unclear risk of bias for both performance and detection bias, as the abstract did not contain enough detail to allow a judgement. Four studies (Ghafouri 2010; Hasegawa 2000; Karan 2002; Viska 2008) were open‐label and were considered to be at high risk of performance and detection bias. In Cronin 2015, also an open‐label study, outcome assessors for the primary outcome (paediatric respiratory assessment measure (PRAM)) at day 4 were unaware of group allocation, but other participant‐reported or influenced outcomes (e.g. decision to re‐present to a healthcare practitioner) may have been affected by knowledge of group allocation, so we rated this study as having unclear risk of detection bias and high risk of performance bias. We considered one study (Qureshi 2001) to be at high risk of performance bias, as the trial was unblinded, but the primary outcome ‐ decision to seek medical care for deteriorating symptoms ‐ was assessed independently of study investigators, and so we rated the risk of detection bias as unclear.
Incomplete outcome data
We assessed 12 studies (Altamimi 2006; Chang 2008; Cronin 2015; Cydulka 1998; Hasegawa 2000; Jones 2002; Karan 2002; Kayani 2002; Lederle 1987; NCT00257933; O’Driscoll 1993; Qureshi 2001) to be at low risk of attrition bias, as they had low and balanced withdrawal, and all participants who withdrew were clearly accounted for in the trial flow. We assessed Aboeed 2014 and Viska 2008, both conference abstracts, as having unclear risk, as they did not describe the number randomised to, or withdrawn from, each treatment arm.
We assessed Langton Hewer 1998 to be at high risk; attrition in the intervention groups was unbalanced (< 10% in the medium‐ and low‐dose groups and 20% in the higher‐dose group), and although all withdrawals were accounted for in the text of the report, this imbalance may have affected the findings. We assessed Ghafouri 2010, a conference abstract, to be at high risk of attrition bias because of unbalanced attrition in intervention groups, and because the reasons for withdrawal were not stated. We assessed Greenberg 2008 also to be at high risk, as approximately half of the participants randomised to each treatment did not complete the trial, and although baseline details are given for those who completed and those who did not, how this high level of attrition may have affected the findings is unclear. Finally, we assessed Kravitz 2011 as having high risk of attrition bias, as 30% (85 out of 285) of all randomised participants did not complete the trial as the result of admission to hospital after they were randomised or loss to follow‐up, and their outcomes remain unknown.
Selective reporting
We assessed 13 studies (Altamimi 2006; Chang 2008; Cronin 2015; Cydulka 1998; Ghafouri 2010; Greenberg 2008; Hasegawa 2000; Jones 2002; Karan 2002; Kayani 2002; Kravitz 2011; Langton Hewer 1998; Qureshi 2001) to be at low risk of reporting bias, although we were able to find prospectively registered protocols only for Chang 2008, Cronin 2015 and Ghafouri 2010.
We assessed Aboeed 2014 and Viska 2008, both conference abstracts, to be at high risk, as they provided minimal details and could not be included in the quantitative synthesis. We assessed NCT00257933 to be at unclear risk, as the trial has not yet been published. Some results are posted on clinicaltrials.gov, and the study authors kindly provided us with an unpublished manuscript, but some listed outcomes are as yet not fully reported (peak flow, clinical asthma score).
We considered Lederle 1987 to be at high risk, as not all outcomes were reported in a way that allowed meta‐analysis, including FEV1 (reported as percentage of baseline value without variance) and diary outcomes (reported narratively in the text with minimal supporting numerical data). Similarly, we assessed O’Driscoll 1993 to be at high risk, as many of the diary outcomes were not reported numerically, and data were displayed graphically with no variance.
Other potential sources of bias
Most studies did not report their funding source, and for those that did, this was not considered to be a likely source of bias. We assessed Cronin 2015 as being at unclear risk of other bias, as investigators allowed participants to enrol more than once in the trial. This may have led to the same participant contributing to outcomes twice; how the trial authors adjusted the analyses to take this into account is not clear, as they simply state that a "descriptive analysis of the patients enrolled multiple times was performed".
Effects of interventions
See: Summary of findings for the main comparison Adults: higher dose/longer course compared with lower dose/shorter course for acute asthma; Summary of findings 2 Adults: prednisolone compared with dexamethasone for acute asthma; Summary of findings 3 Children: higher dose/longer course compared with lower dose/shorter course for acute asthma; Summary of findings 4 Children: prednisolone compared with dexamethasone for acute asthma
Structure of the analysis
We chose to analyse trials in adults and trials in children completely separately in this review.
Structure of the meta‐analysis
We created four main comparison headings within the analysis tree. For each comparison, we chose to meta‐analyse results only when the interventions and outcomes measured were sufficiently similar for pooling to make sense.
Adults: higher dose/longer course versus lower dose/shorter course
This comparison included all studies in adults that compared a higher dose or a longer course with a lower dose or a shorter course of the same oral steroid (Cydulka 1998; Hasegawa 2000; Jones 2002; Karan 2002; Lederle 1987; O’Driscoll 1993; Viska 2008), for example, 40 mg of prednisolone once daily for 10 versus five days, or 36 mg versus 12 mg of prednisolone daily for two weeks.
Adults: prednisolone versus dexamethasone
This comparison included all studies in adults that compared prednisolone with dexamethasone (Aboeed 2014; Kravitz 2011), for example, 40 mg prednisolone daily for five days versus 16 mg of dexamethasone daily for two days.
Children: higher dose/longer course versus lower dose/shorter course
This comparison included all studies in children that compared a higher dose or a longer course with a lower dose of a shorter course of the same oral steroid (Chang 2008; Ghafouri 2010; Kayani 2002; Langton Hewer 1998; NCT00257933), for example, 1 mg/kg daily prednisolone for five versus three days, or 2 mg/kg daily versus 1 mg/kg daily prednisolone for five days.
Children: prednisolone versus dexamethasone
This comparison included all studies in children that compared prednisolone with dexamethasone (Altamimi 2006; Cronin 2015; Greenberg 2008; Qureshi 2001), for example, 1 mg/kg prednisolone twice daily for five days versus dexamethasone 0.6 mg/kg once daily for one day.
Structure of the narrative synthesis
Below, we present the results narratively according to our pre‐specified outcomes. We begin with the primary outcomes: admission/re‐admission to hospital; asthma symptoms; and serious adverse events. Within each outcome, we describe effects of the interventions in adults, followed by effects in children, clearly specifying which of the above comparisons yielded the extracted data. We then describe the secondary outcomes: new exacerbation in the follow‐up period; lung function tests; and all adverse events/side effects, according to the same pattern.
Primary outcomes
Admission/re‐admission to hospital
Overall, our results demonstrated no difference in admission or re‐admission to hospital between participants prescribed a longer course or a higher dose of oral steroids and those prescribed a shorter course or a lower dose, or between those prescribed prednisolone and those prescribed dexamethasone. The requirement for admission at initial presentation was an exclusion criterion for many of the included studies. In those reporting admissions or re‐admissions, events were generally rare, and differences between interventions and populations in the included studies precluded meaningful meta‐analysis, resulting in imprecise estimates and low confidence in the result.
Admission at initial presentation: children
Four studies in children (Altamimi 2006; Cronin 2015; Ghafouri 2010; Qureshi 2001) reported admission at initial presentation. Altamimi 2006, Cronin 2015 and Qureshi 2001 ‐ studies comparing prednisolone and dexamethasone ‐ did not detect a difference in admission rates between intervention groups (Analysis 4.1; odds ratio (OR) 1.08, 95% confidence interval (CI) 0.74 to 1.58; participants = 1007; I2 = 0%), but the confidence intervals include an important reduction and increase in admissions. In addition, one of the studies contributing to this analysis (Qureshi 2001) was considered to be at high risk of selection bias, and another study (Cronin 2015) was open‐label and therefore was at high risk of performance and detection bias for this outcome. We therefore have low confidence in the finding. Ghafouri 2010, a study comparing a longer course versus a shorter course of the same dose of dexamethasone, also reported no difference in admissions at initial presentation (Analysis 3.1; OR 1.66, 95% CI 0.60 to 4.61; participants = 125) but again with wide confidence intervals. It is important to note that admission at initial presentation would have been measured before the differing durations of treatment would have had an impact, and so this result is of limited value.
Re‐admission during follow‐up period: adults
Re‐admission to hospital during the follow‐up period was reported by five studies of adult participants (Hasegawa 2000; Jones 2002; Kravitz 2011; Lederle 1987; O’Driscoll 1993).
In four studies that compared a longer course versus a shorter course of prednisolone (Hasegawa 2000; Jones 2002; Lederle 1987; O’Driscoll 1993), no difference in re‐admissions was found between intervention groups, but events were rare and confidence intervals include the possibility of harm and the possibility of benefit from a longer or a shorter course (Analysis 1.1; OR 1.35, 95% CI 0.38 to 4.79; participants = 142; studies = 4; I2 = 0%). Of note, the study carrying the greatest weight in this analysis (Lederle 1987) likely recruited participants with co‐morbid COPD, so this outcome was additionally downgraded for indirectness of the study population. Similarly, the study comparing prednisolone versus dexamethasone in adults (Kravitz 2011) reported infrequent re‐admissions to hospital and consequently an imprecise result, and was considered to be at high risk of attrition bias (Analysis 2.1; OR 0.35, 95% CI 0.04 to 3.47; participants = 200).
Re‐admission during follow‐up period: children
Re‐admission to hospital during the follow‐up period was reported by eight studies in children (Altamimi 2006; Chang 2008; Cronin 2015; Ghafouri 2010; Kayani 2002; Langton Hewer 1998; NCT00257933; Qureshi 2001).
Three studies in children compared a higher dose versus a lower dose of prednisolone (Kayani 2002; Langton Hewer 1998; NCT00257933), one compared a longer course versus a shorter course of prednisolone (Chang 2008) and one compared a longer course versus a shorter course of dexamethasone (Ghafouri 2010). Again, events were rare, with only nine participants requiring re‐admission across all five studies (with two studies reporting no events), resulting in wide confidence intervals in each of the three studies reporting events (Analysis 3.2). As the interventions were not sufficiently similar, we did not perform a meta‐analysis and our confidence in these estimates is low or very low.
Altamimi 2006, Cronin 2015 and Qureshi 2001 compared prednisolone versus dexamethasone, and although all three studies reported re‐admissions, they were infrequent, resulting in wide confidence intervals (Analysis 4.2; OR 0.44, 95% CI 0.15 to 1.33; participants = 985; I2 = 0%), and our confidence in the finding was further reduced by the risk of selection bias identified in Qureshi 2001 and by lack of blinding in Cronin 2015.
Asthma symptoms
Asthma symptoms were reported by several included studies, but investigators used a variety of measures and time points, limiting meaningful meta‐analysis. In general, individual studies did not detect an important difference between intervention arms but with a high level of imprecision.
Adults
In adults, asthma severity score was reported by Jones 2002 (mean of individuals' mean overall severity 1 to 7; 1 = no symptoms, 7 = worst symptoms) on days six to 21; Analysis 1.2). The result showed modest benefit with a longer course of prednisolone over a shorter course, but the clinical importance of this is not clear (mean difference (MD) ‐0.70, 95% CI ‐1.28 to ‐0.12; participants = 44), and our confidence in this estimate is low. O’Driscoll 1993, a small study comparing a tapered (longer) course of prednisolone versus a non‐tapered (shorter) course, reported the number of participants with complete resolution of asthma symptoms by day 28 but provided insufficient data to allow conclusions (Analysis 1.3; OR 0.55, 95% CI 0.13 to 2.26; participants = 35), and again we have low confidence in this estimate. Kravitz 2011, a trial that compared prednisolone versus dexamethasone, reported the number of participants who had resumed normal activities within three days. Results suggest a modest benefit of dexamethasone over prednisolone (Analysis 2.2; OR 0.44, 95% CI 0.19 to 1.01; participants = 191), but the confidence intervals do not fully exclude no differences, and the one study contributing to this outcome was assessed to be at high risk of attrition bias.
Children
In children, clinical asthma score at discharge was reported by Langton Hewer 1998, a study that compared high‐, medium‐ and low‐dose prednisolone. These findings are inconsistent, have uncertain clinical importance and show no clear benefit of a higher or a lower dose (Analysis 3.3). Chang 2008, a trial of a five‐ versus three‐day course of prednisolone, reported the number of children symptom free at seven days and did not detect a difference between intervention groups (Analysis 3.4; OR 1.22, 95% CI 0.67 to 2.19; participants = 201). We downgraded this outcome once for imprecision, but we are otherwise moderately confident in this estimate. Altamimi 2006, Cronin 2015 and Qureshi 2001 ‐ all trials of prednisolone versus dexamethasone ‐ reported asthma symptoms using different scales. Altamimi 2006 reported both the pulmonary index score (PIS) at day five and the mean number of days for the patient self assessment sheet (PSAS) score to return to normal. Researchers detected no between‐group differences (Analysis 4.3; MD ‐0.10, 95% CI ‐0.45 to 0.25; participants = 110; Analysis 4.4; MD 0.01, 95% CI ‐0.67 to 0.69; participants = 110), but we have low confidence in both estimates as the result of imprecision and lack of clarity about the rigorous validation of the scoring systems used. Cronin 2015 reported the paediatric respiratory assessment measure (PRAM) score at day four as the primary outcome for which the study was powered and detected no between‐group differences (Analysis 4.5; MD 0.00, 95% CI ‐0.36 to 0.36). Qureshi 2001, again a trial of prednisolone versus dexamethasone, reported separately persistent cough, wheeze, chest tightness, night wakening and difficulty maintaining normal activities (Analysis 4.6). This study detected no between‐group differences, but we assessed this trial as having high risk of selection and performance bias.
Serious adverse events
Included studies infrequently reported serious adverse events, and none of the studies in adults specifically reported this outcome. Five studies in children (Altamimi 2006; Chang 2008; Langton Hewer 1998; NCT00257933; Qureshi 2001), including a total of 695 participants, reported that there were no serious adverse events.
Secondary outcomes
New exacerbations during the follow‐up period
New exacerbations during the follow‐up period were reported by seven studies in adults (Cydulka 1998; Hasegawa 2000; Jones 2002; Karan 2002; Kravitz 2011; Lederle 1987; O’Driscoll 1993) and eight studies in children (Altamimi 2006; Chang 2008; Cronin 2015; Ghafouri 2010; Greenberg 2008; Kayani 2002; NCT00257933; Qureshi 2001). New exacerbations were classified in two main ways: those requiring an unscheduled visit to a healthcare provider, and those requiring the prescription of additional oral corticosteroids. Overall, no included study reported a clear, unbiased benefit of one regimen over another, and varied interventions and definitions of an exacerbation prevented a unifying meta‐analysis.
Exacerbation requiring a visit to a healthcare provider: adults
Four small studies in adults (Cydulka 1998; Hasegawa 2000; Karan 2002; O’Driscoll 1993; total n = 96; Analysis 1.4) that compared longer versus shorter courses of prednisolone or stable versus tapered prednisolone reported exacerbations requiring a visit to a healthcare professional during the follow‐up period. Only eight events were reported across the four studies, resulting in insufficient data to ascertain possible differences between interventions for this outcome. Our confidence in these estimates was further reduced by concerns about selection, performance and detection bias in two of the contributing studies (Hasegawa 2000; Karan 2002) and by indirectness of the treatment regimens used, which deviated widely from current standard practice.
Kravitz 2011, a study involving adults that compared prednisolone versus dexamethasone, separately reported exacerbations requiring an emergency department visit and those requiring a visit to a primary healthcare provider. Investigators detected no differences between the two interventions for this outcome, but confidence intervals did not exclude the possibility of risk or harm for either intervention (Analysis 2.3; Analysis 2.4); we assessed this study to be at high risk of attrition bias, further limiting our confidence in this estimate.
Exacerbation requiring a visit to a healthcare provider: children
Five studies in children ‐ one comparing a longer versus a shorter course of dexamethasone (Ghafouri 2010; Analysis 3.7) and four comparing prednisolone versus dexamethasone (Altamimi 2006; Cronin 2015; Greenberg 2008; Qureshi 2001; Analysis 4.8) reported exacerbations requiring an unscheduled visit to a healthcare provider during the follow‐up period. The results reported by Ghafouri 2010 favoured a shorter over a longer course of dexamethasone for this outcome but with wide confidence intervals, which do not exclude the possibility that the longer course may be more beneficial (OR 2.17, 95% CI 0.67 to 7.01; participants = 100). In addition to our serious concerns about imprecision, we considered this study to be at high risk of bias in several domains.
The four studies investigating prednisolone versus dexamethasone favoured prednisolone, but again the confidence intervals did not exclude potential risk or benefit of either steroid for this outcome (Analysis 4.8; OR 0.85, 95% CI 0.54 to 1.34; participants = 981; I2 = 0%). Of note, Qureshi 2001 carried out an intention‐to‐treat analysis for this outcome, assuming that all children excluded because of vomiting or lost to follow‐up had a relapse; this analysis favoured dexamethasone, but confidence intervals did not exclude the possibility of no differences (OR 0.61, 95% CI 0.35 to 1.05), and we assessed this study as having high risk of selection and performance bias. We also rated Cronin 2015 as having high risk of performance and detection bias for this outcome, and we are uncertain about the effect that repeated enrolment of the same participants may have had on this outcome.
Exacerbation requiring additional oral corticosteroids: adults
Three studies in adults (Jones 2002; Lederle 1987; O’Driscoll 1993) that compared longer courses versus shorter courses of prednisolone reported exacerbations requiring an additional course of oral steroids during the follow‐up period. Results favoured a longer course of steroids, but the confidence intervals did not exclude the possibility of no differences or benefit derived from a shorter course (Analysis 1.5; OR 0.62, 95% CI 0.23 to 1.68; participants = 122; I2 = 0%). In addition, as already described, our confidence in the applicability of this finding to a population with asthma is reduced by the likelihood that many of the participants in Lederle 1987 had co‐morbid COPD, and that the higher event rate in this study dominated the analysis.
Viska 2008, a conference abstract, also reported 'relapse'. We did not include this study in the quantitative synthesis, as the total 'n' for each intervention group (higher‐ vs lower‐dose prednisolone) was not given. However, the abstract reported no differences between the two treatment arms for this outcome.
Exacerbation requiring additional oral corticosteroids: children
Finally, five studies in children ‐ two comparing higher versus lower doses of prednisolone (Kayani 2002; NCT00257933), one comparing a longer versus a shorter course of prednisolone (Chang 2008), one comparing a longer versus a shorter course of dexamethasone (Ghafouri 2010) and one comparing prednisolone and dexamethasone (Cronin 2015) ‐ reported exacerbations requiring an additional course of oral steroids. As for previous outcomes, events in Chang 2008, Ghafouri 2010, Kayani 2002 and NCT00257933 were rare, and none of these analyses demonstrated a conclusive benefit of one regimen over the other (Analysis 3.6). Our confidence in these estimates is moderate (Chang 2008) or low (Ghafouri 2010; Kayani 2002; NCT00257933) because of concerns about imprecision and risk of bias. Cronin 2015 detected benefit in favour of prednisolone (Analysis 4.9; OR 0.29, 95% CI 0.10 to 0.81; participants = 242). However, as the study authors discuss, this finding may be related to unblinded clinicians who tended to favour prednisolone over dexamethasone and were more inclined to prescribe additional steroids for those in the dexamethasone intervention group, reducing our confidence in this result.
Lung function tests
Some included studies reported lung function test results, predominantly peak expiratory flow rates (PEFRs) and forced expiratory volume in one second (FEV1), but overall these studies did not identify a conclusive benefit of one steroid regimen over another.
PEFR: adults
Two studies of adult participants that compared longer courses versus shorter courses of prednisolone (Jones 2002; O’Driscoll 1993) reported trough PEFR. Although a combined analysis of results of these two studies did not suggest differences between treatment regimens, the confidence intervals did not rule out a perceivable difference between trial arms (Analysis 1.6; MD ‐4.81, 95% CI ‐45.82 to 36.20; participants = 79; I2 = 0%). Viska 2008, a conference abstract, randomised adult participants to higher‐ versus lower‐dose prednisolone and reported PEFR at four weeks but did not reveal total 'n' for each group and reported no variance, so we were unable to include this study in the quantitative synthesis. Mean PEFR at four weeks (two weeks post treatment) for the higher‐dose group was 272.89 L/min, and for the lower‐dose group 296.11 L/min.
FEV1: adults
Two small studies of stable (higher total dose) versus tapered (lower total dose) prednisolone, given for the same duration (Cydulka 1998; Karan 2002), reported FEV1% predicted at 21 days (exact timing of the test not specified). Again, although investigators detected no differences between treatment regimens, we cannot conclude that the regimens are equivalent because data provided were insufficient (Analysis 1.7; MD ‐1.02, 95% CI ‐4.62 to 2.58; participants = 41; I2 = 0%); our confidence in this result is further reduced by the indirectness of treatment regimens used in these studies and by the unusually small standard deviations reported.
PEFR and FEV1: children
In children, only one study, which compared high‐, medium‐ and low‐dose prednisolone (Langton Hewer 1998), measured FEV1% predicted (Analysis 3.8) and PEFR% predicted (Analysis 3.9) at discharge in a small subgroup of participants who were able to perform these tests. Results were inconsistent (i.e. did not demonstrate a dose‐response relationship) and confidence intervals were overlapping for all three comparisons (high vs medium, high vs low and medium vs low) for both outcomes.
All adverse events/side effects
Similarly to serious adverse events, all adverse events were not frequently reported by the included studies, and when they were reported, benefit of one regimen over another was not generally shown.
Adults
Lederle 1987, a study of long tapering (seven weeks) versus short tapering (seven days) of prednisolone, was the only study including adults that reported adverse events. These were defined as 'steroid side effects', including weight gain, oedema, acne and easy bruising. Findings favoured a shorter taper but with very wide confidence intervals, which did not exclude the possibility of no differences (Analysis 1.9; OR 4.15, 95% CI 0.94 to 18.41; participants = 43). Of note, many participants likely had COPD with reversibility and may represent a distinctly different group from participants in the other included studies. Our confidence in this result is very low.
Children
In children, only one study of a five‐ versus three‐day course of prednisolone (Chang 2008) reported all adverse events. Events were too infrequent to permit conclusions about the relative safety of a longer course versus a shorter course (Analysis 3.10; OR 0.67, 95% CI 0.11 to 4.08; participants = 201). Two studies of higher‐dose versus lower‐dose prednisolone (Kayani 2002; NCT00257933) specifically reported recognised steroid side effects (facial fullness, facial erythema, change in appetite, abdominal pain, diarrhoea, anxiety, euphoria, depression, quiet and reserved manner, hyperactivity and aggressive behaviour). Langton Hewer 1998 also specifically reported 'hyperactivity related to beta‐agonist use', which we combined with findings of the two aforementioned studies in a meta‐analysis. None of the meta‐analyses showed clear benefit of one regimen over another. Of note, analyses of anxiety, hyperactivity and aggressive behaviour demonstrated high levels of heterogeneity, and many showed substantial imprecision (Analysis 3.11).
Finally, Cronin 2015, Greenberg 2008 and Qureshi 2001 ‐ all trials of prednisolone versus dexamethasone ‐ specifically reported the adverse event of vomiting. Findings favoured dexamethasone, but with moderate heterogeneity, and the confidence interval did not exclude the possibility of no difference or modest benefit with use of prednisolone (Analysis 4.10; OR 3.05, 95% CI 0.88 to 10.55; participants = 867; I2 = 53%).
Discusión
Resumen de los resultados principales
Esta revisión incluye 18 estudios que asignaron al azar a 2438 participantes a las comparaciones de interés. Nueve estudios reclutaron sólo los niños y siete adultos solamente. Dos estudios no informaron el rango de edad de los participantes; se supuso que uno fue un estudio en adultos, ya que las dosis de esteroides descritas fueron consistentes con el tratamiento de los adultos (Aboeed 2014); el otro se presentó como un resumen de congreso que no contribuyó a la síntesis cuantitativa (Viska 2008). Los estudios incluidos evaluaron dosis mayores versus inferiores de prednisolona (n = 4); ciclos más largos versus más cortos de prednisolona (n = 3) o dexametasona (n = 1); ciclos decrecientes versus ciclos no decrecientes de prednisolona (n = 4); y prednisolona versus dexametasona (n = 6). Las intervenciones y los resultados informados fueron variados y limitaron el número de metanálisis significativos que fue posible realizar.
En general no se encontraron pruebas convincentes de una diferencia en los resultados entre una dosis mayor o un ciclo más largo y una dosis inferior o un ciclo más corto de prednisolona o dexametasona, o entre la prednisolona y la dexametasona. Para dos de los resultados primarios (ingreso hospitalario y eventos adversos graves) los eventos fueron demasiado poco frecuentes para permitir establecer una conclusión acerca de la superioridad de un tratamiento sobre el otro, o acerca de su equivalencia. Los estudios incluidos informaron los síntomas de asma de varias maneras diferentes y pocas veces utilizaron escalas validadas, lo que también limitó las conclusiones que fue posible establecer. De igual manera, el metanálisis de los resultados secundarios fue obstaculizado por la heterogeneidad entre las intervenciones y las medidas de resultado utilizadas.
En general los estudios incluidos tuvieron una calidad metodológica razonable, pero la mayoría de las veces la generación de la secuencia de asignación al azar, los procedimientos de asignación y el cegamiento de los evaluadores de resultado se describieron de forma insuficiente. En seis estudios los participantes no se cegaron a la asignación a los grupos (Figura 2). La mayoría de los resultados de la revisión se consideraron de calidad baja o muy baja, lo que significa que no hay seguridad en las estimaciones del efecto. El motivo principal para disminuir la calidad fue la imprecisión, pero la falta de direccionalidad y el riesgo de sesgo también redujeron la confianza en algunas estimaciones (Resumen de los hallazgos para la comparación principal; Resumen de los hallazgos 2; Resumen de los hallazgos 3; Resumen de los hallazgos 4).
Compleción y aplicabilidad general de las pruebas
Aunque los esteroides orales se utilizan con frecuencia para las exacerbaciones del asma en todo el mundo, sólo se identificaron 18 estudios de calidad metodológica variable que cumplieron con los criterios de inclusión.
El tratamiento de las exacerbaciones del asma difiere internacionalmente, lo que afecta la definición de un régimen de dosis "alto" o "bajo" o un ciclo "corto" o "largo". Algunas guías definen un rango recomendado para el ciclo de esteroides (GINA 2015; NAEPP 2007); otras recalcan que los ciclos no deben ser menores de cinco días, pero que por lo demás la duración no debe tener límites (BTS/SIGN 2014). Es probable que los regímenes recomendados por las guías difieran en el costo y posiblemente en el cumplimiento, lo que deja a los profesionales sanitarios con dudas acerca de cuál es el plan de preferencia.
La recomendación de utilizar una dosis baja o alta o un ciclo de corta o larga duración podría comprenderse de otro modo en diferentes países si no se presta atención a los estudios individuales de los que se han extraído las pruebas. Además, la práctica ha cambiado con el transcurso del tiempo. Las fechas de los estudios incluidos en esta revisión varían desde 1987 (Lederle 1987) a 2015 (Cronin 2015), y lo que se consideró un "régimen más corto" en un estudio anterior quizás se considere un "régimen más largo" hoy en día. De hecho, aunque muchos de los estudios incluidos compararon regímenes que se utilizan actualmente, otros utilizaron dosis o duraciones del tratamiento poco frecuentes en uno o ambos brazos del ensayo que no se recomiendan en las guías actuales y no se utilizan habitualmente en la práctica actual (Cydulka 1998; Hasegawa 2000; Karan 2002; Lederle 1987; O’Driscoll 1993; Viska 2008), lo que limita la aplicabilidad de las pruebas obtenidas de estos ensayos.
En cuanto a la elección del esteroide, en todas las guías la prednisolona se recomienda como tratamiento de primera línea para los pacientes adultos y los pediátricos, y las pruebas presentadas en esta revisión no son suficientemente consistentes para indicar si la opción de segunda línea habitual, la dexametasona, es mejor o peor que la prednisolona. Es de señalar que esta revisión no consideró otras comparaciones directas de esteroides, ya que el objetivo primario fue evaluar las pruebas de dosis y duraciones diferentes. De hecho, varios estudios incluidos, que compararon dexametasona versus prednisolona, administraron dosis totales de los esteroides muy similares dentro de cada brazo de intervención (p.ej. Aboeed 2014; Greenberg 2008; Kravitz 2011; Tabla 1) y por lo tanto analizaron una interrogante algo diferente. Lo anterior puede explicar por qué los investigadores detectaron pequeñas diferencias entre los brazos. Estos estudios no se combinaron con los que evaluaron una dosis total o una duración diferentes del mismo esteroide.
Una pregunta importante que se analiza en algunos de los estudios incluidos (Aboeed 2014; Altamimi 2006; Cronin 2015; Cydulka 1998; Karan 2002; Lederle 1987; Qureshi 2001) es si la duración o la complejidad del régimen afecta el cumplimiento del participante. Los efectos beneficiosos potenciales de un ciclo de tratamiento más largo se pueden subestimar si el cumplimiento es subóptimo en comparación con un ciclo más corto o menos complejo. En la práctica clínica, lo anterior puede ser un factor que afecte la elección de un médico individual, según la conducta y las necesidades de un paciente particular. Por ejemplo, un médico podría elegir un ciclo más corto o la opción con la menor cantidad de dosis diarias en los pacientes que tienen dificultades para cumplir con los fármacos. Lo anterior puede ser particularmente válido para la comparación directa de dexametasona versus prednisolona descrita en esta revisión, donde factores como la aceptabilidad pueden haber dado lugar a un cumplimiento diferencial con los regímenes de tratamiento. Esta revisión no tuvo como objetivo analizar esta pregunta, pero puede ser un tema importante para los estudios de investigación futuros.
Además, casi todos los estudios incluidos reclutaron participantes de un contexto de departamento de urgencia, lo que limita la aplicabilidad de los resultados a los pacientes con exacerbaciones del asma que se presentan a un profesional de atención primaria.
Se planificó realizar análisis de subgrupos para explorar si la gravedad del asma de base o la gravedad de la exacerbación fueron modificadores importantes del efecto. Sin embargo, lo anterior no fue posible porque esta información no se proporcionó de forma consistente en los estudios incluidos y la heterogeneidad de los estudios limitó el metanálisis.
Calidad de la evidencia
La calidad de las pruebas presentadas en esta revisión se evaluó según los criterios GRADE (Grades of Recommendation, Assessment, Development and Evaluation Working Group) (Higgins 2011) con el uso del programa informático GRADEpro (GRADEpro GDT), y las evaluaciones se presentaron en las tablas "Resumen de los hallazgos".
El Resumen de los hallazgos para la comparación principal presenta una dosis mayor/ciclo más largo versus una dosis inferior/ciclo más corto de esteroides orales en adultos; El Resumen de los hallazgos 2 presenta la prednisolona versus la dexametasona en adultos; Resumen de los hallazgos 3; presenta una dosis mayor/ciclo más largo versus una dosis inferior/ciclo más corto de esteroides orales en niños; y el Resumen de los hallazgos 4 presenta la prednisolona versus la dexametasona en niños. La calidad de la mayoría de los resultados se consideró baja o muy baja, lo que significa que existe una confianza limitada en las estimaciones.
La calidad de todos los resultados se disminuyó al menos una vez por la imprecisión, lo que refleja el tamaño pequeño de la mayoría de los estudios incluidos y el agrupamiento limitado que se pudo realizar. Muchas estimaciones del efecto incluyeron un efecto beneficioso o perjudicial potencialmente importante de la intervención, en particular en los resultados en los que fueron poco frecuentes los eventos como el ingreso hospitalario o las nuevas exacerbaciones durante el período de seguimiento.
También se disminuyó la calidad de varios resultados debido a inquietudes acerca de los posibles sesgos de realización y detección en los estudios incluidos(Cronin 2015; Ghafouri 2010 Karan 2002; Qureshi 2001), la incertidumbre acerca de los procedimientos de asignación(Qureshi 2001) o el sesgo de desgaste(Ghafouri 2010; Kravitz 2011; Langton Hewer 1998). La falta de direccionalidad fue una preocupación en los resultados proporcionados por estudios que utilizaron una intervención que actualmente no se utiliza en la práctica habitual (Lederle 1987; Viska 2008) o que reclutaron una muestra de estudio que probablemente incluyó muchos participantes con EPOC coexistente Viska 2008) la calidad de otros resultados se disminuyó debido a la falta de direccionalidad, ya que hubo inquietudes acerca de la validación rigurosa de las escalas de medición utilizadas (Altamimi 2006).
No se sospechó sesgo de publicación para los resultados evaluados. Los resultados agrupados parecieron ser consistentes, con una heterogeneidad baja en casi todos los resultados, lo que es probable que refleje el enfoque cauteloso para combinar datos en los que los tratamientos, los participantes y las preguntas clínicas fundamentales no fueron suficientemente similares como para que el agrupamiento tuviera sentido.
Sesgos potenciales en el proceso de revisión
Se siguió el procedimiento estándar según el Manual Cochrane para Revisiones Sistemáticas de Intervenciones (Cochrane Handbook for Systematic Reviews of Interventions) (Higgins 2011) para minimizar el sesgo en el proceso de revisión. Se realizó una búsqueda exhaustiva y se considera que es poco probable que no se lograra identificar estudios relevantes. Dos revisores de forma independiente examinaron las búsquedas, extrajeron las características de los estudios y los verificaron de manera puntual para la exactitud; y obtuvieron de forma independiente todos los datos de resultados, que luego verificaron contra el informe original. Dos o más autores de la revisión evaluaron de forma independiente el riesgo de sesgo, mostrando un alto nivel de acuerdo. Estos autores de la revisión resolvieron algunas discrepancias mediante discusión, realizaron evaluaciones GRADE y llegaron a un consenso mediante discusión.
Sin embargo, el enfoque para el análisis requirió de cierta flexibilidad, ya que no fue posible prever en su totalidad la naturaleza de los datos de resultado que se encontrarían. Fue inevitable que las comparaciones precisas utilizadas se realizaran post hoc, lo que introdujo el riesgo de un análisis controlado por los datos. Se considera que este riesgo se disminuyó al analizar a fondo diferentes enfoques para el análisis y al buscar la opinión independiente del editor de contacto para la revisión.
Acuerdos y desacuerdos con otros estudios o revisiones
Se han publicado varias revisiones sistemáticas que analizan la pregunta de la dosis más eficaz de esteroides orales para las exacerbaciones del asma. Una "revisión general" (Krishnan 2009) concluyó que las dosis de corticosteroides de más de 50 a 100 mg por día no ofrecen ventajas sobre las dosis inferiores y que un ciclo en el que no se disminuya gradualmente la dosis administrada durante cinco a diez días es suficiente para la mayoría de los pacientes. Aunque no se consideró que las pruebas presentadas en esta revisión fueran de calidad suficiente para indicar que la administración de una dosis de 50 a 100 mcg por día ofrece alguna ventaja, hay coincidencia en que las dosis altas no han demostrado ser en general más eficaces que las dosis inferiores. Además, una dosis diaria de 50 a 100 mg excede la dosis recomendada por algunas guías actuales (BTS/SIGNO 2014), y un rango de cinco a diez días aún suscita dudas en los profesionales sanitarios, lo que es especialmente importante porque muchos pacientes informan que presentan efectos secundarios desagradables mientras toman esteroides.
Una revisión Cochrane anterior (Manser 2001) evaluó las pruebas para la dosis óptima de los esteroides, administrados por cualquier vía, en los pacientes con exacerbaciones graves del asma que requieren hospitalización. Esta revisión también concluyó que no hubo pruebas que indicaran que las dosis mayores se asociaran con mejores resultados o de hecho con más eventos adversos. Sin embargo, Manser 2001 incluyó una cohorte de pacientes con enfermedad mucho más grave y las dosis administradas en los estudios incluidos sobrepasaron mucho las evaluadas en esta revisión, por ejemplo, las dosis altas se consideraron mayores de 360 mg equivalentes de metilprednisolona por día, la dosis media entre 80 y 360 mg y la dosis baja de 80 mg o menos.
Un metanálisis realizado para analizar la pregunta de si la dexametasona es un tratamiento alternativo equivalente a la prednisolona en los niños con asma agudo(Keeney 2014) incluyó seis estudios, tres de los cuales se incluyeron en la presente revisión. Los otros tres estudios incluidos enKeeney 2014 utilizaron dexametasona administrada por vía intramuscular; por lo tanto, fueron excluidos. También se incluyó Cronin 2015,publicado después deKeeney 2014. Sin embargo, las conclusiones generales de Keeney 2014 son similares a las de la presente revisión; en cuanto a la eficacia, un fármaco no parece ser superior al otro. Los autores del estudio también señalan que la dexametasona se puede asociar con menos episodios de vómitos y mejorar el cumplimiento del tratamiento prescrito, pero que lo anterior se podría esperar en una revisión que incluyera estudios que utilizaran la vía intramuscular para la administración de dexametasona. No fue posible encontrar una revisión sistemática que analizara esta pregunta en pacientes adultos.

Study flow diagram.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Adults: higher dose/longer course vs lower dose/shorter course, Outcome 1 Re‐admission during follow‐up period.

Comparison 1 Adults: higher dose/longer course vs lower dose/shorter course, Outcome 2 Asthma symptoms: asthma severity score.

Comparison 1 Adults: higher dose/longer course vs lower dose/shorter course, Outcome 3 Asthma symptoms: complete resolution.

Comparison 1 Adults: higher dose/longer course vs lower dose/shorter course, Outcome 4 New exacerbation during follow‐up period: requiring visit to healthcare provider.

Comparison 1 Adults: higher dose/longer course vs lower dose/shorter course, Outcome 5 New exacerbation during follow‐up period: oral corticosteroids prescribed.

Comparison 1 Adults: higher dose/longer course vs lower dose/shorter course, Outcome 6 Lung function tests: trough PEFR.

Comparison 1 Adults: higher dose/longer course vs lower dose/shorter course, Outcome 7 Lung function tests: FEV1% predicted.

Comparison 1 Adults: higher dose/longer course vs lower dose/shorter course, Outcome 8 Lung function tests: number of participants achieving personal best at 4 weeks.

Comparison 1 Adults: higher dose/longer course vs lower dose/shorter course, Outcome 9 All adverse events.

Comparison 2 Adults: prednisolone vs dexamethasone, Outcome 1 Re‐admission during follow‐up period.

Comparison 2 Adults: prednisolone vs dexamethasone, Outcome 2 Asthma symptoms: returned to normal activities within 3 days.
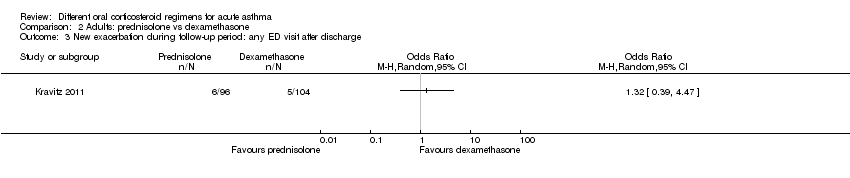
Comparison 2 Adults: prednisolone vs dexamethasone, Outcome 3 New exacerbation during follow‐up period: any ED visit after discharge.

Comparison 2 Adults: prednisolone vs dexamethasone, Outcome 4 New exacerbation during follow‐up period: unscheduled visit to primary healthcare provider.

Comparison 3 Children: higher dose/longer course vs lower dose/shorter course, Outcome 1 Admission at initial presentation.

Comparison 3 Children: higher dose/longer course vs lower dose/shorter course, Outcome 2 Re‐admission during follow‐up period.

Comparison 3 Children: higher dose/longer course vs lower dose/shorter course, Outcome 3 Asthma symptoms: clinical asthma score at discharge.

Comparison 3 Children: higher dose/longer course vs lower dose/shorter course, Outcome 4 Asthma symptoms: symptom free by 7 days.

Comparison 3 Children: higher dose/longer course vs lower dose/shorter course, Outcome 5 Serious adverse events.

Comparison 3 Children: higher dose/longer course vs lower dose/shorter course, Outcome 6 New exacerbation during follow‐up period: oral corticosteroids prescribed.
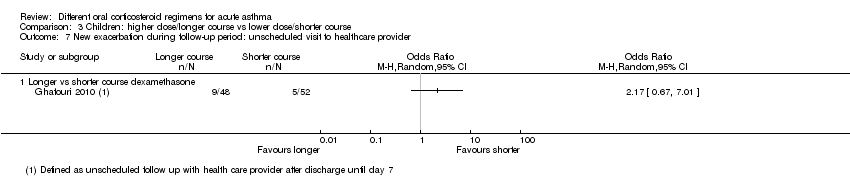
Comparison 3 Children: higher dose/longer course vs lower dose/shorter course, Outcome 7 New exacerbation during follow‐up period: unscheduled visit to healthcare provider.

Comparison 3 Children: higher dose/longer course vs lower dose/shorter course, Outcome 8 Lung function tests: FEV1% predicted at discharge.

Comparison 3 Children: higher dose/longer course vs lower dose/shorter course, Outcome 9 Lung function tests: PEFR% predicted at discharge.

Comparison 3 Children: higher dose/longer course vs lower dose/shorter course, Outcome 10 All adverse events: longer vs short course prednisolone.

Comparison 3 Children: higher dose/longer course vs lower dose/shorter course, Outcome 11 All adverse events: higher‐dose vs lower‐dose prednisolone.

Comparison 4 Children: prednisolone vs dexamethasone, Outcome 1 Admission at initial presentation.

Comparison 4 Children: prednisolone vs dexamethasone, Outcome 2 Re‐admission during follow‐up period.

Comparison 4 Children: prednisolone vs dexamethasone, Outcome 3 Asthma symptoms: PIS.

Comparison 4 Children: prednisolone vs dexamethasone, Outcome 4 Asthma symptoms: PSAS.

Comparison 4 Children: prednisolone vs dexamethasone, Outcome 5 Asthma symptoms: PRAM.
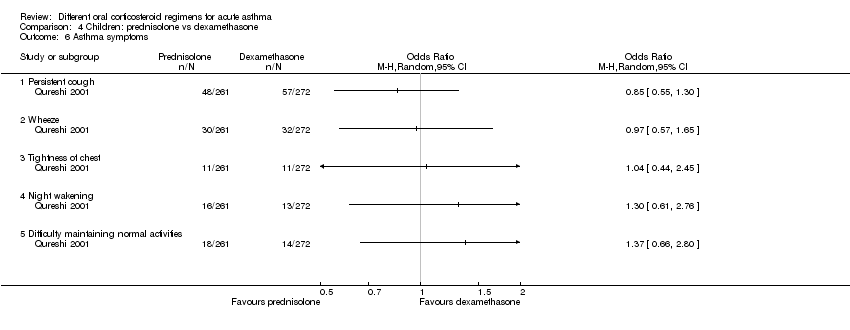
Comparison 4 Children: prednisolone vs dexamethasone, Outcome 6 Asthma symptoms.

Comparison 4 Children: prednisolone vs dexamethasone, Outcome 7 Serious adverse events.

Comparison 4 Children: prednisolone vs dexamethasone, Outcome 8 New exacerbation during follow‐up period: unscheduled visit to healthcare provider.

Comparison 4 Children: prednisolone vs dexamethasone, Outcome 9 New exacerbation during follow‐up period: oral corticosteroids prescribed.

Comparison 4 Children: prednisolone vs dexamethasone, Outcome 10 Adverse event: vomiting.
| Adults: higher dose/longer course compared with lower dose/shorter course for acute asthma | ||||||
| Patient or population: adults with an acute exacerbation of asthma Duration range: 3 to 26 weeks | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Risk with lower dose/shorter course | Risk with higher dose/longer course | |||||
| Re‐admission in follow‐up period | Longer vs shorter course prednisolone | OR 1.35 | 142 | ⊕⊕⊝⊝ | ||
| 74 per 1000 | 97 per 1000 | |||||
| Asthma symptoms Asthma severity score | Longer vs shorter course prednisolone | ‐ | 44 | ⊕⊕⊝⊝ | Higher score = Worse symptoms | |
| Mean asthma severity score was 2.6 | Mean asthma severity score in the longer course group was 0.7 lower (1.28 lower to 0.12 lower) | |||||
| Asthma symptoms Complete resolution by day 28 | Longer vs shorter course prednisolone | OR 0.55 | 35 | ⊕⊕⊝⊝ | ||
| 412 per 1000 | 278 per 1000 | |||||
| New exacerbation in follow‐up period Requiring visit to healthcare provider | Longer vs shorter course prednisolone | OR 0.98 | 55 | ⊕⊝⊝⊝ | ||
| 111 per 1000 | 109 per 1000 | |||||
| Stable (same daily dose for 7 days) vs tapered (tapering daily dose over 7 days) prednisolone | OR 3.56 | 41 | ⊕⊝⊝⊝ | No events were reported in the tapered arm and only 2 events in the stable arm, so we were unable to calculate a baseline risk | ||
| No events | Risk difference in the stable (higher total dose) group was 9% (0 to 26%) | |||||
| New exacerbation in follow‐up period Oral corticosteroids prescribed | Longer vs shorter course prednisolone | OR 0.62 | 122 | ⊕⊕⊝⊝ | Lederle 1987 dominates this analysis, as the event rate was much higher than in the other 2 studies, possibly reflecting co‐morbid COPD in the study population. Result should be interpreted with caution | |
| 241 per 1000 | 165 per 1000 (68 to 348) | |||||
| Lung function tests FEV1% predicted | Stable (same daily dose for 7 days) vs tapered (tapering daily dose over 7 days) prednisolone | ‐ | 41 | ⊕⊝⊝⊝ | Higher percentage = Better lung function | |
| Mean FEV1% predicted was 70.6 | Mean FEV1% predicted in the stable dose (higher total dose) was 1.02 lower (4.62 lower to 2.58 higher) | |||||
| All adverse events | Longer vs shorter course prednisolone | OR 4.15 | 43 | ⊕⊝⊝⊝ | ||
| 143 per 1000 | 409 per 1000 | |||||
| *Risk in the intervention group (and its 95% confidence interval) is based on assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) | ||||||
| GRADE Working Group grades of evidence | ||||||
| aLederle 1987 carried a large proportion of the analysis weight for this outcome because event rates were higher in both groups. This may reflect co‐morbid COPD (participants were older and most had an extensive smoking history). Downgraded once for indirectness bConfidence intervals include no difference and an important benefit of a longer or shorter course. Downgraded once for imprecision cConfidence intervals excluded possible benefit of a shorter course, but the effect was based on only 1 study of 44 people. Downgraded once for imprecision dA 1‐7 scale of symptom severity averaged over days 6‐21 was used, making clinical benefit difficult to interpret. Downgraded once for indirectness eNeither treatment regimen used in the one study in this analysis is consistent with current international guidance. Downgraded once for indirectness fThe study contributing most of the analysis weight was unblinded and uncertainties surrounded the selection procedure. Downgraded once for risk of bias gBoth trials contributing to the analysis used a treatment regimen that was inconsistent with current international guidance. Downgraded once for indirectness hThe effect was derived from 2 very similar studies including 41 people in total. Studies had smaller standard deviations than would be expected given the sample sizes. Downgraded once for imprecision iThe result is based on 1 small study and has wide confidence intervals, which do not exclude the possibility of no difference or an important increase in adverse events in the longer course arm, Downgraded twice for imprecision | ||||||
| Adults: prednisolone compared with dexamethasone for acute asthma | ||||||
| Patient or population: adults with an acute exacerbation of asthma Duration: 2 weeks | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Risk with dexamethasone | Risk with prednisolone | |||||
| Re‐admission during follow‐up period | 29 per 1000 | 10 per 1000 | OR 0.35 | 200 | ⊕⊝⊝⊝ | |
| Asthma symptoms Returned to normal activities within 3 days | 901 per 1000 | 800 per 1000 | OR 0.44 | 191 | ⊕⊕⊝⊝ | |
| New exacerbation during follow‐up period Any ED visit after discharge | 48 per 1000 | 63 per 1000 | OR 1.32 | 200 | ⊕⊝⊝⊝ | |
| New exacerbation during follow‐up period Unscheduled visit to primary healthcare provider | 29 per 1000 | 52 per 1000 | OR 1.85 | 200 | ⊕⊝⊝⊝ | |
| *Risk in the intervention group (and its 95% confidence interval) is based on assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) | ||||||
| GRADE Working Group grades of evidence | ||||||
| aOnly 1 study contributed to this outcome with very few events reported in total, resulting in an imprecise estimate with confidence intervals including both important harms and benefits of either regimen. Downgraded twice for imprecision bOnly contributing study judged to be at high risk of attrition bias because of post‐randomisation exclusions and large numbers lost to follow‐up. Downgraded once for risk of bias cOnly 1 study contributed to this outcome with imprecise estimate and confidence intervals not completely excluding the possibility of no differences. Downgraded once for imprecision | ||||||
| Children: higher dose/longer course compared with lower dose/shorter course for acute asthma | ||||||
| Patient or population: children with an acute exacerbation of asthma Duration range: 1 to 4 weeks | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Risk with lower dose/shorter course | Risk with higher dose/longer course | |||||
| Re‐admission during follow‐up period | Higher‐ vs lower‐dose prednisolone | Not estimable | 98 | ⊕⊝⊝⊝ | Only one 3‐arm study (Langton Hewer 1998) contributed events to this analysis. Two lower‐dose arms pooled for this outcome. OR 1.55 (0.24 to 9.78) favouring lower dose | |
| Not pooled | Not pooled | |||||
| Longer vs shorter course prednisolone | OR 0.33 | 201 | ⊕⊕⊝⊝ | |||
| 10 per 1000 | 3 per 1000 | |||||
| Longer vs shorter course dexamethasone | OR 2.22 | 100 | ⊕⊝⊝⊝ | |||
| 19 per 1000 | 42 per 1000 | |||||
| Asthma symptoms Symptom free by 7 days | Longer vs shorter course prednisolone | OR 1.22 | 201 | ⊕⊕⊕⊝ | One other study (Langton Hewer 1998) randomising 98 children to high‐ vs medium‐ vs low‐dose prednisolone reported clinical asthma score at discharge. Small differences in scores were reported with uncertain clinical importance and no consistent dose‐response effect | |
| 307 per 1000 | 351 per 1000 | |||||
| Serious adverse events | Longer vs shorter course prednisolone | Not estimable | 201 | No events occurred in either trial arm | ||
| 0 per 1000 | 0 per 1000 | |||||
| New exacerbation during follow‐up period Oral corticosteroids prescribed | Higher‐ vs lower‐dose prednisolone | OR 1.38 | 231 | ⊕⊕⊝⊝ | ||
| 17 per 1000 | 24 per 1000 | |||||
| Longer vs shorter course prednisolone | OR 0.61 | 201 | ⊕⊕⊕⊝ | |||
| 79 per 1000 | 50 per 1000 | |||||
| Longer vs shorter course dexamethasone | OR 0.24 | 100 | ⊕⊕⊝⊝ | |||
| 154 per 1000 | 42 per 1000 | |||||
| New exacerbation during follow‐up period Unscheduled visit to healthcare provider | Longer vs shorter course dexamethasone | OR 2.17 | 100 | ⊕⊝⊝⊝ | ||
| 96 per 1000 | 188 per 1000 | |||||
| Lung function tests FEV1% predicted at discharge | High vs medium vs low dose | ‐ | 34 | This outcome includes only 1 small study (Langton Hewer 1998) in which a subset of participants were able to perform PFTs. Reported between‐group differences were small and of uncertain clinical importance with no consistent dose‐response effect. | ||
| ‐ | ‐ | |||||
| All adverse events | Longer vs short course prednisolone | OR 0.67 | 201 | ⊕⊕⊕⊝ | ||
| 30 per 1000 | 20 per 1000 | |||||
| *Risk in the intervention group (and its 95% confidence interval) is based on assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) | ||||||
| GRADE Working Group grades of evidence | ||||||
| aOnly 1 study contributed events to this outcome and was assessed to be at high risk of attrition bias because of unbalanced drop‐out from intervention arms. Downgraded once for risk of bias bThe study contributing events had 3 different dose arms, 1 of which is outside the current dosing guidelines. Two other studies reported no events, but intervention involved much higher doses of prednisolone. Downgraded once for indirectness cOnly 1 study contributed to this analysis. Imprecise estimate with confidence intervals including possibility of important harms or benefits. Downgraded twice for imprecision dOnly contributing study considered at high risk of bias in multiple domains. Downgraded once for risk of bias eOnly 1 study contributed to this outcome, resulting in imprecise estimate and confidence intervals including the possibility of important harms or benefits. Downgraded once for imprecision fOnly 2 studies contributed to this outcome with few events, resulting in imprecise estimate and wide confidence intervals including the possibility of important harms or benefits. Downgraded twice for imprecision gOnly 1 study contributed to this outcome, resulting in imprecise estimate, which does not exclude the possibility of no difference. Downgraded once for imprecision | ||||||
| Children: prednisolone compared with dexamethasone for acute asthma | ||||||
| Patient or population: children with acute exacerbation of asthma Duration range: 1.5 to 3 weeks | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Risk with dexamethasone | Risk with prednisolone | |||||
| Admission at initial presentation | 116 per 1000 | 124 per 1000 | OR 1.08 (0.74 to 1.58) | 1007 | ⊕⊕⊝⊝ | |
| Re‐admission during follow‐up period | 22 per 1000 | 10 per 1000 | OR 0.44 (0.15 to 1.33) | 985 | ⊕⊕⊝⊝ | |
| Asthma symptoms scores Pulmonary Index Score (PIS); Patient Self Assessment Score (PSAS); Paediatric Respiratory Assessment Measure (PRAM) | Not pooled | Not pooled | ‐ | 328 (2 RCTs) | ⊕⊝⊝⊝ | Altamimi 2006 reported PIS and PSAS Cronin 2015 reported PRAM (we extracted the result, which excluded re‐enrolments) No between‐group differences were detected |
| Asthma symptoms Persistent cough, wheeze, chest tightness, night‐time wakening and difficulty maintaining normal activities | Not pooled | Not pooled | ‐ | 533 | The number of people experiencing these symptoms at day 10 was not found to be significantly different between the 2 intervention arms | |
| Serious adverse events | Not pooled | Not pooled | Not estimable | 255 | No events were reported in either study | |
| New exacerbation during follow‐up period Unscheduled visit to healthcare provider | 97 per 1000 | 83 per 1000 | OR 0.85 (0.54 to 1.34) | 981 | ⊕⊕⊝⊝ | |
| *Risk in the intervention group (and its 95% confidence interval) is based on assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) | ||||||
| GRADE Working Group grades of evidence | ||||||
| aThe 2 studies contributing most events to this outcome were considered to be at high or unclear risk of selection (Qureshi 2001) and performance and detection bias (Cronin 2015; Qureshi 2001). In addition, Cronin 2015 allowed 19 participants to enrol more than once in the study. Downgraded once for risk of bias bConfidence intervals include possible harms or benefits of either intervention. Downgraded once for imprecision cThe pulmonary index score may lack rigorous evaluation, so clinical interpretation of this score is limited. Downgraded once for indirectness dConfidence intervals for PIS and PSAS include no difference, but we are unsure whether either end of the confidence intervals includes a clinically important effect. Downgraded once for imprecision eThe PSAS score has been adapted from National Institute of Health guidelines and may lack rigorous evaluation, so clinical interpretation is limited. Downgraded once for indirectness fWe were unable to combine the results of these different scales. Downgraded once for inconsistency | ||||||
| Study ID | Total n | Country | Age range, years | Duration of follow‐up | Comparison | Total dose comparison (converted to prednisolone equivalent) |
| 58 | USA | Not reported | 4 weeks | Prednisone 40 mg once daily for 5 days vs dexamethasone 16 mg once daily for 2 days | 200 mg vs 213 mg | |
| 134 | Canada | 2 to16 | 3 weeks (maximum) | Predisolone 1 mg/kg twice daily for 5 days vs dexamethasone 0.6 mg/kg once daily for 1 day | 200 mg vs 80 mg (based on 20 kg child) | |
| 201 | Australia | 2 to15 | 4 weeks | Prednisolone 1 mg/kg daily for 5 days vs prednisolone 1 mg/kg daily for 3 days | 100 mg vs 60 mg (based on 20 kg child) | |
| 226 | Ireland | 2 to 16 | 2 weeks | Prednisolone 1 mg/kg daily for 3 days vs 0.3 mg/kg dexamethasone once daily for 1 day | 60 mg vs 40 mg (based on 20 kg child) | |
| 15 | USA | 19 to 50 | 3 weeks | Prednisolone 40 mg daily for 8 days vs prednisolone 40 mg daily tapering by 5 mg per day for 8 days | 320 mg vs 180 mg | |
| 125 | USA | 2 to 17 | 1 week | Dexamethasone 0.6 mg/kg once daily for 2 doses (days 1 and 3) versus dexamethasone 0.6 mg/kg once daily for 1 day | 160 mg vs 80 mg (based on 20 kg child) | |
| 167 | USA | 2 to 18 | 1.5 weeks | Prednisolone 1 mg/kg twice daily for 5 days vs dexamethasone 0.6 mg/kg once daily for 2 days | 200 mg vs 160 mg (based on 20 kg child) | |
| 20 | Japan | Not reported | 26 weeks | Prednisolone 0.5 mg/kg daily for 14 days vs prednisolone 0.5 mg/kg once daily for 7 days | 490 mg vs 245 mg (based on 70 kg adult) | |
| 47 | UK | 16 to 60 | 4‐6 weeks | Prednisolone 40 mg once daily for 10 days vs prednisolone 40 mg once daily for 5 days | 400 mg vs 200 mg | |
| 26 | India | 17 to 70 | 3 weeks | Prednisolone 40 mg daily for 8 days vs prednisolone 40 mg daily tapering by 5 mg per day for 8 days | 320 mg vs 180 mg | |
| 88 | USA | 2 to 18 | 4 weeks (maximum) | Prednisolone 2 mg/kg daily for 5 days vs prednisolone 1 mg/kg daily for 5 days | 200 mg vs 100 mg (based on 20 kg child) | |
| 285 | USA | 18 to 45 | 2 weeks | Prednisolone 50 mg once daily for 5 days vs dexamethasone 16 mg once daily for 2 days | 250 mg vs 213 mg | |
| 98 | UK | 1 to 15 | 2 weeks | Prednisolone 2 mg/kg once daily vs prednisolone 1 mg/kg once daily vs prednisolone 0.5 mg/kg once daily while inpatient and for up to 3 days post discharge | 200 mg vs 100 mg vs 50 mg (based on 20 kg child receiving a 5‐day course) | |
| 43 | USA | 30 to 78 | 12 weeks | Prednisolone 45 mg daily reducing to 0 mg daily over 7 weeks vs prednisolone 45 mg daily reducing to 0 mg daily over 7 days | 1575 mg vs 225 mg | |
| 152 | USA | 2 to 18 | 2 weeks | Prednisolone 4 mg/kg daily for 2 days, then 2 mg/kg daily for duration of admission vs prednisolone 2 mg/kg daily for duration of admission | 400 mg vs 200 mg (based on 20 kg child receiving a 5‐day course) | |
| 39 | UK | 16 to 55 | 4‐6 weeks | Prednisolone 40 mg daily for 10 days followed by 7‐day taper vs prednisolone 40 mg daily for 10 days | 540 mg vs 400 mg | |
| 628 | USA | 2 to 18 | 2 weeks | Prednisolone 2 mg/kg initial dose, then 1 mg/kg daily for 5 days vs dexamethasone 0.6 mg/kg once daily for 2 days | 120 mg vs 160 mg (based on 20 kg child) | |
| 86 | Indonesia | "Adults" | 6 weeks | Prednisolone 36 mg daily for 2 weeks vs prednisolone 12 mg daily for 2 weeks | 504 mg vs 168 mg |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Re‐admission during follow‐up period Show forest plot | 4 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Longer vs shorter course prednisolone | 4 | 142 | Odds Ratio (M‐H, Random, 95% CI) | 1.35 [0.38, 4.79] |
| 2 Asthma symptoms: asthma severity score Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.1 Longer vs shorter course prednisolone | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Asthma symptoms: complete resolution Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3.1 Longer vs shorter course prednisolone | 1 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 New exacerbation during follow‐up period: requiring visit to healthcare provider Show forest plot | 4 | Risk Difference (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Longer vs shorter course prednisolone | 2 | 55 | Risk Difference (M‐H, Random, 95% CI) | ‐0.00 [‐0.14, 0.14] |
| 4.2 Stable vs tapered prednisolone | 2 | 41 | Risk Difference (M‐H, Random, 95% CI) | 0.09 [‐0.07, 0.26] |
| 5 New exacerbation during follow‐up period: oral corticosteroids prescribed Show forest plot | 3 | 122 | Odds Ratio (M‐H, Random, 95% CI) | 0.62 [0.23, 1.68] |
| 5.1 Longer vs shorter course prednisolone | 3 | 122 | Odds Ratio (M‐H, Random, 95% CI) | 0.62 [0.23, 1.68] |
| 6 Lung function tests: trough PEFR Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 6.1 Longer vs shorter prednisolone (trough PEFR) | 2 | 79 | Mean Difference (IV, Random, 95% CI) | ‐4.81 [‐45.82, 36.20] |
| 7 Lung function tests: FEV1% predicted Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 7.1 Stable vs tapered prednisolone (FEV1% predicted) | 2 | 41 | Mean Difference (IV, Random, 95% CI) | ‐1.02 [‐4.62, 2.58] |
| 8 Lung function tests: number of participants achieving personal best at 4 weeks Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 8.1 Longer vs shorter course prednisolone | 1 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 All adverse events Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 9.1 Longer vs shorter course prednisolone | 1 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Re‐admission during follow‐up period Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2 Asthma symptoms: returned to normal activities within 3 days Show forest plot | 1 | 191 | Odds Ratio (M‐H, Random, 95% CI) | 0.44 [0.19, 1.01] |
| 3 New exacerbation during follow‐up period: any ED visit after discharge Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4 New exacerbation during follow‐up period: unscheduled visit to primary healthcare provider Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Admission at initial presentation Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.1 Longer vs shorter course dexamethasone | 1 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Re‐admission during follow‐up period Show forest plot | 5 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.1 Higher‐dose vs lower‐dose prednisolone | 3 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Longer vs shorter course prednisolone | 1 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Longer vs shorter course dexamethasone | 1 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Asthma symptoms: clinical asthma score at discharge Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.1 High vs medium dose | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 High vs low dose | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 Medium vs low dose | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Asthma symptoms: symptom free by 7 days Show forest plot | 1 | 201 | Odds Ratio (M‐H, Random, 95% CI) | 1.22 [0.67, 2.19] |
| 4.1 Longer vs shorter course prednisolone | 1 | 201 | Odds Ratio (M‐H, Random, 95% CI) | 1.22 [0.67, 2.19] |
| 5 Serious adverse events Show forest plot | 1 | 201 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.1 Longer vs shorter course prednisolone | 1 | 201 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 New exacerbation during follow‐up period: oral corticosteroids prescribed Show forest plot | 4 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6.1 Higher‐dose vs lower‐dose prednisolone | 2 | 231 | Odds Ratio (M‐H, Random, 95% CI) | 1.38 [0.25, 7.47] |
| 6.2 Longer vs shorter course prednisolone | 1 | 201 | Odds Ratio (M‐H, Random, 95% CI) | 0.61 [0.19, 1.94] |
| 6.3 Longer vs shorter course dexamethasone | 1 | 100 | Odds Ratio (M‐H, Random, 95% CI) | 0.24 [0.05, 1.19] |
| 7 New exacerbation during follow‐up period: unscheduled visit to healthcare provider Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 7.1 Longer vs shorter course dexamethasone | 1 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Lung function tests: FEV1% predicted at discharge Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 8.1 High vs medium dose | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.2 High vs low dose | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.3 Medium vs low dose | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 Lung function tests: PEFR% predicted at discharge Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 9.1 High vs medium dose | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.2 High vs low dose | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.3 Medium vs low dose | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 10 All adverse events: longer vs short course prednisolone Show forest plot | 1 | 201 | Odds Ratio (M‐H, Random, 95% CI) | 0.67 [0.11, 4.08] |
| 11 All adverse events: higher‐dose vs lower‐dose prednisolone Show forest plot | 3 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 11.1 Facial fullness | 2 | 231 | Odds Ratio (M‐H, Random, 95% CI) | 1.28 [0.58, 2.80] |
| 11.2 Facial erythema | 2 | 231 | Odds Ratio (M‐H, Random, 95% CI) | 0.83 [0.33, 2.06] |
| 11.3 Change in appetite | 2 | 231 | Odds Ratio (M‐H, Random, 95% CI) | 0.92 [0.49, 1.72] |
| 11.4 Abdominal pain | 2 | 231 | Odds Ratio (M‐H, Random, 95% CI) | 1.36 [0.57, 3.25] |
| 11.5 Diarrhoea | 2 | 231 | Odds Ratio (M‐H, Random, 95% CI) | 2.43 [0.43, 13.84] |
| 11.6 Anxiety | 2 | 231 | Odds Ratio (M‐H, Random, 95% CI) | 1.75 [0.20, 15.49] |
| 11.7 Euphoria | 2 | 231 | Odds Ratio (M‐H, Random, 95% CI) | 0.79 [0.30, 2.10] |
| 11.8 Depression | 2 | 232 | Odds Ratio (M‐H, Random, 95% CI) | 0.54 [0.16, 1.79] |
| 11.9 Quiet and reserved | 2 | 231 | Odds Ratio (M‐H, Random, 95% CI) | 1.73 [0.69, 4.36] |
| 11.10 Hyperactive | 3 | 318 | Odds Ratio (M‐H, Random, 95% CI) | 0.89 [0.31, 2.52] |
| 11.11 Aggressive behaviour | 2 | 231 | Odds Ratio (M‐H, Random, 95% CI) | 2.06 [0.02, 267.49] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Admission at initial presentation Show forest plot | 3 | 1007 | Odds Ratio (M‐H, Random, 95% CI) | 1.08 [0.74, 1.58] |
| 2 Re‐admission during follow‐up period Show forest plot | 3 | 985 | Odds Ratio (M‐H, Random, 95% CI) | 0.44 [0.15, 1.33] |
| 3 Asthma symptoms: PIS Show forest plot | 1 | 110 | Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.45, 0.25] |
| 4 Asthma symptoms: PSAS Show forest plot | 1 | 110 | Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.67, 0.69] |
| 5 Asthma symptoms: PRAM Show forest plot | 1 | 218 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.36, 0.36] |
| 6 Asthma symptoms Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 6.1 Persistent cough | 1 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 Wheeze | 1 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.3 Tightness of chest | 1 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.4 Night wakening | 1 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.5 Difficulty maintaining normal activities | 1 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Serious adverse events Show forest plot | 2 | 255 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8 New exacerbation during follow‐up period: unscheduled visit to healthcare provider Show forest plot | 4 | 981 | Odds Ratio (M‐H, Random, 95% CI) | 0.85 [0.54, 1.34] |
| 9 New exacerbation during follow‐up period: oral corticosteroids prescribed Show forest plot | 1 | 242 | Odds Ratio (M‐H, Random, 95% CI) | 0.29 [0.10, 0.81] |
| 10 Adverse event: vomiting Show forest plot | 3 | 867 | Odds Ratio (M‐H, Random, 95% CI) | 3.05 [0.88, 10.55] |

