Radical multimodality therapy for malignant pleural mesothelioma
Referencias
References to studies included in this review
References to studies excluded from this review
References to ongoing studies
Additional references
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Randomised clinical trial with two arms: postoperative hemithoracic high‐dose radiotherapy versus no postoperative radiotherapy | |
| Participants | 54 patients with pathologically confirmed mesothelioma underwent three cycles of neoadjuvant chemotherapy (cisplatin 75 mg/m² and pemetrexed 500 mg/m² on day 1 given every 3 weeks) and extrapleural pneumonectomy were randomly assigned (1:1), 27 in each group, to receive high‐dose radiotherapy or not. Male/female: 50/4 Inclusion criteria:
Recruitment: December 2005 to October 2012 | |
| Interventions | All patients had three cycles of neoadjuvant chemotherapy (cisplatin 75 mg/m² and pemetrexed 500 mg/m² on day 1 given every 3 weeks). All patients underwent extrapleural pneumonectomy with complete macroscopic resection. Hemithoracic high‐dose radiotherapy: PTV1 is the entire hemithorax, the thoracotomy channel, and mediastinal nodal stations if affected by disease or violated surgically. PTV2 are areas at high risk for loco‐regional relapse. Three dimensional conformal radio therapy or intensity modulated radiation therapy was permitted with the following schedules: Schedule 1: 25 × 1.8 Gy (45 Gy) to PTV1 followed by 7 × 1.8 Gy (12.6 Gy) to PTV2 (57.6 Gy in total). Schedule 2: 23 × 2 Gy (46 Gy) to PTV1 followed by 5 × 2 Gy (10 Gy) to PTV2 (56 Gy in total). Schedule 3: intensity‐modulated radiotherapy 26 × 1.75 Gy (45.5 Gy) to PTV1 with simultaneously integrated boost 26 × 2.15 Gy (55.9 Gy) to PTV2. No hemithoracic high dose radiotherapy: no radiotherapy, only follow up. Follow‐up included CT scans at 4 months, 8 months, and 12 months after surgery, subsequent follow‐up was done every 6 months or at time of suspicion of relapse. | |
| Outcomes | 1. Survival rate 2. health‐related quality of life 3. Adverse events | |
| Notes | Funding: Swiss Group for Clinical Cancer Research, Swiss State Secretariat for Education, Research and Innovation, | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was done using computer‐generated randomisation sequence balanced according to centre, histology and mediastinal lymph nodes involvement. |
| Allocation concealment (selection bias) | Low risk | Randomisation was done using computer‐generated sequence. |
| Blinding of participants and personnel (performance bias) | High risk | Neither participants nor personnel were masked to treatment allocation (this high risk of bias was considered for endpoints other than overall survival). |
| Blinding of outcome assessment (detection bias) | Unclear risk | The blinding of outcome assessors was unclear. |
| Incomplete outcome data (attrition bias) | Low risk | No missing data in the trial. |
| Selective reporting (reporting bias) | Low risk | No reporting bias was detected. |
| Other bias | Unclear risk | There was unclear risk of other biases. |
| Methods | Randomised clinical trial with two arms: EPP plus postoperative hemithoracic radiotherapy compared with standard (non‐radical) therapy alone following platinum‐based chemotherapy. | |
| Participants | 50 patients with pathologically confirmed mesothelioma underwent induction platinum‐based chemotherapy were randomly assigned: 24 patients to EPP and 26 patients to continued oncological management according to local policy, which could include chemotherapy, palliative radiotherapy, or further surgery. Mean age: 61.5 years Inclusion criteria:
Recruitment: October 2005 to November 2008 | |
| Interventions | All patients had three cycles of platinum‐based chemotherapy with a regimen chosen by the treating physician at the local centre. EPP arm: underwent surgery, followed by postoperative radiotherapy directed at the hemithorax plus continued oncological management and follow‐up and CT scan on first relapse. No‐EPP arm: only continued oncological management and follow‐up and CT scan on first relapse. | |
| Outcomes | 1. Survival rate 2. health‐related quality of life | |
| Notes | Funding: Cancer Research UK (CRUK/04/003), the June Hancock Mesothelioma Research Fund, and Guy’s and We contacted the corresponding author by email (15 April 2017) for some clarifications about adverse events but he could not provide the relevant information. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was done using computer‐generated randomisation sequence. |
| Allocation concealment (selection bias) | Low risk | Randomisation was done using computer‐generated sequence. |
| Blinding of participants and personnel (performance bias) | High risk | Neither participants nor personnel were masked to treatment allocation (this high risk of bias was considered for endpoints other than overall survival). |
| Blinding of outcome assessment (detection bias) | Unclear risk | The blinding of outcome assessors was unclear. |
| Incomplete outcome data (attrition bias) | Low risk | The analysis included summary information from the screening logs on reasons for loss and withdrawal. |
| Selective reporting (reporting bias) | Low risk | No reporting bias was detected. |
| Other bias | Unclear risk | There was unclear risk of other biases. |
EPP: extrapleural pneumonectomy
CT: computerized tomography
Gy: Gray
PTV: planning target volume
TNM: tumour/node/metastasis
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| It didn't match the eligibility criteria as the intervention compared surgery, chemotherapy, immunotherapy and photodynamic therapy with surgery, chemotherapy and immunotherapy. | |
| Non‐randomised study. | |
| Non‐randomised study. | |
| Non‐randomised study. |
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | A study to determine if it is feasible to recruit into a randomised trial comparing (extended) pleurectomy decortication versus no pleurectomy decortication in patients with malignant pleural mesothelioma |
| Methods | Phase III |
| Participants | Participants with histologically confirmed mesothelioma and disease confined to one hemithorax |
| Interventions | Experimental arm: chemotherapy plus (Extended) pleurectomy decortication Standard arm: chemotherapy only |
| Outcomes | Primary Outcome Measures 1. Ability to randomise 50 patients (TimeFrame: 24 months) 2. Ability to randomise 50 patients within the first 24 months or the ability to recruit 25 patients within any 6 month period Secondary Outcome Measures 1. Survival from the time point of randomisation (time frame: follow‐up for up to 5 years) |
| Starting date | May 2015 |
| Contact information | Eric Lim: [email protected] |
| Notes | Sponsor: Royal Brompton & Harefield NHS Foundation Trust |
| Trial name or title | A randomised phase 2 Trial of radical pleurectomy and post‐operative chemotherapy with or without intraoperative porfimer sodium ‐mediated photodynamic therapy for patients with epitheliod malignant pleural mesothelioma |
| Methods | Randomised phase II |
| Participants | Participants with histologically confirmed mesothelioma and disease confined to one hemithorax |
| Interventions | Experimental arm 1: chemotherapy plus radical pleurectomy plus photodynamic therapy Experimental arm 2: chemotherapy plus radical pleurectomy |
| Outcomes | Primary Outcome Measures: Number of adverse events (time frame: 4 years) |
| Starting date | May 2014 |
| Contact information | |
| Notes | Sponsor: Abramson Cancer Center of the University of Pennsylvania |
| Trial name or title | EORTC randomised phase II study of pleurectomy/decortication (P/D) preceded or followed by chemotherapy in patients with early stage malignant pleural mesothelioma. |
| Methods | Randomised phase II |
| Participants | Participants with histologically confirmed mesothelioma |
| Interventions | Experimental: immediate P/D followed by three cycles of pemetrexed 500mg/m2 IV and cisplatin 75 mg/m2 IV, both drugs given on day 1, every three weeks for non‐progressing patients. Active Comparator: delayed P/D three cycles of pemetrexed 500mg/m2 IV and cisplatin 75 mg/m2 IV, both drugs given on day 1, every three weeks followed by P/D, for non‐progressing patients. |
| Outcomes | Primary Outcome Measures 1) Rate of success to complete the full treatment (time frame: 20weeks) Secondary Outcome Measures 1) Loco‐regional failure free survival (time frame: 6 months) 3)Treatment side‐effects (time frame: 36 weeks) |
| Starting date | September 2016 |
| Contact information | |
| Notes | Sponsor: European Organization for Research and Treatment of Cancer (EORTC). |
health‐related quality of life
LC: lung cancer
IV: intravenous
QLQ: health‐related quality of life questionnaire
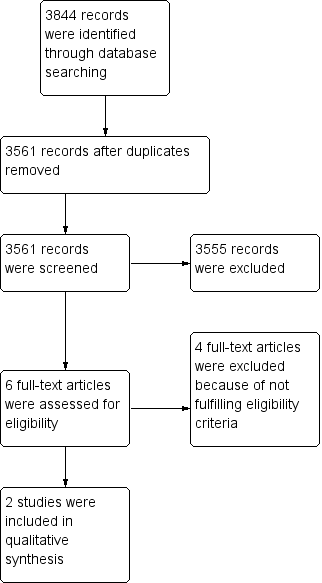
Study flow diagram.
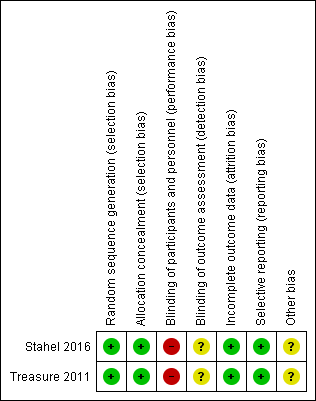
Risk of bias summary: review authors' judgements about each 'Risk of bias' item for each included study (the judgement for performance and detection bias is for endpoints other than overall survival).
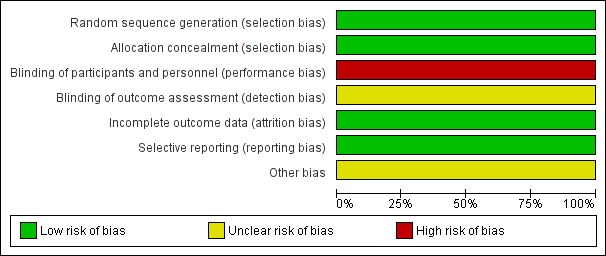
Risk of bias graph: review authors' judgements about each 'Risk of bias' item presented as percentages across all included studies (the judgement for performance and detection bias is for endpoints other than overall survival).
| Combined EPP plus neoadjuvant platinum‐based chemotherapy plus post operative high‐dose hemithoracic radiotherapy compared with combined EPP plus neoadjuvant platinum‐based chemotherapy for malignant pleural mesothelioma | ||||||
| Patient or population: people with malignant pleural mesothelioma Settings: specialist hospital Intervention: combined EPP plus neoadjuvant platinum‐based chemotherapy plus postoperative high‐dose hemithoracic radiotherapy Comparison: combined EPP plus neoadjuvant platinum‐based chemotherapy | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| combined EPP plus chemotherapy | combined EPP plus chemotherapy plus hemithoracic radiotherapy | |||||
| Median overall survival | 20.8 months (95% CI 14.4 to 27.8) | 19.3 months (95% CI 11.5 to 21.8) | ‐ | 54 (1) | ⊕⊕⊕⊝ Moderate1 | |
| Health‐related health‐related quality of life | No changes in the scores for the overall evaluation of life in both groups up to week 14 after randomisation | ‐ | 54 (1) | ⊕⊕⊝⊝ Low2 | ||
| Adverse events | The following adverse events were observed in the radiotherapy arm: anaemia (74%), nausea or vomiting (44%), oesophagitis (29%), fatigue (24%), weight loss (19%), dyspnoea (4%), diarrhoea (4%), and increased alkaline phosphatase concentration (4%). There was no comment on the adverse events in the no radiotherapy arm. | ‐ | 54 (1) | ⊕⊕⊝⊝ Low2 | ||
| Postoperative complications | Postoperative complications included mediastinal shift (11%), major infections (8%), bleeding (6%), bronchial stump fistula(3%), pulmonary embolism, chylothorax, and technical failures (2% each). It was not classified in the trial based on treatment arms | ‐ | 54 (1) | ⊕⊕⊝⊝ Low2 | ||
| Treatment‐related death | None reported. | One patient died of a complicated pneumonia during radiotherapy, which was probably related to treatment. | ‐ | 54 (1) | ⊕⊕⊝⊝ Low2 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Due to imprecision, the quality of the evidence was assessed as moderate. 2Due to imprecision as well as high risk of bias, the quality of the evidence was assessed as low. | ||||||
| Combined platinum‐based chemotherapy plus EPP plus postoperative hemithoracic radiotherapy compared with chemotherapy for malignant pleural mesothelioma | ||||||
| Patient or population: people with malignant pleural mesothelioma Settings: specialist hospital Intervention: combined chemotherapy plus EPP plus postoperative hemithoracic radiotherapy Comparison: chemotherapy | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Chemotherapy | Combined chemotherapy plus EPP plus postoperative hemithoracic radiotherapy | |||||
| Median overall survival | 19.5 months (95% CI 13.4 to time‐not‐yet reached) | 14.4 months (95% CI 5.3 to 18.7) | ‐ | 50 (1) | ⊕⊕⊕⊝ Moderate 1 | |
| Health‐related health‐related quality of life | There were no statistically significant differences between treatment groups | ‐ | 50 (1) | ⊕⊕⊝⊝ Low2 | ||
| Adverse events | 2 serious adverse events | 10 serious adverse events | ‐ | 50 (1) | ⊕⊕⊝⊝ Low2 | |
| Progression‐free survival | 9.0 months (95% CI 7.2 to 14.7) | 7.6 months (95% CI 5.0 to 13.4) | ‐ | 50 (1) | ⊕⊕⊝⊝ Low2 | |
| Treatment‐related death | One perioperative death occurred in the no EPP group (because one of the patients underwent EPP surgery outside the trial). | Three perioperative deaths occurred in patients randomly assigned to EPP. | ‐ | 50 (1) | ⊕⊕⊝⊝ Low2 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Due to imprecision, the quality of the evidence was assessed as moderate. 2Due to imprecision as well as high risk of bias, the quality of the evidence was assessed as low. | ||||||

