Radical multimodality therapy for malignant pleural mesothelioma
Appendices
Appendix 1. Cochrane Central Register of Controlled Trials Register (CENTRAL)
#1 MeSH descriptor: [Mesothelioma] explode all trees
#2 MeSH descriptor: [Pleural Neoplasms] explode all trees
#3 malignant mesothelioma
#4 malignant pleural mesothelioma
#5 pleural neoplasm*
#6 pleural cancer*
#7 #1 or #2 or #3 or #4 or #5 or #6
#8 combin*
#9 multimod*
#10 radical
#11 #8 or #9 or #10
#12 #7 and #11
Appendix 2. MEDLINE (via PubMed) search strategy
#1,"Search mesothelioma[MeSH Terms]"
#2,"Search pleural neoplasms[MeSH Terms]"
#3,"Search ""mesothelioma, malignant""[Supplementary Concept]"
#4,"Search ""malignant pleural mesothelioma""[Other Term]"
#5,"Search malignant mesothelioma[Title/Abstract]"
#6,"Search malignant pleural mesothelioma[Title/Abstract]"
#7,"Search MPM[Title/Abstract]"
#8,"Search pleural neoplas*[Title/Abstract]"
#9,"Search pleural cancer*[Title/Abstract]"
#10,"Search #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9"
#11,"Search ""antineoplastic agents""[MeSH Terms]"
#12,"Search antineoplastic combined chemotherapy protocols[MeSH Terms]"
#13,"Search chemoradiotherapy[MeSH Terms]"
#14,"Search combined modality therapy[MeSH Terms]"
#15,"Search drug therapy[MeSH Terms]"
#16,"Search hyperthermia, induced[MeSH Terms]"
#17,"Search pleura/surgery[MeSH Terms]",1232,06:20:19
#18,"Search pneumonectomy[MeSH Terms]"
#19,"Search radiotherapy[MeSH Terms]"
#20,"Search thoracic surgical procedures[MeSH Terms]"
#21,"Search thoracotomy[MeSH Terms]"
#22,"Search ""pleurectomy""[Other Term]"
#23,"Search ((""decortication""[Other Term] OR ""decortication/pleurectomy""[Other Term] OR ""decortication pleurectomy""[Other Term]))"
#24,"Search radical[Other Term]"
#25,"Search surgery[Other Term]"
#26,"Search adjuvant[Title/Abstract]"
#27,"Search chemoradiotherap*[Title/Abstract]"
#28,"Search chemotherap*[Title/Abstract]"
#29,"Search combination[Title/Abstract]"
#30,"Search cytoreduc*[Title/Abstract]"
#31,"Search decortication[Title/Abstract]"
#32,"Search hypertherm*[Title/Abstract]"
#33,"Search multimod*[Title/Abstract]"
#34,"Search neoadjuvant*[Title/Abstract]"
#35,"Search photochemotherap*[Title/Abstract]"
#36,"Search pleurectom*[Title/Abstract]"
#37,"Search pleuropneumonectom*[Title/Abstract]"
#38,"Search pneumonectom*[Title/Abstract]"
#39,"Search radical[Title/Abstract]"
#40,"Search radiochemotherap*[Title/Abstract]"
#41,"Search radiotherap*[Title/Abstract]"
#42,"Search resection[Title/Abstract]"
#43,"Search surgery[Title/Abstract]"
#44,"Search surgical[Title/Abstract]"
#45,"Search thoracotom*[Title/Abstract]"
#46,"Search trimodal*[Title/Abstract]"
#47,"Search #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33 OR #34 OR #35 OR #36 OR #37 OR #38 OR #39 OR #40 OR #41 OR #42 OR #43 OR #44 OR #45 OR #46"
#48,"Search #10 AND #47"
#49,"Search randomized controlled trial[Publication Type]"
#50,"Search controlled clinical trial[Publication Type]"
#51,"Search randomized[Title/Abstract]"
#52,"Search placebo[Title/Abstract]"
#53,"Search drug therapy[MeSH Subheading]"
#54,"Search randomly[Title/Abstract]"
#55, "Search trial[Title/Abstract]"
#56,"Search groups[Title/Abstract]"
#57,"Search #49 OR #50 OR #51 OR #52 OR #53 OR #54 OR #55 OR #56"
#58,"Search animals[MeSH Terms]"
#59,"Search humans[MeSH Terms]"
#60,"Search #58 NOT #59"
#61,"Search #57 NOT #60"
#62,"Search #48 AND #61"
Appendix 3. Embase (via Ovid) search strategy
#1 'mesothelioma'/exp
#2 'pleura tumor'/exp
#3 'mesothelioma':ab,ti
#4 'malignant mesothelioma':ab,ti
#5 'malignant pleural mesothelioma':ab,ti
#6 'mpm':ab,ti
#7 'pleural neoplas*':ab,ti
#8 'pleural cancer*':ab,ti
#9 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8
#10 'antineoplastic agent'/exp
#11 'chemoradiotherapy'/exp
#12 'multimodality cancer therapy'/exp
#13 'drug therapy'/exp
#14 'hyperthermic therapy'/exp
#15 'pleura'/exp AND 'surgery'/exp
#16 'lung resection'/exp
#17 'radiotherapy'/exp
#18 'thorax surgery'/exp
#19 'thoracotomy'/exp
#20 'adjuvant':ab,ti
#21 'chemoradiotherap*':ab,ti
#22 'chemotherap*':ab,ti
#23 'combination':ab,ti
#24 'cytoreduc*':ab,ti
#25 'decortication':ab,ti
#26 'hypertherm*':ab,ti
#27 'multimod*':ab,ti
#28 'neoadjuvant*':ab,ti
#29 'photochemotherap*':ab,ti
#30 'pleurectom*':ab,ti
#31 'pleuropneumonectom*':ab,ti
#32 'pneumonectom*':ab,ti
#33 'radical':ab,ti
#34 'radiochemotherap*':ab,ti
#35 'radiotherap*':ab,ti
#36 'resection':ab,ti
#37 'surgery':ab,ti
#38 'surgical':ab,ti
#39 'thoracotom*':ab,ti
#40 'trimodal*':ab,ti
#41 #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27OR #28 OR #29 OR #30 OR #31 OR #32 OR #33 OR #34 OR #35 OR #36 OR #37 OR #38 OR #39 OR #40
#42 #9 AND #41
#43 'crossover procedure'/exp OR 'double‐blind procedure'/exp OR 'randomized controlled trial'/exp OR 'single‐blind procedure'/exp OR random* ORfactorial* OR crossover* OR cross NEXT/1 over* OR placebo* OR doubl* NEAR/1 blind* OR singl* NEAR/1 blind* OR assign* OR allocat* ORvolunteer*
#44 #42 AND #43
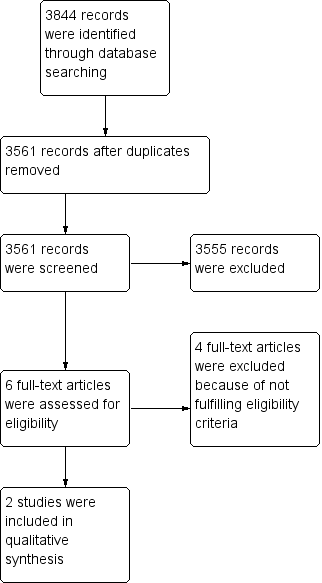
Study flow diagram.
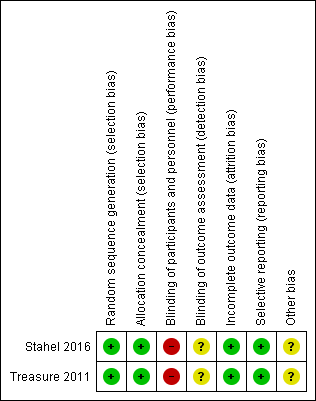
Risk of bias summary: review authors' judgements about each 'Risk of bias' item for each included study (the judgement for performance and detection bias is for endpoints other than overall survival).
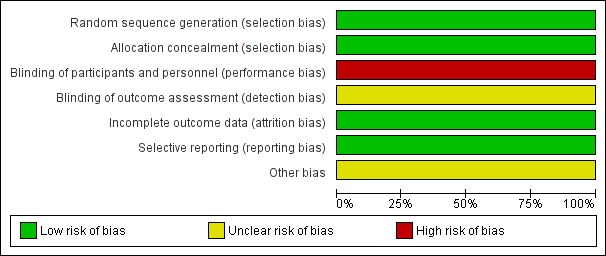
Risk of bias graph: review authors' judgements about each 'Risk of bias' item presented as percentages across all included studies (the judgement for performance and detection bias is for endpoints other than overall survival).
| Combined EPP plus neoadjuvant platinum‐based chemotherapy plus post operative high‐dose hemithoracic radiotherapy compared with combined EPP plus neoadjuvant platinum‐based chemotherapy for malignant pleural mesothelioma | ||||||
| Patient or population: people with malignant pleural mesothelioma Settings: specialist hospital Intervention: combined EPP plus neoadjuvant platinum‐based chemotherapy plus postoperative high‐dose hemithoracic radiotherapy Comparison: combined EPP plus neoadjuvant platinum‐based chemotherapy | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| combined EPP plus chemotherapy | combined EPP plus chemotherapy plus hemithoracic radiotherapy | |||||
| Median overall survival | 20.8 months (95% CI 14.4 to 27.8) | 19.3 months (95% CI 11.5 to 21.8) | ‐ | 54 (1) | ⊕⊕⊕⊝ Moderate1 | |
| Health‐related health‐related quality of life | No changes in the scores for the overall evaluation of life in both groups up to week 14 after randomisation | ‐ | 54 (1) | ⊕⊕⊝⊝ Low2 | ||
| Adverse events | The following adverse events were observed in the radiotherapy arm: anaemia (74%), nausea or vomiting (44%), oesophagitis (29%), fatigue (24%), weight loss (19%), dyspnoea (4%), diarrhoea (4%), and increased alkaline phosphatase concentration (4%). There was no comment on the adverse events in the no radiotherapy arm. | ‐ | 54 (1) | ⊕⊕⊝⊝ Low2 | ||
| Postoperative complications | Postoperative complications included mediastinal shift (11%), major infections (8%), bleeding (6%), bronchial stump fistula(3%), pulmonary embolism, chylothorax, and technical failures (2% each). It was not classified in the trial based on treatment arms | ‐ | 54 (1) | ⊕⊕⊝⊝ Low2 | ||
| Treatment‐related death | None reported. | One patient died of a complicated pneumonia during radiotherapy, which was probably related to treatment. | ‐ | 54 (1) | ⊕⊕⊝⊝ Low2 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Due to imprecision, the quality of the evidence was assessed as moderate. 2Due to imprecision as well as high risk of bias, the quality of the evidence was assessed as low. | ||||||
| Combined platinum‐based chemotherapy plus EPP plus postoperative hemithoracic radiotherapy compared with chemotherapy for malignant pleural mesothelioma | ||||||
| Patient or population: people with malignant pleural mesothelioma Settings: specialist hospital Intervention: combined chemotherapy plus EPP plus postoperative hemithoracic radiotherapy Comparison: chemotherapy | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Chemotherapy | Combined chemotherapy plus EPP plus postoperative hemithoracic radiotherapy | |||||
| Median overall survival | 19.5 months (95% CI 13.4 to time‐not‐yet reached) | 14.4 months (95% CI 5.3 to 18.7) | ‐ | 50 (1) | ⊕⊕⊕⊝ Moderate 1 | |
| Health‐related health‐related quality of life | There were no statistically significant differences between treatment groups | ‐ | 50 (1) | ⊕⊕⊝⊝ Low2 | ||
| Adverse events | 2 serious adverse events | 10 serious adverse events | ‐ | 50 (1) | ⊕⊕⊝⊝ Low2 | |
| Progression‐free survival | 9.0 months (95% CI 7.2 to 14.7) | 7.6 months (95% CI 5.0 to 13.4) | ‐ | 50 (1) | ⊕⊕⊝⊝ Low2 | |
| Treatment‐related death | One perioperative death occurred in the no EPP group (because one of the patients underwent EPP surgery outside the trial). | Three perioperative deaths occurred in patients randomly assigned to EPP. | ‐ | 50 (1) | ⊕⊕⊝⊝ Low2 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Due to imprecision, the quality of the evidence was assessed as moderate. 2Due to imprecision as well as high risk of bias, the quality of the evidence was assessed as low. | ||||||

