Radical multimodality therapy for malignant pleural mesothelioma
Información
- DOI:
- https://doi.org/10.1002/14651858.CD012605.pub2Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 08 enero 2018see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Cáncer de pulmón
- Copyright:
-
- Copyright © 2018 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
Guarantor of the review: Omar Abdel‐Rahman
Conceiving and designing the review: Omar Abdel‐Rahman, Zeinab Elsayed, Hadeer Mohamed and Mostafa Eltobgy
Providing a methodological perspective: Omar Abdel‐Rahman
Providing a clinical perspective: Omar Abdel‐Rahman and Zeinab Elsayed
Writing the review: Omar Abdel‐Rahman, Zeinab Elsayed, Hadeer Mohamed and Mostafa Eltobgy
Sources of support
Internal sources
-
None, Other.
External sources
-
None, Other.
Declarations of interest
Omar Abdel‐Rahman: None known
Zeinab Elsayed: None known
Hadeer Mohamed: None known
Mostafa Eltobgy: None known
Acknowledgements
We would like to thank the Cochrane Lung Cancer group and its supporting editorial team.
Editors: Fergus Macbeth, Renée Manser, Arnaud Scherpereel, Alain Bernard and Virginie Westeel
Managing editor: Corynne Marchal
Information specialists: François Calais, Giorgio Maria Agazzi
Version history
| Published | Title | Stage | Authors | Version |
| 2018 Jan 08 | Radical multimodality therapy for malignant pleural mesothelioma | Review | Omar Abdel‐Rahman, Zeinab Elsayed, Hadeer Mohamed, Mostafa Eltobgy | |
| 2017 Mar 20 | Radical multimodality therapy for malignant pleural mesothelioma | Protocol | Omar Abdel‐Rahman, Zeinab Elsayed, Hadeer Mohamed, Mostafa Eltobgy | |
Differences between protocol and review
We added the following statement to the measure of effect section: "we also used hazard ratios as measure of effect for time‐to‐event outcomes (overall survival)" in order to clarify this point.
Some sections of the background were rewritten to improve clarity of the meaning.
We added the following outcomes to the summary of findings table "postoperative complications and treatment‐related death" in order to clarify the findings of these outcomes.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
- Antineoplastic Agents [*therapeutic use];
- Combined Modality Therapy [*methods];
- Lung Neoplasms [mortality, *therapy];
- Mesothelioma [mortality, *therapy];
- Mesothelioma, Malignant;
- Platinum Compounds [therapeutic use];
- Pneumonectomy [*methods];
- Radiotherapy Dosage;
- Randomized Controlled Trials as Topic;
Medical Subject Headings Check Words
Humans;
PICO
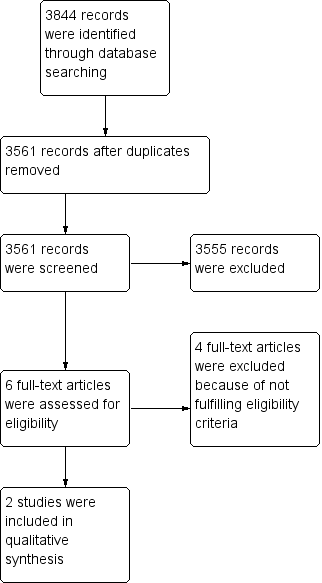
Study flow diagram.
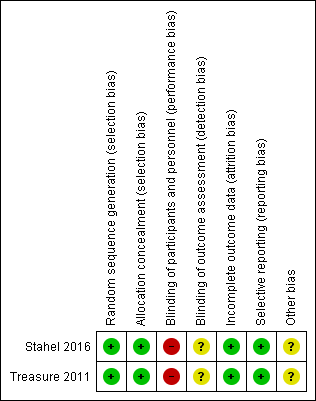
Risk of bias summary: review authors' judgements about each 'Risk of bias' item for each included study (the judgement for performance and detection bias is for endpoints other than overall survival).
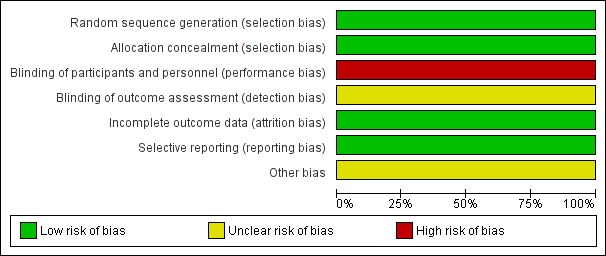
Risk of bias graph: review authors' judgements about each 'Risk of bias' item presented as percentages across all included studies (the judgement for performance and detection bias is for endpoints other than overall survival).
| Combined EPP plus neoadjuvant platinum‐based chemotherapy plus post operative high‐dose hemithoracic radiotherapy compared with combined EPP plus neoadjuvant platinum‐based chemotherapy for malignant pleural mesothelioma | ||||||
| Patient or population: people with malignant pleural mesothelioma Settings: specialist hospital Intervention: combined EPP plus neoadjuvant platinum‐based chemotherapy plus postoperative high‐dose hemithoracic radiotherapy Comparison: combined EPP plus neoadjuvant platinum‐based chemotherapy | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| combined EPP plus chemotherapy | combined EPP plus chemotherapy plus hemithoracic radiotherapy | |||||
| Median overall survival | 20.8 months (95% CI 14.4 to 27.8) | 19.3 months (95% CI 11.5 to 21.8) | ‐ | 54 (1) | ⊕⊕⊕⊝ Moderate1 | |
| Health‐related health‐related quality of life | No changes in the scores for the overall evaluation of life in both groups up to week 14 after randomisation | ‐ | 54 (1) | ⊕⊕⊝⊝ Low2 | ||
| Adverse events | The following adverse events were observed in the radiotherapy arm: anaemia (74%), nausea or vomiting (44%), oesophagitis (29%), fatigue (24%), weight loss (19%), dyspnoea (4%), diarrhoea (4%), and increased alkaline phosphatase concentration (4%). There was no comment on the adverse events in the no radiotherapy arm. | ‐ | 54 (1) | ⊕⊕⊝⊝ Low2 | ||
| Postoperative complications | Postoperative complications included mediastinal shift (11%), major infections (8%), bleeding (6%), bronchial stump fistula(3%), pulmonary embolism, chylothorax, and technical failures (2% each). It was not classified in the trial based on treatment arms | ‐ | 54 (1) | ⊕⊕⊝⊝ Low2 | ||
| Treatment‐related death | None reported. | One patient died of a complicated pneumonia during radiotherapy, which was probably related to treatment. | ‐ | 54 (1) | ⊕⊕⊝⊝ Low2 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Due to imprecision, the quality of the evidence was assessed as moderate. 2Due to imprecision as well as high risk of bias, the quality of the evidence was assessed as low. | ||||||
| Combined platinum‐based chemotherapy plus EPP plus postoperative hemithoracic radiotherapy compared with chemotherapy for malignant pleural mesothelioma | ||||||
| Patient or population: people with malignant pleural mesothelioma Settings: specialist hospital Intervention: combined chemotherapy plus EPP plus postoperative hemithoracic radiotherapy Comparison: chemotherapy | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Chemotherapy | Combined chemotherapy plus EPP plus postoperative hemithoracic radiotherapy | |||||
| Median overall survival | 19.5 months (95% CI 13.4 to time‐not‐yet reached) | 14.4 months (95% CI 5.3 to 18.7) | ‐ | 50 (1) | ⊕⊕⊕⊝ Moderate 1 | |
| Health‐related health‐related quality of life | There were no statistically significant differences between treatment groups | ‐ | 50 (1) | ⊕⊕⊝⊝ Low2 | ||
| Adverse events | 2 serious adverse events | 10 serious adverse events | ‐ | 50 (1) | ⊕⊕⊝⊝ Low2 | |
| Progression‐free survival | 9.0 months (95% CI 7.2 to 14.7) | 7.6 months (95% CI 5.0 to 13.4) | ‐ | 50 (1) | ⊕⊕⊝⊝ Low2 | |
| Treatment‐related death | One perioperative death occurred in the no EPP group (because one of the patients underwent EPP surgery outside the trial). | Three perioperative deaths occurred in patients randomly assigned to EPP. | ‐ | 50 (1) | ⊕⊕⊝⊝ Low2 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Due to imprecision, the quality of the evidence was assessed as moderate. 2Due to imprecision as well as high risk of bias, the quality of the evidence was assessed as low. | ||||||

