Altering the availability or proximity of food, alcohol and tobacco products to change their selection and consumption
Información
- DOI:
- https://doi.org/10.1002/14651858.CD012573Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 01 marzo 2017see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Protocol
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Salud pública
- Copyright:
-
- Copyright © 2017 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
Draft the protocol ‐ all authors
Develop a search strategy ‐ GJH, IS
Search for trials ‐ GJH, PC, IS
Obtain copies of trials ‐ GJH, PC, IS
Select which studies to include ‐ GJH, PC, IS, DO
Extract data from studies ‐ GJH, PC
Enter data into RevMan ‐ GJH, PC
Carry out the analysis ‐ JPTH, GJH, PC
Interpret the analysis ‐ all authors
Draft the final review ‐ all authors
Sources of support
Internal sources
-
Kings College London, UK.
Database access
-
University of Cambridge, UK.
Computer provision, database access
-
University of Bristol, UK.
Computer provision
-
University College London, UK.
Computer provision
External sources
-
Department of Health Policy Research Programme in England ([PR‐UN‐0409‐10109] Policy Research Unit in Behaviour and Health), UK.
Funding for the protocol
Declarations of interest
Gareth Hollands declares no financial or other conflicts of interest.
Patrice Carter declares no financial or other conflicts of interest.
Ian Shemilt declares no financial or other conflicts of interest.
Theresa Marteau declares no financial or other conflicts of interest.
Susan Jebb has received funding from Tanita Ltd (ended Jan 2015), a manufacturer of medical equipment. This funding does not present a conflict with the review topic.
Julian Higgins declares no financial or other conflicts of interest.
David Ogilvie declares no financial or other conflicts of interest.
Acknowledgements
Production of this protocol was funded by the Department of Health Policy Research Programme in England ([PR‐UN‐0409‐10109] Policy Research Unit in Behaviour and Health). The views expressed are those of the authors and not necessarily those of the Department of Health in England. We would like to acknowledge the contributions of Julie Glanville (York Health Economics Consortium, University of York, UK) who reviewed a draft of our MEDLINE search strategy, Claire Stansfield (EPPI‐Centre, UCL Institute of Education, UK) who helped to develop our search strategy for the TRoPHI database, and Patrick Condron (Information Specialist, Cochrane Public Health Group) who reviewed further iterations of our MEDLINE and Embase strategies. We would like to thank Jessie Porter, Rebecca Armstrong, Patrick Condron, Jodie Doyle (Managing Editor), and their colleagues in the Cochrane Public Health Group as well as Maureen Dobbins, Anke Rohwer, Sree Nair, Sadequa Shahrook and other external referees who provided helpful comments on this protocol. We would like to thank colleagues at the EPPI‐Centre, UCL Institute of Education, UK, in particular James Thomas and Sergio Graziosi, for their help in developing technological solutions and managing those processes in order to implement a workable study identification process.
Version history
| Published | Title | Stage | Authors | Version |
| 2019 Sep 04 | Altering the availability or proximity of food, alcohol, and tobacco products to change their selection and consumption | Review | Gareth J Hollands, Patrice Carter, Sumayya Anwer, Sarah E King, Susan A Jebb, David Ogilvie, Ian Shemilt, Julian P T Higgins, Theresa M Marteau | |
| 2019 Aug 27 | Altering the availability or proximity of food, alcohol, and tobacco products to change their selection and consumption | Review | Gareth J Hollands, Patrice Carter, Sumayya Anwer, Sarah E King, Susan A Jebb, David Ogilvie, Ian Shemilt, Julian P T Higgins, Theresa M Marteau | |
| 2017 Mar 01 | Altering the availability or proximity of food, alcohol and tobacco products to change their selection and consumption | Protocol | Gareth J Hollands, Patrice Carter, Ian Shemilt, Theresa M Marteau, Susan A Jebb, Julian Higgins, David Ogilvie | |
PICO
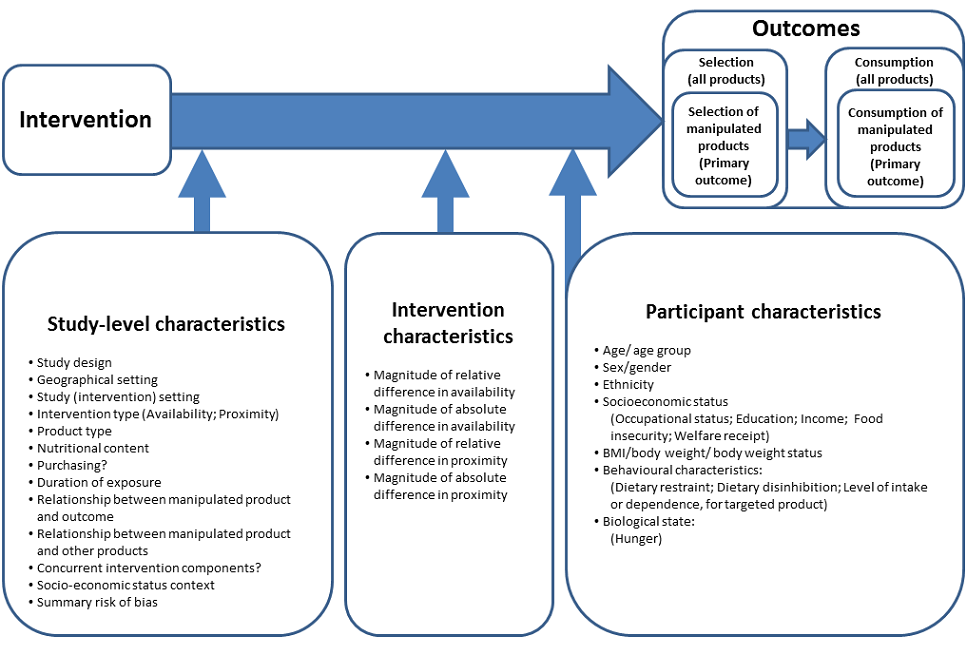
Design‐oriented conceptual model

