Adjunctive systemic antimicrobials for the non‐surgical treatment of chronic and aggressive periodontitis
Abstract
This is a protocol for a Cochrane Review (Intervention). The objectives are as follows:
The focused objective of this review will be to assess the effects of systemically administered antibiotics as an adjunct to scaling and root planing (SRP) in chronic and aggressive periodontitis in comparison to SRP alone or with placebo.
Background
Description of the condition
Periodontal diseases are a group of related diseases affecting the gums. It comprises of both gingivitis and periodontitis (pyorrhoea). Gingivitis refers to an inflammatory condition of the soft tissues surrounding the teeth, and its presence, with other cofactors, may lead to periodontitis (Figure 1). According to AAP 2000, periodontitis is defined as "an inflammatory disease of the supporting tissues of the teeth caused by specific microorganisms, resulting in progressive destruction of the periodontal ligament and alveolar bone with pocket formation, recession, or both".
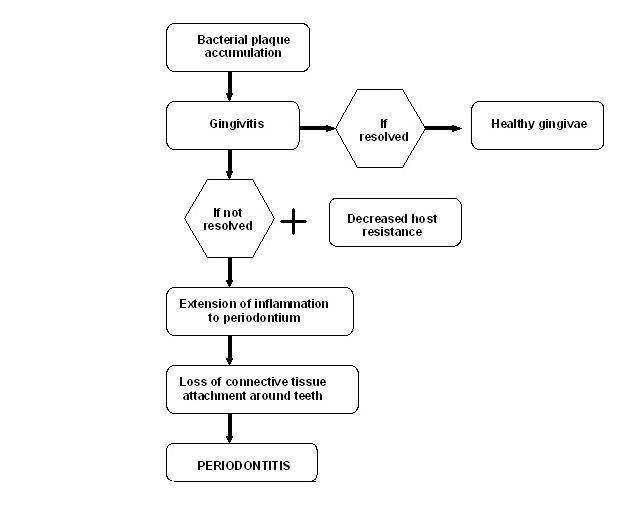
Etiopathogenesis of periodontal disease.
The clinical symptoms of periodontitis include bad breath and painful chewing. The clinical signs include red or swollen gums, bleeding gums, teeth appearing longer or receding gums, attachment loss, deepening of periodontal pockets, furcation involvement of multirooted teeth, alveolar bone loss leading to mobility of teeth and ultimately tooth loss (Page 1998). Thus, periodontitis affects quality of life, not only in terms of reduced functional capacity (i.e. the ability to eat, speak and perform daily activities) but also social and interpersonal relationships (Araújo 2010). The impact on quality of life worsens with an increase in the severity of periodontitis (Chraif 2014; Ferreira 2009; Sundaram 2013).
Periodontitis is multifactorial in origin with pathogenic bacterial flora and genetic predisposition being the predominant aetiological factors. More than 500 microbial species have been identified within periodontal pockets (How 2016; Moore 1994). Most putative periodontal pathogens are Gram‐negative and anaerobic, however Gram‐positive, facultative species of cocci and rods are also found (Moore 1994). Among these species most important ones are Porphyromonas gingivalis,Aggregatibacter actinomycetemcomitans,Treponema denticola,Tannerella forsythia,Fusobacterium and Prevotella intermedia (Feng 2006; Riep 2009). There are also studies that suggest the role of certain viruses like Cytomegalovirus, Epstein‐Barr virus and herpes simplex virus in the aetiology of periodontitis (Contreras 1996; Slots 2007).
Periodontitis is a common chronic oral health problem, contributing to a global health burden on low‐income, middle‐income and high‐income countries (Peterson 2012). The US Centers for Disease Control and Prevention (CDC) data estimate that 47.2% of adults aged 30 years have some form of periodontitis and prevalence increases to about 70.1% at 65 years of age (Eke 2012; Eke 2015).
The prevalence and severity of periodontitis disease vary with the following factors.
-
Patient demographics like age, sex, ethnic background, and socioeconomic status (Beck 1990; Genco 1996; Slade 1995).
-
Systemic conditions: immunocompromised (e.g. HIV‐positive), diabetes mellitus, malnutrition, etc. (Seppälä 1993).
-
Behavioural factors like smoking and oral hygiene practices (Bergström 1990; Vouros 2009).
According to a report by the American Academy of Periodontology (AAP) (Armitage 1999; Geurs 2015), the various forms of periodontitis can be classified on the basis of.
-
Cause.
-
-
Chronic ‐ the most prevalent form in adults (Armitage 1999; Oliver 1991), characterized by a strong association with local factors ‐ plaque and calculus.
-
Aggressive ‐ characterized by (1) rapid rate of periodontal disease progression seen in otherwise healthy individuals, (2) an absence of large accumulation of plaque and calculus, and (3) a family history of aggressive periodontitis suggestive of a genetic trait (Novak 1996; Tonetti 1999).
-
-
Severity ‐ amount of attachment loss (AL) (Eke 2012) (Figure 2).
Figure 2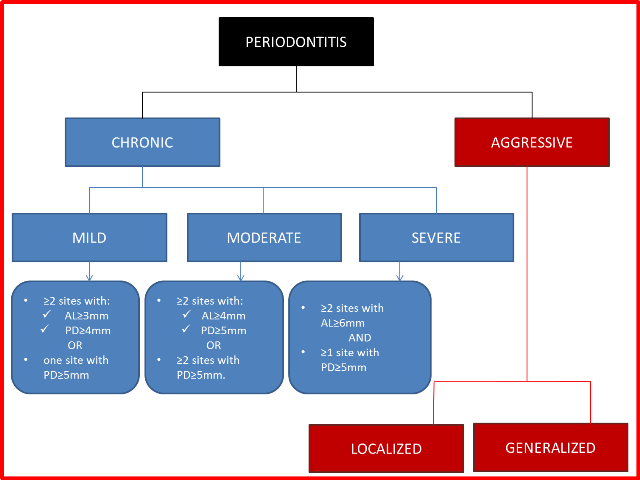
Classification of periodontitis.
AL = attachment loss; PD = pocket depth.
-
-
Mild: ≥ 2 sites with ≥ 3 mm AL or probing pocket depth (PPD) ≥ 4 mm or both, or 1 site with PPD ≥ 5 mm.
-
Moderate: ≥ 2 sites with ≥ 4 mm AL or PPD ≥ 5 mm or both, or ≥ 2 sites with PPD ≥ 5 mm.
-
Severe: AL ≥ 6 mm and ≥ 1 site with PPD ≥ 5 mm.
-
-
Site ‐ both chronic and aggressive forms can be localized or generalized (Geurs 2015).
-
-
Chronic.
-
-
-
-
Localized ‐ affects ≤ 30% of sites.
-
Generalized ‐ affects more than 30% of sites.
-
-
-
-
Aggressive.
-
-
-
-
Localized ‐ first molar/incisor presentation with interproximal attachment loss on at least two permanent teeth, one of which is a first molar, and involving no more than two teeth other than first molars and incisors.
-
Generalized ‐ affects at least three permanent teeth other than first molars and incisors.
-
-
Other types of periodontitis include refractory periodontitis and recurrent periodontitis. Refractory periodontitis is characterized by low plaque scores and low responsiveness to periodontal therapy (Kornman 1982; Magnusson 1996). Recurrent periodontitis is defined as attachment loss that occurs in an individual who was documented to have been successfully treated.
Periodontitis can also occur as a manifestation of systemic diseases (Kinane 2001). On the other hand, it can aggravate other systemic disorders like atherosclerosis, aspiration pneumonia and preterm births (Gendron 2000; Lockhart 2012; Page 1998a; Paquette 2002).
Only chronic and aggressive forms of periodontitis will be considered for this review owing to different pathophysiology and optimum justification for antibiotic prescription in these other mentioned types of periodontitis.
Description of the intervention
Mechanical debridement is the mainstay of non‐surgical periodontal therapy which aims to eliminate local irritating factors. However, it is not possible to eliminate all pathogenic bacteria through instrumentation within the subgingival region (Deas 2016). The remaining subgingival micro‐organisms should therefore be eliminated by using antimicrobials (Mombelli 2012).
Commonly used drugs as per the literature are mentioned below.
‐ For single‐drug therapy.
-
Penicillins.
-
-
Amoxicillin (AMOX) (Rooney 2002).
-
Amoxicillin + clavulanic acid (AMOX + CLAV) (Guzeldemir 2015; Purucker 2001).
-
-
Tetracyclines.
-
-
Doxycycline (DOX) (Akincibay 2008).
-
Minocycline (MIN) (Atilla 1996; Preus 1995).
-
Tetracycline (TTC) (Al‐Joburi 1989; Helldén 1979).
-
-
Macrolides.
-
-
Azithromycin (AZM) (Oteo 2010; Smith 2002).
-
Clindamycin (CLN) (Gordon 1985; Gordon 1990).
-
-
Quinolones.
-
-
Moxifloxacin (Guzeldemir 2015).
-
-
Nitroimidazole.
-
-
Metronidazole (MTZ) (Noyan 1997; Yilmaz 1996).
-
‐ For multiple‐drug therapy.
-
AMOX + MTZ (Akincibay 2008; Guerrero 2005; Rooney 2002).
-
DOX + MTZ (Aitken 1992).
-
DOX + AMOX + CLAV (Matisko 1993).
Despite the suggested benefits of systemic antibiotics, they expose an individual to potential risks like the development of microbial resistance, the emergence of opportunistic fungal infections, allergic reactions and other drug‐dependent systemic side effects (Jorgensen 2000). Hence, judicious prescription of antibiotics is warranted for long‐term health interests.
Subgingival plaque is polymicrobial in nature, hence the administration of an specific antimicrobial based on antibiotic susceptibility is a challenge to the clinicians. Moreover, different policies on antibiotic prescription at various levels compound to the problem.
Antimicrobial resistance (AMR) among anaerobes has consistently increased in the past three decades and the susceptibility of bacteria to antimicrobial agents has become less predictable (Gamboa 2014; Japoni 2011).
How the intervention might work
Systemically administered antibiotics reach the periodontal tissues via the serum, and target micro‐organisms that are inaccessible to scaling instruments (Slots 2002). These antibiotics also help to eradicate infections by suppressing periodontal pathogens that invade subepithelial periodontal tissues and other extra‐dental sites like deep crevices of the tongue. Thus, systemic antibiotic therapy is considered advantageous for eradication and prevention of pathogenic bacteria (Müller 1998).
Antimicrobials administered along with mechanical debridement may have beneficial effect in clinical periodontal parameters. They may aid in reducing the gingival inflammation (López 2000), probing pocket depth (Elter 1997), attachment loss (Elter 1997), and need for periodontal surgery. They may also affect other periodontal parameters.
A range of antibiotics have been prescribed in the literature with differing dosing regimens, alone or in combination. The use of antibiotics has shown statistically significant greater gain in attachment and reduction in depth of periodontal pockets (Feres 2012; Silva 2011). No single antibiotic at concentration achieved in body fluids inhibits all putative periodontopathogens. Slots 2004, thus, suggested the use of multiple‐drug therapy may be far more effective than single‐drug therapy owing to an expanded spectrum of activity and synergistic effects of these drugs on a variety of pathogens. For example, amoxicillin and metronidazole have a synergistic effect on complex periodontopathogens, hence their administration added advantage over single‐drug therapy (Guerrero 2005).
Why it is important to do this review
Beyond the development of short‐term side effects, the indiscriminate use of antibiotics may lead to the development of multidrug resistant bacterial species which may have negative and costly future implications. Development of opportunistic fungal infections is another concern.
The CDC estimates that about one half of antibiotic prescriptions by office‐based physicians are unnecessary (Colgan 2001). Thus, this systematic review will synthesize evidence on whether the use of systemic antibiotics in periodontitis is beneficial.
There is limited and contradicting evidence to support the routine prescription of antibiotics for chronic and aggressive periodontitis.
Azithromycin when compared to placebo exhibited no additional benefit along with non‐surgical periodontal therapy in periodontitis patients (Han 2012; Sampaio 2011) which contradicts the study by Oteo 2010. Similarly, choice of single (Feres 2012) versus a combination of antibiotics (Silva 2011) is debatable for the management of periodontitis.
In a study by Feres 2012, both metronidazole and a combination of it with amoxicillin were effective for patients with chronic periodontitis whereas in a study by Silva 2011, the combination of metronidazole and amoxicillin proved to be more effective than metronidazole alone.
In systematic reviews by Keestra 2015 and Keestra 2015a, it was reported that no specific type of antimicrobial showed statistically significant superiority over the other when used in patients with untreated chronic periodontitis and that an amoxicillin plus metronidazole combination was potent for aggressive periodontitis based on clinical attachment level and probing pocket depth reduction as primary outcomes.
This systematic review will consider the number of closed pockets as the primary outcome which may serve as a better clinical endpoint.
As the literature regarding the use of antimicrobials in periodontal disease is confusing, it becomes imperative to conduct this systematic review.
Objectives
The focused objective of this review will be to assess the effects of systemically administered antibiotics as an adjunct to scaling and root planing (SRP) in chronic and aggressive periodontitis in comparison to SRP alone or with placebo.
Methods
Criteria for considering studies for this review
Types of studies
We will include randomised controlled trials (RCTs) evaluating any antimicrobial used systemically as an adjunct to non‐surgical therapy for the treatment of chronic or aggressive periodontitis. We will exclude quasi‐RCTs, cross‐over and split‐mouth trials.
Types of participants
We will include all individuals with clinically diagnosed untreated periodontitis (chronic/aggressive). Periodontitis and its types are shown in Figure 2.
People with the following conditions will be excluded:
-
necrotizing periodontitis,
-
recurrent periodontitis,
-
refractory periodontitis,
-
periodontitis as a manifestation of systemic disease,
-
pregnancy,
-
systemic disease/condition (e.g. HIV, diabetes).
Types of interventions
Interventions considering the systemic use of antimicrobials as an adjunct to scaling and root planing (SRP) compared with SRP alone or with placebo.
Interventions comparing different antimicrobials administered systemically (different drugs/different dosage regimens/different combinations/at different time periods) as an adjunct to SRP will be included.
Exclusion criteria:
-
studies comparing the use of antimicrobials alone versus SRP with antimicrobial;
-
studies using the application of any local antimicrobial/laser;
-
studies using antibiotics at a subantimicrobial dose (e.g. subdose doxycycline).
Types of outcome measures
All of the following participants' outcomes at different follow‐up periods will be recorded.
Primary outcomes
-
Number of closed pockets (i.e. pocket depth < 4 mm).
Secondary outcomes
-
Clinical/relative attachment level (CAL/RAL).
-
Probing pocket depth (PPD).
-
Bleeding on probing (BOP).
-
Antimicrobial resistance.
-
Adverse effects of antibiotics.
-
Patient‐reported quality of life changes (in terms of function).
Search methods for identification of studies
Cochrane Oral Health's Information Specialist will conduct systematic searches for randomised controlled trials and controlled clinical trials. Due to the Cochrane Embase Project to identify all clinical trials on the database and add them to CENTRAL, only recent months of the Embase database will be searched. Please see the searching page on the Cochrane Oral Health website for more information. No other restrictions will be placed on the language or date of publication when searching the electronic databases.
Electronic searches
We will search the following databases for relevant trials:
-
Cochrane Oral Health's Trials Register;
-
the Cochrane Central Register of Controlled Trials (CENTRAL) in the Cochrane Library;
-
MEDLINE Ovid (from 1946 onwards);
-
Embase Ovid (previous 6 months to date).
The subject strategies for databases will be modelled on the search strategy designed for MEDLINE Ovid in Appendix 1. Where appropriate, this will be combined with subject strategy adaptations of the highly sensitive search strategy designed by Cochrane for identifying randomised controlled trials and controlled clinical trials (as described in the Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0, Box 6.4.c. (Lefebvre 2011)).
Searching other resources
We will search the following trials registries:
-
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (http://clinicaltrials.gov/);
-
World Health Organization International Clinical Trials Registry Platform (apps.who.int/trialsearch).
We will check the bibliographies of included studies and any relevant systematic reviews identified for further references to relevant trials. We will not perform a separate search for adverse effects of interventions. We will consider adverse effects described in included studies only.
Data collection and analysis
Selection of studies
Two pairs of review authors (Shivi Khattri (SK) and Eachempati Prashanti (EP), and Kumbargere N Sumanth (KNS) and Ankita Arora (AA)) will independently screen the titles and abstracts from the electronic searches to identify potentially eligible studies that require further evaluation to determine whether they meet the inclusion criteria for this review.
We will obtain full‐text copies of all eligible and potentially eligible studies and these will be further evaluated by SK and AA to identify those studies that actually meet all the inclusion criteria. From this group, we will record those studies that do not meet the inclusion criteria in the 'Characteristics of excluded studies' table, noting the reason for exclusion. Non‐RCTs will be excluded without giving any reasons for exclusion. We will resolve disagreements by discussion. When resolution is not possible, we will consult an arbiter (KNS). We will assess articles in languages other than English by their abstracts, where possible, and if they appear to be potentially eligible, we will be requesting translators for data extraction.
Data extraction and management
Two review authors (SK and Chandan Kumar Kusum (CKK)) will independently extract the data. The review authors will not be blinded to the authors of the included studies. Disagreement will be resolved by discussion between the two review authors or, if necessary, a third review author (Giovanni Lodi (GL)) will be consulted in order to reach consensus. We will extract data using a customised data extraction form, which will first be pilot tested using a sample of the included studies. All the items in the data extraction form will be designed following guidance from the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We will enter study details into the 'Characteristics of included studies' table in Review Manager (RevMan) software (RevMan 2014).
The following details will be recorded for each included trial.
-
Publication details such as year of publication, language.
-
Demographic details of the report.
-
Inclusion and exclusion criteria.
-
Type of trial, sample size, method of randomisation, allocation concealment, blinding, method of assessing the outcomes and dropouts, if any.
-
Type of intervention.
-
Level of expertise (specialist/general practitioner or dental hygienist or student) and intensity (in minutes or visits) for scaling and root planing.
-
Details of the outcomes reported.
-
Oral hygiene instruction provided/not provided.
-
Duration of follow‐up.
-
Results of the intervention.
-
Funding details.
Assessment of risk of bias in included studies
Two review authors (KNS and SK) will independently assess the risk of bias in the included trials in seven domains:
-
random sequence generation (selection bias);
-
allocation concealment (selection bias);
-
blinding of participants and personnel (performance bias);
-
blinding (outcome assessment) (detection bias);
-
incomplete outcome data (attrition bias);
-
selective outcome reporting (reporting bias);
-
other biases.
For each of these components, we will assign a judgement regarding the risk of bias as either high, low or unclear based on guidance in Higgins 2011. We will contact the trial authors if details are missing in the publications or are unclear. We will resolve disagreements through consensus. We will record our judgements and justifications in 'Risk of bias' tables for each included study and generate a 'Risk of bias' summary graph and figure. We will use these judgements while grading the overall quality of evidence for each comparison and outcome in the 'Summary of findings' tables.
We will summarise the risk of bias according to Higgins 2011 as follows.
| Risk of bias | Interpretation | In outcome | In included studies |
| Low risk of bias | Plausible bias unlikely to seriously alter the results | Low risk of bias for all key domains | Most information is from studies at low risk of bias |
| Unclear risk of bias | Plausible bias that raises some doubt about the results | Unclear risk of bias for one or more key domains | Most information is from studies at low or unclear risk of bias |
| High risk of bias | Plausible bias that seriously weakens confidence in the results | High risk of bias for one or more key domains | The proportion of information from studies at high risk of bias is sufficient to affect the interpretation of results |
Measures of treatment effect
We expect data for our primary outcome to be count data (number of closed pockets). Under secondary outcomes, CAL/RAL, PPD and patient quality of life may be expressed in the form of continuous data. Antimicrobial resistance, BOP, adverse events and patient quality of life may be expressed as dichotomous data. Patient quality of life and BOP might be measured in the form of an ordinal scale.
We will use means and standard deviations (SDs) presented in the studies to calculate mean differences (MD) and 95% confidence interval (CI) to summarize the continuous data. We will use standardized mean difference (SMD) if studies use different scales to measure the same outcome. If data expressed are in ordinal scales, we will explore the possibility of converting them to dichotomous outcomes. If outcomes are reported both at baseline and at follow‐up or at trial endpoints, we will extract both the mean change from baseline and the standard deviation of this mean for each treatment group, as well as the same for endpoint data. The end scores will be preferred a priori. However, change scores for CAL and PPD will be preferred to end scores. Count data will be treated in the same way as continuous outcome data. The intervention effect used will be the mean difference which compares the difference in the mean number of events (standardized to a unit time period) experienced by participants in the intervention group compared to control group.
If the trials provide details on the oral hygiene instructions (OHI) along with SRP, we will use the data as SRP with OHI and SRP without OHI.
Unit of analysis issues
We will consider the participant as the statistical analysis unit considering the mean value of improvement in the CAL and PPD per person in studies with a parallel design. We are not expecting to find any cluster‐randomised trials or split‐mouth designs in our review. If we find any trial with multiple treatment groups, we will combine groups to create a single pair‐wise comparison as recommended by the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). In case of dropouts, we will use what the paper reports and deal with it in the risk of bias assessment. If the search finds trials with repeated observations on participants, we will follow the methods described in Section 9.3.4 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). In case of multiple treatment attempts per participant, we will use the number of participants randomised to calculate the CIs (Higgins 2011). In trials where adverse effects are described as counts, we will follow the methods described in Section 9.2.5 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Dealing with missing data
We plan to contact study authors to obtain missing data. We will use the methods in Section 16.1.3 of the Cochrane Handbook for Systematic Reviews of Interventions to estimate missing SDs (Higgins 2011). Missing SDs will be calculated from the reported values of t, P and Chi2. If it is not possible to calculate the SDs, the outcomes will be described qualitatively.
Assessment of heterogeneity
Where meta‐analyses are performed, we will assess heterogeneity using a Chi2 test, where a P value < 0.1 will indicate statistically significant heterogeneity. We will quantify heterogeneity using the I2 statistic as follows:
-
0% to 40% implies slight heterogeneity;
-
30% to 60% moderate heterogeneity;
-
50% to 90% substantial heterogeneity;
-
75% to 100% considerable heterogeneity.
If there is considerable heterogeneity (I2 > 75%) which cannot be explained by the subgroup analyses, meta‐analysis will not be conducted.
Assessment of reporting biases
If there are more than 10 studies included in a meta‐analysis, we will assess the possible presence of reporting bias by testing for asymmetry in a funnel plot.
If present, we will carry out statistical analysis using the methods described in Section 10.4.3.1 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) for continuous and dichotomous data.
Data synthesis
We will analyze the data using RevMan software (RevMan 2014). If the data available from the studies have similar comparisons and outcomes, we will undertake meta‐analysis. Our general approach will be to use a random‐effects model. With this approach, the CIs for the average intervention effect will be wider than those obtained using a fixed‐effect approach, leading to a more conservative interpretation. We will use all change scores or end scores when available, and combine change and end scores where necessary using the criteria from Section 9.4.5.2 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We will report the results from studies that are not suitable for inclusion in a meta‐analysis using additional tables.
Subgroup analysis and investigation of heterogeneity
If there is significant heterogeneity, we will explore the reasons by performing subgroup analyses based on participant characteristics.
-
Age group.
-
Severity of disease (mild, moderate, severe).
-
Socioeconomic status (low‐, middle‐ and high‐income countries).
-
Oral hygiene practices.
-
Type of antimicrobial drug.
Sensitivity analysis
Provided there are sufficient included studies, we will undertake sensitivity analyses based on risk of bias, including only studies at low risk of bias.
Presentation of main results
We will use the GRADE approach to interpret findings (Schünemann 2011). We will use GRADEprofiler GDT software (GRADE 2014) and import data from RevMan 2014 to create 'Summary of findings' tables for each comparison included in the review. These tables will provide information concerning the overall quality of the evidence from the trials, the magnitude of effect of the interventions examined and the sum of available data on the primary and secondary outcomes. The GRADE approach (Schünemann 2011) considers 'quality' to be a judgement of the extent to which we can be confident that the estimates of effect are correct. A body of evidence from randomised controlled studies is initially graded as high and downgraded by one or two levels on each of five domains after full consideration of: any limitations in the design of the studies, the directness (or applicability) of the evidence, the consistency of results, precision of the results and the possibility of publication bias. A quality level of 'high' reflects confidence that the true effect lies close to that of the estimate of the effect for an outcome. A judgement of 'moderate' quality indicates that the true effect is likely to be close to the estimate of the effect, but acknowledges the possibility that it could be substantially different. 'Low' and 'very low' quality evidence limit our confidence in the effect estimate (Balshem 2011).
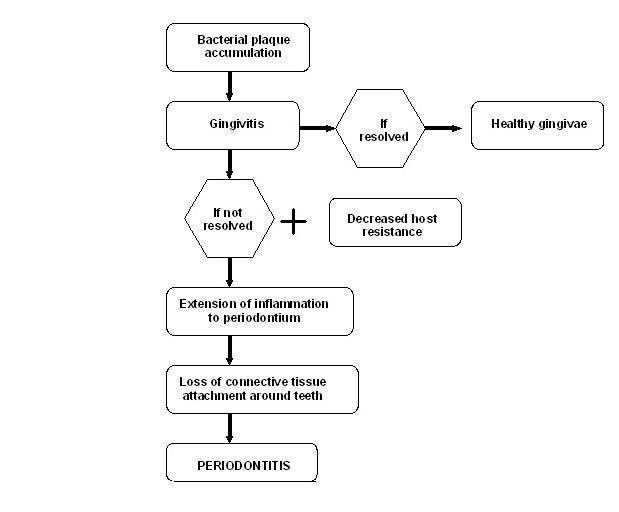
Etiopathogenesis of periodontal disease.
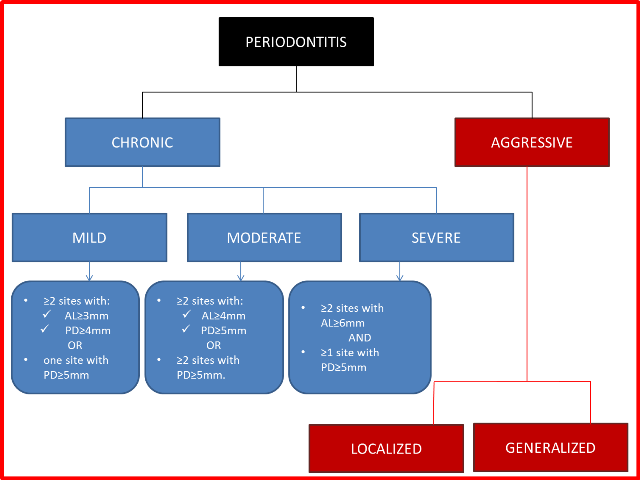
Classification of periodontitis.
AL = attachment loss; PD = pocket depth.

