Adjunctive systemic antimicrobials for the non‐surgical treatment of periodontitis
Abstract
Background
Systemic antimicrobials can be used as an adjunct to mechanical debridement (scaling and root planing (SRP)) as a non‐surgical treatment approach to manage periodontitis. A range of antibiotics with different dosage and combinations are documented in the literature. The review follows the previous classification of periodontitis as all included studies used this classification.
Objectives
To assess the effects of systemic antimicrobials as an adjunct to SRP for the non‐surgical treatment of patients with periodontitis.
Search methods
Cochrane Oral Health's Information Specialist searched the following databases to 9 March 2020: Cochrane Oral Health's Trials Register, CENTRAL, MEDLINE, and Embase. The US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov and the World Health Organization International Clinical Trials Registry Platform were searched for ongoing trials.
Selection criteria
We included randomized controlled trials (RCTs) which involved individuals with clinically diagnosed untreated periodontitis. Trials compared SRP with systemic antibiotics versus SRP alone/placebo, or with other systemic antibiotics.
Data collection and analysis
We selected trials, extracted data, and assessed risk of bias in duplicate. We estimated mean differences (MDs) for continuous data, with 95% confidence intervals (CIs). We assessed the certainty of the evidence using GRADE.
Main results
We included 45 trials conducted worldwide involving 2664 adult participants. 14 studies were at low, 8 at high, and the remaining 23 at unclear overall risk of bias. Seven trials did not contribute data to the analysis. We assessed the certainty of the evidence for the 10 comparisons which reported long‐term follow‐up (≥ 1 year). None of the studies reported data on antimicrobial resistance and patient‐reported quality of life changes.
Amoxicillin + metronidazole + SRP versus SRP in chronic/aggressive periodontitis: the evidence for percentage of closed pockets (MD ‐16.20%, 95% CI ‐25.87 to ‐6.53; 1 study, 44 participants); clinical attachment level (CAL) (MD ‐0.47 mm, 95% CI ‐0.90 to ‐0.05; 2 studies, 389 participants); probing pocket depth (PD) (MD ‐0.30 mm, 95% CI ‐0.42 to ‐0.18; 2 studies, 389 participants); and percentage of bleeding on probing (BOP) (MD ‐8.06%, 95% CI ‐14.26 to ‐1.85; 2 studies, 389 participants) was of very low certainty. Only the results for closed pockets and BOP showed a minimally important clinical difference (MICD) favouring amoxicillin + metronidazole + SRP.
Metronidazole + SRP versus SRP in chronic/aggressive periodontitis: the evidence for percentage of closed pockets (MD ‐12.20%, 95% CI ‐29.23 to 4.83; 1 study, 22 participants); CAL (MD ‐1.12 mm, 95% CI ‐2.24 to 0; 3 studies, 71 participants); PD (MD ‐1.11 mm, 95% CI ‐2.84 to 0.61; 2 studies, 47 participants); and percentage of BOP (MD ‐6.90%, 95% CI ‐22.10 to 8.30; 1 study, 22 participants) was of very low certainty. Only the results for CAL and PD showed an MICD favouring the MTZ + SRP group.
Azithromycin + SRP versus SRP for chronic/aggressive periodontitis: we found no evidence of a difference in percentage of closed pockets (MD 2.50%, 95% CI ‐10.19 to 15.19; 1 study, 40 participants); CAL (MD ‐0.59 mm, 95% CI ‐1.27 to 0.08; 2 studies, 110 participants); PD (MD ‐0.77 mm, 95% CI ‐2.33 to 0.79; 2 studies, 110 participants); and percentage of BOP (MD ‐1.28%, 95% CI ‐4.32 to 1.76; 2 studies, 110 participants) (very low‐certainty evidence for all outcomes).
Amoxicillin + clavulanate + SRP versus SRP for chronic periodontitis: the evidence from 1 study, 21 participants for CAL (MD 0.10 mm, 95% CI ‐0.51 to 0.71); PD (MD 0.10 mm, 95% CI ‐0.17 to 0.37); and BOP (MD 0%, 95% CI ‐0.09 to 0.09) was of very low certainty and did not show a difference between the groups.
Doxycycline + SRP versus SRP in aggressive periodontitis: the evidence from 1 study, 22 participants for CAL (MD ‐0.80 mm, 95% CI ‐1.49 to ‐0.11); and PD (MD ‐1.00 mm, 95% CI ‐1.78 to ‐0.22) was of very low certainty, with the doxycycline + SRP group showing an MICD in PD only.
Tetracycline + SRP versus SRP for aggressive periodontitis: we found very low‐certainty evidence of a difference in long‐term improvement in CAL for the tetracycline group (MD ‐2.30 mm, 95% CI ‐2.50 to ‐2.10; 1 study, 26 participants).
Clindamycin + SRP versus SRP in aggressive periodontitis: we found very low‐certainty evidence from 1 study, 21 participants of a difference in long‐term improvement in CAL (MD ‐1.70 mm, 95% CI ‐2.40 to ‐1.00); and PD (MD ‐1.80 mm, 95% CI ‐2.47 to ‐1.13) favouring clindamycin + SRP.
Doxycycline + SRP versus metronidazole + SRP for aggressive periodontitis: there was very low‐certainty evidence from 1 study, 27 participants of a difference in long‐term CAL (MD 1.10 mm, 95% CI 0.36 to 1.84); and PD (MD 1.00 mm, 95% CI 0.30 to 1.70) favouring metronidazole + SRP.
Clindamycin + SRP versus metronidazole + SRP for aggressive periodontitis: the evidence from 1 study, 26 participants for CAL (MD 0.20 mm, 95% CI ‐0.55 to 0.95); and PD (MD 0.20 mm, 95% CI ‐0.38 to 0.78) was of very low certainty and did not show a difference between the groups.
Clindamycin + SRP versus doxycycline + SRP for aggressive periodontitis: the evidence from 1 study, 23 participants for CAL (MD ‐0.90 mm, 95% CI ‐1.62 to ‐0.18); and PD (MD ‐0.80 mm, 95% CI ‐1.58 to ‐0.02) was of very low certainty and did not show a difference between the groups.
Most trials testing amoxicillin, metronidazole, and azithromycin reported adverse events such as nausea, vomiting, diarrhoea, mild gastrointestinal disturbances, and metallic taste. No serious adverse events were reported.
Authors' conclusions
There is very low‐certainty evidence (for long‐term follow‐up) to inform clinicians and patients if adjunctive systemic antimicrobials are of any help for the non‐surgical treatment of periodontitis. There is insufficient evidence to decide whether some antibiotics are better than others when used alongside SRP. None of the trials reported serious adverse events but patients should be made aware of the common adverse events related to these drugs.
Well‐planned RCTs need to be conducted clearly defining the minimally important clinical difference for the outcomes closed pockets, CAL, PD, and BOP.
PICO
Plain language summary
What are the benefits and risks of using antibiotics as well as cleaning by a dental care professional to treat gum disease?
Why is this question important?
Gum disease is a common condition in which the gums become swollen, sore or infected. It is caused by bacteria that accumulate on gums and teeth. Diseased gums may bleed when people brush their teeth, and may cause bad breath. If gum disease is not treated, teeth can become loose and eventually fall out. This can affect a person’s ability to chew and speak. It can also make people feel self‐conscious about their appearance.
Dental‐care professionals can clean teeth and gums to remove excess bacteria from the mouth. They use special instruments – typically, an ultrasound scraper followed by specialised hand‐held instruments – to scrape bacteria from the teeth, and stop these from affecting the gums.
Antibiotics (medicines that kill bacteria) taken by mouth (orally) can be used alongside professional cleaning, to remove bacteria from the area between the teeth and gums. However, there are potential risks associated with antibiotics, such as allergic reactions and antibiotic resistance (changes in bacteria after exposure to antibiotics, that allow the bacteria to survive future antibiotic treatment).
We conducted a review of the evidence from research studies to find out about the benefits and risks of using antibiotics alongside professional dental cleaning to treat gum disease. We also wanted to know if some antibiotics work better than others in this situation.
How did we identify and evaluate the evidence?
First, we searched for randomized controlled studies (clinical studies where people are randomly put into one of two or more treatment groups), because these studies provide the most robust evidence about the effects of a treatment. We then compared the results, and summarized the evidence from all the studies. Finally, we rated our confidence in the evidence, based on factors such as study methods and sizes, and the consistency of findings across studies.
What did we find?
We found 45 studies that involved a total of 2664 people over the age of 18 who had gum disease. The studies compared professional cleaning plus antibiotics against professional cleaning alone, or compared different antibiotics used alongside professional cleaning against one another.
We cannot tell whether antibiotics reduce gum disease in the long term (one year or more after treatment), or whether some antibiotics are better than others. This is because we have very little confidence in the evidence we found.
We cannot tell whether antibiotics are associated with unwanted effects, because we have too little confidence in the evidence. The most commonly reported unwanted effects were temporary, mild gastrointestinal disturbances, such as nausea, vomiting, diarrhoea, or a metallic taste in the mouth. No serious unwanted effects were reported.
No studies reported on antimicrobial resistance or changes in people’s quality of life.
What does this mean?
We do not know whether:
‐ using antibiotics alongside professional cleaning is beneficial for treating gum disease in the long term (more than one year after treatment);
‐ using antibiotics alongside professional cleaning is associated with unwanted effects; or
‐ some antibiotics are better than others for treating gum disease alongside professional cleaning.
Our confidence in the available evidence is very low. The results of our review are likely to change if more evidence becomes available. Future studies should clearly define what qualifies as a minimally important improvement in gum disease.
How‐up‐to date is this review?
The evidence in this Cochrane Review is current to March 2020.
Authors' conclusions
Summary of findings
| Amoxicillin + metronidazole + SRP compared to SRP for the non‐surgical treatment of periodontitis | ||||||
|---|---|---|---|---|---|---|
| Patient or population: adults with periodontitis (chronic/aggressive) Follow‐up: ≥1 year | ||||||
| Outcomes | Number of participants | Relative effect | Anticipated absolute effects* (95% CI) | Certainty of the evidence | Comments | |
| Risk with control (SRP) | Risk difference with amoxicillin + metronidazole + SRP | |||||
| Percentage of closed pockets | 44 (1 RCTa) | ‐ | The mean percentage of closed pockets was 49.30% | MD 16.20% lower (25.87 lower to 6.53 lower) | ⊕⊝⊝⊝ | Based on our definition of minimally important clinical difference (MICD)f, only the results for percentage of closed pockets and of BOP showed an MICD favouring amoxicillin + metronidazole + SRP |
| CAL (Long‐term improvement in chronic and aggressive periodontitis) | 389 (2 RCTsa,b) | ‐ | The mean CAL was 3.85 mm | MD 0.47 mm lower | ⊕⊝⊝⊝ | |
| Probing pocket depth (Long‐term improvement in chronic and aggressive periodontitis) | 389 (2 RCTsa,b) | ‐ | The mean probing pocket depth was 2.75 mm | MD 0.30 mm lower | ⊕⊝⊝⊝ | |
| Percentage of BOP | 389 | ‐ | The mean percentage of BOP was 29.45% | MD 8.06% lower | ⊕⊝⊝⊝ | |
| Antimicrobial resistance | Included studies have not reported this outcome | |||||
| Adverse events | 389 | Most common adverse events were mild gastrointestinal disturbances, nausea and vomiting, metallic taste, and diarrhoea in the intervention group. Control group reported adverse events like hypothermia, mild gastrointestinal symptoms, diarrhoea, vomiting, cramps, rashes, and weakness. Nausea after alcohol intake was reported in 1 participant from the intervention group | ⊕⊝⊝⊝ | |||
| Patient‐reported quality of life changes | Included studies have not reported this outcome | |||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) BOP: bleeding on probing; CAL: clinical attachment level; CI: confidence interval; MD: mean difference; MICD: minimally important clinical difference; RCT: randomized controlled trial; SRP: scaling and root planing | ||||||
| GRADE Working Group grades of evidence | ||||||
| aBorges 2017. | ||||||
| Metronidazole + SRP compared to SRP for the non‐surgical treatment of periodontitis | ||||||
|---|---|---|---|---|---|---|
| Patient or population: adults with periodontitis (chronic/aggressive) Follow‐up: ≥1 year | ||||||
| Outcomes | Number of participants | Relative effect | Anticipated absolute effects* (95% CI) | Certainty of the evidence | Comments | |
| Risk with control (SRP) | Risk difference with metronidazole + SRP | |||||
| Percentage of closed pockets | 22 (1 RCTa) | ‐ | The mean percentage of closed pockets was 52.80% | MD 12.20% lower | ⊕⊝⊝⊝ | Based on our definition of minimally important clinical difference (MICD)h, only the results for CAL and probing pocket depth showed an MICD favouring metronidazole + SRP |
| CAL | 71 | ‐ | ‐ | MD 1.12 mm lower | ⊕⊝⊝⊝ | |
| Probing pocket depth | 47 | ‐ | The mean probing pocket depth was 4.11 mm | MD 1.11 mm lower | ⊕⊝⊝⊝ | |
| Percentage of BOP | 22 (1 RCTa) | ‐ | The mean percentage of BOP was 44.92% | MD 6.90% lower | ⊕⊝⊝⊝ | |
| Antimicrobial resistance | Included studies have not reported this outcome | |||||
| Adverse events | 71 | No severe adverse effects were reported by any of the participants in 1 study. 2 studies have not reported adverse events | ⊕⊝⊝⊝ | |||
| Patient‐reported quality of life changes | Included studies have not reported this outcome | |||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) | ||||||
| GRADE Working Group grades of evidence | ||||||
| aCarvalho 2004. | ||||||
| Azithromycin + SRP compared to SRP for the non‐surgical treatment of periodontitis | ||||||
|---|---|---|---|---|---|---|
| Patient or population: adults with periodontitis (chronic/aggressive) Follow‐up: ≥1 year | ||||||
| Outcomes | Number of participants | Relative effect | Anticipated absolute effects* (95% CI) | Certainty of the evidence | Comments | |
| Risk with control (SRP) | Risk difference with azithromycin + SRP | |||||
| Percentage of closed pockets | 40 (1 RCTa) | ‐ | The mean percentage of closed pockets was 51.50% | MD 2.50% higher | ⊕⊝⊝⊝ | Based on our definition of minimally important clinical difference (MICD)g, we found no evidence of a difference between intervention and control |
| CAL | 110 | ‐ | The mean CAL was 5.30 mm | MD 0.59 mm lower | ⊕⊝⊝⊝ | |
| Probing pocket depth | 110 | ‐ | The mean probing pocket depth was 4.29 mm | MD 0.77 mm lower | ⊕⊝⊝⊝ | |
| Percentage of BOP | 110 | ‐ | The mean percentage of BOP was 16.96% | MD 1.28% lower | ⊕⊝⊝⊝ | |
| Antimicrobial resistance | Included studies have not reported this outcome | |||||
| Adverse events | 110 | 1 study reported diarrhoea, headache or dizziness, metallic taste, and general unwellness by 4 participants from the intervention group and 3 from the control group. In 1 study none of the participants reported any adverse effects | ⊕⊝⊝⊝ | |||
| Patient‐reported quality of life changes | Included studies have not reported this outcome | |||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) | ||||||
| GRADE Working Group grades of evidence | ||||||
| aSampaio 2011. | ||||||
| Amoxicillin + clavulanate + SRP compared to SRP for the non‐surgical treatment of periodontitis | ||||||
|---|---|---|---|---|---|---|
| Patient or population: adults with periodontitis (chronic) Follow‐up: ≥1 year | ||||||
| Outcomes | Number of participants | Relative effect | Anticipated absolute effects* (95% CI) | Certainty of the evidence | Comments | |
| Risk with control (SRP) | Risk difference with amoxicillin + clavulanate + SRP | |||||
| Percentage of closed pockets | Included study has not reported this outcome | Based on our definition of minimally important clinical difference (MICD)d, we found no evidence of a difference between intervention and control | ||||
| CAL | 21 | ‐ | The mean CAL was 6.80 mm | MD 0.10 mm higher | ⊕⊝⊝⊝ | |
| Probing pocket depth | 21 | ‐ | The mean probing pocket depth was 2.80 mm | MD 0.10 mm higher | ⊕⊝⊝⊝ | |
| Percentage of BOP | 21 | ‐ | The mean percentage of BOP was 0.20% | MD 0% | ⊕⊝⊝⊝ | |
| Antimicrobial resistance | Included study has not reported this outcome | |||||
| Adverse events | 21 | Included study reported diarrhoea in 2 participants from both groups | ⊕⊝⊝⊝ | |||
| Patient‐reported quality of life changes | Included study has not reported this outcome | |||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) | ||||||
| GRADE Working Group grades of evidence | ||||||
| aWinkel 1999. | ||||||
| Doxycycline + SRP compared to SRP for the non‐surgical treatment of periodontitis | ||||||
|---|---|---|---|---|---|---|
| Patient or population: adults with periodontitis (aggressive) Follow‐up: ≥1 year | ||||||
| Outcomes | Number of participants | Relative effect | Anticipated absolute effects* (95% CI) | Certainty of the evidence | Comments | |
| Risk with control (SRP) | Risk difference with doxycycline + SRP | |||||
| Percentage of closed pockets | Included study has not reported this outcome | Based on our definition of minimally important clinical difference (MICD)d, only the results for probing pocket depth showed an MICD favouring doxycycline + SRP | ||||
| CAL | 22 | ‐ | The mean CAL was 5.90 mm | MD 0.80 mm lower | ⊕⊝⊝⊝ | |
| Probing pocket depth | 22 | ‐ | The mean probing pocket depth was 5.20 mm | MD 1 mm lower | ⊕⊝⊝⊝ | |
| Percentage of BOP | Included study has not reported this outcome | |||||
| Antimicrobial resistance | Included study has not reported this outcome | |||||
| Adverse events | Included study has not reported adverse events | |||||
| Patient‐reported quality of life changes | Included study has not reported this outcome | |||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) | ||||||
| GRADE Working Group grades of evidence | ||||||
| aSigusch 2001. | ||||||
| Tetracycline + SRP compared to SRP for the non‐surgical treatment of periodontitis | ||||||
|---|---|---|---|---|---|---|
| Patient or population: adults with periodontitis (aggressive) Follow‐up: ≥1 year | ||||||
| Outcomes | Number of participants | Relative effect | Anticipated absolute effects* (95% CI) | Certainty of the evidence | Comments | |
| Risk with control (SRP) | Risk difference with tetracycline + SRP | |||||
| Percentage of closed pockets | Included study has not reported this outcome | Based on our definition of minimally important clinical difference (MICD)d, the results for CAL showed an MICD favouring tetracycline + SRP | ||||
| CAL | 26 | ‐ | The mean CAL was 4.40 mm | MD 2.30 mm lower | ⊕⊝⊝⊝ | |
| Probing pocket depth | Included study has not reported this outcome | |||||
| Percentage of BOP | Included study has not reported this outcome | |||||
| Antimicrobial resistance | Included study has not reported this outcome | |||||
| Adverse events | Included study has not reported adverse events | |||||
| Patient‐reported quality of life changes | Included study has not reported this outcome | |||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) | ||||||
| GRADE Working Group grades of evidence | ||||||
| aZhang 2006. | ||||||
| Clindamycin + SRP compared to SRP for the non‐surgical treatment of periodontitis | ||||||
|---|---|---|---|---|---|---|
| Patient or population: adults with periodontitis (aggressive) Follow‐up: ≥1 year | ||||||
| Outcomes | Number of participants | Relative effect | Anticipated absolute effects* (95% CI) | Certainty of the evidence | Comments | |
| Risk with control (SRP) | Risk difference with clindamycin + SRP | |||||
| Percentage of closed pockets | Included study has not reported this outcome | Based on our definition of minimally important clinical difference (MICD)d, the results for CAL and probing pocket depth showed an MICD favouring clindamycin + SRP | ||||
| CAL | 21 | ‐ | The mean CAL was 5.90 mm | MD 1.70 mm lower | ⊕⊝⊝⊝ | |
| Probing pocket depth | 21 | ‐ | The mean probing pocket depth was 5.20 mm | MD 1.80 mm lower | ⊕⊝⊝⊝ | |
| Percentage of BOP | Included study has not reported this outcome | |||||
| Antimicrobial resistance | Included study has not reported this outcome | |||||
| Adverse events | Included study has not reported adverse events | |||||
| Patient‐reported quality of life changes | Included study has not reported this outcome | |||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) | ||||||
| GRADE Working Group grades of evidence | ||||||
| aSigusch 2001. | ||||||
| Doxycycline + SRP compared to metronidazole + SRP for the non‐surgical treatment of periodontitis | ||||||
|---|---|---|---|---|---|---|
| Patient or population: adults with periodontitis (aggressive) Follow‐up: ≥1 year | ||||||
| Outcomes | Number of participants | Relative effect | Anticipated absolute effects* (95% CI) | Certainty of the evidence | Comments | |
| Risk with metronidazole + SRP | Risk difference with doxycycline + SRP | |||||
| Percentage of closed pockets | Included study has not reported this outcome | Based on our definition of minimally important clinical difference (MICD)d, the results for CAL and probing pocket depth showed an MICD favouring metronidazole + SRP | ||||
| CAL | 27 | ‐ | The mean CAL was 4 mm | MD 1.10 mm higher | ⊕⊝⊝⊝ | |
| Probing pocket depth | 27 | ‐ | The mean probing pocket depth was 3.20 mm | MD 1 mm higher | ⊕⊝⊝⊝ | |
| Percentage of BOP | Included study has not reported this outcome | |||||
| Antimicrobial resistance | Included study has not reported this outcome | |||||
| Adverse events | Included study has not reported adverse events | |||||
| Patient‐reported quality of life changes | Included study has not reported this outcome | |||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) | ||||||
| GRADE Working Group grades of evidence | ||||||
| aSigusch 2001. | ||||||
| Clindamycin + SRP compared to metronidazole + SRP for the non‐surgical treatment of periodontitis | ||||||
|---|---|---|---|---|---|---|
| Patient or population: adults with periodontitis (aggressive) Follow‐up: ≥1 year | ||||||
| Outcomes | Number of participants | Relative effect | Anticipated absolute effects* (95% CI) | Certainty of the evidence | Comments | |
| Risk with metronidazole + SRP | Risk difference with clindamycin + SRP | |||||
| Percentage of closed pockets | Included study has not reported this outcome | Based on our definition of minimally important clinical difference (MICD)d, we found no evidence of a difference between the groups | ||||
| CAL | 26 | ‐ | The mean CAL was 4 mm | MD 0.20 mm higher | ⊕⊝⊝⊝ | |
| Probing pocket depth | 26 | ‐ | The mean probing pocket depth was 3.20 mm | MD 0.20 mm higher | ⊕⊝⊝⊝ | |
| Percentage of BOP | Included study has not reported this outcome | |||||
| Antimicrobial resistance | Included study has not reported this outcome | |||||
| Adverse events | Included study has not reported adverse events | |||||
| Patient‐reported quality of life changes | Included study has not reported this outcome | |||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) | ||||||
| GRADE Working Group grades of evidence | ||||||
| aSigusch 2001. | ||||||
| Clindamycin + SRP compared to doxycycline + SRP for the non‐surgical treatment of periodontitis | ||||||
|---|---|---|---|---|---|---|
| Patient or population: adults with periodontitis (aggressive) Follow‐up: ≥1 year | ||||||
| Outcomes | Number of participants | Relative effect | Anticipated absolute effects* (95% CI) | Certainty of the evidence | Comments | |
| Risk with doxycycline + SRP | Risk difference with clindamycin + SRP | |||||
| Percentage of closed pockets | Included study has not reported this outcome | Based on our definition of minimally important clinical difference (MICD)d, we found no evidence of a difference between the groups | ||||
| CAL | 23 | ‐ | The mean CAL was 5.10 mm | MD 0.90 mm lower | ⊕⊝⊝⊝ | |
| Probing pocket depth | 23 | ‐ | The mean probing pocket depth was 4.20 mm | MD 0.80 mm lower | ⊕⊝⊝⊝ | |
| Percentage of BOP | Included study has not reported this outcome | |||||
| Antimicrobial resistance | Included study has not reported this outcome | |||||
| Adverse events | Included study has not reported adverse events | |||||
| Patient‐reported quality of life changes | Included study has not reported this outcome | |||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) | ||||||
| GRADE Working Group grades of evidence | ||||||
| aSigusch 2001. | ||||||
Background
Description of the condition
Periodontal diseases are a group of related diseases affecting the periodontal tissues. It comprises of both gingivitis and periodontitis. Dental plaque biofilm‐induced gingivitis is defined at the site level as "an inflammatory lesion resulting from interactions between the dental plaque biofilm and the host's immune‐inflammatory response, which remains contained within the gingiva and does not extend to the periodontal attachment (cementum, periodontal ligament, and alveolar bone). Such inflammation remains confined to the gingiva and does not extend beyond the mucogingival junction and is reversible by reducing levels of dental plaque at and apical to the gingival margin." (Chapple 2018) (Figure 1). Periodontitis is characterized by microbially associated, host‐mediated inflammation that results in loss of periodontal attachment. This is detected as clinical attachment loss by circumferential assessment of the erupted dentition with a standardized periodontal probe with reference to the cemento‐enamel junction (CEJ) (Tonetti 2018).

Etiopathogenesis of periodontal disease.
Case definition of periodontitis (Papapanou 2018): a patient is a 'periodontitis case' if:
-
interdental clinical attachment loss is detectable at ≥ 2 non‐adjacent teeth, or
-
buccal or oral clinical attachment loss ≥ 3 mm with pocketing ≥ 3 mm is detectable at ≥ 2 teeth but the observed clinical attachment loss cannot be ascribed to non‐periodontitis‐related causes such as: a) gingival recession of traumatic origin; b) dental caries extending in the cervical area of the tooth; c) the presence of clinical attachment loss on the distal aspect of a second molar and associated with malposition or extraction of a third molar; d) an endodontic lesion draining through the marginal periodontium; and e) the occurrence of a vertical root fracture.
Its primary features include the loss of periodontal tissue support, manifested through clinical attachment loss and radiographically assessed alveolar bone loss, presence of periodontal pocketing, and gingival bleeding. Periodontitis may lead to tooth loss and disability, negatively affect chewing function and aesthetics and be a source of social inequality. Periodontitis accounts for a substantial proportion of edentulism and masticatory dysfunction, results in significant dental care costs and has a plausible negative impact on general health (Papapanou 2018). Periodontitis affects quality of life, not only in terms of reduced functional capacity (i.e. the ability to eat, speak, and perform daily activities) but also social and interpersonal relationships (Araújo 2010). Not being able to identify one's own symptoms, the patients remain unaware of their prevailing deteriorated periodontal condition.The impact on quality of life worsens with an increase in the severity of periodontitis (Chraif 2014; Ferreira 2009; Sundaram 2013).
Periodontitis is multifactorial in origin with pathogenic bacterial flora and genetic predisposition being the predominant aetiological factors. Common conditions with variable effects on periodontitis include smoking and diabetes mellitus (Jepsen 2018).
More than 500 microbial species have been identified within periodontal pockets (How 2016; Moore 1994). Most putative periodontal pathogens are gram negative and anaerobic, however gram‐positive, facultative species of cocci and rods are also found (Marsh 2011; Moore 1994). Among these species most important ones are Porphyromonas gingivalis, Aggregatibacter actinomycetemcomitans, Treponema denticola, Tannerella forsythia, Fusobacterium and Prevotella intermedia (Feng 2006; Haffajee 2005; Riep 2009). There are also studies that suggest the role of certain viruses like Cytomegalovirus, Epstein Barr virus and Herpes simplex virus in the aetiology of periodontitis (Contreras 1996; Slots 2007).
Periodontitis is a common chronic oral health problem, contributing to a global health burden on low‐income, middle‐income, and high‐income countries (Petersen 2012). The US Centers for Disease Control and Prevention (CDC) data estimate that 47.2% of adults aged 30 years have some form of periodontitis and prevalence increases to about 70.1% at 65 years of age (Eke 2012; Eke 2015).
According to a report by the American Academy of Periodontology and the European Federation of Periodontology following the 2017 World Workshop on the Classification of Periodontal and Peri‐Implant Diseases and Conditions (Caton 2018; Chapple 2018), periodontitis can be classified as.
-
Necrotizing periodontal diseases.
-
Necrotizing gingivitis.
-
Necrotizing periodontitis.
-
Necrotizing stomatitis.
-
-
Periodontitis as manifestation of systemic disease.
-
Periodontitis.
-
Stages: based on severity and complexity of treatment.
-
Stage I: initial periodontitis.
-
Stage II: moderate periodontitis.
-
Stage III: severe periodontitis with potential for additional tooth loss.
-
Stage IV: severe periodontitis with potential for loss of the dentition.
-
-
Extent and severity of distribution: localized; generalized; molar‐incisor distribution.
-
Grades: evidence of risk or rapid progression, anticipated treatment response.
-
Grade A: slow rate of progression.
-
Grade B: moderate rate of progression.
-
Grade C: rapid rate of progression.
-
-
This redesigned disease classification framework built upon a notable change from previous classifications: forms of periodontal disease were defined as one of three distinct forms which included necrotizing periodontitis, periodontitis as a manifestation of systemic conditions, and periodontitis (formerly 'aggressive' and 'chronic' and now grouped under a single category).
This Cochrane Review however, uses the previous classification as all the included studies used the same classification. According to the previous classification by the American Academy of Periodontology (Armitage 1999; Geurs 2015), the various forms of periodontitis can be classified on the basis of.
-
Cause.
-
-
Chronic: the most prevalent form in adults (Armitage 1999; Oliver 1991), characterized by a strong association with local factors ‐ plaque and calculus.
-
Aggressive: characterized by a) rapid rate of periodontal disease progression seen in otherwise healthy individuals, b) an absence of large accumulation of plaque and calculus, and c) a family history of aggressive periodontitis suggestive of a genetic trait (Novak 1996; Tonetti 1999).
-

Classification of periodontitis (Armitage 1999; Geurs 2015).
AL = attachment loss; PD = pocket depth.
-
-
Mild.
-
Moderate.
-
Severe.
-
-
Site: both chronic and aggressive forms can be localized or generalized (Geurs 2015).
This Cochrane Review maintains the distinction between aggressive and chronic periodontitis as all the studies categorized the patients according to the previous classification and the included studies did not use any form of staging as mentioned in the current classification. However, updates of the review might consider using the most recent classification according to its usage in studies conducted in future.
Description of the intervention
Mechanical debridement (scaling and root planing) is the mainstay of non‐surgical periodontal therapy which aims to eliminate local irritating factors. However, it is not possible to eliminate all pathogenic bacteria through instrumentation within the subgingival region (Deas 2016). The remaining subgingival micro‐organisms can therefore be eliminated by using antimicrobials (Mombelli 2012a).
Various drugs prescribed for non‐surgical management of periodontitis documented in the literature are as follows.
-
For single‐drug therapy.
-
-
Penicillins (Purucker 2001; Rooney 2002).
-
Tetracyclines (Akincibay 2008; Al‐Joburi 1989; Atilla 1996; Helldén 1979; Preus 1995).
-
Macrolides (Gordon 1985; Gordon 1990; Oteo 2010; Smith 2002).
-
Quinolones (Guzeldemir 2015).
-
Nitroimidazole (Noyan 1997; Yilmaz 1996).
-
-
For multiple‐drug therapy.
-
-
Amoxicillin (AMOX) + metronidazole (MTZ) (Akincibay 2008; Guerrero 2005; Rooney 2002; van Winkelhoff 1989).
-
Doxycycline (DOX) + MTZ (Aitken 1992).
-
DOX + AMOX + clavulanate (CLAV) (Matisko 1993).
-
Despite the suggested benefits of systemic antibiotics, they expose an individual to potential risks like the development of microbial resistance, the emergence of opportunistic fungal infections, allergic reactions, and other drug‐dependent systemic side effects (Jorgensen 2000). Hence, judicious prescription of antibiotics is warranted for long‐term health interests.
Subgingival plaque is polymicrobial in nature, hence the administration of a specific antimicrobial based on antibiotic susceptibility is a challenge to clinicians. Moreover, different recommendations on antibiotic prescription at various levels compound to the problem.
Antimicrobial resistance (AMR) among micro‐organisms has consistently increased in the past three decades and the susceptibility of bacteria to antimicrobial agents has become less predictable (Gamboa 2014; Japoni 2011).
How the intervention might work
Systemically administered antibiotics reach the periodontal tissues via the serum, and target micro‐organisms that are inaccessible to scaling instruments (Slots 2002). These antibiotics also help to eradicate infections by suppressing periodontal pathogens that invade subepithelial periodontal tissues and other extra‐dental sites like deep crevices of the tongue. Thus, systemic antibiotic therapy is considered advantageous for eradication of pathogenic bacteria (Müller 1998).
Antimicrobials administered along with mechanical debridement may have beneficial effect in clinical periodontal and microbiological parameters. They may aid in reducing the gingival inflammation (López 2000), probing pocket depth (Elter 1997), attachment loss (Elter 1997), and need for periodontal surgery. They may also affect other periodontal parameters. It has been shown that antibiotics may eliminate/suppress the periodontal pathogenic microflora (Mombelli 2012a; Mombelli 2017). However, it is to be noted that scaling and root planing (SRP) is mandatorily done prior to the administration of antibiotics (Jepsen 2016). The rationale for mandatory prior/adjunctive SRP is that biofilms offer resistance to the penetration of antibiotics (Herrera 2008).
A range of antibiotics has been prescribed in the literature with differing dosing regimens, alone or in combination. The use of antibiotics has shown statistically significant greater gain in attachment and reduction in depth of periodontal pockets (Feres 2012; Silva 2011). However, no single antibiotic at concentration achieved in body fluids inhibits all putative periodonto‐pathogens. Slots 2004, thus, suggested that the use of multiple‐drug therapy may be far more effective than single‐drug therapy owing to an expanded spectrum of activity and synergistic effects of these drugs on a variety of pathogens. For example, amoxicillin and metronidazole have a synergistic effect on complex periodonto‐pathogens, hence their administration added advantage over single‐drug therapy (Guerrero 2005).
Why it is important to do this review
The US Centers for Disease Control and Prevention (CDC) estimates that about one half of antibiotic prescriptions by office‐based physicians are unnecessary (Colgan 2001). Thus, this systematic review will synthesize evidence on whether the use of systemic antibiotics in periodontitis is beneficial.
Beyond the development of short‐term side effects, the indiscriminate use of antibiotics may lead to the development of multidrug resistant bacterial species which may have negative and expensive future implications. Development of opportunistic fungal infections is another concern.
Apart from the reasons mentioned above, there is contradicting evidence to support the routine prescription of antibiotics for periodontitis. For example, azithromycin when compared to placebo exhibited no additional benefit along with non‐surgical periodontal therapy in periodontitis patients (Han 2012; Sampaio 2011) which contradicts the study by Oteo 2010. Similarly, choice of single (Feres 2012) versus a combination of antibiotics (Silva 2011) is debatable for the management of periodontitis.
In a study by Feres 2012, both metronidazole and a combination of it with amoxicillin were effective for patients with chronic periodontitis whereas in a study by Silva 2011, the combination of metronidazole and amoxicillin proved to be more effective than metronidazole alone.
In systematic reviews by Keestra 2015 and Keestra 2015a, it was reported that no specific type of antimicrobial showed statistically significant superiority over the other when used in patients with untreated chronic periodontitis, and that an amoxicillin plus metronidazole combination was potent for aggressive periodontitis based on clinical attachment level and probing pocket depth reduction as primary outcomes.
As stated above, the prevalence of periodontitis is high and increases substantially with age. Also, the literature regarding the use of antimicrobials in periodontal disease is confusing, hence the need for this Cochrane Review.
Objectives
The focused objective of this review was to assess the effects of systemic antimicrobials as an adjunct to scaling and root planing (SRP) for the non‐surgical treatment of patients with periodontitis.
Methods
Criteria for considering studies for this review
Types of studies
We included randomized controlled trials (RCTs) evaluating any antimicrobial used systemically as an adjunct to non‐surgical therapy for the treatment of periodontitis (chronic/aggressive). We excluded quasi‐RCTs, cross‐over, and split‐mouth trials.
Types of participants
We included all individuals with clinically diagnosed untreated periodontitis (chronic/aggressive) as per the classification shown in Figure 2.
People with the following conditions were excluded:
-
necrotizing periodontitis,
-
recurrent periodontitis,
-
refractory periodontitis,
-
periodontitis as a manifestation of systemic disease,
-
pregnancy,
-
systemic disease/condition which can predispose to periodontitis (e.g. diabetes).
Types of interventions
Interventions considering the systemic use of antimicrobials as an adjunct to scaling and root planing (SRP) compared with SRP alone or with placebo.
Interventions comparing different antimicrobials administered systemically (different drugs/different dosage regimens/different combinations/at different time periods) as an adjunct to SRP were included.
Exclusion criteria:
-
studies comparing the use of antimicrobials alone versus SRP with antimicrobial;
-
studies using the application of any local antimicrobial/laser;
-
studies using antibiotics at a subantimicrobial dose (e.g. subdose doxycycline).
Types of outcome measures
Primary outcomes
-
Percentage of closed pockets (i.e. pocket depth < 4 mm. The 4 mm non‐bleeding site is referred to as a 'closed pocket' as its risk of future breakdown is significantly reduced relative to sites of pocket depth of 5 mm or greater (Walter 2019)).
Secondary outcomes
-
Clinical/relative attachment level (CAL).
-
Probing pocket depth.
-
Percentage of bleeding on probing (BOP).
-
Antimicrobial resistance.
-
Adverse effects of antibiotics.
-
Long‐term stability of CAL and pocket depth.
-
Patient‐reported quality of life changes (in terms of function, aesthetics, and satisfaction).
Search methods for identification of studies
Cochrane Oral Health's Information Specialist conducted systematic searches for randomized controlled trials and controlled clinical trials. Due to the Cochrane Embase Project to identify all clinical trials on the database and add them to CENTRAL, only recent months of the Embase database was searched. Please see the searching page on the Cochrane Oral Health website for more information. No other restrictions were placed on the language or date of publication when searching the electronic databases.
Electronic searches
Cochrane Oral Health's Information Specialist searched the following databases for relevant trials:
-
Cochrane Oral Health's Trials Register (searched 9 March 2020) (Appendix 1);
-
the Cochrane Central Register of Controlled Trials (CENTRAL; in the Cochrane Register of Studies searched 9 March 2020) (Appendix 2);
-
MEDLINE Ovid (1946 to 9 March 2020) (Appendix 3);
-
Embase Ovid (28 August 2016 to 9 March 2020) (Appendix 4).
Subject strategies were modelled on the search strategy designed for MEDLINE Ovid. Where appropriate, they were combined with subject strategy adaptations of the highly sensitive search strategies designed by Cochrane for identifying randomized controlled trials and controlled clinical trials (as described in the Cochrane Handbook for Systematic Reviews of Interventions, Version 6.1, (Lefebvre 2020)).
Searching other resources
Cochrane Oral Health's Information specialist searched the following trials registries:
-
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (clinicaltrials.gov/) (Appendix 5);
-
World Health Organization International Clinical Trials Registry Platform (apps.who.int/trialsearch) (Appendix 6).
We have checked the bibliographies of included studies and any relevant systematic reviews identified for further references to relevant trials. We have not performed a separate search for adverse effects of interventions. We have considered adverse effects described in the included studies only.
We checked that none of the included studies in this review were retracted due to error or fraud.
Data collection and analysis
Selection of studies
Two pairs of review authors (Shivi Khattri (SK) and Prashanti Eachempati (PE), and Sumanth Kumbargere Nagraj (SKN) and Ankita Arora (AA)) independently screened the titles and abstracts from the electronic searches to identify potentially eligible studies that require further evaluation to determine whether they met the inclusion criteria for this review. The search was designed to be sensitive and include controlled clinical trials, these were filtered out early in the selection process if they were not randomized.
We obtained full‐text copies of all eligible and potentially eligible studies and these were further evaluated by SK and AA to identify those studies that actually met all the inclusion criteria. From this group, we recorded those studies not meeting the inclusion criteria in the 'Characteristics of excluded studies' table, noting the reason for exclusion. Non‐RCTs were excluded without giving any reasons for exclusion. We resolved disagreements by discussion. When resolution was not possible, we consulted an arbiter (SKN). We assessed articles in languages other than English by their abstracts, where possible, and if they appeared to be potentially eligible, we requested translators for data extraction.
Data extraction and management
Two review authors (SK and Chandan Kumar Kusum (CKK)) independently extracted the data. The review authors were not blinded to the authors of the included studies. Disagreement was resolved by discussion between the two review authors or, if necessary, a third review author (Giovanni Lodi (GL)) was consulted in order to reach consensus. We extracted data using a customised data extraction form, which was first pilot tested using a sample of the included studies. All the items in the data extraction form were designed following guidance from the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We entered study details into the 'Characteristics of included studies' table in Review Manager (RevMan) software (Review Manager 2020).
We recorded the following details for each included trial.
-
Publication details such as year of publication, language.
-
Demographic details of the report.
-
Inclusion and exclusion criteria.
-
Type of trial, sample size, method of randomization, allocation concealment, blinding, method of assessing the outcomes and dropouts, if any.
-
Type of intervention.
-
Level of expertise (specialist/general practitioner or dental hygienist or student) and intensity (in minutes or visits) for scaling and root planing.
-
Details of the outcomes reported.
-
Oral hygiene instruction provided/not provided.
-
Duration of follow‐up.
-
Results of the intervention.
-
Funding details.
Assessment of risk of bias in included studies
Two review authors (SKN and SK) independently assessed the risk of bias in the included trials in seven domains:
-
random sequence generation (selection bias);
-
allocation concealment (selection bias);
-
blinding of participants and personnel (performance bias);
-
blinding (outcome assessment) (detection bias);
-
incomplete outcome data (attrition bias);
-
selective outcome reporting (reporting bias);
-
other biases.
For each of these components, we assigned a judgement regarding the risk of bias as either high, low, or unclear based on guidance in Higgins 2011. We contacted the trial authors if details were missing in the publications or were unclear about the methodology. We resolved disagreements through consensus. We recorded our judgements and justifications in 'Risk of bias' tables for each included study and generated a 'Risk of bias' summary graph and figure. We used these judgements while grading the overall certainty of the evidence for each comparison and outcome in the 'Summary of findings' tables.
We summarized the risk of bias according to Higgins 2011 as follows.
| Risk of bias | Interpretation | In outcome | In included studies |
|---|---|---|---|
| Low risk of bias | Plausible bias unlikely to seriously alter the results | Low risk of bias for all key domains | Most information is from studies at low risk of bias |
| Unclear risk of bias | Plausible bias that raises some doubt about the results | Unclear risk of bias for one or more key domains | Most information is from studies at low or unclear risk of bias |
| High risk of bias | Plausible bias that seriously weakens confidence in the results | High risk of bias for one or more key domains | The proportion of information from studies at high risk of bias is sufficient to affect the interpretation of results |
Measures of treatment effect
The primary outcome, percentage of closed pockets was expressed as continuous data. The secondary outcomes, CAL, probing pocket depth and percentage of BOP were also expressed as continuous data. We used means and standard deviations (SD) to calculate the mean differences (MD) and 95% confidence intervals (CI) to summarize both of these data. Adverse events are expressed as descriptive data. We intended for patient‐reported quality of life changes outcome to be measured in the form of an ordinal scale and for antimicrobial resistance data to be treated as dichotomous and presented as risk ratios (RRs) with 95% CIs. However, none of the included trials provided data on patient quality of life or antimicrobial resistance.
We planned to use standardized mean difference (SMD) if studies used different scales to measure the same outcome. If data expressed were in ordinal scales, we wanted to explore the possibility of converting them to dichotomous outcomes. However, we did not come across data collected from different scales or ordinal data in this review. We used mean difference as the intervention effect which compares the difference in the mean number of events (standardized to a unit time period) experienced by participants in the intervention group compared to control group.
If the trials provided details on oral hygiene instructions (OHI) along with SRP, we intended to use the data as SRP with OHI and SRP without OHI.
Unit of analysis issues
We intended to use the mean change scores in the CAL, pocket depth, and BOP in studies with a parallel design. However, we combined change scores and end scores in the analyses.
We did not find any cluster‐randomized trials in our review.
If found any trial with multiple treatment groups, we planned to combine groups to create a single pair‐wise comparison as recommended by the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). However, we could not combine the groups because of lack of homogeneity in the groups (e.g. two treatment arms with different drugs or regimens of the same drug).
If found any trials with repeated observations on participants, we intended to follow the methods described in Section 9.3.4 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). However, the included trials reported different follow‐up durations and hence we could not combine these data in the meta‐analysis.
If adverse effects were described as counts, we intended to follow the methods described in Section 9.2.5 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). However, most of the trials reported adverse events as descriptive data and hence we did not analyze adverse events data.
Dealing with missing data
We contacted study authors to obtain missing data. We used the methods in Section 16.1.3 of the Cochrane Handbook for Systematic Reviews of Interventions to estimate missing SDs (Higgins 2011). Missing SDs were calculated from the reported values of t, P, and Chi2. If data were expressed as graphs, we calculated the data using PlotDigitizer® software. If it was not possible to calculate the SDs, the outcomes were described qualitatively.
Assessment of heterogeneity
Where meta‐analyses were performed, we assessed heterogeneity using a Chi2 test, where a P value < 0.1 indicate statistically significant heterogeneity. We quantified heterogeneity using the I2 statistic as follows:
-
0% to 40% implies slight heterogeneity;
-
30% to 60% moderate heterogeneity;
-
50% to 90% substantial heterogeneity;
-
75% to 100% considerable heterogeneity.
If there was considerable heterogeneity (I2 > 75%) which could not be explained by the subgroup analyses, we downgraded the level of evidence by two levels.
Assessment of reporting biases
If there were more than 10 studies included in a meta‐analysis, we assessed the possible presence of reporting bias by testing for asymmetry in a funnel plot.
If present, we planned to carry out statistical analysis using the methods described in Section 10.4.3.1 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) for continuous and dichotomous data. However, we did not find any reporting bias in our review.
Data synthesis
We analyzed the data using Review Manager software (Review Manager 2020). If the data available from the studies had similar comparisons and outcomes, we undertook meta‐analysis. Our general approach was to use a random‐effects model. With this approach, the CIs for the average intervention effect were wider than those obtained using a fixed‐effect approach, leading to a more conservative interpretation. We combined change scores and end scores in the analysis.
Subgroup analysis and investigation of heterogeneity
If there was significant heterogeneity, we explored the reasons by performing subgroup analyses based on participant smoking status and on severity of disease (chronic and aggressive periodontitis). We intended to use other factors like age, socioeconomic status, and oral hygiene habits of participants but we could not get such data to perform subgroup analyses.
Sensitivity analysis
Provided there were sufficient included studies, we intended to do sensitivity analyses based on risk of bias, including only studies at low risk of bias. However, the majority of the included studies had unclear risk of bias and hence we could not do the sensitivity analysis.
Summary of findings and presentation of main results
We used the GRADE approach to interpret findings (Schünemann 2011) and two review authors (SK and SKN), independently interpreted the findings and through discussions, consensus was arrived. We used GRADEprofiler GDT software (GRADEpro GDT) and imported the data from Review Manager 2020 to create 'Summary of findings' tables and assess the certainty of the evidence for comparisons reporting long‐term follow‐up (≥1 year) for the following outcomes: percentage of closed pockets, clinical attachment level, probing pocket depth, percentage of bleeding on probing, antimicrobial resistance, adverse events, and patient‐reported quality of life changes. These tables provided the information concerning the overall certainty of the evidence from the trials, the magnitude of effect of the interventions examined, and the sum of available data on the primary and secondary outcomes. The GRADE approach (Schünemann 2011) considers certainty to be a judgement of the extent to which we can be confident that the estimates of effect are correct. A body of evidence from randomized controlled studies is initially graded as high and downgraded by one or two levels on each of five domains after full consideration of: any limitations in the design of the studies, the directness (or applicability) of the evidence, the consistency of results, precision of the results, and the possibility of publication bias. A certainty level of high reflects confidence that the true effect lies close to that of the estimate of the effect for an outcome. A judgement of moderate certainty indicates that the true effect is likely to be close to the estimate of the effect, but acknowledges the possibility that it could be substantially different. Low‐ and very low‐certainty evidence limit our confidence in the effect estimate (Balshem 2011).
Results
Description of studies
Results of the search
The electronic search strategies identified 2741 records from English and other language databases and cross‐references of included trials and other systematic reviews. At the end of our screening of titles and abstracts, we removed 2615 records. Two review authors independently and in duplicate assessed the remaining 126 studies to determine their eligibility. We excluded 58 studies (65 reports) for the reasons listed in Characteristics of excluded studies table. Six studies were classified as Studies awaiting classification as the full text could not be obtained. Three are ongoing trials. We identified 45 studies (52 reports) that met the inclusion criteria and included them in this review qualitatively and 38 (42 reports) studies quantitatively (Figure 3).
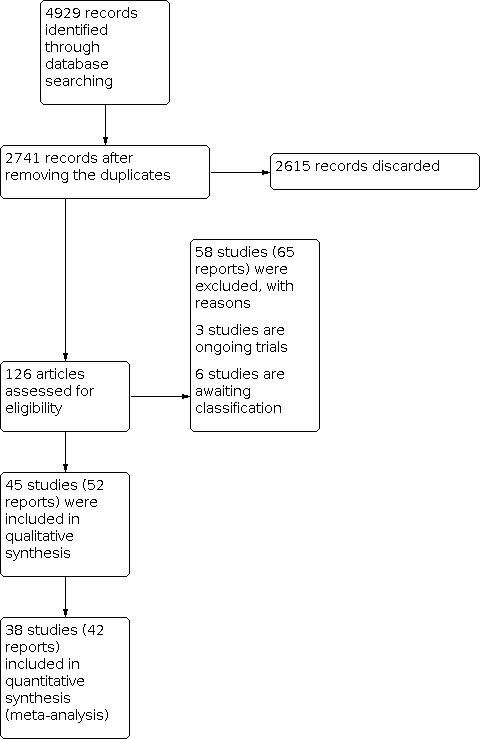
Study flow diagram.
Included studies
We have described these 45 studies in more detail in the Characteristics of included studies table.
Characteristics of the trial participants, design, and settings
Age: the participants in all the included studies, a total of 2664, were 18 years old and above.
Trial arms: of the 45 studies, 14 had multiple treatment arms ranging from three to five. There were nine trials with three arms (Chin Quee 1988; Cosgarea 2016; Dukic 2016; Liaw 2019; Li 2015; Lu 2012; Matarazzo 2008; Sigusch 2000; Silva 2011); four trials with four arms (Carvalho 2004; D'avila 2005; Sigusch 2001; Xajigeorgiou 2006); and one trial with five arms (Borges 2017). Remaining 30 were parallel‐arm studies.
Location: the trials have been done worldwide.
-
Australia: one trial (Liaw 2019).
-
Brazil: 13 trials (Araujo 2019; Borges 2017; Carvalho 2004; Casarin 2012; D'avila 2005; Gomi 2007; Haas 2008; Matarazzo 2008; Rebelatto 2017; Ribeiro 2009; Sampaio 2011; Silva 2011; Taiete 2016).
-
China: four trials (Chin Quee 1988; Li 2015; Lu 2012; Zhang 2006).
-
Colombia: two trials (Ardila 2015; Guzman 2011).
-
Germany: four trials (Cosgarea 2016; Harks 2015; Sigusch 2000; Sigusch 2001).
-
Greece: one trial (Xajigeorgiou 2006).
-
India: four trials (Martande 2016; Pradeep 2011; Pradeep 2014; Pradeep 2015).
-
Iran: one trial (Moeintaghavi 2007).
-
Netherlands: two trials (Winkel 1999; Winkel 2001).
-
Romania: one trial (Boia 2019).
-
Serbia: one trial (Dukic 2016).
-
Switzerland: one trial (Mombelli 2013).
-
Turkey: five trials (Akincibay 2008; Basegmez 2011; Emingil 2012; Han 2012; Yek 2010).
-
UK: three trials (Abu Fanas 1991; Palmer 1996; Smith 2002).
-
USA: two trials (Loesche 1991; Mascarenhas 2005).
Funding: mostly the trials had received funding from the academic institutions, research offices, or local bodies. Eight trials had obtained the funding from pharmaceutical companies (Chin Quee 1988; Dukic 2016; Loesche 1991; Martande 2016; Mascarenhas 2005; Pradeep 2014; Pradeep 2015; Winkel 1999).
Characteristics of the outcomes
Only four studies (Borges 2017; Carvalho 2004; Matarazzo 2008; Sampaio 2011) reported the primary outcome i.e. percentage of closed pockets.
None of the included trials reported on antimicrobial resistance and patient‐reported quality of life changes.
Most studies reported clinical/relative attachment level (CAL), pocket depth, and bleeding on probing (BOP). Five studies (Abu Fanas 1991; Akincibay 2008; Sigusch 2001; Taiete 2016; Xajigeorgiou 2006) did not report BOP outcome.
Adverse events: nine studies (Abu Fanas 1991; Ardila 2015; Carvalho 2004; Emingil 2012; Haas 2008; Han 2012; Lu 2012; Martande 2016; Smith 2002) reported there were no adverse events amongst their study participants. Seven studies (Akincibay 2008; Basegmez 2011; Mascarenhas 2005; Palmer 1996; Sigusch 2000; Sigusch 2001; Zhang 2006) did not investigate adverse events in their trials. The remaining studies reported adverse events either in the intervention or control group or all groups.
Outcome measurement: all included trials used periodontal probes to measure the CAL, probing pocket depth, BOP, and percentage of closed pockets. Only four trials (Borges 2017; Carvalho 2004; Matarazzo 2008; Sampaio 2011) reported the percentage of closed pockets (expressed as percentage). Pocket depth and CAL are measured in millimetres (mm) and BOP is expressed as percentage.
Characteristics of the interventions
The characteristics of the interventions have been summarized in Additional Table 1.
| Serial number | Antibiotic prescribed | Type of periodontitis | Included studies |
|---|---|---|---|
| 1. | AMOX + MTZ | Chronic periodontitis | Boia 2019; Borges 2017; Cosgarea 2016; Dukic 2016; Liaw 2019; Li 2015; Matarazzo 2008; Moeintaghavi 2007; Mombelli 2013; Ribeiro 2009; Silva 2011; Winkel 2001 |
| Aggressive periodontitis | Casarin 2012; Lu 2012; Taiete 2016; Xajigeorgiou 2006; Yek 2010 | ||
| Both chronic and aggressive periodontitis | |||
| 2. | MTZ | Chronic periodontitis | Carvalho 2004; D'avila 2005; Loesche 1991; Matarazzo 2008; Sigusch 2000; Silva 2011 |
| Aggressive periodontitis | |||
| 3. | AZT | Chronic periodontitis | Gomi 2007; Han 2012; Liaw 2019; Martande 2016; Mascarenhas 2005; Sampaio 2011; Smith 2002 |
| Aggressive periodontitis | |||
| 4. | AMOX + CLAV | Chronic periodontitis | |
| AMOX + CLAV versus tetracycline | Aggressive periodontitis | ||
| 5. | Minocycline | Chronic periodontitis | |
| 6. | Spiramycin | Chronic periodontitis | |
| 7. | Tetracycline | Aggressive periodontitis | |
| 8. | Cefixime | Chronic periodontitis | |
| 9 . | Doxycycline | Aggressive periodontitis | |
| 10. | Clindamycin | Aggressive periodontitis | |
| 11. | MTZ versus AMOX + MTZ | Both chronic and aggressive periodontitis | |
| 12. | Doxycycline versus AMOX + MTZ | Aggressive periodontitis | |
| 13. | Doxycycline versus MTZ | Aggressive periodontitis | |
| 14. | Moxifloxacin | Aggressive periodontitis | |
| 15. | Levofloxacin | Chronic periodontitis | |
| 16. | Doxycycline versus clindamycin | Aggressive periodontitis | |
| 17. | Clarithromycin | Both chronic and aggressive periodontitis | |
| 18. | Clindamycin versus MTZ | Aggressive periodontitis | |
| 19. | AMOX+MTZ versus clarithromycin | Aggressive periodontitis |
AMOX = amoxicillin; AZT = azithromycin; CLAV = clavulanate; MTZ = metronidazole.
-
Eighteen trials compared the participants receiving amoxicillin (AMOX) + metronidazole (MTZ) (any dose or duration) + scaling and root planing (SRP) with SRP (control or no AMOX + MTZ) in periodontitis (chronic/aggressive).
-
-
Twelve trials evaluated AMOX + MTZ + SRP in chronic periodontitis patients (Boia 2019; Borges 2017; Cosgarea 2016; Dukic 2016; Liaw 2019; Li 2015; Matarazzo 2008; Moeintaghavi 2007; Mombelli 2013; Ribeiro 2009; Silva 2011; Winkel 2001).
-
Five trials examined AMOX + MTZ + SRP in aggressive periodontitis patients (Casarin 2012; Lu 2012; Taiete 2016; Xajigeorgiou 2006; Yek 2010).
-
One trial examined AMOX + MTZ + SRP in both chronic and aggressive periodontitis patients (Harks 2015).
-
-
Eight trials compared the participants receiving MTZ (any dose or duration) + SRP with SRP (control or no MTZ) in periodontitis (chronic/aggressive).
-
-
Six trials evaluated MTZ + SRP in chronic periodontitis patients (Carvalho 2004; D'avila 2005; Loesche 1991; Matarazzo 2008; Sigusch 2000; Silva 2011).
-
Two trials examined MTZ + SRP in aggressive periodontitis patients (Sigusch 2001; Xajigeorgiou 2006).
-
-
Nine trials compared the participants receiving azithromycin (AZT) (any dose or duration) + SRP with SRP (control or no AZT) in periodontitis (chronic/aggressive).
-
-
Seven trials evaluated the AZT + SRP in chronic periodontitis patients (Gomi 2007; Han 2012; Liaw 2019; Martande 2016; Mascarenhas 2005; Sampaio 2011; Smith 2002).
-
Two trials examined AZT + SRP in aggressive periodontitis patients (Emingil 2012; Haas 2008).
-
-
Other interventions.
-
-
AMOX + clavulanate (CLAV) + SRP: two trials: one compared with SRP (Winkel 1999); one compared with tetracycline + SRP (Abu Fanas 1991).
-
Minocycline + SRP compared to SRP: one trial (Basegmez 2011).
-
Spiramycin + SRP compared to SRP: one trial (Chin Quee 1988).
-
Tetracycline + SRP compared to SRP: one trial (Palmer 1996).
-
Cefixime + SRP compared to SRP: one trial (Dukic 2016).
-
Doxycycline + SRP compared to SRP: three trials (Sigusch 2000; Sigusch 2001; Xajigeorgiou 2006).
-
Clindamycin + SRP compared to SRP: one trial (Sigusch 2001).
-
MTZ + SRP compared to AMOX + MTZ + SRP: three trials (Matarazzo 2008; Silva 2011; Xajigeorgiou 2006).
-
Doxycycline + SRP compared to AMOX + MTZ + SRP: two trials (Akincibay 2008; Xajigeorgiou 2006).
-
Doxycycline + SRP compared to MTZ + SRP: three trials (Sigusch 2000; Sigusch 2001; Xajigeorgiou 2006).
-
Moxifloxacin + SRP compared to SRP: one trial (Ardila 2015).
-
Moxifloxacin + SRP compared to MTZ + ciprofloxacin + SRP: one trial (Guzman 2011).
-
Levofloxacin + SRP compared to SRP: two trials (Pradeep 2014; Pradeep 2015).
-
Doxycycline + SRP compared to clindamycin + SRP: one trial (Sigusch 2001).
-
Clarithromycin + SRP compared to SRP: two trials (Pradeep 2011; Rebelatto 2017).
-
Clindamycin + SRP compared to MTZ + SRP: one trial (Sigusch 2001).
-
AMOX + MTZ + SRP compared to clarithromycin + SRP: one trial (Araujo 2019).
-
Dosing regimens
There is no standardization of the dosing regimens of antibiotics (Additional Table 2).
| Serial number | Dosage | Number of days | Included studies |
|---|---|---|---|
| 1. AMOX + MTZ | |||
| i. | 500 mg AMOX and 500 mg MTZ | 3 | |
| ii. | 500 mg AMOX and 500 mg MTZ | 4 | |
| iii. | 500 mg AMOX and 500 mg MTZ | 7 | |
| iv. | 500 mg AMOX and 400 mg MTZ | 7 | |
| v. | 375 mg AMOX and 500 mg MTZ | 7 | |
| vi. | 375 mg AMOX and 250 mg MTZ | 7 | |
| vii. | 500 mg AMOX and 200 mg MTZ | 7 | |
| viii. | 500 mg AMOX and 250 mg MTZ | 7 | |
| ix. | 500 mg AMOX and 400 mg MTZ | 14 | |
| x. | 500 mg AMOX and 250 mg MTZ | 14 | |
| 2. MTZ alone | |||
| i. | 500 mg thrice/day | 7 | |
| ii. | 500 mg twice/day | 8 | |
| iii. | 400 mg thrice/day | 10 | |
| iv. | thrice/day | 14 | |
| 3. AZT | |||
| i. | 500 mg once/day | 3 | Emingil 2012; Gomi 2007; Haas 2008; Han 2012; Liaw 2019; Martande 2016 |
| ii. | 500 mg once/day | 5 | |
| iii. | 250 mg | Double stat first day followed by once/day for next 4 days | |
| 4. AMOX + CLAV | |||
| i. | 625 mg thrice/day | 10 days | |
| 5. Doxycycline | |||
| i. | 200 mg/day | 8 | |
| ii. | 200 mg 100 mg/day | On first day 14 | |
| 6. Tetracycline | |||
| i. | 250 mg 4 times/day | 14 | |
| ii. | 500 mg/day | Not mentioned | |
| 7. Clarithromycin | |||
| i. | 500 mg twice/day | 3 | |
| ii. | 500 mg twice/day | 7 | |
| 8. Moxifloxacin | |||
| i. | 400 mg/day | 7 | |
| 9. Levofloxacin | |||
| i. | 500 mg once/day | 10 | |
| 10. Clindamycin | |||
| i. | 150 mg 4 times/day | 8 | |
| 11. Cefixime | |||
| i. | 400 mg once/day | 7 | |
AMOX = amoxicillin; AZT = azithromycin; CLAV = clavulanate; MTZ = metronidazole.
AMOX + MTZ has been used in different dosages for different time periods. Trials have prescribed the combination for.
-
Three days: 500 mg AMOX and 500 mg MTZ (Boia 2019).
-
Four days: 500 mg AMOX and 500 mg MTZ (Cosgarea 2016).
-
Seven days: 500 mg AMOX and 500 mg MTZ (Boia 2019; Cosgarea 2016; Xajigeorgiou 2006; Yek 2010); 500 mg AMOX and 400 mg MTZ (Araujo 2019; Borges 2017; Dukic 2016; Harks 2015; Liaw 2019); 375 mg AMOX and 500 mg MTZ (Casarin 2012; Mombelli 2013); 375 mg AMOX and 250 mg MTZ (Ribeiro 2009; Taiete 2016; Winkel 2001); 500 mg AMOX and 200 mg MTZ (Li 2015; Lu 2012); 500 mg AMOX and 250 mg MTZ (Borges 2017).
-
14 days: 500 mg AMOX and 400 mg MTZ (Borges 2017; Matarazzo 2008; Silva 2011); 500 mg AMOX and 250 mg MTZ (Borges 2017).
MTZ alone has also been prescribed in varying dosing regimens.
-
Seven days: 500 mg MTZ thrice/day (Xajigeorgiou 2006).
-
Eight days: 500 mg MTZ twice/day (Sigusch 2000; Sigusch 2001).
-
10 days: 400 mg MTZ thrice/day (Carvalho 2004).
-
14 days: 400 mg MTZ thrice/day (Matarazzo 2008; Silva 2011).
AZT was used according to the following dosing regimens.
-
Three days: 500 mg AZT once/day (Emingil 2012; Gomi 2007; Haas 2008; Han 2012; Liaw 2019; Martande 2016).
-
Five days: 500 mg AZT once/day (Sampaio 2011); 250 mg AZT double stat first day followed by once/day for next four days (Mascarenhas 2005).
AMOX + CLAV was prescribed as 625 mg thrice/day for 10 days (Abu Fanas 1991; Winkel 1999).
Doxycycline was prescribed as 200 mg/day for eight days (Sigusch 2000; Sigusch 2001) and 200 mg on first day followed by 100 mg/day for 14 days (Xajigeorgiou 2006).
Tetracycline was used as 250 mg four times/day for 14 days (Abu Fanas 1991; Palmer 1996) and 500 mg/day (Zhang 2006).
Clarithromycin was reported in dose of 500 mg twice/day for three days (Pradeep 2011; Rebelatto 2017) and seven days (Araujo 2019).
Moxifloxacin was used in one study as a dosage of 400 mg/day for seven days (Ardila 2015).
Levofloxacin was prescribed as 500 mg once/day for 10 days (Pradeep 2014; Pradeep 2015).
Clindamycin was reported in dose of 150 mg four times/day for eight days (Sigusch 2001).
Cefixime was prescribed in the dosage of 400 mg once/day for seven days (Dukic 2016).
Follow‐up period
Trials had different follow‐up periods (Additional Table 3). We classified the results based on the duration of follow‐up as follows.
-
Short term: ≤ 3 months follow‐up.
-
Intermediate term: > 3 months and < 1 year follow‐up.
-
Long term: ≥ 1 year follow‐up.
The studies which followed up only short term were: Akincibay 2008; Boia 2019; Chin Quee 1988; D'avila 2005; Dukic 2016; Li 2015; Liaw 2019; Loesche 1991; Lu 2012; Matarazzo 2008; Moeintaghavi 2007; Mombelli 2013; Palmer 1996; Silva 2011; Winkel 2001.
The studies which followed up long term were: Borges 2017; Carvalho 2004; Harks 2015; Martande 2016; Sampaio 2011; Sigusch 2000; Sigusch 2001; Winkel 1999; Zhang 2006.
The studies which had multiple follow‐up periods were: Abu Fanas 1991; Araujo 2019; Ardila 2015; Basegmez 2011; Borges 2017; Carvalho 2004; Casarin 2012; Cosgarea 2016; Emingil 2012; Gomi 2007; Guzman 2011; Haas 2008; Han 2012; Martande 2016; Mascarenhas 2005; Pradeep 2011; Pradeep 2014; Pradeep 2015; Rebelatto 2017; Ribeiro 2009; Sampaio 2011; Sigusch 2000; Smith 2002; Taiete 2016; Winkel 1999; Xajigeorgiou 2006; Yek 2010; Zhang 2006.
Excluded studies
We excluded 58 studies with reasons (see Characteristics of excluded studies table). We excluded four studies (Boia 2018; Caton 1997; Ehmke 2005; UMIN000012033) as these were not randomized controlled trials (RCTs). 19 studies had used adjunctive local drug delivery or chlorhexidine topical use (ACTRN12617000531314; Cionca 2009; El‐Fadl 2015; Feres 2012; Flemmig 1998; Guentsch 2008; Guerrero 2005; Guzeldemir 2015; NCT00066066; Heller 2011; Jentsch 2016; Kaner 2011; Mestnik 2010; Mombelli 2012; Nepokupnaia 2014; Oliveira 2012; Oteo 2010; Preus 2017; Zhao 2006). The studies using chlorhexidine mouthwash were excluded as it is implicated to have beneficial effect (when administered along with SRP) on the periodontal parameters when compared to SRP alone (Magnusson 1984; da Costa 2017; Oh 2018). In one study, antibiotics were given one month after non‐surgical periodontal therapy (Soder 1990). Seven studies (Ciancio 1981; Ciancio 1984; Di Murro 1986; Eisenberg 1991; Hartmann 1986; Helldean 1979; Muller 1986) had used a split‐mouth study design. Surgical interventions were performed in six studies (Giannopoulou 2006; Kone 2005; Kunihira 1985; Loesche 1992; Sterry 1985; Tinoco 1998). Five studies (Cigana 1989; Counsell 1972; Helovuo 1989; Listgarten 1978; Wang 1996) either did partial SRP or did not do SRP in the participants. Four studies included patients who were not eligible according to the inclusion criteria of this review (Clark 1983; Javed 2014; Re 1988; Rooney 2002). Nine studies either were microbiological studies or have recorded clinical parameters partially (Carvalho 2005; Guerrero 2014; Haas 2012; Feres 1999; Jenkins 1989; Joyston 1986; Shayesteh 2004; Tsarev 1998; Watts 1986). In one study, probiotics were prescribed as intervention (Murugesan 2018) and in another herbal products were used (NCT01499225). In one study Helovuo 1989; SRP was not done so it was excluded. In one study Feres 2017, timings of antibiotics was different in the two groups, hence it was excluded.
Risk of bias in included studies
We documented the risk of bias for included studies based on the full‐text articles. Wherever there was a need for clarification, we tried contacting the authors. Based on the available data, we assessed the risk of bias as low, high, or unclear. 14 trials were at low overall risk of bias (Araujo 2019; Casarin 2012; Cosgarea 2016; Emingil 2012; Guzman 2011; Haas 2008; Han 2012; Martande 2016; Mombelli 2013; Pradeep 2014; Rebelatto 2017; Ribeiro 2009; Silva 2011; Taiete 2016), eight trials at high risk of bias (Chin Quee 1988; Gomi 2007; Harks 2015; Liaw 2019; Mascarenhas 2005; Moeintaghavi 2007; Pradeep 2015; Sigusch 2000), and the remaining 23 trials at unclear risk of bias (Figure 4). See Figure 5 for a summary of the judgements of the risk of bias for each domain in each of the included trials.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
We assessed two trials as at high risk of bias in random sequence generation (Liaw 2019; Mascarenhas 2005).
Twenty‐two trials have unclear risk of selection bias as the method of allocation concealment was not adequately reported (Abu Fanas 1991; Akincibay 2008; Ardila 2015; Basegmez 2011; Carvalho 2004; Chin Quee 1988; D'avila 2005; Dukic 2016; Gomi 2007; Li 2015; Loesche 1991; Lu 2012; Palmer 1996; Pradeep 2011; Pradeep 2015; Sigusch 2000; Sigusch 2001; Smith 2002; Winkel 2001; Xajigeorgiou 2006; Yek 2010; Zhang 2006) and 23 trials have adequately reported the allocation concealment and thus were at low risk (Araujo 2019; Boia 2019; Borges 2017; Casarin 2012; Cosgarea 2016; Emingil 2012; Guzman 2011; Haas 2008; Han 2012; Harks 2015; Liaw 2019; Martande 2016; Mascarenhas 2005; Matarazzo 2008; Moeintaghavi 2007; Mombelli 2013; Pradeep 2014; Rebelatto 2017; Ribeiro 2009; Sampaio 2011; Silva 2011; Taiete 2016; Winkel 1999).
Blinding
None of the studies were reported to have high risk of performance and detection bias. We assessed 21 trials as having unclear risk of performance and detection bias (Abu Fanas 1991; Akincibay 2008; Boia 2019; Carvalho 2004; Chin Quee 1988; D'avila 2005; Dukic 2016; Gomi 2007; Harks 2015; Li 2015; Loesche 1991; Lu 2012; Matarazzo 2008; Palmer 1996; Sigusch 2000; Sigusch 2001; Smith 2002; Winkel 1999; Winkel 2001; Yek 2010; Zhang 2006). The rest of included studies had low risk of performance and detection bias.
Incomplete outcome data
We assessed one trial as at high risk of attrition bias (Harks 2015) and nine trials as having unclear risk of attrition bias (Abu Fanas 1991; Ardila 2015; Borges 2017; Carvalho 2004; Liaw 2019; Loesche 1991; Moeintaghavi 2007; Sigusch 2000; Sigusch 2001). The rest of the studies had low risk of attrition bias.
Selective reporting
We assessed four studies as at high risk of reporting bias (Chin Quee 1988; Gomi 2007; Moeintaghavi 2007; Sigusch 2000) and one trial as having unclear risk of reporting bias (Akincibay 2008).
Other potential sources of bias
Two trials had high risk of other bias (Liaw 2019; Pradeep 2015) and two trials had unclear risk of other bias (Harks 2015; Sampaio 2011). Rest of the studies had low risk of other potential sources of bias.
Effects of interventions
See: Summary of findings 1 Amoxicillin + metronidazole + SRP compared to SRP for the non‐surgical treatment of periodontitis; Summary of findings 2 Metronidazole + SRP compared to SRP for the non‐surgical treatment of periodontitis; Summary of findings 3 Azithromycin + SRP compared to SRP for the non‐surgical treatment of periodontitis; Summary of findings 4 Amoxicillin + clavulanate + SRP compared to SRP for the non‐surgical treatment of periodontitis; Summary of findings 5 Doxycycline + SRP compared to SRP for the non‐surgical treatment of periodontitis; Summary of findings 6 Tetracycline + SRP compared to SRP for the non‐surgical treatment of periodontitis; Summary of findings 7 Clindamycin + SRP compared to SRP for the non‐surgical treatment of periodontitis; Summary of findings 8 Doxycycline + SRP compared to metronidazole + SRP for the non‐surgical treatment of periodontitis; Summary of findings 9 Clindamycin + SRP compared to metronidazole + SRP for the non‐surgical treatment of periodontitis; Summary of findings 10 Clindamycin + SRP compared to doxycycline + SRP for the non‐surgical treatment of periodontitis
We included 45 trials in the review. Seven trials did not contribute data to the analyses. We have presented the results under 18 comparisons. Under each comparison, we analyzed the outcomes based on the duration of follow‐up as short‐term improvement (≤ 3 months), intermediate‐term improvement (> 3 months to < 1 year), and long‐term improvement (≥ 1 year). We did not find the definition of 'minimally important clinical difference' or MICD for the outcomes percentage of closed pockets, CAL, probing pocket depth, and percentage of BOP in the literature. Hence, we decided to use a mean difference (MD) of 1 mm as the MICD for CAL and pocket depth, and 5% for percentage of BOP, and respective 95% confidence interval (CI) not to cross the line of no effect. As the minimum measurement seen on a periodontal probe is 1 mm, we agreed to use this as the MICD for CAL and pocket depth. Most included studies used an inclusion criteria of 20 teeth minimum to be present. Based on this, we calculated the MICD for percentage of closed pockets and percentage of BOP as 5% (BOP of 1 tooth out of 20 teeth). All the analyses have been interpreted according to the MICD values mentioned. Thus, if MD was lesser than MICD, it was interpreted as 'no observable effect' if the CI was not crossing the line of no effect.
1. Amoxicillin (AMOX) + metronidazole (MTZ) + scaling and root planing (SRP) versus SRP in chronic and aggressive periodontitis
Percentage of closed pockets
There was evidence of a difference in percentage of closed pockets at short term, intermediate term, and long term favouring the AMOX + MTZ + SRP group: MD ‐10.05, 95% CI ‐18.78 to ‐1.32; 2 studies, 73 participants; I2 = 0% (Analysis 1.1); MD ‐13.90, 95% CI ‐24.16 to ‐3.64; 1 study, 44 participants; I2 = 0% (Analysis 1.2); and MD ‐16.20, 95% CI ‐25.87 to ‐6.53; 1 study, 44 participants; I2 = 0% (Analysis 1.3), respectively.
CAL
There was no evidence of a difference in CAL at short term, intermediate term, and long term for either AMOX + MTZ + SRP group or SRP group: MD ‐0.21 mm, 95% CI ‐0.41 to ‐0.01; 13 studies, 501 participants; I2 = 33% (Analysis 1.4); MD ‐0.04 mm, 95% CI ‐0.35 to 0.26; 6 studies, 215 participants; I2 = 33% (Analysis 1.5); and MD ‐0.47 mm, 95% CI ‐0.90 to ‐0.05; 2 studies, 389 participants; I2 = 42% (Analysis 1.6), respectively.
The subgroup analysis for chronic periodontitis showed no heterogeneity (0%) and the subgroup analysis for aggressive periodontitis showed substantial heterogeneity (61%). As the data in Yek 2010 appeared to be interchanged between the intervention and control groups in the publication, we did sensitivity analysis by excluding the results of this study and there was 0% heterogeneity after the exclusion.
We checked for reporting bias using the funnel plot. There was symmetrical distribution of studies suggesting no possibility of reporting bias.
Probing pocket depth
There was no evidence of a difference in pocket depth at short term, intermediate term, and long term for either AMOX + MTZ + SRP group or SRP group: MD ‐0.18 mm, 95% CI ‐0.33 to ‐0.04; 15 studies, 562 participants; I2 = 68% (Analysis 1.7); MD ‐0.47 mm, 95% CI ‐0.68 to ‐0.26; 6 studies, 215 participants; I2 = 63% (Analysis 1.8); and MD ‐0.30 mm, 95% CI ‐0.42 to ‐0.18; 2 studies, 389 participants; I2 = 0% (Analysis 1.9), respectively.
The subgroup analysis for chronic periodontitis showed moderate heterogeneity (57%). We explored the possible reason for the heterogeneity by conducting subgroup analyses for chronic periodontitis studies which have included non‐smokers (Borges 2017; Liaw 2019; Silva 2011; Winkel 2001; Moeintaghavi 2007) and studies which have included smokers and non‐smokers (Cosgarea 2016; Dukic 2016; Li 2015; Matarazzo 2008; Mombelli 2013). The subgroup analyses showed negligible heterogeneity which confirmed the smoking status as the reason for the clinical heterogeneity.
We checked for reporting bias using the funnel plot. There was symmetrical distribution of studies suggesting no possibility of reporting bias.
Percentage of BOP
There was no evidence of a difference in percentage of BOP at short term for either AMOX + MTZ + SRP group or SRP group (MD ‐2.05, 95% CI ‐2.98 to ‐1.11; 13 studies, 460 participants; I2 = 90%; Analysis 1.10). There was evidence of a difference in percentage of BOP at intermediate term and long term for the AMOX + MTZ + SRP group: MD ‐5.32, 95% CI ‐12.02 to 1.37; 5 studies, 172 participants; I2 = 99% (Analysis 1.11) and MD ‐8.06, 95% CI ‐14.26 to ‐1.85; 2 studies, 389 participants; I2 = 47% (Analysis 1.12), respectively.
In Analysis 1.10, we performed sensitivity analysis for the chronic periodontitis subgroup by excluding Ribeiro 2009 as the data were extracted from the graph. The heterogeneity was nil and the overall effect estimate was not affected.
In Analysis 1.11, there was considerable heterogeneity (85%) in the subgroup analysis for chronic periodontitis. We conducted sensitivity analysis by removing the results of Ribeiro 2009 whose data were extracted from the graph and the heterogeneity reduced to 54%. The other reason could be that the Borges 2017 and Ribeiro 2009 trials included non‐smokers whereas Cosgarea 2016 included both smokers and non‐smokers.
We checked for reporting bias using the funnel plot for both the short‐ and intermediate‐term results. There was symmetrical distribution of the studies suggesting the minimal possibility of reporting bias.
Adverse events
All the included trials for AMOX + MTZ + SRP reported adverse events, except for Lu 2012 which did not have any adverse effects. Most common adverse events were mild gastrointestinal disturbances, nausea and vomiting, metallic taste and diarrhoea in the intervention group. Control group reported adverse events like hypothermia, mild gastrointestinal symptoms, diarrhoea, vomiting, cramps, rashes, and weakness. Winkel 2001 reported one event of nausea after alcohol intake.
Other outcomes
None of the included trials under this comparison reported other outcomes (antimicrobial resistance and patient‐reported quality of life changes).
2. MTZ + SRP versus SRP in chronic and aggressive periodontitis
Percentage of closed pockets
There was no evidence of a difference in percentage of closed pockets at short‐, intermediate‐, and long‐term follow‐ups for either MTZ + SRP group or SRP group: MD ‐2.56, 95% CI ‐12.33 to 7.22; 2 studies, 51 participants; I2 = 0% (Analysis 2.1); MD ‐7.00, 95% CI ‐23.57 to 9.57; 1 study, 22 participants; I2 = 0% (Analysis 2.2); and MD ‐12.20, 95% CI ‐29.23 to 4.83; 1 study, 22 participants; I2 = 0% (Analysis 2.3), respectively.
CAL
There was no evidence of a difference in CAL at short term and intermediate term for either MTZ + SRP group or SRP group: MD 0.04 mm, 95% CI ‐0.43 to 0.50; 4 studies, 108 participants; I2 = 51% (Analysis 2.4) and MD ‐0.49 mm, 95% CI ‐1.29 to 0.31; 4 studies, 94 participants; I2 = 74% (Analysis 2.5), respectively. However, long‐term follow‐up showed improvement in CAL favouring the use of MTZ + SRP compared to SRP alone (MD ‐1.12 mm, 95% CI ‐2.24 to ‐0.00; 3 studies, 71 participants; I2 = 85%; Analysis 2.6).
In Analysis 2.5, the subgroup analysis for aggressive periodontitis showed considerable heterogeneity (85%). This heterogeneity could be because of Sigusch 2001 excluded smokers and Xajigeorgiou 2006 included both smokers and non‐smokers.
Probing pocket depth
There was no evidence of a difference in pocket depth at short‐term and intermediate‐term follow‐ups for either MTZ + SRP group or SRP group: MD ‐0.10 mm, 95% CI ‐0.37 to 0.16; 4 studies, 108 participants; I2 = 38% (Analysis 2.7) and MD ‐0.59 mm, 95% CI ‐0.95 to ‐0.23; 4 studies, 94 participants; I2 = 25% (Analysis 2.8), respectively. However, there was evidence of minimum improvement in pocket depth at long‐term follow‐up for the MTZ + SRP group when compared to control group (MD ‐1.11 mm, 95% CI ‐2.84 to 0.61; 2 studies, 47 participants; I2 = 95%; Analysis 2.9).
Percentage of BOP
There was evidence of a difference in percentage of BOP at short term (MD ‐9.00, 95% CI ‐16.59 to ‐1.40; 4 studies, 108 participants; I2 = 0%; Analysis 2.10) favouring the MTZ + SRP group. However, the confidence intervals in the intermediate‐term (MD ‐4.62, 95% CI ‐18.83 to 9.58; 2 studies, 45 participants; I2 = 24%; Analysis 2.11) and long‐term (MD ‐6.90, 95% CI ‐22.10 to 8.30; 1 study, 22 participants; I2 = 0%; Analysis 2.12) follow‐ups are crossing the line of no effect.
Adverse events
Sigusch 2001 did not consider this outcome and Carvalho 2004 reported there were no adverse events. Other two studies (Matarazzo 2008; Silva 2011) reported nausea, vomiting, and diarrhoea in the intervention group and in Silva 2011, one participant from the MTZ + SRP group reported that the schedules for taking the drugs disturbed his day‐to‐day activities and, if needed, he would not like to take the drugs again.
Other outcomes
None of the included trials reported other outcomes (antimicrobial resistance and patient‐reported quality of life changes).
3. Azithromycin (AZT) + SRP versus SRP in chronic and aggressive periodontitis
Percentage of closed pockets
There was no evidence of a difference in percentage of closed pockets at intermediate‐term and long‐term follow‐ups for either AZT+ SRP group or SRP group: MD ‐2.90, 95% CI ‐13.91 to 8.11; 1 study, 40 participants; I2 = 0% (Analysis 3.1) and MD 2.50, 95% CI ‐10.19 to 15.19; 1 study, 40 participants; I2 = 0% (Analysis 3.2), respectively.
CAL
There was no evidence of a difference in CAL at short‐term, intermediate‐term, and long‐term follow‐ups for either AZT + SRP group or SRP group: MD ‐0.51 mm, 95% CI ‐0.83 to ‐0.19; 6 studies, 216 participants; I2 = 47% (Analysis 3.3); MD ‐0.43 mm, 95% CI ‐0.77 to ‐0.10; 7 studies, 258 participants; I2 = 57% (Analysis 3.4); and MD ‐0.59 mm, 95% CI ‐1.27 to 0.08; 2 studies, 110 participants; I2 = 70% (Analysis 3.5), respectively.
In Analysis 3.3, chronic periodontitis showed moderate heterogeneity (61%). We explored the reason for this heterogeneity by pooling the studies excluding smokers (Gomi 2007) and studies including smokers and non‐smokers (Han 2012; Liaw 2019; Mascarenhas 2005). However, we could not find the reason for the heterogeneity.
In Analysis 3.4, chronic periodontitis showed moderate heterogeneity (57%). We explored the reason for this heterogeneity by pooling the studies excluding smokers (Gomi 2007; Sampaio 2011) and studies including smokers and non‐smokers (Han 2012; Mascarenhas 2005). The subgroup analysis confirmed the reason for heterogeneity.
Probing pocket depth
There was no evidence of a difference in pocket depth at short‐term, intermediate‐term, and long‐term follow‐ups for either AZT + SRP group or SRP group: MD ‐0.57 mm, 95% CI ‐1.08 to ‐0.07; 6 studies, 216 participants; I2 = 91% (Analysis 3.6); MD ‐0.60 mm, 95% CI ‐1.02 to ‐0.18; 7 studies, 258 participants; I2 = 88% (Analysis 3.7); and MD ‐0.77 mm, 95% CI ‐2.33 to 0.79; 2 studies, 110 participants; I2 = 98% (Analysis 3.8), respectively.
In Analysis 3.6, chronic periodontitis showed considerable heterogeneity (91%). This heterogeneity was due to the pooling of studies including non‐smokers (Gomi 2007) and studies including smokers and non‐smokers (Han 2012; Liaw 2019; Mascarenhas 2005).
In Analysis 3.7, chronic periodontitis showed considerable heterogeneity (88%). We did a subgroup analysis of studies including non‐smokers (Gomi 2007) and studies including smokers and non‐smokers (Han 2012; Mascarenhas 2005; Sampaio 2011). This showed negligible heterogeneity in the analysis including smokers and non‐smokers; however, there was considerable heterogeneity (82%) in the analysis including non‐smokers which could not be explained. Aggressive periodontitis showed considerable heterogeneity (77%) which could be due the pooling of studies excluding smokers who smoke < 10 cigarettes per day (Emingil 2012) and study including smokers and non‐smokers (Haas 2008).
Percentage of BOP
There was no evidence of a difference in percentage of BOP at short‐term, intermediate‐term, and long‐term follow‐ups for either AZT + SRP group or SRP group: MD ‐3.18, 95% CI ‐7.01 to 0.65; 6 studies, 216 participants; I2 = 49% (Analysis 3.9); MD ‐3.32, 95% CI ‐6.23 to ‐0.42; 6 studies, 228 participants; I2 = 30% (Analysis 3.10); and MD ‐1.28, 95% CI ‐4.32 to 1.76; 2 studies, 110 participants; I2 = 25% (Analysis 3.11), respectively.
In Analysis 3.9, chronic periodontitis showed substantial heterogeneity (60%). We did a subgroup analysis of studies including non‐smokers (Gomi 2007) and studies including smokers and non‐smokers (Han 2012; Liaw 2019; Mascarenhas 2005). This showed negligible heterogeneity in the analysis including smokers and non‐smokers; however, there was considerable heterogeneity (88%) in the analysis including non‐smokers which could not be explained.
Adverse events
Two studies (Gomi 2007; Liaw 2019) reported mild gastrointestinal discomfort in four patients from the AZT + SRP group and one patient from the SRP group. Sampaio 2011 reported adverse events (diarrhoea, headache or dizziness, metallic taste, and general unwellness) by four participants from the AZT + SRP group and three from the control group. Mascarenhas 2005 did not mention any details of adverse events and there were no adverse events in Emingil 2012, Haas 2008, Han 2012 and Martande 2016.
Other outcomes
None of the included trials reported other outcomes (antimicrobial resistance and patient‐reported quality of life changes).
4. AMOX + clavulanate (CLAV) + SRP versus SRP in chronic periodontitis
CAL
There was no evidence that one intervention performed better than the other with regards to CAL improvement at short‐, intermediate‐, and long‐term follow‐ups, as the 95% CI crossed the line of no effect: MD 0.10 mm, 95% CI ‐0.43 to 0.63; 1 study, 21 participants (Analysis 4.1); MD 0.10 mm, 95% CI ‐0.45 to 0.65; 1 study, 21 participants (Analysis 4.2); and MD 0.10 mm, 95% CI ‐0.51 to 0.71; 1 study, 21 participants (Analysis 4.3), respectively.
Probing pocket depth
There was no evidence that one intervention performed better than the other with regards to improvement in pocket depth at short‐, intermediate‐, and long‐term follow‐ups, as the 95% CI crossed the line of no effect: MD 0.20 mm, 95% CI ‐0.02 to 0.42; 1 study, 21 participants (Analysis 4.4); MD 0.00 mm, 95% CI ‐0.19 to 0.19; 1 study, 21 participants (Analysis 4.5); and MD 0.10 mm, 95% CI ‐0.17 to 0.37; 1 study, 21 participants (Analysis 4.6), respectively.
Percentage of BOP
There was no evidence that one intervention performed better than the other with regards to improvement in percentage of BOP at short‐, intermediate‐, and long‐term follow‐ups: MD ‐0.10, 95% CI ‐0.19 to ‐0.01; 1 study, 21 participants (Analysis 4.7); MD ‐0.10, 95% CI ‐0.19 to ‐0.01; 1 study, 21 participants (Analysis 4.8); and MD 0.00, 95% CI ‐0.09 to 0.09; 1 study, 21 participants (Analysis 4.9), respectively.
Adverse events
Winkel 1999 reported diarrhoea in two patients from both groups.
Other outcomes
The included trial under this comparison did not report other outcomes (percentage of closed pockets, antimicrobial resistance, and patient‐reported quality of life changes).
5. Doxycycline + SRP versus SRP in aggressive periodontitis
CAL
There was no evidence of a difference in CAL at short‐, intermediate‐, or long‐term follow‐ups for either doxycycline + SRP or SRP group: MD 0.15 mm, 95% CI ‐0.53 to 0.83; 2 studies, 43 participants; I2 = 5% (Analysis 5.1); MD ‐0.61 mm, 95% CI ‐1.14 to ‐0.08; 3 studies, 65 participants; I2 = 0% (Analysis 5.2); and MD ‐0.80 mm, 95% CI ‐1.49 to ‐0.11; 1 study, 22 participants (Analysis 5.3), respectively.
Probing pocket depth
There was no evidence of a difference in pocket depth at short term and intermediate term for either doxycycline + SRP or SRP group: MD 0.10 mm, 95% CI ‐0.29 to 0.50; 2 studies, 43 participants; I2 = 0% (Analysis 5.4); and MD ‐0.43 mm, 95% CI ‐0.88 to 0.01; 3 studies, 65 participants; I2 = 0% (Analysis 5.5). However, there was evidence of a difference in pocket depth at long‐term follow‐up for the doxycycline group compared to the control group (MD ‐1.00 mm, 95% CI ‐1.78 to ‐0.22; 1 study, 22 participants; Analysis 5.6).
As the heterogeneity was substantial in Analysis 5.4, we explored the reason. Sigusch 2001 excluded smokers and Xajigeorgiou 2006 included smokers and non‐smokers and this difference could be reason for the heterogeneity.
Percentage of BOP
There was no evidence of a difference in percentage of BOP at short or intermediate follow‐up for either doxycycline + SRP or SRP group: MD ‐0.09, 95% CI ‐0.29 to 0.11; 1 study, 21 participants (Analysis 5.7) and MD ‐0.01, 95% CI ‐0.21 to 0.19; 1 study, 21 participants (Analysis 5.8), respectively.
Adverse events
None of the trials reported any adverse events in either of the groups.
Other outcomes
None of the included trials reported other outcomes (percentage of closed pockets, antimicrobial resistance, and patient‐reported quality of life changes).
6. Tetracycline + SRP versus SRP in aggressive periodontitis
CAL
There was no evidence that tetracycline + SRP performed better than SRP alone with regards to improvement in CAL at short‐term follow‐up (MD ‐0.92 mm, 95% CI ‐2.38 to 0.54; 2 studies, 64 participants; I2 = 88%; Analysis 6.1). However, there was evidence of a difference in CAL at intermediate‐ and long‐term follow‐ups for the tetracycline group compared to the control group: MD ‐1.90 mm, 95% CI ‐2.17 to ‐1.63; 1 study, 26 participants (Analysis 6.2) and MD ‐2.30 mm, 95% CI ‐2.50 to ‐2.10; 1 study, 26 participants (Analysis 6.3), respectively.
We explored the possible reasons for substantial heterogeneity in the short‐term results. Palmer 1996 included adults and juveniles in the study where 12 years was the minimum age and only adults were included in Zhang 2006. This could be the probable reason for the heterogeneity.
Probing pocket depth
There was no evidence that the tetracycline + SRP group performed better than the SRP group with regards to improvement in pocket depth at short‐term follow‐up (MD ‐0.53 mm, 95% CI ‐1.14 to 0.08; 1 study, 38 participants; Analysis 6.4).
Percentage of BOP
There was evidence that tetracycline + SRP performed better than SRP alone with regards to improvement in percentage of BOP at short‐term follow‐up (MD ‐14.62, 95% CI ‐27.21 to ‐2.03; 1 study, 38 participants; Analysis 6.5).
Other outcomes
Other outcomes (percentage of closed pockets, antimicrobial resistance, adverse events, long‐term stability of pocket depth, and patient‐reported quality of life changes) were not reported in this comparison.
7. Clarithromycin + SRP versus SRP in chronic and aggressive periodontitis
CAL
There was no evidence of a difference in CAL improvement at short and intermediate terms for either clarithromycin + SRP or SRP group: MD ‐0.65 mm, 95% CI ‐1.20 to ‐0.09; 2 studies, 77 participants; I2 = 91% (Analysis 7.1) and MD ‐0.64 mm, 95% CI ‐1.26 to ‐0.03; 2 studies, 77 participants; I2 = 92% (Analysis 7.2), respectively.
Probing pocket depth
There was no evidence of a difference in pocket depth improvement at short and intermediate terms for either clarithromycin + SRP or SRP group: MD ‐0.59 mm, 95% CI ‐1.17 to ‐0.01; 2 studies, 77 participants; I2 = 91% (Analysis 7.3 ) and MD ‐0.67 mm, 95% CI ‐1.33 to ‐0.01; 2 studies, 77 participants; I2 = 93% (Analysis 7.4), respectively.
Percentage of BOP
There was no evidence of a difference in improvement of percentage of BOP at short and intermediate terms for either clarithromycin + SRP or SRP group: MD ‐3.00, 95% CI ‐6.69 to 0.69; 1 study, 40 participants (Analysis 7.5) and MD ‐4.70, 95% CI ‐8.42 to ‐0.98; 1 study, 40 participants (Analysis 7.6), respectively.
Adverse events
Both included trials (Pradeep 2011; Rebelatto 2017) reported adverse events in the clarithromycin + SRP group. Pradeep 2011 reported unpalatable taste and mild gastric intolerance in two patients and Rebelatto 2017 reported mild gastrointestinal discomfort in two patients.
Other outcomes
Other outcomes (percentage of closed pockets, antimicrobial resistance, long‐term stability of pocket depth and CAL, and patient‐reported quality of life changes) were not reported in this comparison.
8. Moxifloxacin + SRP versus SRP in chronic periodontitis
CAL
There was no evidence of a difference in improvement of CAL at short term and intermediate term for either moxifloxacin + SRP or SRP group: MD ‐0.67 mm, 95% CI ‐1.01 to ‐0.33; 1 study, 40 participants (Analysis 8.1) and MD ‐0.63 mm, 95% CI ‐0.95 to ‐0.31; 1 study, 40 participants (Analysis 8.2), respectively.
Probing pocket depth
There was no evidence of a difference in improvement of pocket depth at short term and intermediate term for either moxifloxacin + SRP or SRP group: MD ‐0.55 mm, 95% CI ‐0.87 to ‐0.23; 1 study, 40 participants (Analysis 8.3) and MD ‐0.42 mm, 95% CI ‐0.74 to ‐0.10; 1 study, 40 participants (Analysis 8.4), respectively.
Percentage of BOP
There was no evidence of a difference in improvement of percentage of BOP at short term and intermediate term for either moxifloxacin + SRP or SRP group: MD ‐3.40, 95% CI ‐3.72 to ‐3.08; 1 study, 40 participants (Analysis 8.5) and MD ‐0.80, 95% CI ‐1.12 to ‐0.48; 1 study, 40 participants (Analysis 8.6), respectively.
Adverse events
The included trial (Ardila 2015) had no adverse events amongst the participants.
Other outcomes
Other outcomes (percentage of closed pockets, antimicrobial resistance, long‐term stability of pocket depth and CAL, and patient‐reported quality of life changes) were not reported in this comparison.
9. Levofloxacin + SRP versus SRP in chronic periodontitis
CAL
There was no evidence of a difference in improvement of CAL at short term and intermediate term for either levofloxacin + SRP or SRP group: MD ‐1.02 mm, 95% CI ‐1.39 to ‐0.65; 2 studies, 131 participants; I2 = 43% (Analysis 9.1) and MD ‐1.25 mm, 95% CI ‐1.51 to ‐0.99; 2 studies, 131 participants; I2 = 0%; (Analysis 9.2), respectively.
Probing pocket depth
There was no evidence of a difference in improvement of pocket depth at short term and intermediate term for either levofloxacin + SRP or SRP group: MD ‐1.09 mm, 95% CI ‐1.33 to ‐0.85; 2 studies, 131 participants; I2 = 0% (Analysis 9.3) and MD ‐1.23 mm, 95% CI ‐1.46 to ‐1.00; 2 studies, 131 participants; I2 = 0% (Analysis 9.4), respectively.
Percentage of BOP
There was no evidence of a difference in improvement of percentage of BOP at short term and intermediate term for either levofloxacin + SRP or SRP group: MD ‐1.38, 95% CI ‐3.30 to 0.54; 2 studies, 131 participants; I2 = 0% (Analysis 9.5) and MD ‐0.93, 95% CI ‐2.88 to 1.02; 2 studies, 131 participants; I2 = 0% (Analysis 9.6), respectively.
Adverse events
Both included trials (Pradeep 2014; Pradeep 2015) reported dizziness and diarrhoea in the levofloxacin + SRP group.
Other outcomes
Other outcomes (percentage of closed pockets, antimicrobial resistance, long‐term stability of pocket depth and CAL, and patient‐reported quality of life changes) were not reported in this comparison.
10. Clindamycin + SRP versus SRP in aggressive periodontitis
CAL
There was no evidence of a difference in improvement of CAL at short‐term follow‐up for either clindamycin + SRP or SRP alone group (MD 0.40 mm, 95% CI ‐0.35 to 1.15; 1 study, 21 participants; Analysis 10.1). However, the intermediate‐ and long‐term follow‐ups showed improvement in CAL in the intervention group compared to control group: MD ‐1.30 mm, 95% CI ‐2.14 to ‐0.46; 1 study, 21 participants (Analysis 10.2) and MD ‐1.70 mm, 95% CI ‐2.40 to ‐1.00; 1 study, 21 participants (Analysis 10.3), respectively.
Probing pocket depth
There was no evidence of a difference in improvement of pocket depth at short term for either clindamycin + SRP or SRP group (MD 0.10 mm, 95% CI ‐0.55 to 0.75; 1 study, 21 participants; Analysis 10.4). However, the intermediate‐term and long‐term follow‐ups showed improvement of pocket depth in the clindamycin + SRP group compared to control group: MD ‐1.10 mm, 95% CI ‐1.94 to ‐0.26; 1 study, 21 participants (Analysis 10.5) and MD ‐1.80 mm, 95% CI ‐2.47 to ‐1.13; 1 study, 21 participants (Analysis 10.6), respectively.
Other outcomes
Other outcomes (percentage of closed pockets, percentage of BOP, antimicrobial resistance, adverse events, and patient‐reported quality of life changes) were not reported in this comparison.
11. Cefixime + SRP versus SRP in chronic periodontitis
CAL
There was no evidence of a difference in improvement of CAL at short‐term follow‐up for either group (MD ‐0.19 mm, 95% CI ‐0.77 to 0.39; 1 study, 60 participants; Analysis 11.1).
Probing pocket depth
There was no evidence of a difference in improvement of pocket depth at short‐term follow‐up for either group (MD ‐0.02 mm, 95% CI ‐0.11 to 0.07; 1 study, 60 participants; Analysis 11.2).
Percentage of BOP
There was no evidence of a difference in improvement of percentage of BOP at short‐term follow‐up for either group (MD ‐0.48, 95% CI ‐0.60 to ‐0.37; 1 study, 60 participants; Analysis 11.3).
Adverse events
The only included trial (Dukic 2016) reported nausea, dizziness and/or anorexia in the cefixime + SRP group.
Other outcomes
Other outcomes (percentage of closed pockets, antimicrobial resistance, long‐term stability of pocket depth and CAL, and patient‐reported quality of life changes) were not reported in this comparison.
12. MTZ + SRP versus AMOX + MTZ + SRP for chronic and aggressive periodontitis
CAL
There was no evidence of a difference in improvement of CAL at short‐term (chronic and aggressive periodontitis) and intermediate‐term (aggressive periodontitis) follow‐ups for either group: MD ‐0.07 mm, 95% CI ‐0.40 to 0.26; 3 studies, 84 participants; I2 = 0% (Analysis 12.1) and MD 0.06 mm, 95% CI ‐1.06 to 1.18; 1 study, 22 participants (Analysis 12.2), respectively.
Probing pocket depth
There was no evidence of a difference in improvement of pocket depth at short‐term (chronic and aggressive periodontitis) and intermediate‐term (aggressive periodontitis) follow‐ups for either group: MD 0.10 mm, 95% CI ‐0.12 to 0.32; 3 studies, 84 participants; I2 = 0% (Analysis 12.3) and MD ‐0.26 mm, 95% CI ‐0.83 to 0.31; 1 study, 22 participants (Analysis 12.4), respectively.
Percentage of BOP
There was no evidence of a difference in improvement of percentage of BOP at short‐term follow‐up for either group in chronic and aggressive periodontitis (MD 4.46, 95% CI ‐23.48 to 32.40; 3 studies, 84 participants; I2 = 94%; Analysis 12.5). However, there was evidence of difference in improvement of percentage of BOP at intermediate‐term follow‐up favouring the AMOX + MTZ +SRP group compared to MTZ + SRP group in aggressive periodontitis (MD 20.85, 95% CI 3.31 to 38.39; 1 study, 22 participants; Analysis 12.6).
Adverse events
All three included trials (Matarazzo 2008; Silva 2011; Xajigeorgiou 2006) reported adverse events in both MTZ + SRP and AMOX + MTZ + SRP groups. Matarazzo 2008 reported nausea, vomiting, and diarrhoea in both groups and Silva 2011 reported diarrhoea, headache, metallic taste, vomiting, and irritability in the MTZ + SRP group, and headache, metallic taste, vomiting, and irritability in the AMOX + MTZ + SRP group. In the same study, one subject from the MTZ group reported that the schedules for taking the drugs disturbed his day‐to‐day activities and, if needed, he would not like to take the drugs again. Xajigeorgiou 2006 reported mild gastrointestinal discomfort in two subjects from the AMOX + MTZ + SRP group and a metallic taste in one subject from the MTZ + SRP group.
Other outcomes
Other outcomes ( percentage of closed pockets, antimicrobial resistance, long‐term stability of pocket depth and CAL, and patient‐reported quality of life changes) were not reported in this comparison.
13. Doxycycline + SRP versus AMOX + MTZ + SRP for aggressive periodontitis
CAL
There was no evidence of a difference in improvement of CAL at short term and intermediate term for either group: MD 0.20 mm, 95% CI 0.06 to 0.33; 2 studies, 50 participants; I2 = 0% (Analysis 13.1) and MD 0.17 mm, 95% CI ‐1.28 to 1.62; 1 study, 20 participants (Analysis 13.2), respectively.
Probing pocket depth
There was no evidence of a difference in improvement of pocket depth at short term and intermediate term for either group: MD 0.14 mm, 95% CI ‐0.12 to 0.40; 2 studies, 50 participants; I2 = 0% (Analysis 13.3) and MD 0.23 mm, 95% CI ‐0.41 to 0.87; 1 study, 20 participants (Analysis 13.4), respectively.
Adverse events
Out of two included trials, one trial (Akincibay 2008) did not report this outcome. Xajigeorgiou 2006 reported mild gastrointestinal discomfort in two subjects from the AMOX + MTZ + SRP group.
Other outcomes
Other outcomes (percentage of closed pockets, percentage of BOP, antimicrobial resistance, long‐term stability of pocket depth and CAL, and patient‐reported quality of life changes) were not reported in this comparison.
14. Doxycycline + SRP versus MTZ + SRP in aggressive periodontitis
CAL
There was no evidence of a difference in improvement of CAL at short‐ and intermediate‐term follow‐ups for either group: MD ‐0.12 mm, 95% CI ‐0.72 to 0.49; 2 studies, 49 participants; I2 = 0% (Analysis 14.1) and MD 0.34 mm, 95% CI ‐0.08 to 0.76; 3 studies, 75 participants; I2 = 0% (Analysis 14.2), respectively. However, on long‐term follow‐up, there was an improvement of CAL in the MTZ + SRP group compared to the doxycycline +SRP group (MD 1.10 mm, 95% CI 0.36 to 1.84; 1 study, 27 participants; Analysis 14.3).
Probing pocket depth
There was no evidence of a difference in improvement of pocket depth at short‐ and intermediate‐term follow‐ups for either group: MD ‐0.16 mm, 95% CI ‐0.56 to 0.24; 2 studies, 49 participants; I2 = 3% (Analysis 14.4) and MD 0.38 mm, 95% CI ‐0.00 to 0.76; 3 studies, 75 participants; I2 = 0% (Analysis 14.5), respectively. However, on long‐term follow‐up, there was an improvement in pocket depth in the MTZ + SRP group compared to the doxycycline + SRP group (MD 1.00 mm, 95% CI 0.30 to 1.70; 1 study, 27 participants; Analysis 14.6).
Percentage of BOP
There was no evidence of a difference in improvement of percentage of BOP at short‐ and intermediate‐term follow‐ups for either group: MD ‐0.05, 95% CI ‐0.22 to 0.12; 1 study, 22 participants (Analysis 14.7) and MD ‐0.07, 95% CI ‐0.29 to 0.15; 1 study, 22 participants (Analysis 14.8), respectively.
Other outcomes
Other outcomes (percentage of closed pockets, antimicrobial resistance, adverse events, and patient‐reported quality of life changes) were not reported in this comparison.
15. Clindamycin + SRP versus MTZ + SRP in aggressive periodontitis
CAL
There was no evidence of a difference in improvement of CAL at short‐, intermediate‐, and long‐term follow‐ups for either group: MD 0.40 mm, 95% CI ‐0.22 to 1.02; 1 study, 26 participants (Analysis 15.1); MD 0.10 mm, 95% CI ‐0.60 to 0.80; 1 study, 26 participants (Analysis 15.2); and MD 0.20 mm, 95% CI ‐0.55 to 0.95; 1 study, 26 participants (Analysis 15.3), respectively.
Probing pocket depth
There was no evidence of a difference in improvement of pocket depth at short‐, intermediate‐, and long‐term follow‐ups for either group: MD ‐0.14 mm, 95% CI ‐0.82 to 0.54; 1 study, 26 participants (Analysis 15.4); MD ‐0.10 mm, 95% CI ‐0.79 to 0.59; 1 study, 26 participants (Analysis 15.5); and MD 0.20 mm, 95% CI ‐0.38 to 0.78; 1 study, 26 participants (Analysis 15.6), respectively.
Other outcomes
Other outcomes (percentage of closed pockets, percentage of BOP, antimicrobial resistance, adverse events, and patient‐reported quality of life changes) were not reported in this comparison.
16. AMOX + CLAV + SRP versus tetracycline + SRP in aggressive periodontitis
Probing pocket depth
There was no evidence of a difference in pocket depth at intermediate‐term follow‐up for either group (MD ‐0.40 mm, 95% CI ‐1.11 to 0.31; 1 study, 8 participants; Analysis 16.1).
Percentage of BOP
There was no evidence of a difference in percentage of BOP at intermediate‐term follow‐up for either group (MD ‐1.60, 95% CI ‐14.03 to 10.83; 1 study, 8 participants; Analysis 16.2).
Adverse events
The only included trial (Abu Fanas 1991) reported there were no adverse events.
Other outcomes
Other outcomes (percentage of closed pockets, CAL, antimicrobial resistance, long‐term stability of pocket depth and CAL, and patient‐reported quality of life changes) were not reported in this comparison.
17. Clindamycin + SRP versus doxycycline + SRP in aggressive periodontitis
CAL
There was no evidence of a difference in CAL at short‐term, intermediate‐term, and long‐term follow‐ups for either group: MD 0.50 mm, 95% CI ‐0.26 to 1.26; 1 study, 23 participants (Analysis 17.1); MD ‐0.40 mm, 95% CI ‐1.15 to 0.35; 1 study, 23 participants (Analysis 17.2); and MD ‐0.90 mm, 95% CI ‐1.62 to ‐0.18; 1 study, 23 participants (Analysis 17.3), respectively.
Probing pocket depth
There was no evidence of a difference in pocket depth at short‐term, intermediate‐term, and long‐term follow‐ups for either group: MD 0.20 mm, 95% CI ‐0.36 to 0.76; 1 study, 23 participants (Analysis 17.4); MD ‐0.40 mm, 95% CI ‐1.14 to 0.34; 1 study, 23 participants (Analysis 17.5); and MD ‐0.80 mm, 95% CI ‐1.58 to ‐0.02; 1 study, 23 participants (Analysis 17.6), respectively.
Other outcomes
The only included trial in this comparison (Sigusch 2001) has not reported on any other outcomes (percentage of closed pockets, percentage of BOP, antimicrobial resistance, adverse events, and patient‐reported quality of life changes).
18. AMOX + MTZ + SRP versus clarithromycin + SRP in aggressive periodontitis
CAL
There was no evidence of a difference in CAL at short term and intermediate term for either of the groups: MD ‐0.60 mm, 95% CI ‐0.86 to ‐0.34; 1 study, 46 participants (Analysis 18.1); and MD 0.00 mm, 95% CI ‐0.26 to 0.26; 1 study, 46 participants (Analysis 18.2).
Probing pocket depth
There was no evidence of a difference in probing pocket depth at short term and intermediate term for either of the groups: MD ‐0.20 mm, 95% CI ‐0.35 to ‐0.05; 1 study, 46 participants (Analysis 18.3); and MD ‐0.10 mm, 95% CI ‐0.26 to 0.06; 1 study, 46 participants (Analysis 18.4).
Percentage of BOP
There was no evidence of a difference in percentage of BOP at short term and intermediate term for either of the groups: MD 5.40, 95% CI ‐0.12 to 10.92; 1 study, 46 participants (Analysis 18.5); and MD 4.00, 95% CI ‐1.09 to 9.09; 1 study, 46 participants (Analysis 18.6).
Adverse events
Two patients were reported to have vomiting and unbearable stomach pain in the AMOX + MTZ + SRP group (Araujo 2019).
Other outcomes
Other outcomes (percentage of closed pockets, antimicrobial resistance, long‐term stability of pocket depth and CAL, and patient‐reported quality of life changes) were not reported in this comparison.
Discussion
Summary of main results
summary of findings Table 1; summary of findings Table 2; summary of findings Table 3; summary of findings Table 4; summary of findings Table 5; summary of findings Table 6; summary of findings Table 7; summary of findings Table 8; summary of findings Table 9; and summary of findings Table 10.
We included 45 trials in the review and out of these, 38 are included in the analysis. We assessed the certainty of the evidence for the 10 comparisons which reported long‐term follow‐up (≥1 year). None of the included studies reported data on the secondary outcomes of antimicrobial resistance and patient‐reported quality of life changes.
-
Amoxicillin (AMOX) + metronidazole (MTZ) + scaling and root planing (SRP) versus SRP: the long‐term outcomes measured under this comparison were percentage of closed pockets, clinical attachment level (CAL), probing pocket depth, and percentage of bleeding on probing (BOP) in patients with chronic and aggressive periodontitis. We found very low‐certainty evidence for all the outcomes under this comparison. Based on our definition of minimally important clinical difference (MICD), there was only evidence of a difference favouring AMOX + MTZ + SRP for the outcomes percentage of closed pockets and percentage of BOP. All the included trials under this comparison reported adverse events, except for one trial which did not have any adverse effects.
-
MTZ + SRP versus SRP: we found very low‐certainty evidence for long‐term improvement in chronic periodontitis for the outcomes percentage of closed pockets and percentage of BOP. The evidence was also of very low certainty for the outcomes CAL and probing pocket depth for the long‐term improvement in chronic and aggressive periodontitis. Only the long‐term results for CAL and probing pocket depth showed an MICD favouring the MTZ + SRP group. Two out of three included studies did not report adverse events while no severe adverse effects were reported by any of the participants in the third study.
-
Azithromycin + SRP versus SRP: we found no evidence of a difference in long‐term improvement in CAL, probing pocket depth, and percentage of BOP in patients with chronic and aggressive periodontitis; and in long‐term improvement in percentage of closed pockets in patients with chronic periodontitis (very low‐certainty evidence for all outcomes). One study reported adverse events (mild gastrointestinal discomfort) and in the second none of the individuals reported any adverse effects.
-
AMOX + clavulanate + SRP versus SRP: we found no evidence of a difference in long‐term improvement in CAL, probing pocket depth, and percentage of BOP in patients with chronic periodontitis (very low‐certainty evidence for all outcomes). One study reported diarrhoea in two participants from both groups.
-
Doxycycline + SRP versus SRP: in patients with aggressive periodontitis the evidence was of very low certainty regarding long‐term improvement in CAL and probing pocket depth, with the doxycycline + SRP group showing an MICD in pocket depth only. None of the trials reported any adverse events in either of the groups.
-
Tetracycline + SRP versus SRP: in patients with aggressive periodontitis there was very low‐certainty evidence of a difference in long‐term improvement in CAL for the tetracycline group. Included studies under this comparison did not report any details on adverse events.
-
Clindamycin + SRP versus SRP: in patients with aggressive periodontitis there was very low‐certainty evidence of a difference in long‐term improvement in CAL and probing pocket depth favouring clindamycin + SRP. Included study under this comparison did not report any details on adverse events.
-
Doxycycline + SRP versus MTZ + SRP: in patients with aggressive periodontitis there was very low‐certainty evidence of a difference in long‐term improvement in CAL and probing pocket depth favouring MTZ + SRP. None of the included trials reported adverse events.
-
Clindamycin + SRP versus MTZ + SRP: in patients with aggressive periodontitis we found no evidence of a difference regarding long‐term improvement in CAL and probing pocket depth (very low‐certainty evidence for both outcomes). Included study under this comparison did not report any details on adverse events.
-
Clindamycin + SRP versus doxycycline + SRP: in patients with aggressive periodontitis we found no evidence of a difference regarding long‐term improvement in CAL and probing pocket depth (very low‐certainty evidence for both outcomes). Included study under this comparison did not report any details on adverse events.
Overall completeness and applicability of evidence
We systematically searched for trials according to the previously published protocol. We did an independent Google search and checked all cross references of included articles and other systematic reviews on the topic to be sure that we did not miss any article. Two pairs of review authors did data extraction in duplicate. Trials, which were not included in the meta‐analysis were explained qualitatively. We selected trials with adult participants diagnosed with chronic or aggressive periodontitis or both who have undergone mechanical treatment (SRP) along with systemic antibiotic administration and included all types of interventions with different medications and combination of medications. We included comparisons with placebo and control. All clinically relevant outcomes of interest were analysed. For trials reporting data in graphs, we derived the data using PlotDigitizer® software. When mean and standard error (SE) were given, we calculated the standard deviation (SD) as described in the Cochrane Handbook for Systematic Reviews of Interventions Section 7.7.3.3 (Higgins 2011).
We also included trials in which older terminologies like 'rapidly progressing periodontitis' or 'early onset periodontitis' or 'juvenile periodontitis' were used. Four of the included studies (Abu Fanas 1991; Palmer 1996; Sigusch 2000; Sigusch 2001) used one of these terminologies and we compiled the results from these studies under the heading of aggressive periodontitis.
We could not include results of seven trials in the meta‐analysis. We could not include the results of Basegmez 2011 as outcome measures were given in median and interquartile ranges were not mentioned or 5th and 95th percentile data. Chin Quee 1988 had no data related to the outcomes of interest and hence was not included in the meta‐analysis. Casarin 2012 and D'avila 2005 did not give SD for the outcomes, nor we could impute, and hence we could not include them in the meta‐analysis. Haas 2008; Loesche 1991; and Smith 2002 described data based on the pocket depth (3 mm, 4 mm to 6 mm, and ≥ 7 mm) and overall changes in the outcome was not available.
In multi‐arm trials, we excluded the data from those arms in which the antibiotic dose was subclinical (Jeske 2017) and included only common clinical dosing regimens.
Quality of the evidence
The certainty of the evidence for all comparisons was very low for the long‐term outcomes. We downgraded the trials mainly for three reasons: risk of bias, inconsistency, and imprecision. Most of the trials were downgraded by one level for unclear risk of bias, by two levels for high risk of bias, and downgraded by two levels for imprecision as most were single trials with limited number of participants and low event rates with wide confidence intervals crossing the line of no effect. We downgraded the trials for inconsistency by one level if there was moderate heterogeneity and by two levels if there was either considerable or substantial heterogeneity which could not be explained clinically.
Potential biases in the review process
We have taken steps to minimize bias in every step of the review process. All the mentioned databases, conference proceedings, and trial registries were searched to include all the relevant reports. We tried to contact study authors for missing data through emails. If the reports were very old, we tried to get the contact details of the authors through peer contacts, Google search, and university/hospital websites where they were previously affiliated. Wherever data were not available, we derived the data from the graphs using the PlotDigitizer software to minimize the bias. We tried our best to follow the methodology stated in the protocol. We used standard methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), and ensured compliance with Cochrane standards for the conduct of new reviews of interventions (MECIR 2020). In spite of our comprehensive search strategies, we cannot rule out publication bias occurring due to non‐identification of unpublished trials.
Agreements and disagreements with other studies or reviews
We found more than 15 systematic reviews related to this topic. However, we decided to discuss the findings of systematic reviews which were closely related to our review objectives and had similar inclusion and exclusion criteria. We identified a total of 10 systematic reviews on adjunctive use of various systemic antimicrobials with SRP for chronic/aggressive periodontitis which were published after the year 2014 as mentioned below.
Kolakovic 2014 conducted a systematic review to plot the clinical benefits of the combination of amoxicillin and metronidazole in pocket resolution and avoidance of additional surgical interventions. Mean and standard deviations of "pocket closure" for pocket depth ≤ 3 mm and odds ratios for "avoidance of surgical intervention" for pocket depth ≤ 5 mm from the published literature were assessed statistically. Results showed that the administration of antibiotics resulted in a 3.55 and 4.43 fold higher probability of pocket closure after three months and six months as compared to mechanical therapy alone. However, statistical results failed to show a benefit regarding the possible avoidance of surgical interventions. Large heterogeneity regarding treatment protocol, dose of antibiotic medication, and maintenance was observed amongst the included studies.
In systematic reviews to compare the adjunct use of antibiotics in chronic periodontitis, Keestra 2015 searched the PubMed/MEDLINE database from their earliest records through 16 May 2013 and analyzed mean BOP change, mean CAL gain, and mean probing pocket depth reduction as the outcome measures. They concluded on the additional benefits of systemic antibiotics in the reduction of moderate to deep pockets, and that none of the antibiotics gave statistically significant clinical benefits over the other.
Following the same methodology as mentioned above for aggressive periodontitis too, Keestra 2015a searched records until 20 January 2014 and found that systemic antibiotics demonstrated significant benefits in pocket depth reduction and clinical attachment gain for moderate and deep pockets again. The review highlighted metronidazole + amoxicillin to be the most potent antibiotic combination in aggressive periodontitis.
Rabelo 2015 conducted a systematic review and Bayesian network meta‐analysis from literature searched up to June 2014 of randomized controlled trials comparing the treatment of patients with aggressive periodontitis followed up six months. Results showed additional benefits in clinical attachment gain and pocket depth reduction when SRP was associated with systemic antibiotics. This review concluded metronidazole and metronidazole/amoxicillin to be giving most favourable clinical benefits.
Similarly Smiley 2015 conducted a systematic review comparing adjuncts to SRP (local antibiotics or antimicrobials, systemic antibiotics, combinations of local and systemic antibiotics, agents for bio modification or host modulation, or non‐surgical lasers) versus SRP alone in patients with chronic periodontitis, which is not comparable to our review criteria.
Renatus 2016 conducted a systematic review on the possible benefit of azithromycin as an adjunct to SRP. However, authors took into account both systemic and topical azithromycin in their review, which is not comparable to our review criteria.
Zandbergen 2016 conducted a systematic review to check the effects of concomitant systemic administration of amoxicillin and metronidazole as an adjunct to SRP compared to SRP alone in patients with chronic periodontitis with respect to mean treatment outcome (end scores versus baseline) in terms of pocket depth, CAL, BOP, and plaque indices (PI). The results of the meta‐analysis performed indicated that despite the caveats concerning the heterogeneity of experimental designs, there was moderate to strong evidence that SRP + amoxicillin + metronidazole showed significantly superior clinical outcomes in terms of pocket depth and CAL (especially in initially deep pockets (≥ 6 mm)) compared to SRP alone. It concluded that SRP + amoxicillin + metronidazole might therefore reduce the need for additional periodontal therapy which would assumedly be of a surgical nature in many cases. No major adverse events associated with the intake of amoxicillin + metronidazole were reported.
Jagannathan 2019 did a multivariate meta‐analysis to jointly synthesize six‐month outcomes of azithromycin as adjunctive to SRP in chronic periodontitis, and to investigate three potential sources of heterogeneity (timing of azithromycin, type of SRP (full‐mouth debridement or partial‐mouth debridement), and baseline study‐level mean values of pocket depth/CAL). The analysis concluded that at six months, azithromycin + SRP showed a benefit over the control group for pocket depth when CAL was accounted for. When the role of three potential sources of heterogeneity was investigated, greater pocket depth reduction was associated with azithromycin initiation.
Teughels 2020 conducted a similar systematic review of randomized controlled trials with a follow‐up of at least six months to assess the efficacy of adjunctive systemic antimicrobials, in comparison with subgingival debridement plus a placebo in periodontitis patients, in terms of probing pocket depth reduction. The use of systemic antimicrobials as an adjunct to SRP, specifically metronidazole + amoxicillin, resulted in statistically significant greater probing pocket depth reduction, higher percentage of pocket closure, and reduction in frequency of pockets. The additional probing pocket depth reduction and CAL gain elicited by metronidazole + amoxicillin, and to a lesser extent by metronidazole and azithromycin, were more pronounced in initially deep than in initially moderately deep pockets. These clinical effects were maintained up to 12 months after their use. There was no evidence above two years of follow‐up for the benefits of systemic antimicrobials as adjuncts to SRP. There were no indications that the effect of systemic antimicrobials was different between aggressive and chronic periodontitis patients. Among the different types of systemic antimicrobials, the use of metronidazole + amoxicillin was associated with the largest frequency of side effects. Metronidazole and azithromycin had a significant impact on some outcome measures, but smaller than metronidazole + amoxicillin in terms of magnitude.
Mendes 2020 conducted a systematic review and meta‐analysis to evaluate whether in patients with aggressive periodontitis, metronidazole + amoxicillin in combination with SRP would lead to better results in terms of gain in CAL and reduction in probing depth when compared with SRP alone. It was concluded that there was no statistically significant difference in CAL gain between the use of systemic antibiotics with SRP and the use of SRP alone in the treatment of aggressive periodontitis. However, there was a statistically significant difference in probing depth, favouring the combination of systemic antibiotics and SRP.

Etiopathogenesis of periodontal disease.

Classification of periodontitis (Armitage 1999; Geurs 2015).
AL = attachment loss; PD = pocket depth.

Study flow diagram.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Comparison 1: AMOX + MTZ + SRP versus SRP, Outcome 1: Percentage of closed pockets ‐ short‐term (≤ 3 months) improvement

Comparison 1: AMOX + MTZ + SRP versus SRP, Outcome 2: Percentage of closed pockets ‐ intermediate‐term (> 3 months) improvement

Comparison 1: AMOX + MTZ + SRP versus SRP, Outcome 3: Percentage of closed pockets ‐ long‐term (≥ 1 year) improvement

Comparison 1: AMOX + MTZ + SRP versus SRP, Outcome 4: CAL ‐ short‐term (≤ 3 months) improvement

Comparison 1: AMOX + MTZ + SRP versus SRP, Outcome 5: CAL ‐ intermediate‐term (> 3 months) improvement

Comparison 1: AMOX + MTZ + SRP versus SRP, Outcome 6: CAL ‐ long‐term (≥ 1 year) improvement

Comparison 1: AMOX + MTZ + SRP versus SRP, Outcome 7: Pocket depth ‐ short‐term (≤ 3 months) improvement

Comparison 1: AMOX + MTZ + SRP versus SRP, Outcome 8: Pocket depth ‐ intermediate‐term (> 3 months) improvement

Comparison 1: AMOX + MTZ + SRP versus SRP, Outcome 9: Pocket depth ‐ long‐term (≥ 1 year) improvement

Comparison 1: AMOX + MTZ + SRP versus SRP, Outcome 10: BOP ‐ short‐term (≤ 3 months) improvement

Comparison 1: AMOX + MTZ + SRP versus SRP, Outcome 11: BOP ‐ intermediate‐term (> 3 months) improvement

Comparison 1: AMOX + MTZ + SRP versus SRP, Outcome 12: BOP ‐ long‐term (≥ 1 year) improvement

Comparison 2: MTZ + SRP versus SRP, Outcome 1: Percentage of closed pockets ‐ short‐term (≤ 3 months) improvement in chronic periodontitis

Comparison 2: MTZ + SRP versus SRP, Outcome 2: Percentage of closed pockets ‐ intermediate‐term (> 3 months) improvement in chronic periodontitis

Comparison 2: MTZ + SRP versus SRP, Outcome 3: Percentage of closed pockets ‐ long‐term (≥ 1 year) improvement

Comparison 2: MTZ + SRP versus SRP, Outcome 4: CAL ‐ short‐term (≤ 3 months) improvement

Comparison 2: MTZ + SRP versus SRP, Outcome 5: CAL ‐ intermediate‐term (> 3 months) improvement

Comparison 2: MTZ + SRP versus SRP, Outcome 6: CAL ‐ long‐term (≥ 1 year) improvement

Comparison 2: MTZ + SRP versus SRP, Outcome 7: Pocket depth ‐ short‐term (≤ 3 months) improvement

Comparison 2: MTZ + SRP versus SRP, Outcome 8: Pocket depth ‐ intermediate‐term (> 3 months) improvement

Comparison 2: MTZ + SRP versus SRP, Outcome 9: Pocket depth ‐ long‐term (≥ 1 year) improvement

Comparison 2: MTZ + SRP versus SRP, Outcome 10: BOP ‐ short‐term (≤ 3 months) improvement

Comparison 2: MTZ + SRP versus SRP, Outcome 11: BOP ‐ intermediate‐term (> 3 months) improvement

Comparison 2: MTZ + SRP versus SRP, Outcome 12: BOP ‐ long‐term (≥ 1 year) improvement

Comparison 3: AZT + SRP versus SRP, Outcome 1: Percentage of closed pockets ‐ intermediate‐term (> 3 months) improvement

Comparison 3: AZT + SRP versus SRP, Outcome 2: Percentage of closed pockets ‐ long‐term (≥ 1 year) improvement

Comparison 3: AZT + SRP versus SRP, Outcome 3: CAL ‐ short‐term (≤ 3 months) improvement

Comparison 3: AZT + SRP versus SRP, Outcome 4: CAL ‐ intermediate‐term (> 3 months) improvement

Comparison 3: AZT + SRP versus SRP, Outcome 5: CAL ‐ long‐term (≥ 1 year) improvement

Comparison 3: AZT + SRP versus SRP, Outcome 6: Pocket depth ‐ short‐term (≤ 3 months) improvement

Comparison 3: AZT + SRP versus SRP, Outcome 7: Pocket depth ‐ intermediate‐term (> 3 months) improvement

Comparison 3: AZT + SRP versus SRP, Outcome 8: Pocket depth ‐ long‐term (≥ 1 year) improvement

Comparison 3: AZT + SRP versus SRP, Outcome 9: BOP ‐ short‐term (≤ 3 months) improvement

Comparison 3: AZT + SRP versus SRP, Outcome 10: BOP ‐ intermediate‐term (> 3 months) improvement

Comparison 3: AZT + SRP versus SRP, Outcome 11: BOP ‐ long‐term (≥ 1 year) improvement

Comparison 4: AMOX + CLAV + SRP versus SRP in chronic periodontitis, Outcome 1: CAL ‐ short‐term (≤ 3 months) improvement

Comparison 4: AMOX + CLAV + SRP versus SRP in chronic periodontitis, Outcome 2: CAL ‐ intermediate‐term (> 3 months) improvement

Comparison 4: AMOX + CLAV + SRP versus SRP in chronic periodontitis, Outcome 3: CAL ‐ long‐term (≥ 1 year) improvement

Comparison 4: AMOX + CLAV + SRP versus SRP in chronic periodontitis, Outcome 4: Pocket depth ‐ short‐term (≤ 3 months) improvement

Comparison 4: AMOX + CLAV + SRP versus SRP in chronic periodontitis, Outcome 5: Pocket depth ‐ intermediate‐term (> 3 months) improvement

Comparison 4: AMOX + CLAV + SRP versus SRP in chronic periodontitis, Outcome 6: Pocket depth ‐ long‐term (≥ 1 year) improvement

Comparison 4: AMOX + CLAV + SRP versus SRP in chronic periodontitis, Outcome 7: BOP ‐ short‐term (≤ 3 months) improvement

Comparison 4: AMOX + CLAV + SRP versus SRP in chronic periodontitis, Outcome 8: BOP ‐ intermediate‐term (> 3 months) improvement

Comparison 4: AMOX + CLAV + SRP versus SRP in chronic periodontitis, Outcome 9: BOP ‐ long‐term (≥ 1 year) improvement

Comparison 5: Doxycycline + SRP versus SRP in aggressive periodontitis, Outcome 1: CAL ‐ short‐term (≤ 3 months) improvement

Comparison 5: Doxycycline + SRP versus SRP in aggressive periodontitis, Outcome 2: CAL ‐ intermediate‐term (> 3 months) improvement

Comparison 5: Doxycycline + SRP versus SRP in aggressive periodontitis, Outcome 3: CAL ‐ long‐term (≥ 1 year) improvement

Comparison 5: Doxycycline + SRP versus SRP in aggressive periodontitis, Outcome 4: Pocket depth ‐ short‐term (≤ 3 months) improvement

Comparison 5: Doxycycline + SRP versus SRP in aggressive periodontitis, Outcome 5: Pocket depth ‐ intermediate‐term (> 3 months) improvement

Comparison 5: Doxycycline + SRP versus SRP in aggressive periodontitis, Outcome 6: Pocket depth ‐ long‐term (≥ 1 year) improvement

Comparison 5: Doxycycline + SRP versus SRP in aggressive periodontitis, Outcome 7: BOP ‐ short‐term (≤ 3 months) improvement

Comparison 5: Doxycycline + SRP versus SRP in aggressive periodontitis, Outcome 8: BOP ‐ intermediate‐term (> 3 months) improvement

Comparison 6: Tetracycline + SRP versus SRP in aggressive periodontitis, Outcome 1: CAL ‐ short‐term (≤ 3 months) improvement

Comparison 6: Tetracycline + SRP versus SRP in aggressive periodontitis, Outcome 2: CAL ‐ intermediate‐term (> 3 months) improvement

Comparison 6: Tetracycline + SRP versus SRP in aggressive periodontitis, Outcome 3: CAL ‐ long‐term (≥ 1 year) improvement

Comparison 6: Tetracycline + SRP versus SRP in aggressive periodontitis, Outcome 4: Pocket depth ‐ short‐term (≤ 3 months) improvement

Comparison 6: Tetracycline + SRP versus SRP in aggressive periodontitis, Outcome 5: BOP ‐ short‐term (≤ 3 months) improvement

Comparison 7: Clarithromycin + SRP versus SRP, Outcome 1: CAL ‐ short‐term (≤ 3 months) improvement

Comparison 7: Clarithromycin + SRP versus SRP, Outcome 2: CAL ‐ intermediate‐term (> 3 months) improvement

Comparison 7: Clarithromycin + SRP versus SRP, Outcome 3: Pocket depth ‐ short‐term (≤ 3 months) improvement

Comparison 7: Clarithromycin + SRP versus SRP, Outcome 4: Pocket depth ‐ intermediate‐term (> 3 months) improvement

Comparison 7: Clarithromycin + SRP versus SRP, Outcome 5: BOP ‐ short‐term (≤ 3 months) improvement

Comparison 7: Clarithromycin + SRP versus SRP, Outcome 6: BOP ‐ intermediate‐term (> 3 months) improvement

Comparison 8: Moxifloxacin + SRP versus SRP in aggressive periodontitis, Outcome 1: CAL ‐ short‐term (≤ 3 months) improvement

Comparison 8: Moxifloxacin + SRP versus SRP in aggressive periodontitis, Outcome 2: CAL ‐ intermediate‐term (> 3 months) improvement

Comparison 8: Moxifloxacin + SRP versus SRP in aggressive periodontitis, Outcome 3: Pocket depth ‐ short‐term (≤ 3 months) improvement

Comparison 8: Moxifloxacin + SRP versus SRP in aggressive periodontitis, Outcome 4: Pocket depth ‐ intermediate‐term (> 3 months) improvement

Comparison 8: Moxifloxacin + SRP versus SRP in aggressive periodontitis, Outcome 5: BOP ‐ short‐term (≤ 3 months) improvement

Comparison 8: Moxifloxacin + SRP versus SRP in aggressive periodontitis, Outcome 6: BOP ‐ intermediate‐term (> 3 months) improvement

Comparison 9: Levofloxacin + SRP versus SRP in chronic periodontitis, Outcome 1: CAL ‐ short‐term (≤ 3 months) improvement

Comparison 9: Levofloxacin + SRP versus SRP in chronic periodontitis, Outcome 2: CAL ‐ intermediate‐term (> 3 months) improvement

Comparison 9: Levofloxacin + SRP versus SRP in chronic periodontitis, Outcome 3: Pocket depth ‐ short‐term (≤ 3 months) improvement

Comparison 9: Levofloxacin + SRP versus SRP in chronic periodontitis, Outcome 4: Pocket depth ‐ intermediate‐term (> 3 months) improvement

Comparison 9: Levofloxacin + SRP versus SRP in chronic periodontitis, Outcome 5: BOP ‐ short‐term (≤ 3 months) improvement

Comparison 9: Levofloxacin + SRP versus SRP in chronic periodontitis, Outcome 6: BOP ‐ intermediate‐term (> 3 months) improvement

Comparison 10: Clindamycin + SRP versus SRP in aggressive periodontitis, Outcome 1: CAL ‐ short‐term (≤ 3 months) improvement

Comparison 10: Clindamycin + SRP versus SRP in aggressive periodontitis, Outcome 2: CAL ‐ intermediate‐term (> 3 months) improvement

Comparison 10: Clindamycin + SRP versus SRP in aggressive periodontitis, Outcome 3: CAL ‐ long‐term (≥ 1 year) improvement

Comparison 10: Clindamycin + SRP versus SRP in aggressive periodontitis, Outcome 4: Pocket depth ‐ short‐term (≤ 3 months) improvement

Comparison 10: Clindamycin + SRP versus SRP in aggressive periodontitis, Outcome 5: Pocket depth ‐ intermediate‐term (> 3 months) improvement

Comparison 10: Clindamycin + SRP versus SRP in aggressive periodontitis, Outcome 6: Pocket depth ‐ long‐term (≥ 1 year) improvement

Comparison 11: Cefixime + SRP versus SRP in chronic periodontitis, Outcome 1: CAL ‐ short‐term (≤ 3 months) improvement

Comparison 11: Cefixime + SRP versus SRP in chronic periodontitis, Outcome 2: Pocket depth ‐ short‐term (≤ 3 months) improvement

Comparison 11: Cefixime + SRP versus SRP in chronic periodontitis, Outcome 3: BOP ‐ short‐term (≤ 3 months) improvement

Comparison 12: MTZ + SRP versus AMOX + MTZ + SRP, Outcome 1: CAL ‐ short‐term (≤ 3 months) improvement

Comparison 12: MTZ + SRP versus AMOX + MTZ + SRP, Outcome 2: CAL ‐ intermediate‐term (> 3 months) improvement

Comparison 12: MTZ + SRP versus AMOX + MTZ + SRP, Outcome 3: Pocket depth ‐ short‐term (≤ 3 months) improvement

Comparison 12: MTZ + SRP versus AMOX + MTZ + SRP, Outcome 4: Pocket depth ‐ intermediate‐term (> 3 months) improvement

Comparison 12: MTZ + SRP versus AMOX + MTZ + SRP, Outcome 5: BOP ‐ short‐term (≤ 3 months) improvement

Comparison 12: MTZ + SRP versus AMOX + MTZ + SRP, Outcome 6: BOP ‐ intermediate‐term (> 3 months) improvement

Comparison 13: Doxycycline + SRP versus AMOX + MTZ + SRP in aggressive periodontitis, Outcome 1: CAL ‐ short‐term (≤ 3 months) improvement

Comparison 13: Doxycycline + SRP versus AMOX + MTZ + SRP in aggressive periodontitis, Outcome 2: CAL ‐ intermediate‐term (> 3 months) improvement

Comparison 13: Doxycycline + SRP versus AMOX + MTZ + SRP in aggressive periodontitis, Outcome 3: Pocket depth ‐ short‐term (≤ 3 months) improvement

Comparison 13: Doxycycline + SRP versus AMOX + MTZ + SRP in aggressive periodontitis, Outcome 4: Pocket depth ‐ intermediate‐term (> 3 months) improvement

Comparison 14: Doxycycline + SRP versus MTZ + SRP in aggressive periodontitis, Outcome 1: CAL ‐ short‐term (≤ 3 months) improvement

Comparison 14: Doxycycline + SRP versus MTZ + SRP in aggressive periodontitis, Outcome 2: CAL ‐ intermediate‐term (> 3 months) improvement

Comparison 14: Doxycycline + SRP versus MTZ + SRP in aggressive periodontitis, Outcome 3: CAL ‐ long‐term (≥ 1 year) improvement

Comparison 14: Doxycycline + SRP versus MTZ + SRP in aggressive periodontitis, Outcome 4: Pocket depth ‐ short‐term (≤ 3 months) improvement

Comparison 14: Doxycycline + SRP versus MTZ + SRP in aggressive periodontitis, Outcome 5: Pocket depth ‐ intermediate‐term (> 3 months) improvement

Comparison 14: Doxycycline + SRP versus MTZ + SRP in aggressive periodontitis, Outcome 6: Pocket depth ‐ long‐term (≥ 1 year) improvement

Comparison 14: Doxycycline + SRP versus MTZ + SRP in aggressive periodontitis, Outcome 7: BOP ‐ short‐term (≤ 3 months) improvement

Comparison 14: Doxycycline + SRP versus MTZ + SRP in aggressive periodontitis, Outcome 8: BOP ‐ intermediate‐term (> 3 months) improvement

Comparison 15: Clindamycin + SRP versus MTZ + SRP in aggressive periodontitis, Outcome 1: CAL ‐ short‐term (≤ 3 months) improvement

Comparison 15: Clindamycin + SRP versus MTZ + SRP in aggressive periodontitis, Outcome 2: CAL ‐ intermediate‐term (> 3 months) improvement

Comparison 15: Clindamycin + SRP versus MTZ + SRP in aggressive periodontitis, Outcome 3: CAL ‐ long‐term (≥ 1 year) improvement

Comparison 15: Clindamycin + SRP versus MTZ + SRP in aggressive periodontitis, Outcome 4: Pocket depth ‐ short‐term (≤ 3 months) improvement

Comparison 15: Clindamycin + SRP versus MTZ + SRP in aggressive periodontitis, Outcome 5: Pocket depth ‐ intermediate‐term (> 3 months) improvement

Comparison 15: Clindamycin + SRP versus MTZ + SRP in aggressive periodontitis, Outcome 6: Pocket depth ‐ long‐term (≥ 1 year) improvement

Comparison 16: AMOX + CLAV + SRP versus tetracycline + SRP in aggressive periodontitis, Outcome 1: Pocket depth ‐ intermediate‐term (> 3 months) improvement

Comparison 16: AMOX + CLAV + SRP versus tetracycline + SRP in aggressive periodontitis, Outcome 2: BOP ‐ intermediate‐term (> 3 months) improvement

Comparison 17: Clindamycin + SRP versus doxycycline + SRP in aggressive periodontitis, Outcome 1: CAL ‐ short‐term (≤ 3 months) improvement

Comparison 17: Clindamycin + SRP versus doxycycline + SRP in aggressive periodontitis, Outcome 2: CAL ‐ intermediate‐term (> 3 months) improvement

Comparison 17: Clindamycin + SRP versus doxycycline + SRP in aggressive periodontitis, Outcome 3: CAL ‐ long‐term (≥ 1 year) improvement

Comparison 17: Clindamycin + SRP versus doxycycline + SRP in aggressive periodontitis, Outcome 4: Pocket depth ‐ short‐term (≤ 3 months) improvement

Comparison 17: Clindamycin + SRP versus doxycycline + SRP in aggressive periodontitis, Outcome 5: Pocket depth ‐ intermediate‐term (> 3 months) improvement

Comparison 17: Clindamycin + SRP versus doxycycline + SRP in aggressive periodontitis, Outcome 6: Pocket depth ‐ long‐term (≥ 1 year) improvement

Comparison 18: AMOX + MZT versus clarithromycin + SRP, Outcome 1: CAL ‐ short‐term (≤ 3 months) improvement

Comparison 18: AMOX + MZT versus clarithromycin + SRP, Outcome 2: CAL ‐ intermediate‐term (> 3 months) improvement

Comparison 18: AMOX + MZT versus clarithromycin + SRP, Outcome 3: Pocket depth ‐ short‐term (≤ 3 months) improvement

Comparison 18: AMOX + MZT versus clarithromycin + SRP, Outcome 4: Pocket depth ‐ intermediate‐term (> 3 months) improvement

Comparison 18: AMOX + MZT versus clarithromycin + SRP, Outcome 5: BOP ‐ short‐term (≤ 3 months) improvement

Comparison 18: AMOX + MZT versus clarithromycin + SRP, Outcome 6: BOP ‐ intermediate‐term (> 3 months) improvement
| Amoxicillin + metronidazole + SRP compared to SRP for the non‐surgical treatment of periodontitis | ||||||
|---|---|---|---|---|---|---|
| Patient or population: adults with periodontitis (chronic/aggressive) Follow‐up: ≥1 year | ||||||
| Outcomes | Number of participants | Relative effect | Anticipated absolute effects* (95% CI) | Certainty of the evidence | Comments | |
| Risk with control (SRP) | Risk difference with amoxicillin + metronidazole + SRP | |||||
| Percentage of closed pockets | 44 (1 RCTa) | ‐ | The mean percentage of closed pockets was 49.30% | MD 16.20% lower (25.87 lower to 6.53 lower) | ⊕⊝⊝⊝ | Based on our definition of minimally important clinical difference (MICD)f, only the results for percentage of closed pockets and of BOP showed an MICD favouring amoxicillin + metronidazole + SRP |
| CAL (Long‐term improvement in chronic and aggressive periodontitis) | 389 (2 RCTsa,b) | ‐ | The mean CAL was 3.85 mm | MD 0.47 mm lower | ⊕⊝⊝⊝ | |
| Probing pocket depth (Long‐term improvement in chronic and aggressive periodontitis) | 389 (2 RCTsa,b) | ‐ | The mean probing pocket depth was 2.75 mm | MD 0.30 mm lower | ⊕⊝⊝⊝ | |
| Percentage of BOP | 389 | ‐ | The mean percentage of BOP was 29.45% | MD 8.06% lower | ⊕⊝⊝⊝ | |
| Antimicrobial resistance | Included studies have not reported this outcome | |||||
| Adverse events | 389 | Most common adverse events were mild gastrointestinal disturbances, nausea and vomiting, metallic taste, and diarrhoea in the intervention group. Control group reported adverse events like hypothermia, mild gastrointestinal symptoms, diarrhoea, vomiting, cramps, rashes, and weakness. Nausea after alcohol intake was reported in 1 participant from the intervention group | ⊕⊝⊝⊝ | |||
| Patient‐reported quality of life changes | Included studies have not reported this outcome | |||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) BOP: bleeding on probing; CAL: clinical attachment level; CI: confidence interval; MD: mean difference; MICD: minimally important clinical difference; RCT: randomized controlled trial; SRP: scaling and root planing | ||||||
| GRADE Working Group grades of evidence | ||||||
| aBorges 2017. | ||||||
| Metronidazole + SRP compared to SRP for the non‐surgical treatment of periodontitis | ||||||
|---|---|---|---|---|---|---|
| Patient or population: adults with periodontitis (chronic/aggressive) Follow‐up: ≥1 year | ||||||
| Outcomes | Number of participants | Relative effect | Anticipated absolute effects* (95% CI) | Certainty of the evidence | Comments | |
| Risk with control (SRP) | Risk difference with metronidazole + SRP | |||||
| Percentage of closed pockets | 22 (1 RCTa) | ‐ | The mean percentage of closed pockets was 52.80% | MD 12.20% lower | ⊕⊝⊝⊝ | Based on our definition of minimally important clinical difference (MICD)h, only the results for CAL and probing pocket depth showed an MICD favouring metronidazole + SRP |
| CAL | 71 | ‐ | ‐ | MD 1.12 mm lower | ⊕⊝⊝⊝ | |
| Probing pocket depth | 47 | ‐ | The mean probing pocket depth was 4.11 mm | MD 1.11 mm lower | ⊕⊝⊝⊝ | |
| Percentage of BOP | 22 (1 RCTa) | ‐ | The mean percentage of BOP was 44.92% | MD 6.90% lower | ⊕⊝⊝⊝ | |
| Antimicrobial resistance | Included studies have not reported this outcome | |||||
| Adverse events | 71 | No severe adverse effects were reported by any of the participants in 1 study. 2 studies have not reported adverse events | ⊕⊝⊝⊝ | |||
| Patient‐reported quality of life changes | Included studies have not reported this outcome | |||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) | ||||||
| GRADE Working Group grades of evidence | ||||||
| aCarvalho 2004. | ||||||
| Azithromycin + SRP compared to SRP for the non‐surgical treatment of periodontitis | ||||||
|---|---|---|---|---|---|---|
| Patient or population: adults with periodontitis (chronic/aggressive) Follow‐up: ≥1 year | ||||||
| Outcomes | Number of participants | Relative effect | Anticipated absolute effects* (95% CI) | Certainty of the evidence | Comments | |
| Risk with control (SRP) | Risk difference with azithromycin + SRP | |||||
| Percentage of closed pockets | 40 (1 RCTa) | ‐ | The mean percentage of closed pockets was 51.50% | MD 2.50% higher | ⊕⊝⊝⊝ | Based on our definition of minimally important clinical difference (MICD)g, we found no evidence of a difference between intervention and control |
| CAL | 110 | ‐ | The mean CAL was 5.30 mm | MD 0.59 mm lower | ⊕⊝⊝⊝ | |
| Probing pocket depth | 110 | ‐ | The mean probing pocket depth was 4.29 mm | MD 0.77 mm lower | ⊕⊝⊝⊝ | |
| Percentage of BOP | 110 | ‐ | The mean percentage of BOP was 16.96% | MD 1.28% lower | ⊕⊝⊝⊝ | |
| Antimicrobial resistance | Included studies have not reported this outcome | |||||
| Adverse events | 110 | 1 study reported diarrhoea, headache or dizziness, metallic taste, and general unwellness by 4 participants from the intervention group and 3 from the control group. In 1 study none of the participants reported any adverse effects | ⊕⊝⊝⊝ | |||
| Patient‐reported quality of life changes | Included studies have not reported this outcome | |||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) | ||||||
| GRADE Working Group grades of evidence | ||||||
| aSampaio 2011. | ||||||
| Amoxicillin + clavulanate + SRP compared to SRP for the non‐surgical treatment of periodontitis | ||||||
|---|---|---|---|---|---|---|
| Patient or population: adults with periodontitis (chronic) Follow‐up: ≥1 year | ||||||
| Outcomes | Number of participants | Relative effect | Anticipated absolute effects* (95% CI) | Certainty of the evidence | Comments | |
| Risk with control (SRP) | Risk difference with amoxicillin + clavulanate + SRP | |||||
| Percentage of closed pockets | Included study has not reported this outcome | Based on our definition of minimally important clinical difference (MICD)d, we found no evidence of a difference between intervention and control | ||||
| CAL | 21 | ‐ | The mean CAL was 6.80 mm | MD 0.10 mm higher | ⊕⊝⊝⊝ | |
| Probing pocket depth | 21 | ‐ | The mean probing pocket depth was 2.80 mm | MD 0.10 mm higher | ⊕⊝⊝⊝ | |
| Percentage of BOP | 21 | ‐ | The mean percentage of BOP was 0.20% | MD 0% | ⊕⊝⊝⊝ | |
| Antimicrobial resistance | Included study has not reported this outcome | |||||
| Adverse events | 21 | Included study reported diarrhoea in 2 participants from both groups | ⊕⊝⊝⊝ | |||
| Patient‐reported quality of life changes | Included study has not reported this outcome | |||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) | ||||||
| GRADE Working Group grades of evidence | ||||||
| aWinkel 1999. | ||||||
| Doxycycline + SRP compared to SRP for the non‐surgical treatment of periodontitis | ||||||
|---|---|---|---|---|---|---|
| Patient or population: adults with periodontitis (aggressive) Follow‐up: ≥1 year | ||||||
| Outcomes | Number of participants | Relative effect | Anticipated absolute effects* (95% CI) | Certainty of the evidence | Comments | |
| Risk with control (SRP) | Risk difference with doxycycline + SRP | |||||
| Percentage of closed pockets | Included study has not reported this outcome | Based on our definition of minimally important clinical difference (MICD)d, only the results for probing pocket depth showed an MICD favouring doxycycline + SRP | ||||
| CAL | 22 | ‐ | The mean CAL was 5.90 mm | MD 0.80 mm lower | ⊕⊝⊝⊝ | |
| Probing pocket depth | 22 | ‐ | The mean probing pocket depth was 5.20 mm | MD 1 mm lower | ⊕⊝⊝⊝ | |
| Percentage of BOP | Included study has not reported this outcome | |||||
| Antimicrobial resistance | Included study has not reported this outcome | |||||
| Adverse events | Included study has not reported adverse events | |||||
| Patient‐reported quality of life changes | Included study has not reported this outcome | |||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) | ||||||
| GRADE Working Group grades of evidence | ||||||
| aSigusch 2001. | ||||||
| Tetracycline + SRP compared to SRP for the non‐surgical treatment of periodontitis | ||||||
|---|---|---|---|---|---|---|
| Patient or population: adults with periodontitis (aggressive) Follow‐up: ≥1 year | ||||||
| Outcomes | Number of participants | Relative effect | Anticipated absolute effects* (95% CI) | Certainty of the evidence | Comments | |
| Risk with control (SRP) | Risk difference with tetracycline + SRP | |||||
| Percentage of closed pockets | Included study has not reported this outcome | Based on our definition of minimally important clinical difference (MICD)d, the results for CAL showed an MICD favouring tetracycline + SRP | ||||
| CAL | 26 | ‐ | The mean CAL was 4.40 mm | MD 2.30 mm lower | ⊕⊝⊝⊝ | |
| Probing pocket depth | Included study has not reported this outcome | |||||
| Percentage of BOP | Included study has not reported this outcome | |||||
| Antimicrobial resistance | Included study has not reported this outcome | |||||
| Adverse events | Included study has not reported adverse events | |||||
| Patient‐reported quality of life changes | Included study has not reported this outcome | |||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) | ||||||
| GRADE Working Group grades of evidence | ||||||
| aZhang 2006. | ||||||
| Clindamycin + SRP compared to SRP for the non‐surgical treatment of periodontitis | ||||||
|---|---|---|---|---|---|---|
| Patient or population: adults with periodontitis (aggressive) Follow‐up: ≥1 year | ||||||
| Outcomes | Number of participants | Relative effect | Anticipated absolute effects* (95% CI) | Certainty of the evidence | Comments | |
| Risk with control (SRP) | Risk difference with clindamycin + SRP | |||||
| Percentage of closed pockets | Included study has not reported this outcome | Based on our definition of minimally important clinical difference (MICD)d, the results for CAL and probing pocket depth showed an MICD favouring clindamycin + SRP | ||||
| CAL | 21 | ‐ | The mean CAL was 5.90 mm | MD 1.70 mm lower | ⊕⊝⊝⊝ | |
| Probing pocket depth | 21 | ‐ | The mean probing pocket depth was 5.20 mm | MD 1.80 mm lower | ⊕⊝⊝⊝ | |
| Percentage of BOP | Included study has not reported this outcome | |||||
| Antimicrobial resistance | Included study has not reported this outcome | |||||
| Adverse events | Included study has not reported adverse events | |||||
| Patient‐reported quality of life changes | Included study has not reported this outcome | |||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) | ||||||
| GRADE Working Group grades of evidence | ||||||
| aSigusch 2001. | ||||||
| Doxycycline + SRP compared to metronidazole + SRP for the non‐surgical treatment of periodontitis | ||||||
|---|---|---|---|---|---|---|
| Patient or population: adults with periodontitis (aggressive) Follow‐up: ≥1 year | ||||||
| Outcomes | Number of participants | Relative effect | Anticipated absolute effects* (95% CI) | Certainty of the evidence | Comments | |
| Risk with metronidazole + SRP | Risk difference with doxycycline + SRP | |||||
| Percentage of closed pockets | Included study has not reported this outcome | Based on our definition of minimally important clinical difference (MICD)d, the results for CAL and probing pocket depth showed an MICD favouring metronidazole + SRP | ||||
| CAL | 27 | ‐ | The mean CAL was 4 mm | MD 1.10 mm higher | ⊕⊝⊝⊝ | |
| Probing pocket depth | 27 | ‐ | The mean probing pocket depth was 3.20 mm | MD 1 mm higher | ⊕⊝⊝⊝ | |
| Percentage of BOP | Included study has not reported this outcome | |||||
| Antimicrobial resistance | Included study has not reported this outcome | |||||
| Adverse events | Included study has not reported adverse events | |||||
| Patient‐reported quality of life changes | Included study has not reported this outcome | |||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) | ||||||
| GRADE Working Group grades of evidence | ||||||
| aSigusch 2001. | ||||||
| Clindamycin + SRP compared to metronidazole + SRP for the non‐surgical treatment of periodontitis | ||||||
|---|---|---|---|---|---|---|
| Patient or population: adults with periodontitis (aggressive) Follow‐up: ≥1 year | ||||||
| Outcomes | Number of participants | Relative effect | Anticipated absolute effects* (95% CI) | Certainty of the evidence | Comments | |
| Risk with metronidazole + SRP | Risk difference with clindamycin + SRP | |||||
| Percentage of closed pockets | Included study has not reported this outcome | Based on our definition of minimally important clinical difference (MICD)d, we found no evidence of a difference between the groups | ||||
| CAL | 26 | ‐ | The mean CAL was 4 mm | MD 0.20 mm higher | ⊕⊝⊝⊝ | |
| Probing pocket depth | 26 | ‐ | The mean probing pocket depth was 3.20 mm | MD 0.20 mm higher | ⊕⊝⊝⊝ | |
| Percentage of BOP | Included study has not reported this outcome | |||||
| Antimicrobial resistance | Included study has not reported this outcome | |||||
| Adverse events | Included study has not reported adverse events | |||||
| Patient‐reported quality of life changes | Included study has not reported this outcome | |||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) | ||||||
| GRADE Working Group grades of evidence | ||||||
| aSigusch 2001. | ||||||
| Clindamycin + SRP compared to doxycycline + SRP for the non‐surgical treatment of periodontitis | ||||||
|---|---|---|---|---|---|---|
| Patient or population: adults with periodontitis (aggressive) Follow‐up: ≥1 year | ||||||
| Outcomes | Number of participants | Relative effect | Anticipated absolute effects* (95% CI) | Certainty of the evidence | Comments | |
| Risk with doxycycline + SRP | Risk difference with clindamycin + SRP | |||||
| Percentage of closed pockets | Included study has not reported this outcome | Based on our definition of minimally important clinical difference (MICD)d, we found no evidence of a difference between the groups | ||||
| CAL | 23 | ‐ | The mean CAL was 5.10 mm | MD 0.90 mm lower | ⊕⊝⊝⊝ | |
| Probing pocket depth | 23 | ‐ | The mean probing pocket depth was 4.20 mm | MD 0.80 mm lower | ⊕⊝⊝⊝ | |
| Percentage of BOP | Included study has not reported this outcome | |||||
| Antimicrobial resistance | Included study has not reported this outcome | |||||
| Adverse events | Included study has not reported adverse events | |||||
| Patient‐reported quality of life changes | Included study has not reported this outcome | |||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) | ||||||
| GRADE Working Group grades of evidence | ||||||
| aSigusch 2001. | ||||||
| Serial number | Antibiotic prescribed | Type of periodontitis | Included studies |
|---|---|---|---|
| 1. | AMOX + MTZ | Chronic periodontitis | Boia 2019; Borges 2017; Cosgarea 2016; Dukic 2016; Liaw 2019; Li 2015; Matarazzo 2008; Moeintaghavi 2007; Mombelli 2013; Ribeiro 2009; Silva 2011; Winkel 2001 |
| Aggressive periodontitis | Casarin 2012; Lu 2012; Taiete 2016; Xajigeorgiou 2006; Yek 2010 | ||
| Both chronic and aggressive periodontitis | |||
| 2. | MTZ | Chronic periodontitis | Carvalho 2004; D'avila 2005; Loesche 1991; Matarazzo 2008; Sigusch 2000; Silva 2011 |
| Aggressive periodontitis | |||
| 3. | AZT | Chronic periodontitis | Gomi 2007; Han 2012; Liaw 2019; Martande 2016; Mascarenhas 2005; Sampaio 2011; Smith 2002 |
| Aggressive periodontitis | |||
| 4. | AMOX + CLAV | Chronic periodontitis | |
| AMOX + CLAV versus tetracycline | Aggressive periodontitis | ||
| 5. | Minocycline | Chronic periodontitis | |
| 6. | Spiramycin | Chronic periodontitis | |
| 7. | Tetracycline | Aggressive periodontitis | |
| 8. | Cefixime | Chronic periodontitis | |
| 9 . | Doxycycline | Aggressive periodontitis | |
| 10. | Clindamycin | Aggressive periodontitis | |
| 11. | MTZ versus AMOX + MTZ | Both chronic and aggressive periodontitis | |
| 12. | Doxycycline versus AMOX + MTZ | Aggressive periodontitis | |
| 13. | Doxycycline versus MTZ | Aggressive periodontitis | |
| 14. | Moxifloxacin | Aggressive periodontitis | |
| 15. | Levofloxacin | Chronic periodontitis | |
| 16. | Doxycycline versus clindamycin | Aggressive periodontitis | |
| 17. | Clarithromycin | Both chronic and aggressive periodontitis | |
| 18. | Clindamycin versus MTZ | Aggressive periodontitis | |
| 19. | AMOX+MTZ versus clarithromycin | Aggressive periodontitis | |
| AMOX = amoxicillin; AZT = azithromycin; CLAV = clavulanate; MTZ = metronidazole. | |||
| Serial number | Dosage | Number of days | Included studies |
|---|---|---|---|
| 1. AMOX + MTZ | |||
| i. | 500 mg AMOX and 500 mg MTZ | 3 | |
| ii. | 500 mg AMOX and 500 mg MTZ | 4 | |
| iii. | 500 mg AMOX and 500 mg MTZ | 7 | |
| iv. | 500 mg AMOX and 400 mg MTZ | 7 | |
| v. | 375 mg AMOX and 500 mg MTZ | 7 | |
| vi. | 375 mg AMOX and 250 mg MTZ | 7 | |
| vii. | 500 mg AMOX and 200 mg MTZ | 7 | |
| viii. | 500 mg AMOX and 250 mg MTZ | 7 | |
| ix. | 500 mg AMOX and 400 mg MTZ | 14 | |
| x. | 500 mg AMOX and 250 mg MTZ | 14 | |
| 2. MTZ alone | |||
| i. | 500 mg thrice/day | 7 | |
| ii. | 500 mg twice/day | 8 | |
| iii. | 400 mg thrice/day | 10 | |
| iv. | thrice/day | 14 | |
| 3. AZT | |||
| i. | 500 mg once/day | 3 | Emingil 2012; Gomi 2007; Haas 2008; Han 2012; Liaw 2019; Martande 2016 |
| ii. | 500 mg once/day | 5 | |
| iii. | 250 mg | Double stat first day followed by once/day for next 4 days | |
| 4. AMOX + CLAV | |||
| i. | 625 mg thrice/day | 10 days | |
| 5. Doxycycline | |||
| i. | 200 mg/day | 8 | |
| ii. | 200 mg 100 mg/day | On first day 14 | |
| 6. Tetracycline | |||
| i. | 250 mg 4 times/day | 14 | |
| ii. | 500 mg/day | Not mentioned | |
| 7. Clarithromycin | |||
| i. | 500 mg twice/day | 3 | |
| ii. | 500 mg twice/day | 7 | |
| 8. Moxifloxacin | |||
| i. | 400 mg/day | 7 | |
| 9. Levofloxacin | |||
| i. | 500 mg once/day | 10 | |
| 10. Clindamycin | |||
| i. | 150 mg 4 times/day | 8 | |
| 11. Cefixime | |||
| i. | 400 mg once/day | 7 | |
| AMOX = amoxicillin; AZT = azithromycin; CLAV = clavulanate; MTZ = metronidazole. | |||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1.1 Percentage of closed pockets ‐ short‐term (≤ 3 months) improvement Show forest plot | 2 | 73 | Mean Difference (IV, Random, 95% CI) | ‐10.05 [‐18.78, ‐1.32] |
| 1.2 Percentage of closed pockets ‐ intermediate‐term (> 3 months) improvement Show forest plot | 1 | 44 | Mean Difference (IV, Random, 95% CI) | ‐13.90 [‐24.16, ‐3.64] |
| 1.3 Percentage of closed pockets ‐ long‐term (≥ 1 year) improvement Show forest plot | 1 | 44 | Mean Difference (IV, Random, 95% CI) | ‐16.20 [‐25.87, ‐6.53] |
| 1.4 CAL ‐ short‐term (≤ 3 months) improvement Show forest plot | 13 | 501 | Mean Difference (IV, Random, 95% CI) | ‐0.21 [‐0.41, ‐0.01] |
| 1.4.1 Chronic periodontitis | 9 | 380 | Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐0.44, 0.05] |
| 1.4.2 Aggressive periodontitis | 4 | 121 | Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐0.62, 0.22] |
| 1.5 CAL ‐ intermediate‐term (> 3 months) improvement Show forest plot | 6 | 215 | Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐0.35, 0.26] |
| 1.5.1 Chronic periodontitis | 3 | 127 | Mean Difference (IV, Random, 95% CI) | ‐0.29 [‐0.69, 0.10] |
| 1.5.2 Aggressive periodontitis | 3 | 88 | Mean Difference (IV, Random, 95% CI) | 0.13 [‐0.27, 0.53] |
| 1.6 CAL ‐ long‐term (≥ 1 year) improvement Show forest plot | 2 | 389 | Mean Difference (IV, Random, 95% CI) | ‐0.47 [‐0.90, ‐0.05] |
| 1.6.1 Chronic periodontitis | 1 | 44 | Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.82, 0.62] |
| 1.6.2 Chronic and aggressive periodontitis | 1 | 345 | Mean Difference (IV, Random, 95% CI) | ‐0.60 [‐0.80, ‐0.40] |
| 1.7 Pocket depth ‐ short‐term (≤ 3 months) improvement Show forest plot | 15 | 562 | Mean Difference (IV, Random, 95% CI) | ‐0.18 [‐0.33, ‐0.04] |
| 1.7.1 Chronic periodontitis | 11 | 441 | Mean Difference (IV, Random, 95% CI) | ‐0.21 [‐0.35, ‐0.06] |
| 1.7.2 Aggressive periodontitis | 4 | 121 | Mean Difference (IV, Random, 95% CI) | ‐0.13 [‐0.64, 0.38] |
| 1.8 Pocket depth ‐ intermediate‐term (> 3 months) improvement Show forest plot | 6 | 215 | Mean Difference (IV, Random, 95% CI) | ‐0.47 [‐0.68, ‐0.25] |
| 1.8.1 Chronic periodontitis | 3 | 127 | Mean Difference (IV, Random, 95% CI) | ‐0.57 [‐0.79, ‐0.35] |
| 1.8.2 Aggressive periodontitis | 3 | 88 | Mean Difference (IV, Random, 95% CI) | ‐0.31 [‐0.57, ‐0.05] |
| 1.9 Pocket depth ‐ long‐term (≥ 1 year) improvement Show forest plot | 2 | 389 | Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐0.42, ‐0.18] |
| 1.9.1 Chronic periodontitis | 1 | 44 | Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐0.63, 0.03] |
| 1.9.2 Chronic and aggressive periodontitis | 1 | 345 | Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐0.43, ‐0.17] |
| 1.10 BOP ‐ short‐term (≤ 3 months) improvement Show forest plot | 13 | 460 | Mean Difference (IV, Random, 95% CI) | ‐2.05 [‐2.98, ‐1.11] |
| 1.10.1 Chronic periodontitis ‐ percentage of BOP | 10 | 354 | Mean Difference (IV, Random, 95% CI) | ‐8.24 [‐12.00, ‐4.49] |
| 1.10.2 Chronic periodontitis ‐ BOP in Muhlemann score | 1 | 60 | Mean Difference (IV, Random, 95% CI) | ‐0.44 [‐0.56, ‐0.32] |
| 1.10.3 Aggressive periodontitis ‐ percentage of BOP | 1 | 25 | Mean Difference (IV, Random, 95% CI) | ‐0.60 [‐3.03, 1.83] |
| 1.10.4 Aggressive periodontitis ‐ BOP score | 1 | 21 | Mean Difference (IV, Random, 95% CI) | ‐0.11 [‐0.29, 0.07] |
| 1.11 BOP ‐ intermediate‐term (> 3 months) improvement Show forest plot | 5 | 172 | Mean Difference (IV, Random, 95% CI) | ‐5.32 [‐12.02, 1.37] |
| 1.11.1 Chronic periodontitis | 3 | 127 | Mean Difference (IV, Random, 95% CI) | ‐9.25 [‐15.48, ‐3.03] |
| 1.11.2 Aggressive periodontitis | 1 | 24 | Mean Difference (IV, Random, 95% CI) | ‐0.70 [‐2.74, 1.34] |
| 1.11.3 Aggressive periodontitis ‐ BOP score | 1 | 21 | Mean Difference (IV, Random, 95% CI) | 0.00 [‐0.17, 0.17] |
| 1.12 BOP ‐ long‐term (≥ 1 year) improvement Show forest plot | 2 | 389 | Mean Difference (IV, Random, 95% CI) | ‐8.06 [‐14.26, ‐1.85] |
| 1.12.1 Chronic periodontitis | 1 | 44 | Mean Difference (IV, Random, 95% CI) | ‐13.18 [‐22.86, ‐3.50] |
| 1.12.2 Chronic and aggressive periodontitis | 1 | 345 | Mean Difference (IV, Random, 95% CI) | ‐6.10 [‐8.87, ‐3.33] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 2.1 Percentage of closed pockets ‐ short‐term (≤ 3 months) improvement in chronic periodontitis Show forest plot | 2 | 51 | Mean Difference (IV, Random, 95% CI) | ‐2.56 [‐12.33, 7.22] |
| 2.2 Percentage of closed pockets ‐ intermediate‐term (> 3 months) improvement in chronic periodontitis Show forest plot | 1 | 22 | Mean Difference (IV, Random, 95% CI) | ‐7.00 [‐23.57, 9.57] |
| 2.3 Percentage of closed pockets ‐ long‐term (≥ 1 year) improvement Show forest plot | 1 | 22 | Mean Difference (IV, Random, 95% CI) | ‐12.20 [‐29.23, 4.83] |
| 2.4 CAL ‐ short‐term (≤ 3 months) improvement Show forest plot | 4 | 108 | Mean Difference (IV, Random, 95% CI) | 0.04 [‐0.43, 0.50] |
| 2.4.1 Chronic periodontitis | 3 | 85 | Mean Difference (IV, Random, 95% CI) | ‐0.19 [‐0.53, 0.15] |
| 2.4.2 Aggressive periodontitis | 1 | 23 | Mean Difference (IV, Random, 95% CI) | 0.81 [0.07, 1.55] |
| 2.5 CAL ‐ intermediate‐term (> 3 months) improvement Show forest plot | 4 | 94 | Mean Difference (IV, Random, 95% CI) | ‐0.49 [‐1.29, 0.31] |
| 2.5.1 Chronic periodontitis | 2 | 46 | Mean Difference (IV, Random, 95% CI) | ‐0.25 [‐1.28, 0.78] |
| 2.5.2 Aggressive periodontitis | 2 | 48 | Mean Difference (IV, Random, 95% CI) | ‐0.70 [‐2.11, 0.71] |
| 2.6 CAL ‐ long‐term (≥ 1 year) improvement Show forest plot | 3 | 71 | Mean Difference (IV, Random, 95% CI) | ‐1.12 [‐2.24, ‐0.00] |
| 2.6.1 Chronic periodontitis | 2 | 46 | Mean Difference (IV, Random, 95% CI) | ‐0.71 [‐2.30, 0.87] |
| 2.6.2 Aggressive periodontitis | 1 | 25 | Mean Difference (IV, Random, 95% CI) | ‐1.90 [‐2.62, ‐1.18] |
| 2.7 Pocket depth ‐ short‐term (≤ 3 months) improvement Show forest plot | 4 | 108 | Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.37, 0.16] |
| 2.7.1 Chronic periodontitis | 3 | 85 | Mean Difference (IV, Random, 95% CI) | ‐0.21 [‐0.44, 0.02] |
| 2.7.2 Aggressive periodontitis | 1 | 23 | Mean Difference (IV, Random, 95% CI) | 0.28 [‐0.17, 0.73] |
| 2.8 Pocket depth ‐ intermediate‐term (> 3 months) improvement Show forest plot | 4 | 94 | Mean Difference (IV, Random, 95% CI) | ‐0.59 [‐0.95, ‐0.23] |
| 2.8.1 Chronic periodontitis | 2 | 46 | Mean Difference (IV, Random, 95% CI) | ‐0.45 [‐1.04, 0.15] |
| 2.8.2 Aggressive periodontitis | 2 | 48 | Mean Difference (IV, Random, 95% CI) | ‐0.79 [‐1.25, ‐0.34] |
| 2.9 Pocket depth ‐ long‐term (≥ 1 year) improvement Show forest plot | 2 | 47 | Mean Difference (IV, Random, 95% CI) | ‐1.11 [‐2.84, 0.61] |
| 2.9.1 Chronic periodontitis | 1 | 22 | Mean Difference (IV, Random, 95% CI) | ‐0.24 [‐0.71, 0.23] |
| 2.9.2 Aggressive periodontitis | 1 | 25 | Mean Difference (IV, Random, 95% CI) | ‐2.00 [‐2.58, ‐1.42] |
| 2.10 BOP ‐ short‐term (≤ 3 months) improvement Show forest plot | 4 | 108 | Mean Difference (IV, Random, 95% CI) | ‐9.00 [‐16.59, ‐1.40] |
| 2.10.1 Chronic periodontitis | 3 | 85 | Mean Difference (IV, Random, 95% CI) | ‐10.33 [‐18.89, ‐1.78] |
| 2.10.2 Aggressive periodontitis | 1 | 23 | Mean Difference (IV, Random, 95% CI) | ‐4.00 [‐20.53, 12.53] |
| 2.11 BOP ‐ intermediate‐term (> 3 months) improvement Show forest plot | 2 | 45 | Mean Difference (IV, Random, 95% CI) | ‐4.62 [‐18.83, 9.58] |
| 2.11.1 Chronic periodontitis | 1 | 22 | Mean Difference (IV, Random, 95% CI) | ‐9.57 [‐23.21, 4.07] |
| 2.11.2 Aggressive periodontitis | 1 | 23 | Mean Difference (IV, Random, 95% CI) | 6.00 [‐16.93, 28.93] |
| 2.12 BOP ‐ long‐term (≥ 1 year) improvement Show forest plot | 1 | 22 | Mean Difference (IV, Random, 95% CI) | ‐6.90 [‐22.10, 8.30] |
| 2.12.1 Chronic periodontitis | 1 | 22 | Mean Difference (IV, Random, 95% CI) | ‐6.90 [‐22.10, 8.30] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 3.1 Percentage of closed pockets ‐ intermediate‐term (> 3 months) improvement Show forest plot | 1 | 40 | Mean Difference (IV, Random, 95% CI) | ‐2.90 [‐13.91, 8.11] |
| 3.2 Percentage of closed pockets ‐ long‐term (≥ 1 year) improvement Show forest plot | 1 | 40 | Mean Difference (IV, Random, 95% CI) | 2.50 [‐10.19, 15.19] |
| 3.3 CAL ‐ short‐term (≤ 3 months) improvement Show forest plot | 6 | 216 | Mean Difference (IV, Random, 95% CI) | ‐0.51 [‐0.83, ‐0.19] |
| 3.3.1 Chronic periodontitis | 4 | 114 | Mean Difference (IV, Random, 95% CI) | ‐0.47 [‐0.97, 0.04] |
| 3.3.2 Agressive periodontitis | 2 | 102 | Mean Difference (IV, Random, 95% CI) | ‐0.58 [‐1.10, ‐0.06] |
| 3.4 CAL ‐ intermediate‐term (> 3 months) improvement Show forest plot | 7 | 258 | Mean Difference (IV, Random, 95% CI) | ‐0.43 [‐0.77, ‐0.10] |
| 3.4.1 Chronic periodontitis | 4 | 132 | Mean Difference (IV, Random, 95% CI) | ‐0.27 [‐0.76, 0.23] |
| 3.4.2 Aggressive periodontitis | 3 | 126 | Mean Difference (IV, Random, 95% CI) | ‐0.60 [‐0.98, ‐0.22] |
| 3.5 CAL ‐ long‐term (≥ 1 year) improvement Show forest plot | 2 | 110 | Mean Difference (IV, Random, 95% CI) | ‐0.59 [‐1.27, 0.08] |
| 3.5.1 Chronic periodontitis | 1 | 40 | Mean Difference (IV, Random, 95% CI) | ‐0.25 [‐0.77, 0.27] |
| 3.5.2 Aggressive periodontitis | 1 | 70 | Mean Difference (IV, Random, 95% CI) | ‐0.94 [‐1.47, ‐0.41] |
| 3.6 Pocket depth ‐ short‐term (≤ 3 months) improvement Show forest plot | 6 | 216 | Mean Difference (IV, Random, 95% CI) | ‐0.57 [‐1.08, ‐0.07] |
| 3.6.1 Chronic periodontitis | 4 | 114 | Mean Difference (IV, Random, 95% CI) | ‐0.61 [‐1.21, ‐0.01] |
| 3.6.2 Aggressive periodontitis | 2 | 102 | Mean Difference (IV, Random, 95% CI) | ‐0.54 [‐1.61, 0.53] |
| 3.7 Pocket depth ‐ intermediate‐term (> 3 months) improvement Show forest plot | 7 | 258 | Mean Difference (IV, Random, 95% CI) | ‐0.60 [‐1.02, ‐0.18] |
| 3.7.1 Chronic periodontitis | 4 | 132 | Mean Difference (IV, Random, 95% CI) | ‐0.37 [‐0.82, 0.07] |
| 3.7.2 Aggressive periodontitis | 3 | 126 | Mean Difference (IV, Random, 95% CI) | ‐0.93 [‐2.08, 0.22] |
| 3.8 Pocket depth ‐ long‐term (≥ 1 year) improvement Show forest plot | 2 | 110 | Mean Difference (IV, Random, 95% CI) | ‐0.77 [‐2.33, 0.79] |
| 3.8.1 Chronic periodontitis | 1 | 40 | Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.27, 0.31] |
| 3.8.2 Aggressive periodontitis | 1 | 70 | Mean Difference (IV, Random, 95% CI) | ‐1.57 [‐1.91, ‐1.23] |
| 3.9 BOP ‐ short‐term (≤ 3 months) improvement Show forest plot | 6 | 216 | Mean Difference (IV, Random, 95% CI) | ‐3.18 [‐7.01, 0.65] |
| 3.9.1 Chronic periodontitis | 4 | 114 | Mean Difference (IV, Random, 95% CI) | ‐6.65 [‐10.41, ‐2.89] |
| 3.9.2 Aggressive periodontitis | 2 | 102 | Mean Difference (IV, Random, 95% CI) | ‐1.19 [‐3.63, 1.26] |
| 3.10 BOP ‐ intermediate‐term (> 3 months) improvement Show forest plot | 6 | 228 | Mean Difference (IV, Random, 95% CI) | ‐3.32 [‐6.23, ‐0.42] |
| 3.10.1 Chronic periodontitis | 3 | 102 | Mean Difference (IV, Random, 95% CI) | ‐4.14 [‐9.27, 1.00] |
| 3.10.2 Aggressive periodontitis | 3 | 126 | Mean Difference (IV, Random, 95% CI) | ‐1.79 [‐5.27, 1.69] |
| 3.11 BOP ‐ long‐term (≥ 1 year) improvement Show forest plot | 2 | 110 | Mean Difference (IV, Random, 95% CI) | ‐1.28 [‐4.32, 1.76] |
| 3.11.1 Chronic periodontitis | 1 | 40 | Mean Difference (IV, Random, 95% CI) | 0.54 [‐3.62, 4.70] |
| 3.11.2 Aggressive periodontitis | 1 | 70 | Mean Difference (IV, Random, 95% CI) | ‐2.60 [‐5.95, 0.75] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 4.1 CAL ‐ short‐term (≤ 3 months) improvement Show forest plot | 1 | 21 | Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.43, 0.63] |
| 4.2 CAL ‐ intermediate‐term (> 3 months) improvement Show forest plot | 1 | 21 | Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.45, 0.65] |
| 4.3 CAL ‐ long‐term (≥ 1 year) improvement Show forest plot | 1 | 21 | Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.51, 0.71] |
| 4.4 Pocket depth ‐ short‐term (≤ 3 months) improvement Show forest plot | 1 | 21 | Mean Difference (IV, Random, 95% CI) | 0.20 [‐0.02, 0.42] |
| 4.5 Pocket depth ‐ intermediate‐term (> 3 months) improvement Show forest plot | 1 | 21 | Mean Difference (IV, Random, 95% CI) | 0.00 [‐0.19, 0.19] |
| 4.6 Pocket depth ‐ long‐term (≥ 1 year) improvement Show forest plot | 1 | 21 | Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.17, 0.37] |
| 4.7 BOP ‐ short‐term (≤ 3 months) improvement Show forest plot | 1 | 21 | Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.19, ‐0.01] |
| 4.8 BOP ‐ intermediate‐term (> 3 months) improvement Show forest plot | 1 | 21 | Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.19, ‐0.01] |
| 4.9 BOP ‐ long‐term (≥ 1 year) improvement Show forest plot | 1 | 21 | Mean Difference (IV, Random, 95% CI) | 0.00 [‐0.09, 0.09] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 5.1 CAL ‐ short‐term (≤ 3 months) improvement Show forest plot | 2 | 43 | Mean Difference (IV, Random, 95% CI) | 0.15 [‐0.53, 0.83] |
| 5.1.1 Aggressive periodontitis | 2 | 43 | Mean Difference (IV, Random, 95% CI) | 0.15 [‐0.53, 0.83] |
| 5.2 CAL ‐ intermediate‐term (> 3 months) improvement Show forest plot | 3 | 65 | Mean Difference (IV, Random, 95% CI) | ‐0.61 [‐1.14, ‐0.08] |
| 5.2.1 Aggressive periodontitis | 3 | 65 | Mean Difference (IV, Random, 95% CI) | ‐0.61 [‐1.14, ‐0.08] |
| 5.3 CAL ‐ long‐term (≥ 1 year) improvement Show forest plot | 1 | 22 | Mean Difference (IV, Random, 95% CI) | ‐0.80 [‐1.49, ‐0.11] |
| 5.3.1 Aggressive periodontitis | 1 | 22 | Mean Difference (IV, Random, 95% CI) | ‐0.80 [‐1.49, ‐0.11] |
| 5.4 Pocket depth ‐ short‐term (≤ 3 months) improvement Show forest plot | 2 | 43 | Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.29, 0.50] |
| 5.4.1 Aggressive periodontitis | 2 | 43 | Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.29, 0.50] |
| 5.5 Pocket depth ‐ intermediate‐term (> 3 months) improvement Show forest plot | 3 | 65 | Mean Difference (IV, Random, 95% CI) | ‐0.43 [‐0.88, 0.01] |
| 5.5.1 Aggressive Periodontitis | 3 | 65 | Mean Difference (IV, Random, 95% CI) | ‐0.43 [‐0.88, 0.01] |
| 5.6 Pocket depth ‐ long‐term (≥ 1 year) improvement Show forest plot | 1 | 22 | Mean Difference (IV, Random, 95% CI) | ‐1.00 [‐1.78, ‐0.22] |
| 5.6.1 Aggressive periodontitis | 1 | 22 | Mean Difference (IV, Random, 95% CI) | ‐1.00 [‐1.78, ‐0.22] |
| 5.7 BOP ‐ short‐term (≤ 3 months) improvement Show forest plot | 1 | 21 | Mean Difference (IV, Random, 95% CI) | ‐0.09 [‐0.29, 0.11] |
| 5.7.1 Aggressive periodontitis | 1 | 21 | Mean Difference (IV, Random, 95% CI) | ‐0.09 [‐0.29, 0.11] |
| 5.8 BOP ‐ intermediate‐term (> 3 months) improvement Show forest plot | 1 | 21 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.21, 0.19] |
| 5.8.1 Aggressive periodontitis | 1 | 21 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.21, 0.19] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 6.1 CAL ‐ short‐term (≤ 3 months) improvement Show forest plot | 2 | 64 | Mean Difference (IV, Random, 95% CI) | ‐0.92 [‐2.38, 0.54] |
| 6.2 CAL ‐ intermediate‐term (> 3 months) improvement Show forest plot | 1 | 26 | Mean Difference (IV, Random, 95% CI) | ‐1.90 [‐2.17, ‐1.63] |
| 6.3 CAL ‐ long‐term (≥ 1 year) improvement Show forest plot | 1 | 26 | Mean Difference (IV, Random, 95% CI) | ‐2.30 [‐2.50, ‐2.10] |
| 6.4 Pocket depth ‐ short‐term (≤ 3 months) improvement Show forest plot | 1 | 38 | Mean Difference (IV, Random, 95% CI) | ‐0.53 [‐1.14, 0.08] |
| 6.5 BOP ‐ short‐term (≤ 3 months) improvement Show forest plot | 1 | 38 | Mean Difference (IV, Random, 95% CI) | ‐14.62 [‐27.21, ‐2.03] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 7.1 CAL ‐ short‐term (≤ 3 months) improvement Show forest plot | 2 | 77 | Mean Difference (IV, Random, 95% CI) | ‐0.65 [‐1.20, ‐0.09] |
| 7.1.1 Chronic periodontitis | 1 | 37 | Mean Difference (IV, Random, 95% CI) | ‐0.92 [‐1.11, ‐0.73] |
| 7.1.2 Aggressive periodontitis | 1 | 40 | Mean Difference (IV, Random, 95% CI) | ‐0.35 [‐0.64, ‐0.06] |
| 7.2 CAL ‐ intermediate‐term (> 3 months) improvement Show forest plot | 2 | 77 | Mean Difference (IV, Random, 95% CI) | ‐0.64 [‐1.26, ‐0.03] |
| 7.3 Pocket depth ‐ short‐term (≤ 3 months) improvement Show forest plot | 2 | 77 | Mean Difference (IV, Random, 95% CI) | ‐0.59 [‐1.17, ‐0.01] |
| 7.4 Pocket depth ‐ intermediate‐term (> 3 months) improvement Show forest plot | 2 | 77 | Mean Difference (IV, Random, 95% CI) | ‐0.67 [‐1.33, ‐0.01] |
| 7.5 BOP ‐ short‐term (≤ 3 months) improvement Show forest plot | 1 | 40 | Mean Difference (IV, Random, 95% CI) | ‐3.00 [‐6.69, 0.69] |
| 7.6 BOP ‐ intermediate‐term (> 3 months) improvement Show forest plot | 1 | 40 | Mean Difference (IV, Random, 95% CI) | ‐4.70 [‐8.42, ‐0.98] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 8.1 CAL ‐ short‐term (≤ 3 months) improvement Show forest plot | 1 | 40 | Mean Difference (IV, Random, 95% CI) | ‐0.67 [‐1.01, ‐0.33] |
| 8.2 CAL ‐ intermediate‐term (> 3 months) improvement Show forest plot | 1 | 40 | Mean Difference (IV, Random, 95% CI) | ‐0.63 [‐0.95, ‐0.31] |
| 8.3 Pocket depth ‐ short‐term (≤ 3 months) improvement Show forest plot | 1 | 40 | Mean Difference (IV, Random, 95% CI) | ‐0.55 [‐0.87, ‐0.23] |
| 8.4 Pocket depth ‐ intermediate‐term (> 3 months) improvement Show forest plot | 1 | 40 | Mean Difference (IV, Random, 95% CI) | ‐0.42 [‐0.74, ‐0.10] |
| 8.5 BOP ‐ short‐term (≤ 3 months) improvement Show forest plot | 1 | 40 | Mean Difference (IV, Random, 95% CI) | ‐3.40 [‐3.72, ‐3.08] |
| 8.6 BOP ‐ intermediate‐term (> 3 months) improvement Show forest plot | 1 | 40 | Mean Difference (IV, Random, 95% CI) | ‐0.80 [‐1.12, ‐0.48] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 9.1 CAL ‐ short‐term (≤ 3 months) improvement Show forest plot | 2 | 131 | Mean Difference (IV, Random, 95% CI) | ‐1.02 [‐1.39, ‐0.65] |
| 9.2 CAL ‐ intermediate‐term (> 3 months) improvement Show forest plot | 2 | 131 | Mean Difference (IV, Random, 95% CI) | ‐1.25 [‐1.51, ‐0.99] |
| 9.3 Pocket depth ‐ short‐term (≤ 3 months) improvement Show forest plot | 2 | 131 | Mean Difference (IV, Random, 95% CI) | ‐1.09 [‐1.33, ‐0.85] |
| 9.4 Pocket depth ‐ intermediate‐term (> 3 months) improvement Show forest plot | 2 | 131 | Mean Difference (IV, Random, 95% CI) | ‐1.23 [‐1.46, ‐1.00] |
| 9.5 BOP ‐ short‐term (≤ 3 months) improvement Show forest plot | 2 | 131 | Mean Difference (IV, Random, 95% CI) | ‐1.38 [‐3.30, 0.54] |
| 9.6 BOP ‐ intermediate‐term (> 3 months) improvement Show forest plot | 2 | 131 | Mean Difference (IV, Random, 95% CI) | ‐0.93 [‐2.88, 1.02] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 10.1 CAL ‐ short‐term (≤ 3 months) improvement Show forest plot | 1 | 21 | Mean Difference (IV, Random, 95% CI) | 0.40 [‐0.35, 1.15] |
| 10.2 CAL ‐ intermediate‐term (> 3 months) improvement Show forest plot | 1 | 21 | Mean Difference (IV, Random, 95% CI) | ‐1.30 [‐2.14, ‐0.46] |
| 10.3 CAL ‐ long‐term (≥ 1 year) improvement Show forest plot | 1 | 21 | Mean Difference (IV, Random, 95% CI) | ‐1.70 [‐2.40, ‐1.00] |
| 10.4 Pocket depth ‐ short‐term (≤ 3 months) improvement Show forest plot | 1 | 21 | Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.55, 0.75] |
| 10.5 Pocket depth ‐ intermediate‐term (> 3 months) improvement Show forest plot | 1 | 21 | Mean Difference (IV, Random, 95% CI) | ‐1.10 [‐1.94, ‐0.26] |
| 10.6 Pocket depth ‐ long‐term (≥ 1 year) improvement Show forest plot | 1 | 21 | Mean Difference (IV, Random, 95% CI) | ‐1.80 [‐2.47, ‐1.13] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 11.1 CAL ‐ short‐term (≤ 3 months) improvement Show forest plot | 1 | 60 | Mean Difference (IV, Random, 95% CI) | ‐0.19 [‐0.77, 0.39] |
| 11.2 Pocket depth ‐ short‐term (≤ 3 months) improvement Show forest plot | 1 | 60 | Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.11, 0.07] |
| 11.3 BOP ‐ short‐term (≤ 3 months) improvement Show forest plot | 1 | 60 | Mean Difference (IV, Random, 95% CI) | ‐0.48 [‐0.60, ‐0.37] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 12.1 CAL ‐ short‐term (≤ 3 months) improvement Show forest plot | 3 | 84 | Mean Difference (IV, Random, 95% CI) | ‐0.07 [‐0.40, 0.26] |
| 12.1.1 Chronic periodontitis | 2 | 62 | Mean Difference (IV, Random, 95% CI) | ‐0.13 [‐0.49, 0.23] |
| 12.1.2 Agressive periodontitis | 1 | 22 | Mean Difference (IV, Random, 95% CI) | 0.30 [‐0.56, 1.16] |
| 12.2 CAL ‐ intermediate‐term (> 3 months) improvement Show forest plot | 1 | 22 | Mean Difference (IV, Random, 95% CI) | 0.06 [‐1.06, 1.18] |
| 12.3 Pocket depth ‐ short‐term (≤ 3 months) improvement Show forest plot | 3 | 84 | Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.12, 0.32] |
| 12.3.1 Chronic periodontitis | 2 | 62 | Mean Difference (IV, Random, 95% CI) | 0.12 [‐0.14, 0.39] |
| 12.3.2 Aggressive periodontitis | 1 | 22 | Mean Difference (IV, Random, 95% CI) | 0.03 [‐0.38, 0.44] |
| 12.4 Pocket depth ‐ intermediate‐term (> 3 months) improvement Show forest plot | 1 | 22 | Mean Difference (IV, Random, 95% CI) | ‐0.26 [‐0.83, 0.31] |
| 12.5 BOP ‐ short‐term (≤ 3 months) improvement Show forest plot | 3 | 84 | Mean Difference (IV, Random, 95% CI) | 4.46 [‐23.48, 32.40] |
| 12.5.1 Chronic periodontitis | 2 | 62 | Mean Difference (IV, Random, 95% CI) | ‐8.11 [‐22.40, 6.17] |
| 12.5.2 Aggressive periodontitis | 1 | 22 | Mean Difference (IV, Random, 95% CI) | 28.78 [20.29, 37.27] |
| 12.6 BOP ‐ intermediate‐term (> 3 months) improvement Show forest plot | 1 | 22 | Mean Difference (IV, Random, 95% CI) | 20.85 [3.31, 38.39] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 13.1 CAL ‐ short‐term (≤ 3 months) improvement Show forest plot | 2 | 50 | Mean Difference (IV, Random, 95% CI) | 0.20 [0.06, 0.33] |
| 13.2 CAL ‐ intermediate‐term (> 3 months) improvement Show forest plot | 1 | 20 | Mean Difference (IV, Random, 95% CI) | 0.17 [‐1.28, 1.62] |
| 13.3 Pocket depth ‐ short‐term (≤ 3 months) improvement Show forest plot | 2 | 50 | Mean Difference (IV, Random, 95% CI) | 0.14 [‐0.12, 0.40] |
| 13.4 Pocket depth ‐ intermediate‐term (> 3 months) improvement Show forest plot | 1 | 20 | Mean Difference (IV, Random, 95% CI) | 0.23 [‐0.41, 0.87] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 14.1 CAL ‐ short‐term (≤ 3 months) improvement Show forest plot | 2 | 49 | Mean Difference (IV, Random, 95% CI) | ‐0.12 [‐0.72, 0.49] |
| 14.2 CAL ‐ intermediate‐term (> 3 months) improvement Show forest plot | 3 | 75 | Mean Difference (IV, Random, 95% CI) | 0.34 [‐0.08, 0.76] |
| 14.3 CAL ‐ long‐term (≥ 1 year) improvement Show forest plot | 1 | 27 | Mean Difference (IV, Random, 95% CI) | 1.10 [0.36, 1.84] |
| 14.4 Pocket depth ‐ short‐term (≤ 3 months) improvement Show forest plot | 2 | 49 | Mean Difference (IV, Random, 95% CI) | ‐0.16 [‐0.56, 0.24] |
| 14.5 Pocket depth ‐ intermediate‐term (> 3 months) improvement Show forest plot | 3 | 75 | Mean Difference (IV, Random, 95% CI) | 0.38 [‐0.00, 0.76] |
| 14.6 Pocket depth ‐ long‐term (≥ 1 year) improvement Show forest plot | 1 | 27 | Mean Difference (IV, Random, 95% CI) | 1.00 [0.30, 1.70] |
| 14.7 BOP ‐ short‐term (≤ 3 months) improvement Show forest plot | 1 | 22 | Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐0.22, 0.12] |
| 14.8 BOP ‐ intermediate‐term (> 3 months) improvement Show forest plot | 1 | 22 | Mean Difference (IV, Random, 95% CI) | ‐0.07 [‐0.29, 0.15] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 15.1 CAL ‐ short‐term (≤ 3 months) improvement Show forest plot | 1 | 26 | Mean Difference (IV, Random, 95% CI) | 0.40 [‐0.22, 1.02] |
| 15.2 CAL ‐ intermediate‐term (> 3 months) improvement Show forest plot | 1 | 26 | Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.60, 0.80] |
| 15.3 CAL ‐ long‐term (≥ 1 year) improvement Show forest plot | 1 | 26 | Mean Difference (IV, Random, 95% CI) | 0.20 [‐0.55, 0.95] |
| 15.4 Pocket depth ‐ short‐term (≤ 3 months) improvement Show forest plot | 1 | 26 | Mean Difference (IV, Random, 95% CI) | ‐0.14 [‐0.82, 0.54] |
| 15.5 Pocket depth ‐ intermediate‐term (> 3 months) improvement Show forest plot | 1 | 26 | Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.79, 0.59] |
| 15.6 Pocket depth ‐ long‐term (≥ 1 year) improvement Show forest plot | 1 | 26 | Mean Difference (IV, Random, 95% CI) | 0.20 [‐0.38, 0.78] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 16.1 Pocket depth ‐ intermediate‐term (> 3 months) improvement Show forest plot | 1 | 8 | Mean Difference (IV, Random, 95% CI) | ‐0.40 [‐1.11, 0.31] |
| 16.2 BOP ‐ intermediate‐term (> 3 months) improvement Show forest plot | 1 | 8 | Mean Difference (IV, Random, 95% CI) | ‐1.60 [‐14.03, 10.83] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 17.1 CAL ‐ short‐term (≤ 3 months) improvement Show forest plot | 1 | 23 | Mean Difference (IV, Random, 95% CI) | 0.50 [‐0.26, 1.26] |
| 17.2 CAL ‐ intermediate‐term (> 3 months) improvement Show forest plot | 1 | 23 | Mean Difference (IV, Random, 95% CI) | ‐0.40 [‐1.15, 0.35] |
| 17.3 CAL ‐ long‐term (≥ 1 year) improvement Show forest plot | 1 | 23 | Mean Difference (IV, Random, 95% CI) | ‐0.90 [‐1.62, ‐0.18] |
| 17.4 Pocket depth ‐ short‐term (≤ 3 months) improvement Show forest plot | 1 | 23 | Mean Difference (IV, Random, 95% CI) | 0.20 [‐0.36, 0.76] |
| 17.5 Pocket depth ‐ intermediate‐term (> 3 months) improvement Show forest plot | 1 | 23 | Mean Difference (IV, Random, 95% CI) | ‐0.40 [‐1.14, 0.34] |
| 17.6 Pocket depth ‐ long‐term (≥ 1 year) improvement Show forest plot | 1 | 23 | Mean Difference (IV, Random, 95% CI) | ‐0.80 [‐1.58, ‐0.02] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 18.1 CAL ‐ short‐term (≤ 3 months) improvement Show forest plot | 1 | 46 | Mean Difference (IV, Random, 95% CI) | ‐0.60 [‐0.86, ‐0.34] |
| 18.2 CAL ‐ intermediate‐term (> 3 months) improvement Show forest plot | 1 | 46 | Mean Difference (IV, Random, 95% CI) | 0.00 [‐0.26, 0.26] |
| 18.3 Pocket depth ‐ short‐term (≤ 3 months) improvement Show forest plot | 1 | 46 | Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐0.35, ‐0.05] |
| 18.4 Pocket depth ‐ intermediate‐term (> 3 months) improvement Show forest plot | 1 | 46 | Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.26, 0.06] |
| 18.5 BOP ‐ short‐term (≤ 3 months) improvement Show forest plot | 1 | 46 | Mean Difference (IV, Random, 95% CI) | 5.40 [‐0.12, 10.92] |
| 18.6 BOP ‐ intermediate‐term (> 3 months) improvement Show forest plot | 1 | 46 | Mean Difference (IV, Random, 95% CI) | 4.00 [‐1.09, 9.09] |

