Retiro del tratamiento biológico o inmunosupresor en pacientes con enfermedad de Crohn inactiva
Información
- DOI:
- https://doi.org/10.1002/14651858.CD012540.pub2Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 12 mayo 2018see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Salud digestiva
- Copyright:
-
- Copyright © 2018 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
Ray Boyapati: study conception and design, acquisition of data, analysis and interpretation of data, drafting and critical revision of the manuscript.
Joana Torres: study conception and design, acquisition of data, analysis and interpretation of data, drafting and critical revision of the manuscript.
Carolina Palmela: acquisition of data.
Claire E Parker: study conception and design, acquisition of data, analysis and interpretation of data, drafting and critical revision of the manuscript.
Orli M Silverberg: acquisition of data.
Sonam D Upadhyaya: acquisition of data.
Tran M. Nguyen: designed search strategies, ran searches, acquisition of data.
Jean‐Frédéric Colombel: study conception and design, analysis and interpretation of data and critical revision of the manuscript.
Declarations of interest
Ray Boyapati: has no known conflicts of interest.
Joana Torres has served as a consultant for Takeda, and Abbvie; and has received travel support from Ferring and Abbvie. All of these activities are outside the submitted work.
Carolina Palmelahas received consultancy fees from Laboratorios Vitoria and travel grant from Abbvie and MSD. All of these activities are outside the submitted work.
Claire E Parker: has no known conflicts of interest.
Orli M Silverberg: has no known conflicts of interest.
Sonam D Upadhyaya: has no known conflicts of interest.
Tran M. Nguyen: has no known conflicts of interest.
Jean‐Frédéric Colombel has received consulting fees from AbbVie, Amgen, Boehringer‐Ingelheim, Arena Pharmaceuticals, Celgene Corporation, Celltrion, Enterome, Eli Lilly, Ferring Pharmaceuticals, Genentech, Janssen and Janssen, Medimmune, Merck & Co., Nextbiotix, Novartis Pharmaceuticals Corporation, Otsuka Pharmaceutical Development & Commercialization, Inc., Pfizer, Protagonist, Second Genome, Gilead, Seres Therapeutics, Shire, Takeda, Theradiag; Grants/Grants pending from Abbvie, Takeda, Janssen Pharmaceuticals; Payments for lectures from AbbVie, Ferring, Takeda, Celgene Corporation; stocks and stock options from Intestinal Biotech Development and Genfit. All of these activities are outside the submitted work.
Acknowledgements
Funding for the Cochrane IBD Group (May 1, 2017 ‐ April 30, 2022) has been provided by Crohn's and Colitis Canada (CCC).
Version history
| Published | Title | Stage | Authors | Version |
| 2018 May 12 | Withdrawal of immunosuppressant or biologic therapy for patients with quiescent Crohn's disease | Review | Ray K Boyapati, Joana Torres, Carolina Palmela, Claire E Parker, Orli M Silverberg, Sonam D Upadhyaya, Tran M Nguyen, Jean‐Frédéric Colombel | |
| 2017 Feb 02 | Withdrawal of drug therapy for patients with quiescent Crohn's disease | Protocol | Ray Boyapati, Joana Torres, Carolina Palmela, Claire E Parker, Orli M Silverberg, Sonam D Upadhyaya, Reena Khanna, Vipul Jairath, Brian G Feagan, Jean‐Frédéric Colombel | |
Differences between protocol and review
The title of this review in its original protocol: "Withdrawal of drug therapy for patients with quiescent Crohn's disease", was changed to:"Withdrawal of immunosuppressant or biologic therapy for patients with quiescent Crohn's disease". A description of the factors assessed by the NOS was added to the methods section. We also decided to perform subgroup analyses based on study design (RCTs versus observational studies) as detailed in the most current version of the methods section. The subgroup analysis for pediatric studies was removed because the study participants included were defined as adults (> 18 years). Finally, a random‐effects model and not a fixed‐effect model was used for analyzing the data.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
- *Withholding Treatment;
- Azathioprine [*therapeutic use];
- Combined Modality Therapy [methods];
- Crohn Disease [complications, drug therapy, *therapy];
- Feasibility Studies;
- Gastrointestinal Agents [*therapeutic use];
- Immunosuppressive Agents [*therapeutic use];
- Infliximab [*therapeutic use];
- Randomized Controlled Trials as Topic;
- Recurrence;
- Remission Induction;
- Secondary Prevention [methods];
Medical Subject Headings Check Words
Adult; Humans;
PICO

Study flow diagram.
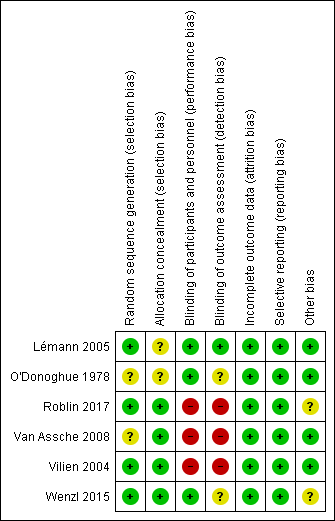
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
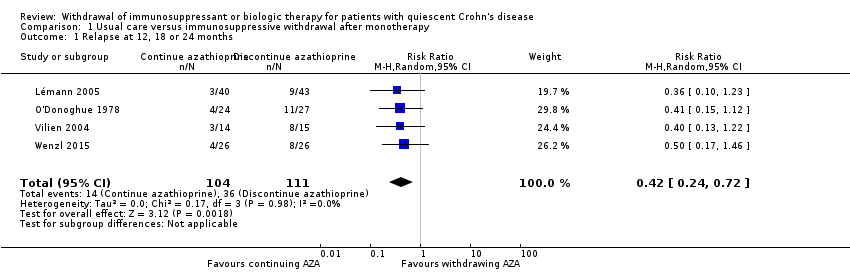
Comparison 1 Usual care versus immunosuppressive withdrawal after monotherapy, Outcome 1 Relapse at 12, 18 or 24 months.

Comparison 1 Usual care versus immunosuppressive withdrawal after monotherapy, Outcome 2 New CD‐related complications.

Comparison 1 Usual care versus immunosuppressive withdrawal after monotherapy, Outcome 3 Adverse events.

Comparison 1 Usual care versus immunosuppressive withdrawal after monotherapy, Outcome 4 Serious adverse events.
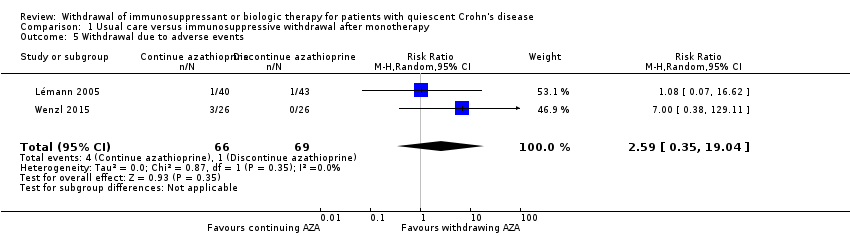
Comparison 1 Usual care versus immunosuppressive withdrawal after monotherapy, Outcome 5 Withdrawal due to adverse events.
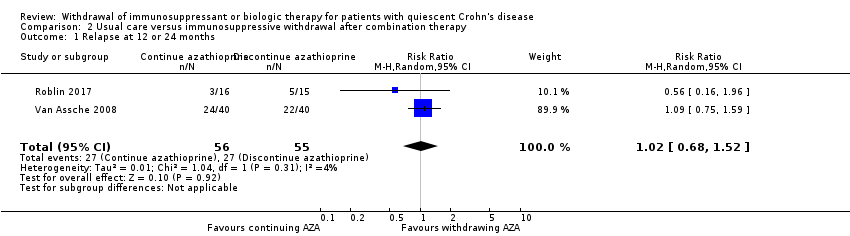
Comparison 2 Usual care versus immunosuppressive withdrawal after combination therapy, Outcome 1 Relapse at 12 or 24 months.

Comparison 2 Usual care versus immunosuppressive withdrawal after combination therapy, Outcome 2 Adverse events.
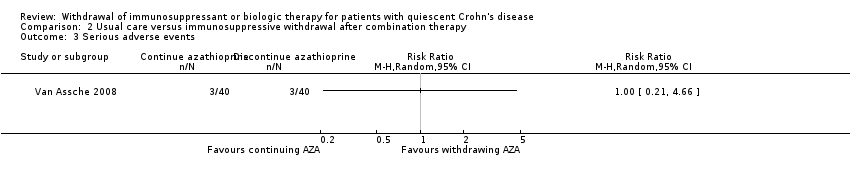
Comparison 2 Usual care versus immunosuppressive withdrawal after combination therapy, Outcome 3 Serious adverse events.
| Usual care compared to immunosuppressive withdrawal after monotherapy for patients with quiescent Crohn's disease | ||||||
| Patient or population: Patients with quiescent Crohn's disease | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
| Risk with immunosuppressive withdrawal after monotherapy | Risk with usual care | |||||
| Relapse at 12, 18 or 24 months | Study population | RR 0.42 | 215 | ⊕⊕⊝⊝ | Sparse data (50 events) | |
| 324 per 1,000 | 136 per 1,000 | |||||
| New CD‐related complications | Study population | RR 0.34 | 135 | ⊕⊝⊝⊝ | Very sparse data (5 events) | |
| 58 per 1,000 | 20 per 1,000 | |||||
| Adverse events | Study population | RR 0.88 | 186 | ⊕⊕⊝⊝ | Sparse data (45 events) | |
| 240 per 1,000 | 211 per 1,000 | |||||
| Serious adverse events | Study population | RR 3.29 | 134 | ⊕⊝⊝⊝ | Very sparse data (2 events) | |
| 0 per 1,000 | 0 per 1,000 | |||||
| Withdrawal due to adverse events | Study population | RR 2.59 | 135 | ⊕⊝⊝⊝ | Very sparse data (5 events) | |
| 14 per 1,000 | 38 per 1,000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded one level due to high risk of bias for blinding in one study and unclear risk of bias in three studies in the pooled analysis 2 Downgraded one level due to sparse data 3 Downgraded one level due to unclear risk of bias in the two studies in the pooled analysis 4 Downgraded two levels due to very sparse data 5 Downgraded one level due to unclear risk of bias in the three studies in the pooled analysis | ||||||
| Usual care compared to immunosuppressive withdrawal after combination therapy for patients with quiescent Crohn's disease | ||||||
| Patient or population: Patients with quiescent Crohn's disease | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
| Risk with immunosuppressive withdrawal after combination therapy | Risk with usual care | |||||
| Relapse at 12 or 24 months | Study population | RR 1.02 | 111 | ⊕⊕⊝⊝ | Sparse data (54 events) | |
| 491 per 1,000 | 501 per 1,000 | |||||
| Adverse events | Study population | RR 1.11 | 111 | ⊕⊕⊝⊝ | Sparse data (51 events) | |
| 455 per 1,000 | 505 per 1,000 | |||||
| Serious adverse events | Study population | RR 1.00 | 80 | ⊕⊝⊝⊝ | Very sparse data (6 events) | |
| 75 per 1,000 | 75 per 1,000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded one level due to high risk of bias for blinding 2 Downgraded one level due to sparse data 3 Downgraded two levels due to very sparse data | ||||||
| Comparison 1: Usual care versus immunosuppressive withdrawal after monotherapy | |||
| Outcome | Random Effects RR (95% CI) | Fixed Effect RR (95% CI) | Impact |
| 1.1 Relapse at 12, 18 or 24 months | 0.42 (0.24‐0.72) | 0.42 (0.24‐0.72) | No change |
| 1.2 New CD‐related complications | 0.34 (0.06‐2.08) | 0.34 (0.06‐2.08) | No change |
| 1.3 Adverse events | 0.88 (0.67‐1.17) | 0.97 (0.71‐1.32) | Minimal |
| 1.4 Serious adverse events | 3.29 (0.35‐30.80) | 3.29 (0.35‐30.80) | No change |
| 1.5 Withdrawal due to adverse events | 2.59 (0.35‐19.04) | 3.10 (0.49‐19.41) | Minimal |
| Comparison 2: Usual care versus Immunosuppressive withdrawal after combination therapy | |||
| Outcome | Random Effects RR (95% CI) | Fixed Effect RR (95% CI) | |
| 2.1 Relapse at 12 or 24 months | 1.02 (0.68‐1.52) | 0.99 (0.69‐1.43) | Minimal |
| 2.2 Adverse events | 1.11 (0.44‐2.81) | 1.04 (0.73‐1.47) | Minimal |
| 2.3 Serious adverse events | No pooling | No pooling | No pooling |
| Study | Length of remission prior to drug withdrawal | Definition of remission prior to drug withdrawal |
| Minimum 6 months | CDAI ≤ 150 and fecal calprotectin levels < 250 μg/g | |
| Mean 62 months (standard deviation 26 months); Minimum 42 months | Clinical remission (CDAI ≤ 150) and no need for medical/surgical therapy in the previous 42 months | |
| Minimum 6 months | Clinical remission not otherwise specified | |
| Minimum 6 months | Clinical response to infliximab and disease control | |
| Not specified | Clinical remission: physician's global assessment | |
| Minimum 12 months | Clinical remission, no need for new medical therapy in the previous 12 months |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Relapse at 12, 18 or 24 months Show forest plot | 4 | 215 | Risk Ratio (M‐H, Random, 95% CI) | 0.42 [0.24, 0.72] |
| 2 New CD‐related complications Show forest plot | 2 | 135 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.06, 2.08] |
| 3 Adverse events Show forest plot | 3 | 186 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.67, 1.17] |
| 4 Serious adverse events Show forest plot | 2 | 134 | Risk Ratio (M‐H, Random, 95% CI) | 3.29 [0.35, 30.80] |
| 5 Withdrawal due to adverse events Show forest plot | 2 | 135 | Risk Ratio (M‐H, Random, 95% CI) | 2.59 [0.35, 19.04] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Relapse at 12 or 24 months Show forest plot | 2 | 111 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.68, 1.52] |
| 2 Adverse events Show forest plot | 2 | 111 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.44, 2.81] |
| 3 Serious adverse events Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |

