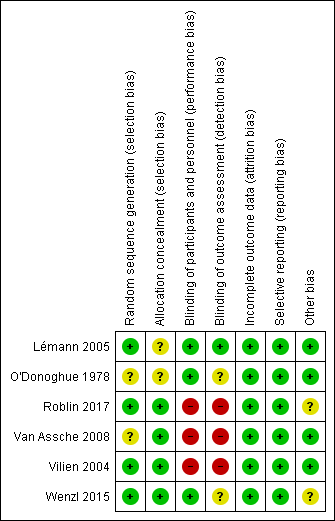
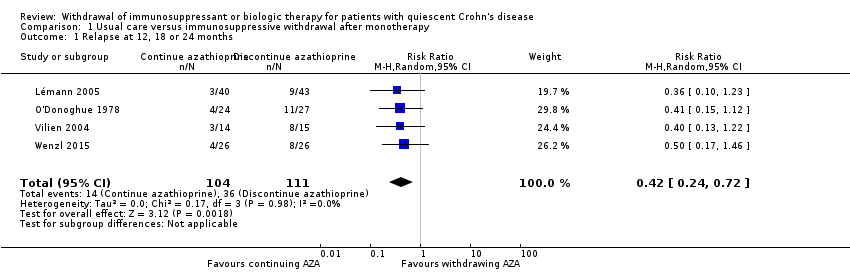
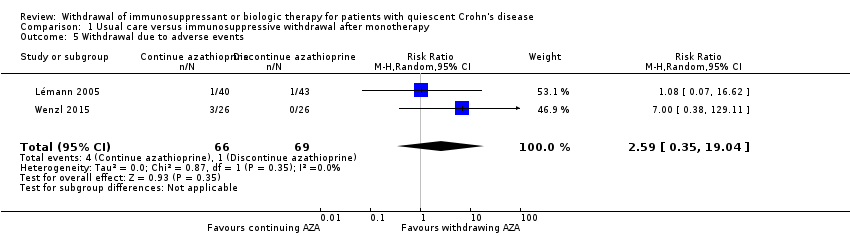
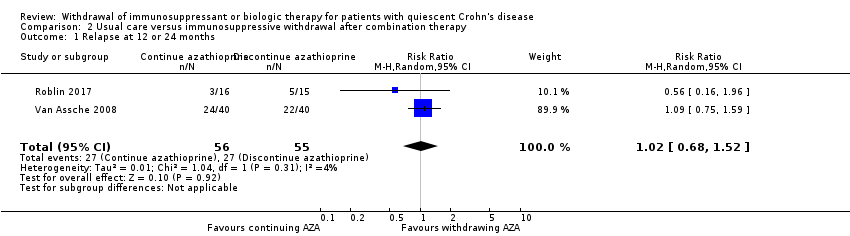
Contenido relacionado
Revisiones y protocolos relacionados
Nilesh Chande, Cassandra M Townsend, Claire E Parker, John K MacDonald | 26 octubre 2016
Teuta Gjuladin‐Hellon, Zipporah Iheozor‐Ejiofor, Morris Gordon, Anthony K Akobeng | 6 agosto 2019
John WD McDonald, Yongjun Wang, David J Tsoulis, John K MacDonald, Brian G Feagan | 6 agosto 2014
Nilesh Chande, Petrease H Patton, David J Tsoulis, Benson S Thomas, John K MacDonald | 30 octubre 2015
Zipporah Iheozor‐Ejiofor, Morris Gordon, Andrew Clegg, Suzanne C Freeman, Teuta Gjuladin‐Hellon, John K MacDonald, Anthony K Akobeng | 12 septiembre 2019
Ramesh Srinivasan, Anthony K Akobeng | 15 abril 2009
Petrease H Patton, Claire E Parker, John K MacDonald, Nilesh Chande | 22 julio 2016
Roberto Candia, Gonzalo A Bravo-Soto, Hugo Monrroy, Cristian Hernandez, Geoffrey C Nguyen | 3 agosto 2020
Wee‐Chian Lim, Yongjun Wang, John K MacDonald, Stephen Hanauer | 3 julio 2016
Cassandra M Townsend, Tran M Nguyen, Jeremy Cepek, Mohamad Abbass, Claire E Parker, John K MacDonald, Reena Khanna, Vipul Jairath, Brian G Feagan | 16 mayo 2020