Fármacos antiepilépticos para el dolor crónico no relacionado con el cáncer en niños y adolescentes
Referencias
References to studies included in this review
References to studies excluded from this review
Additional references
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Allocation: randomised Blinding: double‐blind Controlled: placebo Centre: multi Arm: 2 arms, parallel groups Imputation: BOCF Study dates: 55‐month trial (May 2010 to December 2014) | |
| Participants | Inclusion criteria: adolescents with fibromyalgia, Yunus and Masi diagnostic criteria, mean daily pain rating NRS score of ≥ 4 (0 to 10). Exclusion criteria: pain due to other conditions, systemic inflammatory MSK disorders, rheumatic diseases other than fibromyalgia, serious active infections, untreated endocrine disorders, prior participation in a clinical trial of pregabalin, history of failed treatment with pregabalin, mental health conditions, active malignancy, immunocompromised, or history of drug abuse. Baseline characteristics N = 107 Age: 12 to 17 years Gender: male (15); female (92) Number randomised: intervention (54); placebo (53) Number completed: intervention (44); placebo (36) Setting and location: 36 centres in the USA (28), India (5), Taiwan (2), and Czech Republic (1) | |
| Interventions | Intervention group (N = 54): flexible‐dose pregabalin 75 to 450 mg/day Control group (N = 53): flexible‐dose placebo 75 to 450 mg/day Study duration: 15 weeks' duration including 4 phases: doses were optimised over 3 weeks based on efficacy and tolerability to 75, 150, 300, 450 mg/day. Remaining at that dose for 12 weeks. | |
| Outcomes | Primary outcomes
Secondary outcomes
| |
| Notes | Sources of funding: sponsored by Pfizer. Medical writing support was provided. Trial registrations: NCT01020474; NCT01020526. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Subjects were randomized 1:1 to receive pregabalin or matched placebo according to a computer‐generated pseudo‐random code using the method of random permuted blocks." |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information provided. |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "this was a double‐blind study", "pregabalin or matched placebo was administered twice daily" |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "Under this approach, a blinded assessment of the change in mean pain score of the 95 subjects who had been randomized at that point was conducted" |
| Incomplete outcome data (attrition bias) | Low risk | Comment: all participants were accounted for. |
| Selective reporting (reporting bias) | Low risk | Comment: all planned outcomes listed in the methods were reported in the results. |
| Size | High risk | Comment: total participants > 50 per treatment arm randomised. Participants < 50 per treatment arm completed. |
| Other bias | Unclear risk | Comment: see notes above for details on Pfizer funding. |
| Methods | Allocation: randomised Blinding: double‐blind Controlled: active comparator Centre: single Arm: 2 arms, parallel groups Imputation: BOCF Study dates: 38‐month trial (April 2006 to July 2010) | |
| Participants | Inclusion criteria: CRPS‐I or neuropathic pain and recommendation for pharmacological treatment with gabapentin or amitriptyline by a clinic physician during the patient's intake appointment. Exclusion criteria: unable to speak English, lactose intolerant, pregnant, previously using either gabapentin or amitriptyline for the treatment of CRPS‐I or neuropathic pain or if they were unable to swallow a size “0” gelatin capsule. Children were also excluded if study medications were contraindicated by additional health conditions or the treatment of such conditions, including the regular use of any of the following medications or classes of medications: anticholinergics, antihypertensives, anticonvulsants, H2 receptor antagonists, antidepressants, sympathomimetics, thyroid replacements, antacids, and analgesics. Baseline characteristics N = 34 Age: 7 to 18 years Gender: male (6); female (28) Number randomised: intervention (17); active comparator (17) Number completed: intervention (15); active comparator (14) Setting and location: Chronic Pain Clinic (The Hospital for Sick Children, Toronto, Canada) | |
| Interventions | Intervention group (N = 17): oral gabapentin 900 mg/day (300 mg x 3). Control group (N = 17): oral amitriptyline 10 mg/day. Study duration: 6 weeks. | |
| Outcomes | Primary outcomes
Secondary outcomes
| |
| Notes | Sources of funding: Canadian Institutes of Health Research (CIHR) New Emerging Team (NET) Grant (GHL‐63209). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The randomization sequence generation was completed by the research support service pharmacist (not involved in patient care) and the allocation list was concealed from the participants and the study team" Quote: "Since some neuropathic pain conditions disproportionately affect boys and girls, randomization was stratified by sex to ensure that equivalent numbers of boys and girls were randomized to each treatment group. The randomization sequence of 1:1 ratio of amitriptyline to gabapentin was a block 4 design with the possible sequence combinations (e.g., AABB, ABAB) assigned a number and then a point on a page of printed random numbers picked" |
| Allocation concealment (selection bias) | Low risk | Quote: "The research support pharmacy held the allocation sequence schedule, with a copy of participant‐specific medications in sealed manila envelopes available to the research coordinator for emergency purposes or unblinding at the end of the study period" |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "To maintain blinding due to differences in dosing frequency for the two study drugs, participants were prescribed one capsule at night (∼20:00 h) for the first 3 days, then added a second capsule in the morning (∼08:00 h) for the next 3 days and then added a third capsule mid‐afternoon (∼14:00 h) for the remainder of the trial. Children randomized to the amitriptyline group received amitriptyline in the evening pill and placebo in the morning and afternoon pills; while children randomized to gabapentin received 300 mg of gabapentin in each pill." Quote: "Both study and placebo medications were made to be similar in composition, odour, colour and taste by over encapsulating the untouched original dosage form with a larger opaque hard gelatin capsule (7.34 ml in length) and filling any space with lactose powder." |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: insufficient information provided. |
| Incomplete outcome data (attrition bias) | Low risk | Comment: all participants were accounted for. |
| Selective reporting (reporting bias) | Unclear risk | Comment: not all planned outcomes were reported; disruption of school, social, and sports not reported. |
| Size | High risk | Comment: total participants < 50 per treatment arm randomised and completed. |
| Other bias | Low risk | Comment: no other potential sources of bias. |
BOCF: baseline observation carried forward; CPRS‐I: complex regional pain syndrome type 1; MSK: musculoskeletal; NRS: numerical rating scale
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Participants: adult population | |
| Participants: adult population | |
| Participants: adult population | |
| Participants: adult population | |
| Participants: adult population | |
| Participants: adult population |
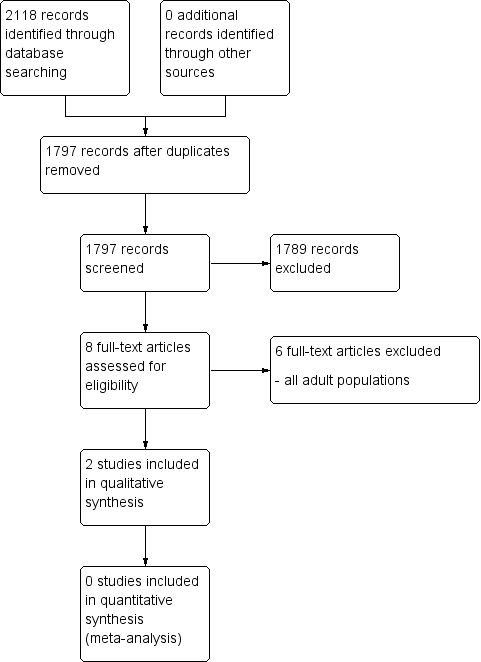
Study flow diagram.
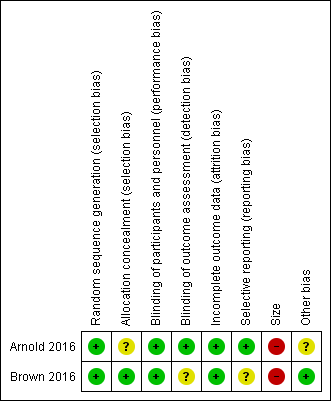
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
| Gabapentin compared with amitriptyline for chronic non‐cancer pain | ||||||
| Patient or population: children and adolescents (birth to 17 years of age) with chronic non‐cancer pain Settings: primary care Intervention: gabapentin Comparison: amitriptyline | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Amitriptyline | Gabapentin | |||||
| Participant‐reported pain relief of 30% or greater | No data | No data | N/A | N/A | ‐ | No evidence to support or refuteb |
| Participant‐reported pain relief of 50% or greater | No data | No data | N/A | N/A | ‐ | No evidence to support or refuteb |
| Patient Global Impression of Change: much improved or very much improved | No data | No data | N/A | N/A | ‐ | No evidence to support or refuteb |
| Any adverse events | 1/17 | 2/17 | N/A | 34 participants (1 study) | ⊕⊝⊝⊝ | |
| Serious adverse events | 0/17 | 0/17 | N/A | 34 participants (1 study) | ⊕⊝⊝⊝ | |
| Withdrawals due to adverse events | 1/17 | 2/17 | N/A | 34 participants (1 study) | ⊕⊝⊝⊝ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; N/A: not applicable; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: We are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of effect, but there is a possibility that it is substantially different. Low quality: Our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low quality: We have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
| aDowngraded three levels due to too few data and number of events were too small to be meaningful. bNo data available for this outcome, and therefore no GRADE rating has been applied and there is no evidence to support or refute. | ||||||
| Pregabalin compared with placebo for chronic non‐cancer pain | ||||||
| Patient or population: children and adolescents (birth to 17 years of age) with chronic non‐cancer pain Settings: multicentre, USA (28 primary care centres), India (5 primary care centres), Taiwan (2 primary care centres), and Czech Republic (1 primary care centre) Intervention: pregabalin Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Placebo | Pregabalin | |||||
| Participant‐reported pain relief of 30% or greater** | 16/51 | 18/54 | N/A | 107 participants (1 study) | ⊕⊝⊝⊝ | |
| Participant‐reported pain relief of 50% or greater** | 4/51 | 9/54 | N/A | 107 participants (1 study) | ⊕⊝⊝⊝ | |
| Patient Global Impression of Change: much improved or very much improved** | 15/51 | 29/54 | N/A | 107 participants (1 study) | ⊕⊝⊝⊝ | |
| Adverse events | 34/53 | 38/54 | N/A | 107 participants (1 study) | ⊕⊝⊝⊝ | |
| Serious adverse events | 0/53 | 1/54 | N/A | 107 participants (1 study) | ⊕⊝⊝⊝ | |
| Withdrawals due to adverse events | 4/53 | 4/54 | N/A | 107 participants (1 study) | ⊕⊝⊝⊝ | |
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: We are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of effect, but there is a possibility that it is substantially different. Low quality: Our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low quality: We have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
| aDowngraded three levels due to too few data and number of events were too small to be meaningful. bNo data available for this outcome, and therefore no GRADE rating has been applied and there is no evidence to support or refute. | ||||||

