Fármacos antiepilépticos para el dolor crónico no relacionado con el cáncer en niños y adolescentes
Resumen
Antecedentes
El dolor es una característica común en la niñez y la adolescencia en todo el mundo, y en el caso de muchos jóvenes, el dolor es crónico. En las guías de la Organización Mundial de la Salud (OMS) sobre los tratamientos farmacológicos del dolor persistente en niños se reconoce que el dolor en este grupo etario es un problema de gran interés y significación para la salud pública en la mayoría de los países del mundo. Anteriormente, el dolor se desestimaba en la mayoría de los casos y con frecuencia no se trataba, los criterios sobre el dolor en niños han cambiado con el transcurso del tiempo y en la actualidad su alivio se considera importante.
Se diseñó una serie de siete revisiones sobre dolor crónico no relacionado con el cáncer y dolor por cáncer (en que se estudiaron antidepresivos, antiepilépticos, antiinflamatorios no esteroideos, opiáceos y paracetamol) para examinar la evidencia sobre el dolor en niños mediante el uso de intervenciones farmacológicas en niños y adolescentes.
Como se trata de la principal causa de morbilidad en el mundo actual, las enfermedades crónicas (y el dolor asociado) es un problema de salud significativo. El dolor crónico (definido como el dolor que dura tres meses o más) se puede presentar en la población pediátrica en diversas clasificaciones fisiopatológicas (nociceptivo, neuropático o idiopático), en relación con enfermedades genéticas, dolor por daño en los nervios, dolor musculoesquelético crónico, dolor abdominal crónico y otras causas desconocidas.
Los fármacos antiepilépticos (anticonvulsivantes), desarrollados originalmente para tratar las convulsiones en los pacientes con epilepsia, se han utilizado en los últimos años como analgésicos en adultos para muchas afecciones dolorosas crónicas, y ahora se recomiendan para el tratamiento del dolor crónico en la lista de la OMS de medicamentos esenciales. Los efectos secundarios comprobados de los fármacos antiepiléticos van desde sudoración, cefalea, fiebre, náuseas y dolor abdominal hasta efectos más graves como trastornos mentales y motores.
Objetivos
Evaluar la eficacia analgésica y los eventos adversos de los fármacos antiepilépticos administrados para tratar el dolor no relacionado con el cáncer en niños y adolescentes, desde el nacimiento hasta los 17 años de edad, en cualquier ámbito.
Métodos de búsqueda
Se hicieron búsquedas en el Registro Cochrane Central de Ensayos Controlados (Cochrane Central Register of Controlled Trials; CENTRAL) vía Cochrane Register of Studies Online, MEDLINE vía Ovid y Embase vía Ovid desde su inicio hasta el 6 de septiembre de 2016. También se realizaron búsquedas en las listas de referencias de estudios y revisiones recuperados, así como en registros de ensayos clínicos en línea.
Criterios de selección
Ensayos controlados aleatorizados, con o sin cegamiento, del tratamiento, por cualquier vía, del dolor crónico no relacionado con el cáncer en niños y adolescentes, que compararan un fármaco antiepiléptico con placebo o un comparador activo.
Obtención y análisis de los datos
Dos autores de la revisión, de forma independiente, evaluaron la elegibilidad de los estudios. Se programó usar datos dicotómicos para calcular el cociente de riesgos y el número necesario a tratar para un evento adicional, con el uso de métodos estándar siempre que se dispusiera de datos. Se evaluó la evidencia mediante GRADE y se crearon dos tablas "Resumen de los hallazgos".
Resultados principales
Se incluyeron dos estudios con un total de 141 participantes (siete a 18 años de edad) con dolor neuropático crónico, síndrome de dolor regional complejo tipo 1 (SDRC‐I) o fibromialgia. Un estudio investigó pregabalina versus placebo en pacientes con fibromialgia (107 participantes), y el otro investigó gabapentina versus amitriptilina en pacientes con SDRC‐I o dolor neuropático (34 participantes). No fue posible realizar un análisis cuantitativo.
Hubo variación en el riesgo de sesgo de los dos estudios incluidos por las siguientes razones: problemas con la asignación al azar (riesgo bajo a incierto), cegamiento de los evaluadores de resultados (riesgo bajo a incierto), sesgo de informe (riesgo bajo a cierto), tamaño de las poblaciones de estudio (alto riesgo) y financiamiento de la industria en el dominio "otros" (riesgo bajo a incierto). Se consideró bajo el riesgo de sesgo en los dominios restantes (generación de la secuencia, cegamiento de los participantes y el personal y deserción).
Resultados primarios
Un estudio (gabapentina 900 mg/día versus amitriptilina 10 mg/día, 34 participantes, durante seis semanas) no informó sobre los resultados primarios de esta revisión.
El segundo estudio (pregabalina 75 a 450 mg/día versus placebo 75 a 450 mg/día, 107 participantes, durante 15 semanas) no informó cambios significativos en las puntuaciones de dolor para el alivio del dolor del 30% o mayor entre pregabalina 18/54 (33,3%) y placebo 16/51 (31,4%), P = 0,83 (evidencia de calidad muy baja). Este estudio también informó el Patient Global Impression of Change: 53,1% de los pacientes que recibieron pregabalina y 29,5% que recibieron placebo percibieron "una mejoría importante o muy importante" (evidencia de calidad muy baja).
Resultados secundarios
En un estudio pequeño, los eventos adversos fueron poco frecuentes: gabapentina dos participantes (dos eventos adversos); amitriptilina un participante (un evento adverso) (ensayo de seis semanas). El segundo estudio informó un mayor número de eventos adversos: pregabalina 38 participantes (167 eventos adversos); placebo 34 participantes (132 eventos adversos) (ensayo de 15 semanas) (evidencia de calidad muy baja).
Los retiros debido a eventos adversos fueron poco frecuentes en ambos estudios: pregabalina (cuatro participantes), placebo (cuatro participantes), gabapentina (dos participantes) y amitriptilina (un participante) (evidencia de calidad muy baja).
Se informaron eventos adversos graves en ambos estudios. Un estudio informó sólo un evento adverso grave (colelitiasis y depresión grave que derivó en hospitalización en el grupo de pregabalina) y el otro estudio no informó eventos adversos graves (evidencia de calidad muy baja).
Hubo pocos datos, o no hubo, de los otros resultados secundarios.
Calidad de la evidencia
La calidad de la evidencia se disminuyó en tres niveles a muy baja debido a la escasa cantidad de datos y a que el número de eventos fue demasiado pequeño para que fuera significativo.
Conclusiones de los autores
Esta revisión identificó sólo dos estudios pequeños, con datos insuficientes para el análisis.
Como no fue posible realizar un metanálisis, no se pueden hacer comentarios sobre la eficacia ni los efectos perjudiciales de la administración de fármacos antiepiléticos para el tratamiento del dolor crónico no relacionado con el cáncer en niños y adolescentes. De manera similar, no es posible hacer observaciones sobre los resultados secundarios restantes de esta revisión: Carer Global Impression of Change; necesidad de analgesia de rescate; duración y calidad del sueño; aceptabilidad del tratamiento; funcionamiento físico y calidad de vida de vida.
A partir de ensayos controlados aleatorizados en adultos se sabe que algunos antiepilépticos, como la gabapentina y la pregabalina, pueden ser efectivos en algunas afecciones de dolor crónico.
PICOs
Resumen en términos sencillos
Fármacos antiepilépticos para el dolor crónico no relacionado con el cáncer en niños y adolescentes
Conclusión
No hay seguridad acerca de si los fármacos antiepiléticos brindan alivio del dolor para el dolor crónico no relacionado con el cáncer en niños y adolescentes. No se encontró evidencia que indique que un tipo de fármaco antiepiléptico es más efectivo que otro.
Antecedentes
Los niños pueden presentar dolor crónico o recurrente relacionado con trastornos genéticos, daño en los nervios, dolor muscular o articular, dolor abdominal u otras razones desconocidas. El dolor crónico se define como dolor que dura tres meses o más y se acompaña frecuentemente de cambios en la funcionalidad y el modo de vida, así como por signos y síntomas de depresión y ansiedad.
Los fármacos antiepiléticos (anticonvulsivantes) se desarrollaron originalmente para tratar la epilepsia, aunque se ha demostrado que algunos de estos fármacos brindan alivio del dolor en algunas afecciones dolorosas crónicas en adultos.
Características de los estudios
En septiembre de 2016 se buscaron ensayos clínicos en que se usaran fármacos antiepiléticos para tratar el dolor crónico. Se incluyeron dos estudios con un total de 141 participantes (siete a 18 años de edad) con dolor neuropático crónico, síndrome de dolor regional complejo tipo 1 (SDRC‐I) o fibromialgia, de más de tres meses de evolución.
Resultados clave
Un estudio analizó pregabalina versus placebo para pacientes con fibromialgia, y no halló cambios significativos en las puntuaciones de dolor. El otro estudio evaluó la gabapentina en comparación con la amitriptilina, pero no informó los resultados específicos de dolor de esta revisión.
Los efectos secundarios fueron poco frecuentes y sólo hubo reacciones leves (como náuseas, mareos, somnolencia, cansancio y molestias abdominales): 38 con pregabalina, dos con gabapentina, uno con amitriptilina y 34 con placebo. Sólo 11 participantes se retiraron debido a estos efectos secundarios leves (cuatro pregabalina, dos gabapentina, uno amitriptilina, cuatro placebo).
Calidad de la evidencia
La calidad de la evidencia de los estudios se calificó con el uso de cuatro niveles: muy baja, baja, moderada o alta. La evidencia de calidad muy baja significa que hay muy poca seguridad en cuanto a los resultados. La evidencia de alta calidad significan que existe mucha seguridad en cuanto a los resultados.
La evidencia disponible en esta revisión fue de calidad muy baja debido a la falta de datos y el pequeño tamaño de los estudios.
Authors' conclusions
Summary of findings
| Gabapentin compared with amitriptyline for chronic non‐cancer pain | ||||||
| Patient or population: children and adolescents (birth to 17 years of age) with chronic non‐cancer pain Settings: primary care Intervention: gabapentin Comparison: amitriptyline | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Amitriptyline | Gabapentin | |||||
| Participant‐reported pain relief of 30% or greater | No data | No data | N/A | N/A | ‐ | No evidence to support or refuteb |
| Participant‐reported pain relief of 50% or greater | No data | No data | N/A | N/A | ‐ | No evidence to support or refuteb |
| Patient Global Impression of Change: much improved or very much improved | No data | No data | N/A | N/A | ‐ | No evidence to support or refuteb |
| Any adverse events | 1/17 | 2/17 | N/A | 34 participants (1 study) | ⊕⊝⊝⊝ | |
| Serious adverse events | 0/17 | 0/17 | N/A | 34 participants (1 study) | ⊕⊝⊝⊝ | |
| Withdrawals due to adverse events | 1/17 | 2/17 | N/A | 34 participants (1 study) | ⊕⊝⊝⊝ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; N/A: not applicable; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: We are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of effect, but there is a possibility that it is substantially different. Low quality: Our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low quality: We have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
| aDowngraded three levels due to too few data and number of events were too small to be meaningful. bNo data available for this outcome, and therefore no GRADE rating has been applied and there is no evidence to support or refute. | ||||||
| Pregabalin compared with placebo for chronic non‐cancer pain | ||||||
| Patient or population: children and adolescents (birth to 17 years of age) with chronic non‐cancer pain Settings: multicentre, USA (28 primary care centres), India (5 primary care centres), Taiwan (2 primary care centres), and Czech Republic (1 primary care centre) Intervention: pregabalin Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Placebo | Pregabalin | |||||
| Participant‐reported pain relief of 30% or greater** | 16/51 | 18/54 | N/A | 107 participants (1 study) | ⊕⊝⊝⊝ | |
| Participant‐reported pain relief of 50% or greater** | 4/51 | 9/54 | N/A | 107 participants (1 study) | ⊕⊝⊝⊝ | |
| Patient Global Impression of Change: much improved or very much improved** | 15/51 | 29/54 | N/A | 107 participants (1 study) | ⊕⊝⊝⊝ | |
| Adverse events | 34/53 | 38/54 | N/A | 107 participants (1 study) | ⊕⊝⊝⊝ | |
| Serious adverse events | 0/53 | 1/54 | N/A | 107 participants (1 study) | ⊕⊝⊝⊝ | |
| Withdrawals due to adverse events | 4/53 | 4/54 | N/A | 107 participants (1 study) | ⊕⊝⊝⊝ | |
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: We are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of effect, but there is a possibility that it is substantially different. Low quality: Our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low quality: We have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
| aDowngraded three levels due to too few data and number of events were too small to be meaningful. bNo data available for this outcome, and therefore no GRADE rating has been applied and there is no evidence to support or refute. | ||||||
Background
Pain is a common feature of childhood and adolescence around the world, and for many young people, that pain is chronic. The World Health Organization (WHO) guidelines for pharmacological treatments for persisting pain in children acknowledge that pain in children is a major public health concern of high significance in most parts of the world (WHO 2012). While in the past, pain was largely dismissed and was frequently left untreated, views on children's pain have changed over time, and relief of pain is now seen as important. Since the 1970s, studies comparing child and adult pain management have revealed a variety of responses to pain, fuelling the need for a more in‐depth focus on paediatric pain (Caes 2016).
Infants (zero to 12 months), children (1 to 9 years), and adolescents (10 to 18 years), WHO 2012, account for 27% (1.9 billion) of the world's population (United Nations 2015); the proportion of those aged 14 years and under ranges from 12% (in Hong Kong) to 50% (in Niger) (World Bank 2014). However, little is known about the pain management needs of this population. For example, in the Cochrane Library, approximately 12 reviews produced by the Cochrane Pain, Palliative and Supportive Care Review Group in the past 18 years have been specifically concerned with children and adolescents, compared to over 100 reviews specific to adults. Additional motivating factors for investigating children's pain include the vast amount of unmanaged pain in the paediatric population and the development of new technologies and treatments. We convened an international group of leaders in paediatric pain to design a suite of seven reviews in chronic pain and cancer pain (looking at antidepressants, antiepileptic drugs, non‐steroidal anti‐inflammatory drugs, opioids, and paracetamol as priority areas) in order to review the evidence under a programme grant for children's pain utilising pharmacological interventions in children and adolescents (Appendix 1).
This review is based on a template for reviews of pharmacotherapies used to relieve pain in infants, children, and adolescents. The aim is for all reviews to use the same methods, based on new criteria for what constitutes reliable evidence (Appendix 2) (Moore 2010a; Moore 2012). This review focused on antiepileptic drugs to treat chronic non‐cancer pain.
Description of the condition
This review focused on chronic non‐cancer pain experienced by children and adolescents as a result of any type of chronic disease that occurs throughout the global paediatric population. Children's level of pain can be mild, moderate, or severe, and pain management is an essential element of patient management during all care stages of chronic disease.
As the leading cause of morbidity in the world today, chronic disease (and its associated pain) is a major health concern. Chronic pain can arise in the paediatric population in a variety of pathophysiological classifications: nociceptive, neuropathic, or idiopathic. Chronic pain is pain that lasts three months or longer and can be accompanied by changes in lifestyle, personality, and functional abilities, as well as by signs and symptoms of depression (Ripamonti 2008).
Whilst diagnostic and perioperative procedures performed to treat chronic diseases are a known common cause of pain in these patients, this review did not cover perioperative pain or adverse effects of treatments such as mucositis.
Description of the intervention
Evidence for the use of antiepileptic (anticonvulsant) drugs, which were originally developed and manufactured to treat convulsions and convulsive disorders in people with epilepsy, to provide pain relief in many chronic painful conditions has existed since the 1960s (Blom 1962; Wiffen 2013). Phenytoin was used as long ago as the 1940s to treat trigeminal neuralgia (Ryder 2005). Antiepileptic drugs are now recommended for the treatment of both convulsive disorders and chronic pain in the WHO list of essential medicines (WHO 2015).
Antiepileptic drugs are available worldwide and have been found to reduce the chronic pain associated with fibromyalgia, neuropathic pain, and other kinds of chronic pain. Antiepileptic drugs include, but are not limited to, carbamazepine, clonazepam, diazepam, ethosuximide, lorazepam, magnesium sulphate, midazolam, phenobarbital, phenytoin, and valproic acid (sodium valproate) (WHO 2015).
Routes of administration include oral tablets, oral liquids, gels, rectal solutions, parenteral formulations, intravenous injections, and intramuscular injections (WHO 2015). Recommended doses for children vary depending on the drug, age of the child, and painful condition.
Known side effects of antiepileptic drugs range from sweating, headache, elevated temperature, nausea, and abdominal pain to more serious adverse effects including mental or motor function impairment, physical or congenital abnormalities if taken during pregnancy, and on rare occasions death from haematological reactions.
The use of antiepileptic drugs to treat chronic pain in children is likely to be outside the product licence in most countries.
How the intervention might work
Different antiepileptic drugs have different mechanisms of action, not all of which are well understood, especially in terms of how a given drug produces pain relief in any particular individual with any particular chronic pain condition.
In general, antiepileptic drugs are thought to reduce the ability of the neuron to fire at high frequency (Chong 2000). The two standard explanations are enhanced gamma‐aminobutyric acid (GABA) inhibition (valproate, clonazepam), or a stabilising effect on neuronal cell membranes, possibly by modulating ion channels. A third possibility is action via N‐methyl‐D‐aspartate (NMDA) receptor sites (Dickenson 2007).
The mechanisms of action of specific drugs are as follows.
-
Gabapentin is thought to act by binding to calcium channels and modulating calcium influx. This mode of action confers antiepileptic, analgesic, and sedative effects. Recent research indicates that gabapentin acts by blocking new synapse formation (Eroglu 2009). Clear‐cut explanations are not available.
-
Pregabalin has a mechanism of action similar to gabapentin, binding to calcium channels and modulating calcium influx as well as influencing GABAergic neurotransmission. It is more potent than gabapentin and is therefore used at lower doses. Again, clear‐cut explanations are not available.
-
Lamotrigine is chemically unrelated to other antiepileptic agents. It is thought to exert its antiepileptic effect via sodium channels. There is some evidence that agents that block sodium channels are useful in the treatment of neuropathic pain (McCleane 2000). More recently it has been shown that neuronal alpha‐4‐beta2‐nicotinic acetylcholine receptors may be a target for lamotrigine, and this may mediate its antiepileptic effects (Zheng 2010).
-
Lacosamide is a functionalised amino acid molecule that selectively enhances the slow inactivation of voltage‐gated sodium channels and interacts with the collapsin‐response mediator protein‐2 (Beydoun 2009; Errington 2008). Voltage‐gated sodium channels play an important role in the excitability of nociceptors.
-
Carbamazepine and its keto analogue oxcarbazepine are also thought to work by blocking voltage‐gated sodium channels, making the cells less excitable.
-
There is no consensus as to how phenytoin exerts any analgesic effects. It may involve voltage‐gated sodium channel blockade.
-
Valproate is thought to influence GABAergic neurotransmission. It is also thought to block sodium and calcium channels. Although their mechanism of action in pain relief is not yet fully understood, increasing levels of GABA and stabilisation of cell membranes probably result in a reduction of pain signals being processed in the brain. A number of other putative mechanisms of action have been suggested based on the effects on signal transduction in neurons (Toth 2005).
-
It has been suggested that clonazepam works by antagonising hyperexcitability of neurotransmission through the enhancement of inhibitory GABAergic signalling pathways.
-
Topiramate has been shown to block activity‐dependent, voltage‐gated sodium channels; enhance the action of GABAreceptors; inhibit L‐type voltage‐gated calcium channels; presynaptically reduce glutamate release; and postsynaptically block kainate/α‐amino‐3‐hydroxy‐5‐methyl‐4‐isoxazolepropionic acid (AMPA) receptors (Chong 2003).
Why it is important to do this review
The paediatric population is at risk of inadequate management of pain (AMA 2013). Some conditions that would be aggressively treated in adult patients are being managed with insufficient analgesia in younger populations (AMA 2013). Although there have been repeated calls for best evidence to treat children's pain, such as Eccleston 2003, there are no easily available summaries of the most effective paediatric pain relief.
This review formed part of a Programme Grant addressing the unmet needs of people with chronic pain, commissioned by the National Institute for Health Research (NIHR) in the UK. This topic was identified in June 2015 during consultation with experts in paediatric pain. Please see Appendix 1 for full details of the meeting. The standards used to assess evidence in chronic pain trials have changed substantially in recent years, with particular attention being paid to trial duration, withdrawals, and statistical imputation following withdrawal, all of which can substantially alter estimates of efficacy. The most important change was to encourage a move from using average pain scores, or average change in pain scores, to the number of people who have a large decrease in pain (by at least 50%). Pain intensity reduction of 50% or more has been shown to correlate with improvements in comorbid symptoms, function, and quality of life (Moore 2011a). These standards are set out in the reference guide for pain studies (AUREF 2012).
Objectives
To assess the analgesic efficacy and adverse events of antiepileptic drugs used to treat chronic non‐cancer pain in children and adolescents aged between birth and 17 years, in any setting.
Methods
Criteria for considering studies for this review
Types of studies
We included only randomised controlled trials, with or without blinding, and participant‐ or observer‐reported outcomes.
Full journal publication was required, with the exception of online clinical trial results, summaries of otherwise unpublished clinical trials, and abstracts with sufficient data for analysis. We included studies published in any language. We excluded abstracts (usually meeting reports) or unpublished data, non‐randomised studies, studies of experimental pain, case reports, and clinical observations.
Types of participants
We included studies of infants, children, and adolescents, aged from birth to 17 years old, with chronic or recurrent pain (lasting for three months or longer), arising from genetic conditions, neuropathy, or other conditions. These included but were not limited to chronic musculoskeletal pain and chronic abdominal pain.
We excluded studies of perioperative pain, acute pain, cancer pain, headache, migraine, and pain associated with primary disease or its treatment.
We planned to include studies of participants with more than one type of chronic pain, in which case we would analyse results according to the primary condition.
Types of interventions
We included studies reporting interventions prescribing antiepileptic drugs for the relief of chronic non‐cancer pain; by any route, in any dose, with comparison to a placebo or any active comparator.
Types of outcome measures
In order to be eligible for inclusion in this review, studies had to report pain assessment, as well as meeting the other selection criteria.
We included trials measuring pain intensity and pain relief assessed using validated tools such as numerical rating scale (NRS), visual analogue scale (VAS), Faces Pain Scale ‐ Revised (FPS‐R), Colour Analogue Scale (CAS), or any other validated numerical rating scale.
We were particularly interested in Pediatric Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials (PedIMMPACT) definitions for moderate and substantial benefit in chronic pain studies (PedIMMPACT 2008). These were defined as: at least 30% pain relief over baseline (moderate); at least 50% pain relief over baseline (substantial); much or very much improved on Patient Global Impression of Change scale (PGIC) (moderate); very much improved on PGIC (substantial).
These outcomes differ from those used in most earlier reviews, concentrating as they do on dichotomous outcomes where pain responses do not follow a normal (Gaussian) distribution. People with chronic pain desire high levels of pain relief, ideally more than 50% pain intensity reduction, and ideally having no worse than mild pain (Moore 2013a; O'Brien 2010).
We also recorded any reported adverse events. We planned to report the timing of outcome assessments.
Primary outcomes
-
Participant‐reported pain relief of 30% or greater
-
Participant‐reported pain relief of 50% or greater
-
PGIC much or very much improved
In the absence of self reported pain, we considered the use of 'other‐reported' pain, typically by an observer such as a parent, carer, or healthcare professional (Stinson 2006; von Baeyer 2007).
Secondary outcomes
We identified the following with reference to the PedIMMPACT recommendations, which suggest core outcome domains and measures for consideration in paediatric acute and chronic/recurrent pain clinical trials (PedIMMPACT 2008),
-
Carer Global Impression of Change
-
Requirement for rescue analgesia
-
Sleep duration and quality
-
Acceptability of treatment
-
Physical functioning as defined by validated scales
-
Quality of life as defined by validated scales
-
Any adverse events
-
Withdrawals due to adverse events
-
Any serious adverse event. Serious adverse events typically include any untoward medical occurrence or effect that at any dose results in death, is life‐threatening, requires hospitalisation or prolongation of existing hospitalisation, results in persistent or significant disability or incapacity, is a congenital anomaly or birth defect, is an 'important medical event' that may jeopardise the patient, or may require an intervention to prevent one of the above characteristics or consequences.
Search methods for identification of studies
We developed the search strategy with our Information Specialist based on previous strategies used by the Cochrane Pain, Palliative and Supportive Care Review Group and carried out the searches.
Electronic searches
We searched the following databases:
-
Cochrane Central Register of Controlled Trials (CENTRAL) (via the Cochrane Register of Studies Online) searched on 6 September 2016;
-
MEDLINE (via Ovid) 1947 to week 2 September 2016, searched 6 September 2016;Embase (via Ovid) 1974 to week 2 September 2016, searched 6 September 2016.
We used medical subject headings (MeSH) or equivalent and text word terms. We restricted our search to randomised controlled trials and clinical trials. There were no language or date restrictions. We restricted our search to published papers only and excluded conference abstracts and meeting reports. The focus of the key words in our search terms was on chronic pain and anticonvulsant pharmacological agents. We tailored searches to individual databases. The search strategies for MEDLINE, Embase, and CENTRAL are in Appendix 3, Appendix 4, and Appendix 5, respectively.
Searching other resources
We searched ClinicalTrials.gov (www.clinicaltrials.gov) and the World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) (apps.who.int/trialsearch/) for ongoing trials, on 6 September 2017. In addition, we checked reference lists of reviews and retrieved articles for additional studies, and performed citation searches on key articles. We planned to contact experts in the field for unpublished and ongoing trials. We planned to contact study authors for additional information where necessary.
Data collection and analysis
We planned to perform separate analyses according to particular chronic pain conditions. We planned to combine different chronic pain conditions in analyses for exploratory purposes only.
Selection of studies
Two review authors independently determined study eligibility by reading the abstract of each study identified by the search. Review authors independently eliminated studies that clearly did not satisfy the inclusion criteria, and obtained full copies of the remaining studies. Two review authors independently read these studies to select those that met the inclusion criteria, a third review author adjudicating in the event of disagreement. We did not anonymise the studies in any way before assessment. We included a PRISMA flow chart (Figure 1) to illustrate the results of the search and the process of screening and selecting studies for inclusion in the review (Moher 2009), as recommended in section 11.2.1 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We included studies in the review irrespective of whether measured outcome data are reported in a ‘usable’ way.

Study flow diagram.
Data extraction and management
We obtained full copies of the studies, and two review authors independently carried out data extraction. Where these data were available, data extraction included information about the pain condition, number of participants treated, drug and dosing regimen, study design (placebo or active control), study duration and follow‐up, analgesic outcome measures and results, withdrawals, and adverse events (participants experiencing any adverse event or serious adverse event). We planned to collate multiple reports of the same study, so that each study rather than each report was the unit of interest in the review. We collected characteristics of the included studies in sufficient detail to populate a ‘Characteristics of included studies’ table.
We used a template data extraction form and checked for agreement before entry into Cochrane's statistical software Review Manager 5 (RevMan 2014).
If a study had more than two intervention arms, we planned to only include the intervention and control groups that met the eligibility criteria. If multi‐arm studies were included, we planned to analyse multiple intervention groups in an appropriate way that avoided arbitrary omission of relevant groups and double‐counting of participants.
Assessment of risk of bias in included studies
Two review authors independently assessed the risk of bias for each study, using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
We completed a 'Risk of bias' table for each included study using the Cochrane 'Risk of bias' tool in Review Manager 5 (RevMan 2014).
We assessed the following for each study. Any disagreements were resolved by discussion between review authors or by consulting a third review author when necessary.
-
Random sequence generation (checking for possible selection bias). We assessed the method used to generate the allocation sequence as: low risk of bias (i.e. any truly random process, e.g. random number table; computer random number generator); or unclear risk of bias (when the method used to generate the sequence was not clearly stated). We excluded studies that used a non‐random process and were therefore at high risk of bias (e.g. odd or even date of birth; hospital or clinic record number).
-
Allocation concealment (checking for possible selection bias). The method used to conceal allocation to interventions prior to assignment determines whether intervention allocation could have been foreseen in advance of, or during, recruitment, or changed after assignment. We assessed the methods as: low risk of bias (e.g. telephone or central randomisation; consecutively numbered, sealed, opaque envelopes); or unclear risk of bias (when the method was not clearly stated). We excluded studies that did not conceal allocation and were therefore at a high risk of bias (e.g. open list).
-
Blinding of participants and personnel (checking for possible performance bias). We assessed any methods used to blind the participants and personnel from knowledge of which intervention a participant received. We assessed the methods as: low risk of bias (study states that the participants and personnel involved were blinded to treatment groups); unclear risk of bias (study does not state whether or not participants and personnel were blinded to treatment groups); or high risk of bias (participants or personnel were not blinded) (as stated in Types of studies, we still included trials with or without blinding, and participant‐ or observer‐reported outcomes).
-
Blinding of outcome assessment (checking for possible detection bias). We assessed any methods used to blind the outcome assessors from knowledge of which intervention a participant received. We assessed the methods as: low risk of bias (e.g. study states that it was single‐blinded and describes the method used to achieve blinding of the outcome assessor); unclear risk of bias (study states that outcome assessors were blinded but does not provide an adequate description of how this was achieved); or high risk of bias (outcome assessors were not blinded) (as stated in Types of studies, we still included trials with or without blinding, and participant‐ or observer‐reported outcomes).
-
Incomplete outcome data (checking for possible attrition bias due to the amount, nature, and handling of incomplete outcome data). We assessed the methods used to deal with incomplete data as: low risk of bias (i.e. less than 10% of participants did not complete the study or 'baseline observation carried forward' (BOCF) analysis was used, or both); unclear risk of bias (used 'last observation carried forward' (LOCF) analysis); or high risk of bias (used 'completer' analysis).
-
Selective reporting (checking for possible reporting bias). We assessed the methods used to report the outcomes of the study as: low risk of bias (if all planned outcomes in the protocol or methods were reported in the results); unclear risk of bias (if there was not a clear distinction between planned outcomes and reported outcomes); high risk of bias (if some planned outcomes from the protocol or methods were clearly not reported in the results).
-
Size of study (checking for possible biases confounded by small size (Dechartres 2013; Dechartres 2014; McQuay 1998; Nüesch 2010; Thorlund 2011)). We assessed studies as being at low risk of bias (200 participants or more per treatment arm); unclear risk of bias (50 to 199 participants per treatment arm); or high risk of bias (fewer than 50 participants per treatment arm).
-
Other bias. We assessed studies for any additional sources of bias such as early stopping or baseline imbalances as low, unclear, or high, and provided rationale.
Measures of treatment effect
Where dichotomous data were available, we planned to calculate a risk ratio (RR) with 95% confidence interval (CI) and meta‐analyse the data as appropriate. We planned to calculate numbers needed to treat for an additional beneficial outcome (NNTBs) where appropriate (McQuay 1998); for unwanted effects the NNTB becomes the number needed to treat for an additional harmful outcome (NNTH) and would be calculated in the same manner. Where continuous data were reported, we planned to use appropriate methods to combine these data in the meta‐analysis.
Unit of analysis issues
We planned to accept randomisation to the individual participant only. We planned to split the control treatment arm between active treatment arms in a single study if the active treatment arms were not combined for analysis. We only accepted studies with minimum 10 participants per treatment arm.
Dealing with missing data
We planned to use intention‐to‐treat analysis where the intention‐to‐treat population consisted of participants who were randomised, took at least one dose of the assigned study medication, and provided at least one postbaseline assessment. We would assign missing participants zero improvement wherever possible.
Assessment of heterogeneity
We planned to identify and measure heterogeneity as recommended in Chapter 9 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We planned to deal with clinical heterogeneity by combining studies that examined similar conditions. We planned to undertake and present a meta‐analysis only if we judged participants, interventions, comparisons, and outcomes to be sufficiently similar to ensure a clinically meaningful answer. We planned to assess statistical heterogeneity visually and by using the I² statistic (L'Abbé 1987). When I² was greater than 50%, we planned to consider the possible reasons.
Assessment of reporting biases
We assessed the risk of reporting bias, as recommended in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
The aim of this review is to use dichotomous outcomes of known utility and of value to patients (Hoffman 2010; Moore 2010b; Moore 2010c; Moore 2010d; Moore 2013a). The review did not depend on what the authors of the original studies chose to report or not, though clearly difficulties would arise in studies failing to report any dichotomous results. We extracted and used continuous data, which probably reflected efficacy and utility poorly, and is useful for illustrative purposes only (Appendix 6; Appendix 7).
We planned to assess publication bias using a method designed to detect the amount of unpublished data with a null effect required to make any result clinically irrelevant (usually taken to mean a number needed to treat (NNT) of 10 or higher) (Moore 2008).
Data synthesis
We planned to use a fixed‐effect model for meta‐analysis. We planned to use a random‐effects model for meta‐analysis if there was significant clinical heterogeneity and we considered it appropriate to combine studies. We planned to conduct our analysis using the primary outcomes of pain and adverse events, and to calculate the NNTHs for adverse events. We planned to use the Cochrane software program Review Manager 5 (RevMan 2014).
Quality of the evidence
To analyse data, two review authors independently rated the quality of each outcome. We planned to use the GRADE approach to assess the quality of the body of evidence related to each of the key outcomes, and to report our judgement in a 'Summary of findings' table per Chapter 12 of the Cochrane Handbook (Appendix 8) (Higgins 2011).
In addition, there may be circumstances where the overall rating for a particular outcome would need to be adjusted as recommended by GRADE guidelines (Guyatt 2013a). For example, if there were so few data that the results were highly susceptible to the random play of chance, or if studies used LOCF imputation in circumstances where there were substantial differences in adverse event withdrawals, one would have no confidence in the result, and would need to downgrade the quality of the evidence by three levels, to very low quality. In addition, in circumstances where no data were reported for an outcome, we planned to report that there was no evidence to support or refute (Guyatt 2013b).
'Summary of findings' table
We included a 'Summary of findings' table as set out in the Cochrane Pain, Palliative and Supportive Care Review Group’s author guide (AUREF 2012), and recommended in section 4.6.6 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We planned to justify and document all assessments of the quality of the body of evidence.
In an attempt to interpret reliability of the findings for this systematic review, we attempted to assess the summarised data using the GRADE guidelines (Appendix 8) to rate the quality of the body of evidence of each of the key outcomes listed in Types of outcome measures per Chapter 12 of the Cochrane Handbook (Guyatt 2011; Higgins 2011), as appropriate. Utilising the explicit criteria against study design, risk of bias, imprecision, inconsistency, indirectness, and magnitude of effect, we planned to summarise the evidence in an informative, transparent and, succinct 'Summary of findings' table or 'Evidence profile' table (Guyatt 2011).
Subgroup analysis and investigation of heterogeneity
We planned to perform subgroup analyses where a minimum number of data were available (at least 200 participants per treatment arm). We planned to analyse according to age group; geographical location or country; type of control group; baseline measures; frequency, dose, and duration of drugs; and nature of drug.
We planned to investigate whether the results of subgroups were significantly different by inspecting the overlap of confidence intervals and by performing the test for subgroup differences available in Review Manager 5.
Sensitivity analysis
We did not plan to carry out any sensitivity analysis because the evidence base is known to be too small to allow reliable analysis; we did not plan to pool results from chronic pain of different origins in the primary analyses. We planned to examine details of dose escalation schedules in the unlikely circumstance that this could provide some basis for a sensitivity analysis.
Results
Description of studies
Results of the search
A PRISMA flow diagram of the search results is shown in Figure 1.
The three main database searches revealed 2118 records of titles and abstracts, of which 321 duplicates were removed. Our searches of ClinicalTrials.gov and the WHO ICTRP yielded no additional eligible studies.
We screened the remaining 1797 titles and abstracts for eligibility, of which 1789 were removed as ineligible studies.
We retrieved the full‐text reports of the eight remaining studies. We deemed six as ineligible. We identified no ongoing studies. Two studies fulfilled the eligibility criteria and provided data. As they investigated different antiepileptic drugs and type of chronic pain condition, we could enter neither study into a quantitative meta‐analysis.
Included studies
See Characteristics of included studies.
Arnold 2016 investigated 107 participants in a multicentre (36 centres), randomised, double‐blind, placebo‐controlled, parallel‐group study. Participants had a diagnosis of fibromyalgia according to Yunus and Masi criteria with a mean daily pain score of ≥ 4 out of 10. Ages ranged from 12 to 17 years, and 86% were female. The flexible dose of oral pregabalin was administered as 75 to 450 mg/day, and flexible‐dose placebo tablets were administered in a similar regimen. The study duration was 15 weeks, whereby 4 doses were optimised over 3 weeks based on efficacy and tolerability to 75, 150, 300, 450 mg/day, remaining at that dose for 12 weeks. People were excluded if they had pain due to other conditions, systemic inflammatory musculoskeletal disorders, rheumatic diseases other than fibromyalgia, serious active infections, untreated endocrine disorders, prior participation in a clinical trial of pregabalin, history of failed treatment with pregabalin, unstable mental health conditions, active malignancy, immunocompromised, or history of drug abuse. Authors used a baseline observation carried forward (BOCF) method for missing data.
Brown 2016 investigated 34 participants in a single‐centre, randomised, double‐blind, controlled by active comparator, parallel‐group study. Participants had a diagnosis of complex regional pain syndrome type 1 (CRPS‐I) or neuropathic pain and were recommended for pharmacological treatment with either gabapentin or amitriptyline by their clinical physician. Ages ranged from 7 to 18 years, and 82% were female. A fixed dose of oral gabapentin was administered as 900 mg/day (300 mg at 3 separate times), and fixed‐dose oral amitriptyline was administered as a single evening dose of 10 mg/day with placebo capsules administered to this group at the other two dosing time points. People were excluded if they were lactose intolerant; pregnant; previously using either gabapentin or amitriptyline for the treatment of CRPS‐I or neuropathic pain; or had health conditions requiring the regular use of anticholinergics, antihypertensives, anticonvulsants, H2 receptor antagonists, antidepressants, sympathomimetics, thyroxine replacement, or antacids that might interact adversely with one of the study medications. Authors used a baseline observation carried forward (BOCF) method for missing data.
Excluded studies
We excluded six studies in this review. Upon reading the full texts of Kalita 2014, Ogawa 2010, Pramod 2011, Ries 2003, To 2002, and Yilmaz 2015, we discovered the age ranges were on average 18 years and above, with a small number of participants who were 15 to 17 years old. All six studies were randomised controlled trials investigating the use of antiepileptic drugs to treat chronic pain, but unfortunately primarily in adult participants.
Risk of bias in included studies
A summary of the 'Risk of bias' assessment is available in Figure 2. See Characteristics of included studies for full details of 'Risk of bias' assessments.
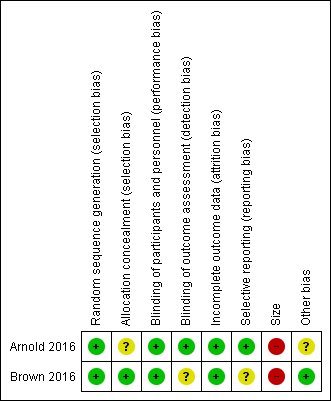
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Random sequence generation
Both studies used computer‐generated randomisation methods, with a 1:1 ratio of sequence to randomise participants. We judged both studies as at low risk of selection bias for random sequence generation.
Allocation concealment
Brown 2016 adequately described the methods used to conceal treatment allocation to the participants. We judged this study as at low risk of selection bias for allocation concealment.
Arnold 2016 did not adequately describe the methods used to conceal treatment allocation to the participants. We judged this study as at unclear risk of selection bias for allocation concealment.
Blinding
Performance bias
Both studies adequately described the methods used to maintain blinding in both participants and study personnel from knowledge of the treatment groups (Arnold 2016; Brown 2016). We judged both studies as at low risk of performance bias.
Detection bias
Arnold 2016 described adequate methods used to blind outcome assessors. We judged this study as at low risk of detection bias.
Brown 2016 did not provide adequate information regarding how the outcomes were assessed. We judged this study as at unclear risk of detection bias.
Incomplete outcome data
Both studies adequately accounted for all participants from the recruitment stage, through randomisation until follow‐up, including counting all withdrawals (Arnold 2016; Brown 2016). We judged both studies as at low risk of attrition bias.
Neither study displayed an unclear or high risk of attrition bias.
Selective reporting
Arnold 2016 reported on all planned outcomes as initially listed in the methods section. We judged this study as at low risk of reporting bias.
Brown 2016 did not report on all planned outcomes as initially listed in the methods section. Some secondary outcomes, for example disruption of school, social, and sports, were not reported. We judged this study as at unclear risk of selection bias.
Other potential sources of bias
Size
Arnold 2016 investigated a total of 107 participants, however fewer than 50 participants per treatment arm completed the study. We judged this study as at high risk of bias for size.
Brown 2016 investigated a total of 34 participants, and fewer than 50 participants per treatment arm for both randomisation and completion of the study. We also judged this study as at high risk of bias for size.
Other
We found no other potential sources of bias in Brown 2016. We judged this study as at low risk of other bias. Arnold 2016 reported funding for the study and medical writing support from Pfizer as well as the majority of the author team to be Pfizer employees. We judged this study as at unclear risk of other bias.
Effects of interventions
See: Summary of findings for the main comparison Gabapentin compared with amitriptyline for chronic non‐cancer pain; Summary of findings 2 Pregabalin compared with placebo for chronic non‐cancer pain
Results and outcomes of the individual studies are shown in Appendix 6 and Appendix 7.
Comparison 1: Antiepileptic drugs versus active comparator
One study, Brown 2016, investigated antiepileptic drugs compared with an active comparator (gabapentin versus amitriptyline) in people with CRPS‐I or neuropathic pain.
Primary outcomes
No data were reported for our primary outcomes: participant‐reported pain relief of 30% or greater; participant‐reported pain relief of 50% or greater; and PGIC much or very much improved.
Due to the lack of evidence, we were unable to judge the quality (no evidence to support or refute).
Secondary outcomes
Sleep duration and quality
Brown 2016 reported the average decrease in sleep score on a 5‐point Likert scale mean (±standard deviation) (m (±SD)). For completed participants, the mean decrease in sleep score for 14 gabapentin participants was 0.46 (±1.60), and for 12 amitriptyline participants 1.25 (±1.86); P = 0.75. For all participants, the mean decrease in sleep score for 17 gabapentin participants was 0.38 (±1.45), and for 17 amitriptyline participants 0.88 (±1.69); P = 0.77 (very low‐quality evidence).
Any adverse event
Brown 2016 reported the number of participants who experienced at least 1 adverse event: 1 for gabapentin (1 event) and 2 for amitriptyline (1 event per participant) (p = 0.77). Adverse events in all studies, across active treatment and comparator groups, were considered to be a mild reaction, such as nausea, dizziness, drowsiness, tiredness, and abdominal discomfort (very low‐quality evidence).
Withdrawals due to adverse events
Total withdrawals were low: 2 out of 17 in the gabapentin group and 1 out of 17 in the amitriptyline group (very low‐quality evidence) (Brown 2016).
Withdrawals due to adverse events were low: 2 out of 17 in the gabapentin group and 1 out of 17 in the amitriptyline group (very low‐quality evidence) (Brown 2016).
Serious adverse events
Brown 2016 reported no serious adverse events for gabapentin (0 out of 17) and amitriptyline (0 out of 17) groups (very low‐quality evidence).
Other secondary outcomes
No data were reported for our remaining secondary outcomes: Carer Global Impression of Change; requirement for rescue analgesia; acceptability of treatment; physical functioning as defined by validated scales; and quality of life as defined by validated scales (no evidence to support or refute).
Quality of the evidence
There is no evidence to support or refute the use of antiepileptic drugs versus an active comparator across our primary outcomes.
The quality of evidence for antiepileptic drugs versus an active comparator across our secondary outcomes is very low‐quality, due to too few data and the fact that the number of events was too small to be meaningful.
Comparison 2: Antiepileptic drugs versus placebo
One study, Arnold 2016, investigated antiepileptic drugs compared with a placebo (pregabalin versus placebo).
Primary outcomes
Participant‐reported pain relief of 30% or greater
Arnold 2016 reported no significant change in pain scores for pain relief of 30% or greater between pregabalin 18/54 (33.3%) and placebo 16/51 (31.4%), p = 0.83. The authors of Arnold 2016 reported in their conclusions that "pregabalin did not significantly improve the mean pain score in adolescents with FM [fibromyalgia]". For mean pain scores, see Appendix 6 (very low‐quality evidence).
Participant‐reported pain relief of 50% of greater
Arnold 2016 reported no significant change in pain scores for pain relief of 50% or greater between pregabalin 9/54 (16.7%) and placebo 4/51 (7.8%), p = 0.179 (very low‐quality evidence).
PGIC much or very much improved
Arnold 2016 reported much or very much improved pain scores for pregabalin 53.1% and placebo 29.5% (p = 0.013) (very low‐quality evidence).
Secondary outcomes
Carer Global Impression of Change
Arnold 2016 reported Parent GIC much or very much improved responses for pregabalin responders (51.0%) compared with placebo responders (25.0%) (p = 0.011) (very low‐quality evidence).
Sleep duration and quality
Arnold 2016 reported the average decrease in sleep score on an 11‐point numerical rating scale m (±SD) at baseline: pregabalin: 5.8 (1.6) and placebo: 5.6 (2.5); at week 8 treatment difference: ‐1.01 (95% confidence interval (CI) ‐1.73 to ‐0.30), P = 0.006; at week 10 treatment difference: P = 0.037; at week 15 treatment difference: ‐0.17 (95% CI ‐0.95 to 0.61). The overall treatment difference was ‐0.48 (95% CI ‐1.02 to 0.06), p = 0.081 (very low‐quality evidence).
Physical functioning
Arnold 2016 reported an FIQ‐C (Fibromyalgia Impact Questionnaire for children) total score of least squares mean difference between gabapentin and amitriptyline: ‐2.46 (95% CI ‐6.87 to 1.95), p = 0.270 (very low‐quality evidence).
Any adverse events
Arnold 2016 reported total adverse events as 167 events in the pregabalin group and 132 events in the placebo group. Arnold 2016 also reported the number of participants who experienced an adverse event as 38 (70.4%) in the pregabalin group and 34 (64.2%) in the placebo group (very low‐quality evidence).
Withdrawals due to adverse events
Arnold 2016 reported total withdrawals as 10 (19%) pregabalin and 17 (32%) placebo. Total withdrawals due to adverse events in Arnold 2016 were low: 4 (7%) pregabalin and 4 (8%) placebo (very low‐quality evidence).
Serious adverse events
Arnold 2016 reported serious adverse events experienced by one participant (2%) for the pregabalin group (cholelithiasis and major depression resulting in hospitalisation), and none for the placebo group (very low‐quality evidence).
Other secondary outcomes
No data were reported for our remaining secondary outcomes: requirement for rescue analgesia; acceptability of treatment; physical functioning as defined by validated scales; and quality of life as defined by validated scales (no evidence to support or refute).
Quality of the evidence
The quality of evidence for antiepileptic drugs versus placebo across our primary outcomes is very low‐quality, due to too few data and the fact that the number of events was too small to be meaningful.
The quality of evidence for antiepileptic drugs versus placebo across some of our secondary outcomes is very low‐quality, due to too few data and the fact that the number of events was too small to be meaningful. For the remaining secondary outcomes, there was no evidence to support or refute the use of antiepileptic drugs to treat chronic non‐cancer pain in children and adolescents.
Discussion
Summary of main results
We included two studies in this review that reported data from 141 participants (aged 7 to 18 years), comparing gabapentin versus amitriptyline, or pregabalin versus placebo.
The two studies were not comparable by pain condition or by type of antiepileptic drug, therefore we were unable to complete any quantitative analysis of our outcomes.
Risk of bias for the two included studies varied, due to issues with randomisation (low to unclear risk), blinding of outcome assessors (low to unclear risk), reporting bias (low to unclear risk), the size of the study populations (high risk), and industry funding in the 'other' domain (low to unclear risk). We judged the remaining domains of sequence generation, blinding of participants and personnel, and attrition as low risk of bias.
There is no evidence from randomised controlled trials to suggest that antiepileptic drugs are effective in treating chronic non‐cancer pain in children or adolescents, nor do we have evidence to suggest that one antiepileptic drug is more effective than another. We were unable to comment on harm.
Overall completeness and applicability of evidence
This review identified only a small number of studies (two), with insufficient data for meta‐analysis. Arnold 2016 investigated pregabalin versus placebo for people with fibromyalgia, and Brown 2016 investigated gabapentin versus amitriptyline in people with CRPS‐I or neuropathic pain.
As we could undertake no meta‐analysis, we are unable to comment on efficacy or harm from the use of antiepileptic drugs to treat chronic non‐cancer pain in children and adolescents. Similarly, we cannot comment on our remaining secondary outcomes: Carer Global Impression of Change, requirement for rescue analgesia, sleep duration and quality, acceptability of treatment, physical functioning, and quality of life.
We know from adult randomised controlled trials that some antiepileptics, such as gabapentin and pregabalin, can be effective in certain chronic neuropathic pain conditions (Wiffen 2013). However, we suggest that these drugs be used with caution in children and only by those with suitable expertise.
The suite of reviews
This review is part of a suite of reviews on pharmacological interventions for chronic pain and cancer‐related pain in children and adolescents (Appendix 1). Taking a broader view on this suite of reviews, some pharmacotherapies (investigated in our other reviews) are likely to provide more data than others. The results were thus as expected considering that randomised controlled trials in children are known to be limited. The results have the potential to inform policymaking decisions for the funding of future clinical trials into antiepileptic drug treatment of child and adolescent pain, therefore any results (large or small) are important in order to capture a snapshot of the current evidence for antiepileptic drugs.
Quality of the evidence
Of the two included studies, only one clearly described randomisation methods, and only one was clearly described as double‐blind, however both studies provided information about withdrawals, dropouts, and adverse events.
The two studies recruited participants with adequate baseline pain and used clinically useful outcome measures. Only Arnold 2016 reported our prespecified primary outcomes of pain relief at 30% or greater and 50% or greater. Brown 2016 reported a difference between mean pain scores, with limited data to arrive at any results.
The studies themselves were of moderate quality, however the number of studies and sample sizes for the comparisons were somewhat limited, given what is known about study size and estimates of effect for outcomes derived from studies with few participants and events (Dechartres 2013; Dechartres 2014; McQuay 1998; Nüesch 2010; Thorlund 2011).
There was no evidence to support or refute the use of antiepileptic drugs, versus an active comparator, for primary outcomes. The evidence was very low‐quality for secondary outcomes for this comparison due to too few data and the fact that the number of events was too small to be meaningful.
The quality of evidence for antiepileptic drugs versus placebo was very low‐quality (for primary and secondary outcomes), due to too few data and the fact that the number of events was too small to be meaningful.
Potential biases in the review process
We carried out extensive searches of major databases using broad search criteria, and also searched two large clinical trial registries. We consider it to be unlikely that we have missed relevant studies.
Agreements and disagreements with other studies or reviews
We were not able to identify any published systematic reviews on this topic.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
| Gabapentin compared with amitriptyline for chronic non‐cancer pain | ||||||
| Patient or population: children and adolescents (birth to 17 years of age) with chronic non‐cancer pain Settings: primary care Intervention: gabapentin Comparison: amitriptyline | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Amitriptyline | Gabapentin | |||||
| Participant‐reported pain relief of 30% or greater | No data | No data | N/A | N/A | ‐ | No evidence to support or refuteb |
| Participant‐reported pain relief of 50% or greater | No data | No data | N/A | N/A | ‐ | No evidence to support or refuteb |
| Patient Global Impression of Change: much improved or very much improved | No data | No data | N/A | N/A | ‐ | No evidence to support or refuteb |
| Any adverse events | 1/17 | 2/17 | N/A | 34 participants (1 study) | ⊕⊝⊝⊝ | |
| Serious adverse events | 0/17 | 0/17 | N/A | 34 participants (1 study) | ⊕⊝⊝⊝ | |
| Withdrawals due to adverse events | 1/17 | 2/17 | N/A | 34 participants (1 study) | ⊕⊝⊝⊝ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; N/A: not applicable; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: We are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of effect, but there is a possibility that it is substantially different. Low quality: Our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low quality: We have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
| aDowngraded three levels due to too few data and number of events were too small to be meaningful. bNo data available for this outcome, and therefore no GRADE rating has been applied and there is no evidence to support or refute. | ||||||
| Pregabalin compared with placebo for chronic non‐cancer pain | ||||||
| Patient or population: children and adolescents (birth to 17 years of age) with chronic non‐cancer pain Settings: multicentre, USA (28 primary care centres), India (5 primary care centres), Taiwan (2 primary care centres), and Czech Republic (1 primary care centre) Intervention: pregabalin Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Placebo | Pregabalin | |||||
| Participant‐reported pain relief of 30% or greater** | 16/51 | 18/54 | N/A | 107 participants (1 study) | ⊕⊝⊝⊝ | |
| Participant‐reported pain relief of 50% or greater** | 4/51 | 9/54 | N/A | 107 participants (1 study) | ⊕⊝⊝⊝ | |
| Patient Global Impression of Change: much improved or very much improved** | 15/51 | 29/54 | N/A | 107 participants (1 study) | ⊕⊝⊝⊝ | |
| Adverse events | 34/53 | 38/54 | N/A | 107 participants (1 study) | ⊕⊝⊝⊝ | |
| Serious adverse events | 0/53 | 1/54 | N/A | 107 participants (1 study) | ⊕⊝⊝⊝ | |
| Withdrawals due to adverse events | 4/53 | 4/54 | N/A | 107 participants (1 study) | ⊕⊝⊝⊝ | |
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: We are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of effect, but there is a possibility that it is substantially different. Low quality: Our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low quality: We have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
| aDowngraded three levels due to too few data and number of events were too small to be meaningful. bNo data available for this outcome, and therefore no GRADE rating has been applied and there is no evidence to support or refute. | ||||||

