Fármacos antiepilépticos para el dolor crónico no relacionado con el cáncer en niños y adolescentes
Información
- DOI:
- https://doi.org/10.1002/14651858.CD012536.pub2Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 05 agosto 2017see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Dolor y cuidados paliativos
- Copyright:
-
- Copyright © 2020 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
TC and PW registered the title.
TC, PW, and Christopher Eccleston wrote the template protocol for the suite of children's reviews, of which this review is a part.
All authors contributed to writing the protocol, and all authors agreed on the final version.
All authors were responsible for data extraction, analysis, and writing of the Discussion for the full review.
All authors will be responsible for the completion of updates.
Sources of support
Internal sources
-
No sources of support supplied
External sources
-
National Institute for Health Research (NIHR), UK.
NIHR Programme Grant, Award Reference Number: 13/89/29 (Addressing the unmet need of chronic pain: providing the evidence for treatments of pain)
Declarations of interest
PW: none known.
TC: none known.
LH: none known.
JC: none known; JC is a specialist paediatric pain physician and treats patients with complex pain.
RH: none known; RH is a specialist paediatric pain clinician and treat patients with chronic pain.
EK has received consulting fees for attending a research strategy meeting from Pfizer, Inc. (2015) and for protocol and research consultation from Mallinckrodt Pharmaceuticals, Inc. (2014), AstraZeneca, Inc. (2014), and Collegium Pharma (2016); EK is a specialist paediatric pain clinician and treats patients with chronic pain.
SL: none known; SL is a specialist paediatric pain clinician and treats patients with chronic pain.
NS (Sethna) has received grants from Gebauer Company for the conduct of animal studies using a topical anaesthetic (2015). NS has offered consultant expertise to Pfizer in designing a multicentre study for use of gabapentin in treatment of neuropathic pain in children (2015). NS is a co‐investigator with an ongoing multicentre Phase 3 trial of an experimental drug SMNRX [antisense oligonucleotide] for treatment of infants and children with spinal muscle atrophy (2012 to present). NS is an anaesthesiologist and manages paediatric patients with chronic pain.
NS (Schechter): none known; NS is a developmental paediatrician and treats children and adolescents with pain; NS directs the Chronic Pain Clinic at Boston Children’s Hospital and is on the faculty at Harvard Medical School.
CW: none known; CW is a paediatrician and anaesthesiologist; CW specialises in pain and treats children and adults presenting chronic pain; CW also treats patients in palliative care.
This review was identified in a 2019 audit as not meeting the current definition of the Cochrane Commercial Sponsorship policy. At the time of its publication it was compliant with the interpretation of the existing policy. As with all reviews, new and updated, at update this review will be revised according to 2020 policy update.
Acknowledgements
We acknowledge the contribution of Christopher Eccleston to the template protocol.
We thank Tonya Palermo and Andrew Moore for peer reviewing the protocol and review.
Cochrane Review Group funding acknowledgement: The National Institute for Health Research (NIHR) is the largest single funder of the Cochrane Pain, Palliative and Supportive Care Review Group. Disclaimer: The views and opinions expressed herein are those of the authors and do not necessarily reflect those of the NIHR, National Health Service (NHS), or the Department of Health.
Version history
| Published | Title | Stage | Authors | Version |
| 2017 Aug 05 | Antiepileptic drugs for chronic non‐cancer pain in children and adolescents | Review | Tess E Cooper, Philip J Wiffen, Lauren C Heathcote, Jacqui Clinch, Richard Howard, Elliot Krane, Susan M Lord, Navil Sethna, Neil Schechter, Chantal Wood | |
| 2017 Feb 14 | Antiepileptic drugs for chronic non‐cancer pain in children and adolescents | Protocol | Philip J Wiffen, Tess E Cooper, Lauren C Heathcote, Jacqui Clinch, Richard Howard, Elliot Krane, Susan M Lord, Navil Sethna, Neil Schechter, Chantal Wood | |
Differences between protocol and review
We did not consider studies with fewer than 10 participants per treatment arm for inclusion in this review, as is standard practice for this group.
In the protocol we stated the age inclusion criterion as birth to 17 years old. One trial reported participants as being between 7 and 18 years of age. We decided to include the study rather than miss data on the participants who were under 18 years of age, who constituted most of the participants in the study.
Notes
A restricted search in March 2019 did not identify any potentially relevant studies likely to change the conclusions. Therefore, this review has now been stabilised following discussion with the authors and editors. The review will be re‐assessed for updating in five years. If appropriate, we will update the review before this date if new evidence likely to change the conclusions is published, or if standards change substantially which necessitates major revisions.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
- Amines [adverse effects, *therapeutic use];
- Amitriptyline [adverse effects, *therapeutic use];
- Anticonvulsants [adverse effects, *therapeutic use];
- Chronic Pain [*drug therapy];
- Complex Regional Pain Syndromes [*drug therapy];
- Cyclohexanecarboxylic Acids [adverse effects, *therapeutic use];
- Fibromyalgia [*drug therapy];
- Gabapentin;
- Neuralgia [*drug therapy];
- Pregabalin [adverse effects, *therapeutic use];
- Randomized Controlled Trials as Topic;
- gamma‐Aminobutyric Acid [adverse effects, *therapeutic use];
Medical Subject Headings Check Words
Adolescent; Child; Humans;
PICO
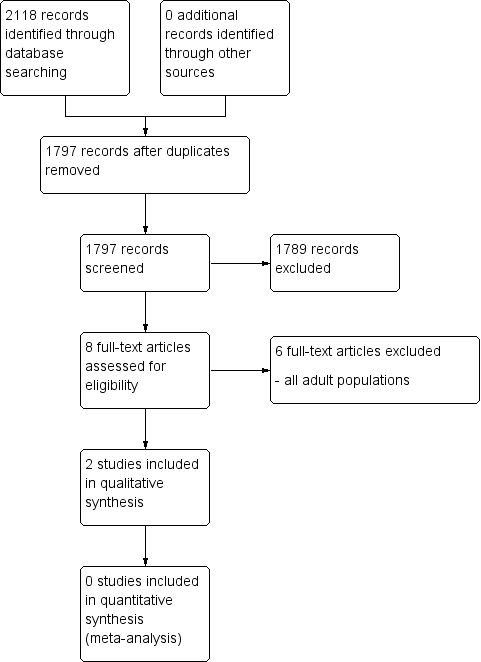
Study flow diagram.
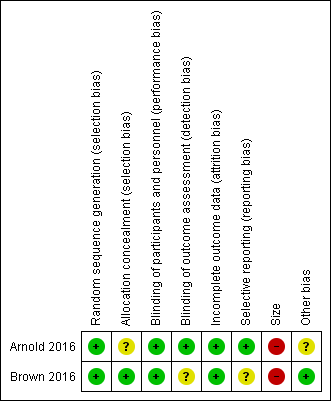
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
| Gabapentin compared with amitriptyline for chronic non‐cancer pain | ||||||
| Patient or population: children and adolescents (birth to 17 years of age) with chronic non‐cancer pain Settings: primary care Intervention: gabapentin Comparison: amitriptyline | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Amitriptyline | Gabapentin | |||||
| Participant‐reported pain relief of 30% or greater | No data | No data | N/A | N/A | ‐ | No evidence to support or refuteb |
| Participant‐reported pain relief of 50% or greater | No data | No data | N/A | N/A | ‐ | No evidence to support or refuteb |
| Patient Global Impression of Change: much improved or very much improved | No data | No data | N/A | N/A | ‐ | No evidence to support or refuteb |
| Any adverse events | 1/17 | 2/17 | N/A | 34 participants (1 study) | ⊕⊝⊝⊝ | |
| Serious adverse events | 0/17 | 0/17 | N/A | 34 participants (1 study) | ⊕⊝⊝⊝ | |
| Withdrawals due to adverse events | 1/17 | 2/17 | N/A | 34 participants (1 study) | ⊕⊝⊝⊝ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; N/A: not applicable; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: We are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of effect, but there is a possibility that it is substantially different. Low quality: Our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low quality: We have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
| aDowngraded three levels due to too few data and number of events were too small to be meaningful. bNo data available for this outcome, and therefore no GRADE rating has been applied and there is no evidence to support or refute. | ||||||
| Pregabalin compared with placebo for chronic non‐cancer pain | ||||||
| Patient or population: children and adolescents (birth to 17 years of age) with chronic non‐cancer pain Settings: multicentre, USA (28 primary care centres), India (5 primary care centres), Taiwan (2 primary care centres), and Czech Republic (1 primary care centre) Intervention: pregabalin Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Placebo | Pregabalin | |||||
| Participant‐reported pain relief of 30% or greater** | 16/51 | 18/54 | N/A | 107 participants (1 study) | ⊕⊝⊝⊝ | |
| Participant‐reported pain relief of 50% or greater** | 4/51 | 9/54 | N/A | 107 participants (1 study) | ⊕⊝⊝⊝ | |
| Patient Global Impression of Change: much improved or very much improved** | 15/51 | 29/54 | N/A | 107 participants (1 study) | ⊕⊝⊝⊝ | |
| Adverse events | 34/53 | 38/54 | N/A | 107 participants (1 study) | ⊕⊝⊝⊝ | |
| Serious adverse events | 0/53 | 1/54 | N/A | 107 participants (1 study) | ⊕⊝⊝⊝ | |
| Withdrawals due to adverse events | 4/53 | 4/54 | N/A | 107 participants (1 study) | ⊕⊝⊝⊝ | |
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: We are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of effect, but there is a possibility that it is substantially different. Low quality: Our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low quality: We have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
| aDowngraded three levels due to too few data and number of events were too small to be meaningful. bNo data available for this outcome, and therefore no GRADE rating has been applied and there is no evidence to support or refute. | ||||||

