Autocuidado para la bronquiectasia
Resumen
Antecedentes
La bronquiectasia es un trastorno respiratorio a largo plazo con una tasa de diagnóstico cada vez mayor. Se asocia con síntomas persistentes, exacerbaciones infectivas repetidas, y una reducción en la calidad de vida, lo cual impone una carga en los individuos y los sistemas de asistencia sanitaria. Los objetivos principales del tratamiento terapéutico son la reducción de las exacerbaciones y la mejoría en la calidad de vida. Las intervenciones de autocuidado son potencialmente importantes para capacitar a los pacientes con bronquiectasia en el control de la afección de forma más efectiva y la búsqueda de atención en el momento adecuado. Las intervenciones de autocuidado son beneficiosas en el tratamiento de otras enfermedades de las vías respiratorias como el asma y la EPOC (enfermedad pulmonar obstructiva crónica) y se han identificado como una prioridad de investigación para la bronquiectasia.
Objetivos
Evaluar la eficacia, la relación costo‐efectividad y los efectos adversos de las intervenciones de autocuidado para los adultos y los niños con bronquiectasia no relacionada con la fibrosis quística.
Métodos de búsqueda
Se realizaron búsquedas en el registro especializado de ensayos del Grupo Cochrane de Vías Respiratorias (Cochrane Airways Group), registros de ensayos clínicos, listas de referencias de estudios incluidos y artículos de revisión y en sitios web de fabricantes relevantes hasta el 13 de diciembre de 2017.
Criterios de selección
Se incluyeron todos los ensayos controlados aleatorios de cualquier duración que incorporaran a adultos o niños con un diagnóstico de bronquiectasia no relacionada con la fibrosis quística y que evaluaran las intervenciones de autocuidado administradas de cualquier forma. Las intervenciones de autocuidado incluían al menos dos de los siguientes ítems: educación del paciente, técnicas de depuración de las vías respiratorias, adherencia a la medicación, ejercicio (incluida la rehabilitación pulmonar) y planes de acción.
Obtención y análisis de los datos
Dos autores de revisión cribaron las búsquedas, extrajeron de forma independiente las características de los estudios y los datos de resultados y evaluaron el riesgo de sesgo para cada estudio incluido. Los resultados primarios fueron la calidad de vida relacionada con la salud, la frecuencia de las exacerbaciones y los eventos adversos graves. Los resultados secundarios fueron el número de participantes que ingresaron al hospital en al menos una ocasión, la función pulmonar, los síntomas, la autoeficacia y los costos económicos. Se utilizó un modelo de efectos aleatorios para los análisis y los métodos Cochrane estándar desde el principio hasta el final.
Resultados principales
Se incluyeron dos estudios con un total de 84 participantes: un ECA de 12 meses de la rehabilitación temprana en adultos con una media de edad de 72 años realizados en dos centros en Inglaterra (Reino Unido) y un ECA de seis meses de prueba de concepto de un programa especializado en pacientes (EPP, por sus siglas en inglés) en adultos con una media de edad de 60 años en un único centro de enfermedades respiratorias regional en Irlanda del Norte (Reino Unido). El EPP se administró en formato grupal una vez a la semana durante ocho semanas mediante materiales estandarizados de EPP más educación específica de la enfermedad incluidas las técnicas de depuración de las vías respiratorias, el manejo de los síntomas, las exacerbaciones, la promoción de la salud y el apoyo disponible. No se encontró ningún estudio que incluyera niños. La agregación de datos no fue posible y los resultados se informan de manera narrativa en la revisión.
Para los resultados primarios, ambos estudios informaron la calidad de vida relacionada con la salud, según lo medido por el St George's Respiratory Questionnaire (SGRQ), aunque no hubo evidencia clara del beneficio. En un estudio, las puntuaciones totales medias del SGRQ no fueron significativamente diferentes al momento del seguimiento de seis semanas, tres meses y 12 meses (diferencia de medias [DM] a los 12 meses ‐10,27; intervalo de confianza [IC] del 95%: ‐45,15 a 24,61). En el segundo estudio, no hubo diferencias significativas en el SGRQ. Las puntuaciones totales no fueron significativamente diferentes entre los grupos (seis meses, DM 3,20; IC del 95%: ‐6,64 a 13,04). La evidencia para este resultado se consideró baja o muy baja. Ninguno de los estudios incluidos informó los datos sobre las exacerbaciones que requirieron antibióticos. Para los eventos adversos graves, un estudio informó más muertes en el grupo de intervención comparado con el grupo de control, (intervención: 4 de 8; control: 2 de 12), aunque la interpretación es limitada por la tasa baja de eventos y el número pequeño de participantes en cada grupo.
Para los resultados secundarios, no hubo evidencia de un beneficio en cuanto a la frecuencia de los ingresos al hospital o el VEF1 L, basado en evidencia de muy baja calidad. Un estudio informó la autoeficacia mediante la escala Chronic Disease Self‐Efficacy, que comprende 10 componentes. Todas las escalas mostraron un beneficio significativo de la intervención aunque los efectos sólo se mantuvieron hasta el final del estudio en la escala Managing Depression. Se informan más detalles en la revisión principal. Sobre la base de la calidad del estudio en general, esta evidencia se consideró de baja calidad. Ningún estudio informó datos sobre los síntomas respiratorios, los costos económicos ni los eventos adversos.
Conclusiones de los autores
Hay evidencia insuficiente para determinar si las intervenciones de autocuidado benefician a los pacientes con bronquiectasia. A falta de evidencia de alta calidad, es aconsejable que los profesionales se adhieran a las guías internacionales actuales que preconizan el autocuidado para los pacientes con bronquiectasia.
Los estudios futuros deben procurar definir claramente y justificar la naturaleza específica del autocuidado, medir los resultados clínicamente importantes e incluir a niños así como a adultos.
PICO
Resumen en términos sencillos
Autocuidado para la bronquiectasia no relacionada con la fibrosis quística
Antecedentes
La bronquiectasia es un trastorno respiratorio que puede ocurrir tanto en niños como en adultos y se está diagnosticando con una frecuencia cada vez mayor. Es un trastorno a largo plazo, en el que los pacientes tienen infecciones respiratorias recurrentes y síntomas que incluyen tos, producción de moco y exacerbaciones recurrentes que reducen la calidad de vida. Los objetivos principales del tratamiento son reducir el riesgo de exacerbaciones mediante el uso de diversos tratamientos que incluyen antibióticos, inhaladores y ejercicios de fisioterapia. Es importante que los pacientes/cuidadores se adhieran a los tratamientos, y las estrategias de autocuidado pueden ayudar a los pacientes a hacerlo al enseñarles acerca de su enfermedad, los tratamientos disponibles, el ejercicio y qué hacer si cambia la enfermedad. El objetivo de la revisión es evaluar la efectividad y la relación calidad‐precio de las intervenciones de autocuidado para adultos y niños con bronquiectasia no relacionada con la fibrosis quística.
Pregunta de la revisión
Se evaluaron los efectos beneficcualqui iosos y perjudiciales posibles de las estrategias de autocuidado, incluida la educación del paciente, las técnicas de depuración de las vías respiratorias, la educación dirigida hacia un aumento de la adherencia a la medicación, el ejercicio (incluida la rehabilitación pulmonar) y los planes de acción para los niños y los adultos con bronquiectasia.
Características de los estudios
Se realizó una búsqueda el 13 de diciembre de 2017 y se encontraron sólo dos estudios del Reino Unido con 84 participantes y que compararon un enfoque de autocuidado con la atención normal para los adultos con bronquiectasia. Un estudio consideró el impacto de un programa especializado de autocuidado del paciente y el otro, que incluyó sólo a un número pequeño de participantes con bronquiectasia, consideró el autocuidado en combinación con ejercicios para mejorar la función pulmonar. Ningún estudio incluyó a niños.
Resultados principales
La calidad de vida relacionada con la salud no mejoró en ninguno de los estudios. Aunque hubo más muertes en el grupo que recibió autocuidado en un estudio, los números fueron muy pequeños y no se conoce si la diferencia es significativa. El número de ingresos al hospital y la función pulmonar no mostraron ningún beneficio del autocuidado. En uno de los estudios, los pacientes que recibieron autocuidado se sintieron más capacitados para controlar su afección. No hubo información sobre el impacto del autocuidado en los síntomas de la bronquiectasia, los eventos adversos ni los ahorros de costos potenciales que surgieron del autocuidado más efectivo. No hay ningún estudio que considere el autocuidado en los niños.
En general no hay información suficiente para evaluar si las estrategias para apoyar el autocuidado pueden ayudar a los pacientes con bronquiectasia y se necesitan estudios adicionales. Los estudios futuros necesitarán considerar con qué frecuencia ocurren las exacerbaciones, con qué frecuencia se prescriben antibióticos y por cuánto tiempo, si los pacientes tienen una mejor calidad de vida y el impacto del autocuidado en los costos de la atención. También es importante considerar el autocuidado para la bronquiectasia en los niños.
Calidad de los resultados
Esta revisión se basa en sólo dos ensayos pequeños y la calidad de los estudios es muy deficiente. Debido a que sólo hay dos estudios que consideran enfoques muy específicos al autocuidado no es posible establecer con algún grado de certeza si las estrategias de autocuidado funcionan para los pacientes con bronquiectasia, aunque hasta que haya evidencia adicional disponible se recomienda la adherencia a las guías internacionales actuales que preconizan el autocuidado para los pacientes con bronquiectasia.
Authors' conclusions
Summary of findings
| Self‐management compared to usual care for bronchiectasis | ||||||
| Patient or population: people with non‐cystic fibrosis bronchiectasis | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
| Risk with usual care | Risk with self‐management | |||||
| Health‐related quality of life | The mean health‐related quality of life was 56.02 points | MD 10.27 lower | ‐ | 20 | ⊕⊝⊝⊝ | No clear benefit or harm from self‐management (very low‐quality evidence) |
| Health‐related quality of life Follow up: range post‐intervention to 6 months | The mean health‐related quality of life was 44.7 points | MD 3.2 higher | ‐ | 60 | ⊕⊕⊝⊝ | No clear benefit or harm from self‐management |
| Exacerbations requiring antibiotics | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| Serious adverse events: mortality | ‐ | ‐ | not estimable | 20 | ||
| Hospital admissions (number admitted at least once) | ‐ | ‐ | not estimable | 20 | ‐ | |
| Lung function assessed with: FEV1 L | The mean FEV1 was 1.03 L | MD 0.3 higher | ‐ | 20 | ⊕⊝⊝⊝ | No clear benefit or harm from self‐management |
| Self‐efficacy assessed with: CDSS Follow‐up: postintervention to 6 months | ‐ | ‐ | not estimable | 60 | ⊕⊕⊝⊝ | Six out of ten scales showed significant improvements over time with the intervention. We elected not to include all 10 scales in the table but graded the evidence based on overall quality of the study |
| Economic costs | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1One point deducted for the unblinded nature of the comparison. | ||||||

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Background
Description of the condition
Bronchiectasis, also referred to as non‐cystic fibrosis (non‐CF) bronchiectasis, is a persistent respiratory condition characterised by abnormal dilation of the airways (Pasteur 2010; Chang 2015). Pathological processes include weakness and destruction of the structural components of the bronchial wall, which together with the loss of ciliated epithelium, and increase in number and hypertrophy of mucus‐secreting glands, causes mucus to accumulate, which in turn creates a conducive environment for bacteria and leads to a ‘vicious cycle’ of bacterial infection (Cole 1986), inflammatory mediator release, airway damage and further infection (Welsh 2015). Chronic infection is associated with a variety of pathogens (Martinez‐García 2007; Murray 2011; Chalmers 2012; Tunney 2013), contributing to persistent symptoms and repeated exacerbations (Murray 2011).
Causes of bronchiectasis include a wide range of factors such as damage by serious infection (including Mycobacterium tuberculosis), necrotising pneumonia, immune deficiency, allergic bronchopulmonary aspergillosis, and recurrent aspiration, although the majority of cases are idiopathic (Pasteur 2000; Goeminne 2012; Lonni 2015). Diagnosis is based on clinico‐radiographic assessments, requiring identification of one or more abnormally dilated bronchi using high‐resolution computerised tomography (HRCT) scanning and appropriate symptoms, including chronic productive or wet cough and recurrent lower respiratory tract infections, together with a range of other symptoms such as breathlessness, wheeze, chest pain (in stable patients) and lethargy (Pasteur 2010; Chang 2015). Factors associated with disease severity include frequency of hospital admissions and mortality, poor lung function, bacterial colonisation, high Medical Research Council (MRC) dyspnoea score and frequency of exacerbations (Chalmers 2014; Martinez‐García 2014). The impact on people's quality of life is significant and health status is poor with progressive deterioration. Severity may be assessed with tools such as the Bronchiectasis Severity Index (Chalmers 2014), or FACED (FEV1, Age, Chronic colonisation, Extension (number of lobes), Dyspnoea) (Martinez‐García 2014), to identify high‐risk individuals, though they have limited value as outcome measures because of the non‐modifiable nature of components such as lung function.
Estimates of the prevalence of bronchiectasis vary considerably. Although it has previously been considered a relatively rare disease (Kolbe 1996), more recent studies have suggested an increasing prevalence, particularly in those over 75 years (Weycker 2005), and higher prevalence rates in low‐income and middle‐income countries (Habesoglu 2011). Co‐morbidity may also influence detection and prevalence, with one UK study showing that 29% of people with COPD scanned by HRCT had bronchiectasis (O'Brien 2000). Prevalence rates per 100,000 were estimated at 0.5 in Finland and 3.7 in New Zealand though these data are more than 10 years old (European Lung White Book 2013). Higher prevalence rates have been observed in ethnic populations such as amongst indigenous Australians (up to 14 per 1000) and Native Alaskan children (up to 20.5 per 1000) (Singleton 2000; Chang 2002). Higher prevalence rates are also observed in women and people aged over 60 years (Chang 2003; Seitz 2012). Recent data suggest that incidence and prevalence in the UK may be higher than previously estimated (Quint 2016). Over a nine‐year period to 2013, point prevalence rates per 100,000 rose from 350.5 to 566.1 in women and from 301.2 to 485.5 in men. This reflects an increase of more than 60% with approximately 263,000 adults living with bronchiectasis in 2013. Similarly, the incidence rates per 100,000 person‐years rose from 21.2 to 35.2 in women and from 18.2 to 26.9 in men, a 63% increase in new cases to over 15,000 in 2013. However, these increases may be due to improved diagnosis resulting from easier access to high‐quality CT scanners, rather than a true rise in prevalence (Goeminne 2016).
Mortality rates for bronchiectasis in England and Wales rose by 3% per year between 2001 to 2007 (Roberts 2010), and hospitalisations also increased by 3% per year over a nine‐year period in the US (Seitz 2010). Average bronchiectasis mortality rates per 100,000 general population in Europe are estimated at 0.3 in 27 of the 28 countries in the EU (ranging from 0.01 in Germany to 1.18 in the UK) and 0.2 in nine non‐EU countries (ranging from 0.01 in Azerbaijan to 0.67 in Kyrgyzstan), based on 2005 to 2009 data (European Lung White Book 2013). The recent UK study reported higher age‐adjusted bronchiectasis mortality rates, with estimates 2.26 times higher in women and 2.14 times higher in men compared to the general population (Quint 2016).
The main aims of therapeutic management are: preservation of lung function, reduction of symptoms and exacerbations, minimising complications, and improvement in quality of life (Pasteur 2010; Saleh 2014; Chang 2015).
Description of the intervention
Taylor 2014 describes a taxonomy in which long‐term conditions are diagnosed and brought under control by professionals; thereafter the individual self‐manages the condition with support, to achieve stable maintenance. Self‐management support empowers the person with the condition by enabling them to modify treatment or behaviour, or to seek professional advice and has been defined as “increasing the capacity, confidence, and efficacy of the individual” (Kennedy 2013). Self‐management interventions are defined as structured programmes for individuals, designed to improve self‐health behaviours and self‐management skills (Lorig 2003). Self‐management programmes should ideally include training with feedback to improve problem solving, decision making, resource utilisation, formation of patient‐provider partnerships, action planning and self‐tailoring (Lorig 2003). People become more confident at managing their own health and this in turn supports the development and maintenance of beneficial health behaviours (Lorig 2003; Bourbeau 2004).
Self‐management support is delivered in a range of ways, all of which aim to equip the individual with knowledge, ability, and confidence, to take appropriate action. The support can take the form of specific techniques employed to help people choose healthy behaviours, but it can also be a fundamental alteration of the patient‐caregiver relationship into a collaborative partnership (de Silva 2011). Interventions can range from individualised support such as the provision of educational material, to larger but localised whole‐system approaches. An example of a whole‐system approach involved practitioners trained to offer a range of resources, such as a tool to assess the support needs of patients, guidebooks on self‐management, and a web‐based directory of local self‐management resources (Kennedy 2013). There are also extensive generic programmes such as the ‘Expert Patients Programme’ (Department of Health 2001).
Self‐management support increasingly includes a mutually agreed, individualised plan, which incorporates behavioural elements including goal setting and problem solving. Recent work conducted by the Richmond Group of Charities and The King's Fund suggests that clients and professionals should co‐create a personalised self‐management plan which could include patient and career education, medicines' management advice and support, use of telecare and telehealth to aid self‐monitoring, psychological interventions (e.g. coaching), telephone‐based health coaching, symptom management and patient access to their own records (Naylor 2015). Self‐management support and interventions can therefore vary significantly. All approaches aim to enable the individual to develop the knowledge and confidence to appropriately manage their long‐term condition, and to seek professional support when needed.
The components of self‐management programmes may need to be condition‐specific; for example, education may be particularly beneficial for diabetes, but cognitive and behavioural interventions may work well for people with depression (de Silva 2011). The principal aims of management in bronchiectasis are to maintain and improve pulmonary function and to improve quality of life by reducing symptoms and exacerbations (Pasteur 2010; Chang 2015). British Thoracic Society guidelines recommend a range of therapeutic strategies including physiotherapy for airway clearance, pulmonary rehabilitation for significant dyspnoea, bronchodilators for reversible airflow obstruction and a range of antibiotic therapy to reduce bacterial load. The latter may include short‐term courses for exacerbations, prophylactic therapy for frequent exacerbators (≥ 3 exacerbations requiring antibiotics per year) and combination therapy for people with multiple airway pathogens (Pasteur 2010). Recommendations are often based on a small number of short trials that are insufficient to draw firm conclusions about benefits and harms (Welsh 2015).
Bronchiectasis impacts upon physical and psychosocial well‐being and there is the potential to improve self‐management through self‐regulation of medication, adherence to airway clearance techniques and patient education about management of the condition (Wilson 1997; Lavery 2007). Current guidelines recommend airway clearance techniques, adherence to medication, action plans, exercise (including pulmonary rehabilitation), and patient education as potential components of self‐management interventions for bronchiectasis (Pasteur 2010; Chang 2015). The educational component focuses on understanding the basic principles of disease management and early recognition of an exacerbation to facilitate timely intervention (Pasteur 2010). In COPD, self‐management programmes that include action plans have been shown to accelerate appropriate treatment‐seeking behaviours (Walters 2010), and studies including action plans should therefore be considered separately.
How the intervention might work
Studies of long‐term chronic conditions suggest that self‐management support may improve self‐efficacy, health status, psychological well‐being, coping strategies and physical functioning (Farrell 2004; Griffiths 2005; Siu 2007; Challis 2010). Benefits may be attributable to enhanced adherence to medication, the adoption of appropriate behaviours, and reduced stress and anxiety, though this may also be associated with increased use of healthcare resources (Naylor 2015). Self‐management programmes for COPD, defined above, have improved quality of life and reduced breathlessness and hospital admissions (Zwerink 2014), though there is currently no consensus on the most effective components of self‐management interventions (Effing 2012). The evidence of effectiveness in cystic fibrosis is less clear, with interpretation of observed increases in knowledge and changes in behaviour hampered by small, poor‐quality trials (Savage 2014).
The objectives of care in bronchiectasis are to treat identifiable underlying causes, control symptoms, reduce the number of exacerbations, prevent deterioration in pulmonary function, improve quality of life and minimise complications (Pasteur 2010; Chalmers 2016). The potential benefits from self‐management in individuals with bronchiectasis may include: reduction in symptoms and subsequent improvement in quality of life; and reduction in the number and severity of exacerbations, together with potential reduction in hospital admissions, length of stay, and disease and health status decline.
Non‐adherence to therapy may be a significant problem in bronchiectasis with up to 50% of people with severe chest infections not completing prescribed courses of antibiotics, other medicines and airway clearance (McCullough 2014). People who do not adhere to therapy have a shorter time to first exacerbation (Haworth 2014); and a higher annual exacerbation rate compared to those who are adherent (McCullough 2014). Similar to reports from cystic fibrosis (Sawicki 2009), treatment burden may increase with the emergence of new treatments, which may in turn lead to more problems with adherence. Non‐adherence to antibiotic therapy and airway clearance procedures may be attributable to a range of factors including beliefs about their potential risks and benefits, a younger age and (for antibiotics) a higher number of prescribed medications (McCullough 2015). It is likely that patient self‐management programmes may help to improve adherence to prescribed therapy and reduce the negative consequences of poor adherence. With the rise of antimicrobial resistance, adherence to frontline antibiotic therapy may be particularly important for people with bronchiectasis (O'Neill 2016).
Why it is important to do this review
Bronchiectasis is a chronic disease which causes both persistent day‐to‐day symptoms such as cough and breathlessness, and intercurrent exacerbations. The long‐term management of bronchiectasis focuses on reducing these features of the disease.
Self‐management interventions have been shown to be beneficial in the management of other airways diseases associated with management of day‐to‐day respiratory symptoms and respiratory exacerbations such as asthma and COPD (Zwerink 2014; Peytremann‐Bridevaux 2015). Guidelines recommend self‐management plans for these diseases and patient education is one of the factors in bronchiectasis management recently prioritised by the European EMBARC group (Aliberti 2016).
This review aims to summarise the evidence for self‐management strategies for people with bronchiectasis and will seek to provide guidance for both current recommendations and possible future research needs.
Objectives
To assess the efficacy, cost‐effectiveness and adverse effects of self‐management interventions for adults and children with non‐cystic fibrosis bronchiectasis.
Methods
Criteria for considering studies for this review
Types of studies
We included parallel and cluster‐randomised controlled trials (RCTs) of any duration. We included studies reported as full‐text, those published as abstract only, and unpublished data.
Types of participants
Adults (> 18 years) and children with a diagnosis of non‐cystic fibrosis bronchiectasis confirmed by plain film chest radiograph, bronchography or high‐resolution computed tomography with at least three months of daily sputum expectoration. We excluded participants with a diagnosis of cystic fibrosis (CF), sarcoidosis or active allergic bronchopulmonary aspergillosis. We also excluded studies of other long‐term health conditions unless results for people with bronchiectasis were reported separately.
Types of interventions
Self‐management interventions were defined as structured interventions for individuals with bronchiectasis designed to improve self‐health behaviours and self‐management skills. We specified that interventions should include collaborative interaction between participants and healthcare providers, involving goal setting and feedback, with at least two points of contact, and that specific programmes should include at least two of the following components: patient education, airway clearance techniques, adherence to medication, exercise (including pulmonary rehabilitation), and action plans (Pasteur 2010; Chang 2015). Self‐management interventions that included action plans were to be considered separately (Hagger 2014). We excluded interventions solely comprising participant education or those focused only on exercise, such as pulmonary rehabilitation delivered in a care setting. We included pulmonary rehabilitation interventions only when they explicitly included self‐management strategies within the programme. We included studies of self‐management interventions delivered in any form (e.g. Internet, mobile device, face‐to‐face, paper) with the following comparisons.
-
Self‐management versus usual care
-
Self‐management versus an alternate form of self‐management (e.g. paper‐based booklet versus mobile app)
For comparisons between different types of self‐management programmes we included co‐interventions, including types of exercise interventions, provided that they were evenly distributed between groups.
Types of outcome measures
We included all outcomes irrespective of follow‐up duration, but planned to evaluate the impact of follow‐up in subgroup analyses if sufficient data were available.
Primary outcomes
-
Health‐related quality of life using measures validated for people with bronchiectasis in a clinical setting (e.g. Bronchiectasis Severity Index (BSI; St. George's Respiratory Questionnaire (SGRQ))
-
Exacerbations (requiring antibiotic therapy) measured as frequency, proportion with one or more, or duration
-
Serious adverse events (i.e. any adverse even that results in death or is life‐threatening)
Secondary outcomes
-
Frequency of hospital admissions measured
-
Lung function (forced expiratory volume in one second (FEV1) litres or percent of predicted)
-
Symptoms (e.g. dyspnoea, cough, wheeze), for example using the Leicester Cough Questionnaire (LCQ)
-
Self‐efficacy (e.g. Chronic Disease Self‐Efficacy Scale)
-
Economic costs (e.g. direct: costs of care such as cost‐benefit or cost‐effectiveness; indirect: days lost from work or full‐time education)
-
Adverse events (e.g. pneumonia)
Reporting of one or more of the outcomes above was not an inclusion criterion for the review.
We recognise the limitation of using self‐efficacy as a primary outcome, and it may be viewed as an intermediate or process outcome. Research is needed to establish if, in bronchiectasis, improvements in self‐efficacy leads to long‐term improvements in clinically important endpoints (Lavery 2011).
Search methods for identification of studies
Electronic searches
We identified studies from the Cochrane Airways Trials Register, which is maintained by the Information Specialist for the Group. The Cochrane Airways Trials Register contains studies identified from several sources:
-
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL), through the Cochrane Register of Studies Online (crso.cochrane.org);
-
weekly searches of MEDLINE Ovid SP 1946 to date;
-
weekly searches of Embase Ovid SP 1974 to date;
-
Monthly searches of PsycINFO Ovid SP 1967 to date;
-
Monthly searches of CINAHL EBSCO (Cumulative Index to Nursing and Allied Health Literature) 1937 to date;
-
Monthly searches of AMED EBSCO (Allied and Complementary Medicine);
-
handsearches of the proceedings of major respiratory conferences.
Studies contained in the Trials Register are identified through search strategies based on the scope of Cochrane Airways. Details of these strategies, as well as a list of handsearched conference proceedings are in Appendix 1. See Appendix 2 for search terms used to identify studies for this review.
We also conducted a search of ClinicalTrials.gov (www.ClinicalTrials.gov) and the World Health Organization (WHO) trials portal (www.who.int/ictrp/en/). We searched for studies in any language in all databases from their inception to November 2016.
Searching other resources
We examined the reference lists of all primary studies and review articles for additional references and searched relevant manufacturers' websites for trial information. We searched the ‘grey’ literature at OpenGrey (www.opengrey.eu/) and searched for errata or retractions from included studies published in full‐text on PubMed (www.ncbi.nlm.nih.gov/pubmed).
Data collection and analysis
Selection of studies
Two review authors (CK and SG) independently screened titles and abstracts for inclusion of all potential studies and classified them as 'retrieve' (eligible or potentially eligible/unclear) or 'do not retrieve'. Two review authors (CK and S Grundy) independently screened the full‐text of retrieved studies to identify those for inclusion and record reasons for exclusion of the ineligible studies. We resolved disagreements through discussion or through a third review author (SS). We identified and excluded duplicates and collated multiple reports of the same study so that each study, rather than each report, was the unit of interest in the review. We recorded the selection process in sufficient detail to complete a PRISMA flow diagram and a 'Characteristics of excluded studies' table (Moher 2009).
Data extraction and management
We used a data collection form, piloted on at least one study in the review, to record study characteristics and outcome data. One review author (DL) extracted the following characteristics from the included studies.
-
Methods: study design, total duration of study, details of any 'run in' period, number of study centres and location, study setting, withdrawals, and date of study
-
Participants: number, mean age, age range, gender, severity of condition, diagnostic criteria, baseline lung function, smoking history, inclusion criteria, and exclusion criteria
-
Interventions: intervention, comparison, concomitant medications, and excluded medications
-
Outcomes: primary and secondary outcomes specified and collected, and time points reported
-
Notes: funding for trial, and notable conflicts of interest of trial authors
Two review authors (DL and CK) independently extracted outcome data from the included studies. We noted outcome data that were not reported in a usable way in the 'Characteristics of included studies' table. We resolved disagreements by consensus or by involving a third review author (SS). One review author (DJWE) transferred data into the Review Manager 5 (RevMan 5) file (RevMan 2014). We validated data entry by comparing the data presented in the systematic review with the study reports. A second review author (CK) spot‐checked study characteristics for accuracy against the trial report.
Assessment of risk of bias in included studies
Two review authors (CK and DL) independently assessed the risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a). We resolved disagreements by discussion or by involving another review author (SS). We assessed the risk of bias according to the following domains.
-
Random sequence generation
-
Allocation concealment
-
Blinding of participants and personnel
-
Blinding of outcome assessment
-
Incomplete outcome data
-
Selective outcome reporting
-
Other bias
We graded each potential source of bias as high, low or unclear and provided a quote from the study report together with a justification for our judgement in the 'Risk of bias' table. We summarised the risk of bias judgements across different studies for each of the domains listed and considered blinding separately for different key outcomes where necessary (e.g. for unblinded outcome assessment, risk of bias for all‐cause mortality may be very different than for a patient‐reported quality‐of‐life scale). Where information on risk of bias related to unpublished data or correspondence with a trialist, we noted this in the 'Risk of bias' table.
When considering treatment effects, we took into account the risk of bias for studies that contributed to that outcome.
Assessment of bias in conducting the systematic review
We conducted the review according to the published protocol and reported any deviations from it in the 'Differences between protocol and review' section of the systematic review.
Measures of treatment effect
We estimated intervention effects using odds ratios with 95% confidence intervals (CI) for dichotomous data and mean difference or standardised mean difference with 95% CI for continuous data. Where standard deviations (SD) were not reported but other measures of variance around mean differences, such as standard error, CIs, or P values were reported, we calculated these according to Section 7.3 in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b). In this review it was likely that different scales may have been used to measure the same outcome (for example, Bronchiectasis‐Quality of Life (B‐QoL) and St. George's Respiratory Questionnaire (SGRQ)). In this case, we planned to use the standardised mean difference (SMD) and its 95% CI, ensuring a consistent direction of effect by reversing scaling where necessary, supported by a statement in the text on direction of interpretation.
We planned to undertake meta‐analyses only where this was meaningful, that is, where the treatments, participants and the underlying clinical question were similar enough for pooling to make sense. We planned to narratively describe skewed data reported as medians and interquartile ranges.
Unit of analysis issues
In this review the unit of analysis was the participant. For all dichotomous data, we reported the proportion of participants that contributed to each outcome compared with the total number of randomised participants.
Cross‐over trials
Cross‐over trials were not appropriate for this intervention as it was not possible to avoid carry‐over of knowledge acquisition from the first phase. However, if we had identified eligible cross‐over studies we planned to only include data from the first pre cross‐over phase.
Cluster‐randomised trials
Large‐scale trials are uncommon in bronchiectasis and it was therefore unlikely that we would identify eligible RCTs randomising at group level (e.g. by primary care practice). We planned to analyse eligible cluster‐RCTs in accordance with methods described in Section 16.3 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011c), using average cluster size and an estimate of the intraclass correlation coefficient (ICC) to adjust sample sizes to the 'effective sample size'. Where appropriate, we planned to combine single RCTs with cluster‐RCTs if we considered the designs and interventions sufficiently similar and the effect of the intervention was unlikely to be influenced by the method of randomisation.
Multiple‐arm trials
Where trials included multiple arms we planned to describe all study groups in the 'Characteristics of included studies' table, but to only include the arms that met our review criteria in the analyses. If multiple comparisons (e.g. self‐management A versus self‐management B versus self‐management C versus usual care) were combined in the same meta‐analysis, we planned to divide the usual care (control) group by the number of intervention arms to avoid 'double‐counting'.
Dealing with missing data
We contacted investigators of included studies to provide unreported data such as missing outcomes, missing data, means or SDs and noted differential dropout between study groups along with reasons for withdrawal. Where a particular outcome included substantial loss to follow‐up (≥ 50%), we planned to report this in the text and mark the data with an asterisk. We used available cases for data analysis and did not impute missing data. Where studies included analyses based on the imputation of missing values, we planned to include data at low risk of bias and report data separately for those at higher risk of bias in the text of the review. Multiple imputation methods that included sensitivity analyses pre‐specified in published protocols were considered at low risk of bias (Little 2012; Gewandter 2014). Imputation of missing data related to trial outcomes, using methods such as last observation carried forward, were not considered appropriate. For example, completion of missing data (e.g. relating to an efficacy outcome) following an intervention‐related death would be inappropriate (Gewandter 2014).
Where missing data were thought to introduce a high risk of bias (substantial loss to follow‐up or inappropriate imputation), we planned to explore the impact of including such studies using a sensitivity analysis.
Assessment of heterogeneity
In this review, the specific nature of the intervention, population, outcomes and methodological quality had the potential to vary considerably between studies. We therefore planned to assess potential sources of variability between studies in the following ways.
-
Clinical variability: to compare the distribution of participants, interventions, and outcomes across the included studies. To discuss and agree potential clinical heterogeneity by consensus.
-
Methodological variability: to compare study designs and study quality using 'Risk of bias' criteria.
-
Statistical heterogeneity (where variability in the effects of interventions is greater than expected by chance alone): to evaluate the statistical significance of heterogeneity using the Chi² test (P = 0.10 is significant). However, this test may be unreliable, lacking power to detect important heterogeneity with few or small studies and the potential to detect clinically insignificant heterogeneity with large numbers of studies. It is also possible for trials to show large consistent effects in the face of significant heterogeneity. Therefore, in addition to assessing evidence of heterogeneity using the Chi² test as above, we also planned to quantify the magnitude of heterogeneity using the Tau² (random‐effects model only), and I² statistics (Higgins 2003) with the following interpretation thresholds, based on recommendations in Section 9.5 of the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2011):
-
0% to 40%: might not be important;
-
30% to 60%: may represent moderate heterogeneity;
-
50% to 90%: may represent substantial heterogeneity;
-
75% to 100%: considerable heterogeneity.
-
We planned to report substantial heterogeneity (> 50%) and to explore possible causes by prespecified subgroup analysis.
Assessment of reporting biases
We planned to compare the results of data from published and unpublished studies as a direct test of publication bias and, if there were a sufficient number of studies (10 or more), to explore potential bias arising from small‐study effects using Egger’s method to test for asymmetry in funnel plots (Egger 1997). If smaller studies had shown larger intervention effects compared to larger studies, we planned to evaluate potential causes (for example, poor methodological quality; differences in populations or interventions) and to report studies at high risk of bias in the text of the review.
Data synthesis
We planned to include studies in meta‐analyses where the study designs, interventions and outcomes were similar. Where substantial heterogeneity (> 50%) was identified we planned to report outcomes in the text, giving direction and size of the effect along with the strength of the evidence (risk of bias). It was likely that included studies would vary by population, design and outcomes, therefore we considered meta‐analysis using a random‐effects model the most appropriate. However, where there are few studies or the effects of interventions across studies are not randomly distributed (for example, with publication bias), the random‐effects model estimates may be unreliable or biased. It was likely that this review would only include a small number of low‐powered studies and we therefore planned to use a fixed‐effect model and to evaluate the impact of model choice using a sensitivity analysis. We aimed to synthesise and report dichotomous and continuous data separately for a given outcome, should the need arise (e.g. exacerbation/no exacerbation or exacerbation duration). Where both end‐of‐study point estimates and change from baseline scores were reported we analysed these separately. We performed the analyses using RevMan 5 (RevMan 2014).
'Summary of findings' table
We created a 'Summary of findings' table using the following primary and secondary outcomes: health‐related quality of life, hospital admissions, serious adverse events, exacerbations, lung function, self‐efficacy and economic costs. We tabulated the quality of each outcome with the five GRADE criteria (study limitations, consistency of effect, imprecision, indirectness and publication bias) (GRADE 2004) using methods and recommendations described in Section 8.5 (Higgins 2011a) and Chapter 12 (Schünemann 2011) of the Cochrane Handbook for Systematic Reviews of Interventions and GRADEpro GDT software (GRADEpro GDT 2015). We justified all decisions to downgrade or upgrade the quality of studies using footnotes and we included comments to aid the reader's understanding where necessary.
Subgroup analysis and investigation of heterogeneity
We planned to carry out the following subgroup analyses.
-
Age: adults (> 18 years) versus children
-
Duration of follow‐up (less than 12 months versus 12 months or longer)
We planned to use the following outcomes in subgroup analyses.
-
Health‐related quality of life
-
Hospital admissions
-
Adverse events
We planned to use the formal test for subgroup interactions in RevMan 5 (RevMan 2014).
Sensitivity analysis
We planned to carry out the following sensitivity analyses.
-
To exclude studies at high risk of selection bias
-
Analyses using a random‐effects model
-
Missing data (studies with > 50% or those using inappropriate imputation)
Results
Description of studies
Results of the search
Following comprehensive electronic searches of bibliographic databases we identified ninety‐two records and an additional eight records through searches of clinicaltrials.gov and the WHO trials portal. Of a total of sixty records (40 duplicates removed), forty‐three were excluded following screening of titles and abstracts. We examined the full‐text articles of 17 records (11 studies) and excluded 12 records (8 studies; see Excluded studies). The remaining five records reported the results of three studies; two studies were included in the review and one was an ongoing study (Characteristics of ongoing studies). The PRISMA flow diagram in Figure 2 shows the study selection process.
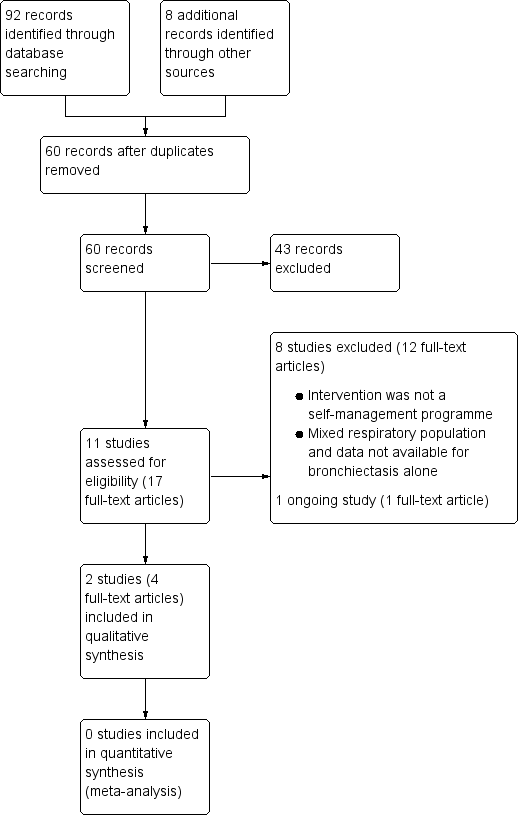
Study flow diagram
Included studies
Two studies met the inclusion criteria for this review and contributed data to the narrative synthesis (Lavery 2011; Greening 2014). The two studies randomised a total of 84 adult participants with bronchiectasis. Greening 2014 recruited and reported data for participants with any respiratory disease (n = 389), but the study authors kindly provided disaggregated data for the subset of participants with a primary diagnosis of bronchiectasis that are reported in the review. Both studies were reported as full‐text, peer‐reviewed articles. In Greening 2014 the complex rehabilitation intervention included a self‐management programme. As this component of the complex intervention was substantial, we have included the trial in this review; however, we are mindful that because the self‐management programme was included alongside other interventional components (described in Characteristics of included studies) the findings from this trial in relation to the predefined inclusion criteria for this review should be interpreted with caution.
Methods
Greening 2014 was a prospective, 12‐month RCT conducted in an acute cardiorespiratory unit in a teaching hospital and an acute medical unit in an affiliated teaching district general hospital, both located in England (UK) Characteristics of included studies.
Lavery 2011 was a proof‐of‐concept, six‐month RCT in a single regional respiratory centre in Northern Ireland (UK) Characteristics of included studies.
Participants
Greening 2014 enrolled adults with a diagnosis of chronic respiratory disease, aged 40 years or over (bronchiectasis group mean age = 72 (intervention = 78, control = 68) with self‐reported breathlessness on exertion (MRC dyspnoea grade 3 or worse). Individuals with concomitant acute cardiac events or more than four emergency admissions to hospital (any cause) during the previous 12 months were excluded. Eligible patients were enrolled within 48 hours of admission to hospital for an exacerbation of chronic respiratory disease. The study included people with COPD, chronic asthma, interstitial lung disease and bronchiectasis but the study authors kindly provided disaggregated data for the cohort of participants with bronchiectasis (n = 20); baseline characteristics are presented in the Characteristics of included studies.
In Lavery 2011 64 adult participants were randomised (with 32 participants in each arm of the study), and four were withdrawn before the study reached completion (two in each arm of the study). In both groups the mean age was 60 years, with an inclusion criterion of 18 years or over, and with a primary diagnosis of bronchiectasis based on respiratory physician assessment, including a computed tomographic scan Characteristics of included studies
Interventions
Greening 2014 randomised individuals on a 1:1 basis to receive early rehabilitation plus self‐management (six weeks' duration) and usual care, versus usual care alone. Early rehabilitation comprised supervised volitional (strength and aerobic training) and non‐volitional (neuromuscular electrical stimulation) techniques and a self‐management programme. The progressive exercise programme was individually tailored and delivered/supported by physiotherapists and nurses during the stay on an acute medical ward, and after discharge was supported by weekly telephone consultations with the pulmonary rehabilitation team, consisting of physiotherapists and nurses. The self‐management programme comprised the SPACE (Self management programme of Activity, Coping and Education) manual for COPD, a structured programme of exercise, education, and psychosocial support. Motivational interviewing techniques were used to familiarise participants with the manual, which was used throughout the participants’ inpatient stay and during telephone discussions. Usual care comprised standard care from the ward physiotherapy team, including physiotherapist‐delivered techniques for airway clearance, assessment and supervision of mobility and smoking cessation advice; usual care did not include a supervised progressive exercise programme during admission or immediately after discharge.
In Lavery 2011 the intervention was usual care plus an expert patient programme (EPP); disease‐specific EPP was delivered in group format during one session per week (2.5 hours) for eight weeks (two weeks of disease‐specific education; six weeks of standardised EPP). The disease‐specific component included causes of bronchiectasis, disease process, medical investigations, dealing with symptoms, airway clearance techniques, exacerbations, health promotion and available support. Participants in the control group received usual care; review at a specialist respiratory clinic on a three‐monthly basis to monitor spirometry, inflammatory blood markers, and sputum microbiologic assessment. Inhaled therapy and antibiotics were prescribed where required and treatment was adjusted to the needs of the participant as necessary, including hospital admission.
Outcomes
The primary outcome for the study reported by Greening 2014 was the readmission rate at 12 months. Secondary outcomes included number of hospital days, mortality, lung function, physical performance and health‐related quality of life, measured using the St George's Respiratory Questionnaire (SGRQ); secondary outcomes were assessed at baseline, discharge from hospital, six‐week, and three‐ and 12‐month follow‐up.
In Lavery 2011 the primary outcome was the Chronic Disease Self‐efficacy Scales (CDSS), which measures the confidence of an individual to perform items related to self‐management. Secondary outcomes included the Revised Illness Perception Questionairre (IPQ‐R), which explains health behaviour in terms of coping; SGRQ was used to measure quality of life; two standard EPP questionnaires relating to self‐rated health, ability to manage their condition and adherence to medication. Other outcome measures included FEV1, frequency of antibiotic therapy and sputum microbiology. Secondary measures were recorded at baseline, post‐intervention (eight weeks) and at three‐ and six‐month follow‐up.
Excluded studies
We excluded a total of eight studies (Newall 2005; Liaw 2011; Mandal 2012; Gurses 2013; Lee 2014; Mazzoleni 2014 ;Hester 2016; Aksamit 2017; ); three studies were excluded because both intervention and control groups received common self‐management components (Newall 2005; Mandal 2012; Lee 2014), resulting in a single component of difference between the two groups, which failed to meet our inclusion criteria of at least two elements in the self‐management component; the intervention in one study was solely an information resource, which also did not meet our criteria (Hester 2016); in three studies the intervention was not self‐management (Liaw 2011; Gurses 2013;Aksamit 2017; ); in one study with a mixed population of participants, data were not available for bronchiectasis participants alone (Mazzoleni 2014).
Risk of bias in included studies
'Risk of bias' assessments and supporting evidence are presented in the Characteristics of included studies. Figure 1 provides a summary of risk of bias judgements presented by study and domain. Figure 3 depicts the risk of bias for each domain, presented as percentage values across both included studies. Overall, the two included studies were well reported and of high methodological quality; we considered the studies to have a low risk of bias for the majority of domains (see below).

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Both studies used appropriate methods for random sequence generation and allocation concealment. We judged both studies to be at low risk of selection bias.
Blinding
Blinding of the participants and personnel (i.e. those delivering the intervention) to treatment allocation was not feasible due to the nature of the interventions. Thus, we considered both studies to be at low risk of performance bias for objective outcomes (i.e. exacerbations, serious adverse events, serious adverse events, lung function, frequency of hospital admissions, lung function, economic costs, adverse events) and at high risk of performance bias for subjective outcomes (i.e. health‐related quality of life; symptoms, self‐efficacy). In both studies, outcome assessors were blinded to treatment allocation; we considered the risk of detection bias to be low for both studies.
Incomplete outcome data
We considered both studies to have a low risk of attrition bias based on low and balanced rates of participant withdrawal, which were documented adequately in the study reports.
Selective reporting
Both studies were pre‐registered on appropriate clinical trials registries, where predefined outcomes of interest were listed. Both studies reported all pre‐defined outcomes of interest and were considered to be at low risk of reporting bias.
Other potential sources of bias
The study by Lavery 2011 was a proof‐of‐concept RCT that did not include a power calculation. There was therefore a risk that negative findings were influenced by an inadequate sample size and we considered this study at high risk of 'other bias', though this was not related to the methodological quality of the study.
The authors of Greening 2014 provided disaggregated data for participants with bronchiectasis. The number of participants with bronchiectasis was low (n = 20) and there appeared to be a baseline imbalance in disease severity; participants in the intervention group showed a trend towards higher MRC dyspnoea grades (baseline and stable state) and worse baseline lung function (lower FEV1); however, statistical comparisons were not available. In addition, mortality rates were imbalanced between groups in the subset of bronchiectasis participants. We therefore considered the study by Greening 2014 to be at high risk for 'other bias', although we would stress that this was not related to the methodological quality of the overall study, and likely a result of our use of disaggregated data with an insufficient sample size.
Effects of interventions
The different nature of the interventions in the two included studies (a complex rehabilitation intervention including self‐management and a single expert patient programme) meant that meta‐analysis of the data was not appropriate and the results were therefore included narratively.
Primary outcomes
Health‐related quality of life
The authors of Greening 2014 provided health‐related quality of life data for the subset of participants with bronchiectasis. Mean SGRQ Total scores in both groups improved over time but were not significantly different at six weeks', three and 12 months' follow‐up (6 weeks MD ‐12.70, 95% CI ‐30.39 to 4.99; 3 months MD ‐9.15, 95% CI ‐28.08 to 9.78; 12 months MD ‐10.27, 95% CI ‐45.15 to 24.61; Analysis 1.1). The mean difference between groups at each time point exceeds the minimum clinically important difference (MCID) of four units (Jones 2005) but confidence intervals indicate imprecision in the effect, potentially attributable to the low sample size (max n = 13). The study authors did not formally report the change from baseline to 12 months and it was not possible to calculate the change value as more than half of the participants withdrew during the study. However, results should be interpreted with caution due to the small sample size and unequal numbers of participants in each group (n = 20).
In Lavery 2011 there were no differences in SGRQ Total scores between groups postintervention, or at three or six months' follow‐up (postintervention: MD ‐6.50, 95% CI ‐16.59 to 3.59; 3 months, MD ‐2.60, 95% CI ‐12.97 to 7.77, 6 months, MD 3.20, 95% CI ‐6.64 to 13.04; Analysis 2.1). The study authors reported marginal worsening in SGRQ Total scores over the six‐month follow‐up in the intervention group (from 46.9, 95% CI 40.2 to 53.6 to 47.9, 95% CI 40.7 to 55.1) and an improvement of 6.9 units in the control group (from 51.6, 95% CI 45.0 to 58.1 to 44.7, 95% CI 37.6 to 51.7).
There is therefore no indication of benefit in health‐related quality of life, based on SGRQ total scores, attributable to the intervention. Using GRADE criteria we judged the quality of the evidence for this outcome to be either low (Lavery 2011) or very low (Greening 2014) summary of findings Table for the main comparison.
Exacerbations (requiring antibiotic therapy)
Neither of the included studies reported data on exacerbations requiring antibiotic therapy.
Serious adverse events
Greening 2014 provided mortality data for the subset of participants with bronchiectasis. During the 12 months of follow‐up, four of eight participants (50%) died in the early rehabilitation group and two of 12 participants (16.7%) died in the usual care group. However, results should be interpreted with caution due to the low event rate, unequal numbers of participants in each group and small sample size (n = 20).
Lavery 2011 did not evaluate serious adverse events.
It was not possible to assess this outcome using GRADE criteria.
Secondary outcomes
Frequency of hospital admissions
The study authors provided data on hospital admissions for the subset of participants with bronchiectasis. In the early rehabilitation group (n = 8), six participants (75%) were readmitted to hospital during 12 months of follow‐up; three were readmitted once; one was readmitted twice; and two were readmitted three times. In the usual care group (n = 12), six participants (50%) were readmitted to hospital during the 12 months of follow‐up; four were readmitted once; one was readmitted three times; and one was readmitted seven times. The mean (SD) number of admissions per participant per 12 months in the early rehabilitation group and usual care groups, respectively, were 1.38 (1.19) and 1.17 (2.04); there did not appear to be a significant difference between groups. However, results should be interpreted with caution due to the low event rate, unequal numbers of participants in each group and small sample size (n = 20).
Lavery 2011 did not evaluate the frequency of hospital admissions.
It was not possible to assess this outcome using GRADE criteria.
Lung function
The study authors provided data on FEV1 (L) for the subset of participants with bronchiectasis. Baseline measurements were recorded when individuals were in a stable state. There were no significant differences between groups at discharge, six weeks', three or 12 months' follow‐up (discharge MD ‐0.13, 95% CI ‐0.60 to 0.34; 6 weeks MD 0.07, 95% CI ‐0.55 to 0.69; 3 months MD 0.15, 95% CI ‐0.55 to 0.85; 12 months MD 0.30, 95% CI ‐1.11 to 1.71; Analysis 1.2). Change in lung function was not formally reported in the paper and it was not possible to calculate the change value as more than half of the participants withdrew during the study. The results should be interpreted with caution due the low sample size at each time point (n = 17 at discharge; n = 8 at one year), the unequal numbers of participants in each group (intervention = 8; control = 12) and the small size of the overall sample (n = 20).
Lavery 2011 reported no significant differences between the control and intervention groups with regard to lung function (P > 0.05) but did not report further details.
Using GRADE criteria we judged the quality of the evidence for this outcome to be very low summary of findings Table for the main comparison.
Symptoms
Neither of the included studies reported data on symptoms.
Self‐efficacy
Greening 2014 did not examine self‐efficacy.
Lavery 2011 reported data on self‐efficacy using an outcome measure found to be valid and reliable when tested in individuals with long term‐health conditions. The Chronic Disease Self‐efficacy Scale (CDSS) measures the confidence of an individual to perform self‐management tasks on 10 component scales using 1 to 10 Likert responses. All scales showed significant benefit postintervention (exercise, MD 2.10, 95% CI 0.89 to 3.31, Analysis 2.2; disease information, MD 1.50, 95% CI 0.07 to 2.93, Analysis 2.3; obtaining help, MD 1.10, 95% CI 0.05 to 2.15, Analysis 2.4; communicating with physician, MD 1.10, 95% CI 0.14 to 2.06, Analysis 2.5; managing disease, MD 1.10, 95% CI 0.17 to 2.03, Analysis 2.6; doing chores, MD 2.00, 95% CI 0.78 to 3.22, Analysis 2.7; social activity, MD 2.00, 95% CI 0.84 to 3.16, Analysis 2.8; Managing symptoms, MD 1.90, 95% CI 0.78 to 3.02, Analysis 2.9; Managing breathlessness, MD 1.50, 95% CI 0.32 to 2.68, Analysis 2.10; Managing depression, MD 2.00, 95% CI 0.91 to 3.09, Analysis 2.11), but the effect was only sustained by the six‐month endpoint on the Managing Depression scale (6 months, MD 1.30, 95% CI 0.09 to 2.51, Analysis 2.11). The study authors report a significant improvement in self‐efficacy over time with the intervention on six of the 10 subscales of the CDSS (exercise, disease information, doing chores, social activity, managing symptoms and managing depression) though they acknowledge that the greatest impact was immediately postintervention. The authors report that four subscales did not show a significant difference between groups over time (managing the disease, obtaining help, communicating with physician, managing breathlessness). There is no Minimum Clinically Important Difference value for the CDSS.
As the CDSS comprises 10 component scales we opted not to include them in the 'Summary of findings' tables. However using GRADE criteria, we judged the quality of evidence for this outcome as very low, based on the overall quality of the study summary of findings Table for the main comparison.
Economic costs
Neither of the included studies reported data on economic costs.
Adverse events
Neither of the included studies reported data on adverse events.
Discussion
Summary of main results
There is insufficient evidence to draw clear conclusions as to whether self‐management interventions for people with bronchiectasis have a significant impact on the primary outcomes of this review, namely: health‐related quality of life; frequency of exacerbations and serious adverse events.
Only two studies fulfilled the inclusion criteria for the review. The first evaluated the effect of a combined early rehabilitation and self‐management programme on 389 participants admitted to hospital with acute exacerbations of chronic respiratory disease (Greening 2014). However, we were only able to use a subset of 20 participants with bronchiectasis in the review. The second study evaluated the impact of an expert patient self‐management programme on 64 participants with bronchiectasis (Lavery 2011). It is important to note that the Greening study recruited participants at an exacerbation, whilst Lavery recruited participants in stable condition. Merging results of these two self‐management plans therefore is limited by the different clinical conditions of participants and should be considered as an additional limitation. Participants receiving self‐management did not show any significant benefit from the intervention in terms of health‐related quality of life by the endpoint in either study. There was a trend towards a higher mortality rate in one very small study but no clear difference was seen.. Neither of the included studies measured exacerbation frequency. In terms of our secondary outcomes, there was no impact on the frequency of hospital admissions in one study and no impact on lung function in either of our included studies. One study reported self‐efficacy using the CDSS and showed evidence of improvement following an expert patient programme in six of the ten scales that comprised the outcome measure. Neither of the included studies reported symptoms, adverse events and economic costs.
It is important to note that (Greening 2014) was a compound intervention incorporating a substantial self‐management component and we included it in the review for that reason. However, the findings from this trial in relation to the predefined inclusion criteria for this review should be interpreted with caution as we cannot isolate the effects of self‐management from the other intervention components. In addition, the self‐management component was based on a model used with people with COPD and was not specifically tailored to people with bronchiectasis. All of the results from this study should be interpreted with caution as the disaggregated data were not powered to detect differences and there were imbalances between groups.
Overall there is inadequate published data to establish with any degree of certainty whether self‐management strategies for people with bronchiectasis have either a positive or negative impact.
Overall completeness and applicability of evidence
The paucity of data available for inclusion in this review mandates caution when extrapolating the findings to similar populations or other settings. We identified only two adult studies in the review, looking at different interventions. We did not identify any trials of self‐management programmes for children, nor any trials comparing one type of self‐management strategy with another. Neither of the studies included in the review measured the frequency of exacerbations requiring antibiotics, an important marker of disease activity in bronchiectasis, symptoms, adverse events or economic costs. As an exercise component was included in the compound intervention in one of the studies, it would have been useful to have included exercise performance as an outcome measure in the review. As it stands, the evidence for the benefits and harms of self‐management interventions is incomplete and of limited applicability to clinical practice.
Quality of the evidence
With guidance from the GRADE criteria we considered the quality of the evidence, where data were available for formal comparison, to be low or very low, and where data were available for narrative inclusion we would draw similar conclusions. We are aware of the limitations of the two included studies, in particular the small amount of disaggregated data available for inclusion from Greening 2014, and have summarised them in Characteristics of included studies, Figure 2 and Figure 3. It was not possible to consider publication bias through the construction of a funnel plot due to the very small number of included studies.
Potential biases in the review process
We are aware of the potential for publication bias in this systematic review, as there is inevitably the concern that we may not have found relevant unpublished trials. However, we have received excellent support from Cochrane Airways, with comprehensive and systematic database searches, and we endeavoured to address any study selection bias with two review authors independently evaluating trials for inclusion. Throughout we were careful to ensure that this process was consistent with our predefined inclusion criteria.
Agreements and disagreements with other studies or reviews
The need for patient self‐management has been identified and included in bronchiectasis guidelines and research prioritisation (Pasteur 2010; Aliberti 2016). However, uptake remains unclear with a UK audit showing that only 33% of 97 institutions provided individualised self‐management plans, and a single‐centre UK audit suggesting that effective personal management was achievable without a formal self‐management plan (Hill 2013; Ali 2015). Aliberti 2016 suggest in their research priorities in bronchiectasis that future studies should be conducted to determine the effectiveness of patient self‐management and adherence to treatment; this review supports this by revealing how little evidence currently exists. Due to the limited data available from our review it is not possible to assess whether effects of self‐management may be observed in people with bronchiectasis but planned future studies may help to resolve the uncertainty (Hester 2016).

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
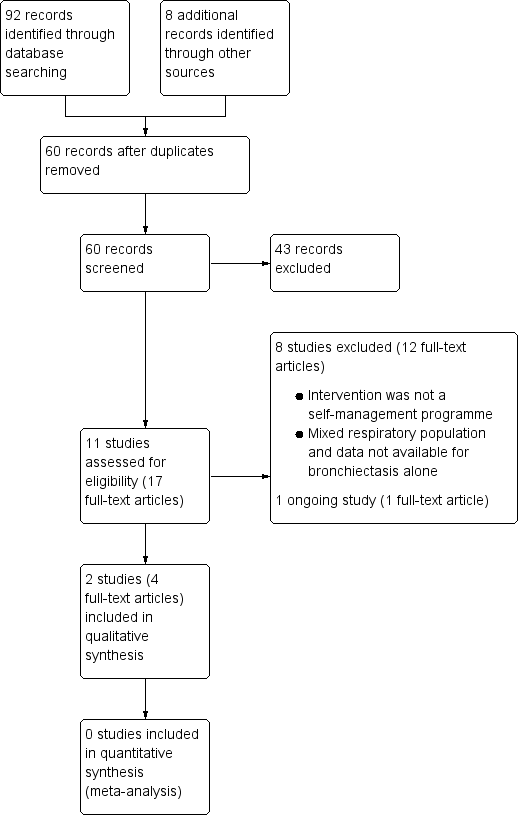
Study flow diagram

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
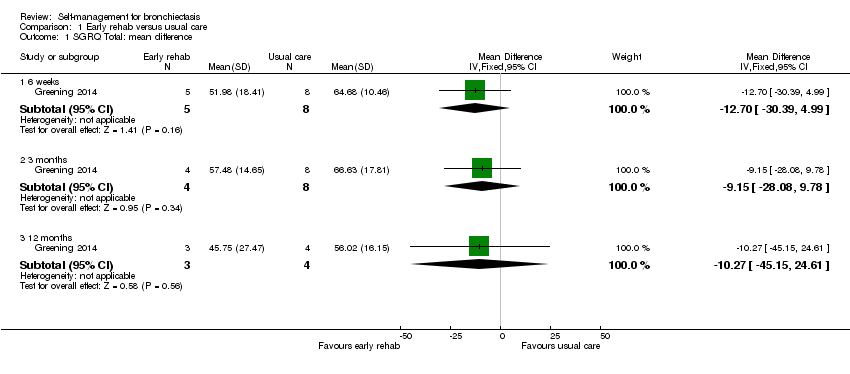
Comparison 1 Early rehab versus usual care, Outcome 1 SGRQ Total: mean difference.

Comparison 1 Early rehab versus usual care, Outcome 2 FEV1 L: mean difference.
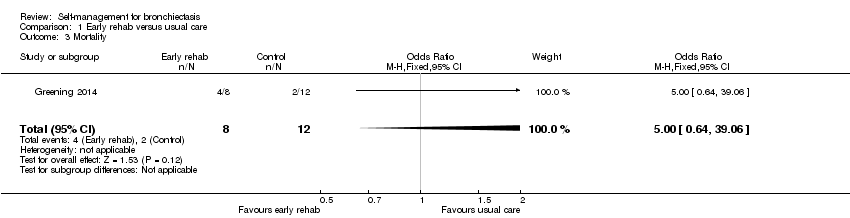
Comparison 1 Early rehab versus usual care, Outcome 3 Mortality.
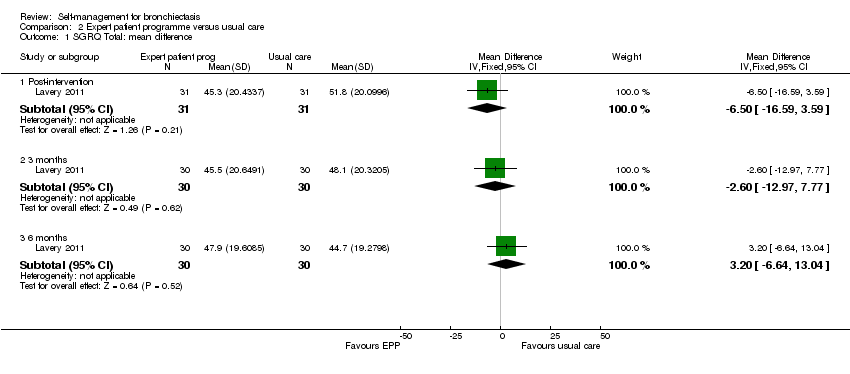
Comparison 2 Expert patient programme versus usual care, Outcome 1 SGRQ Total: mean difference.

Comparison 2 Expert patient programme versus usual care, Outcome 2 Self‐efficacy: Exercise.
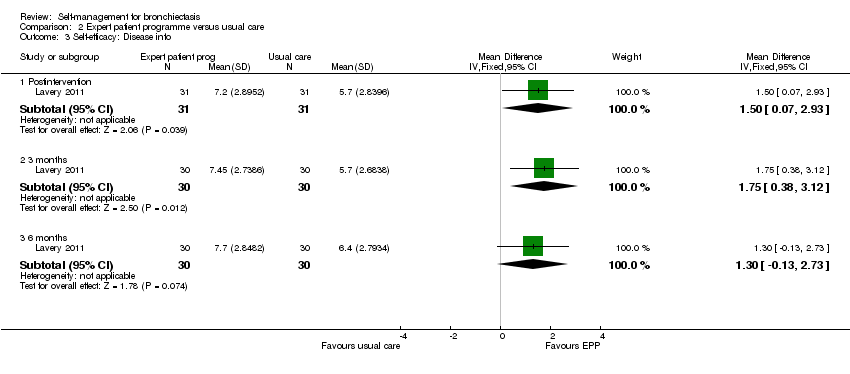
Comparison 2 Expert patient programme versus usual care, Outcome 3 Self‐efficacy: Disease info.

Comparison 2 Expert patient programme versus usual care, Outcome 4 Self‐efficacy: Obtain help.
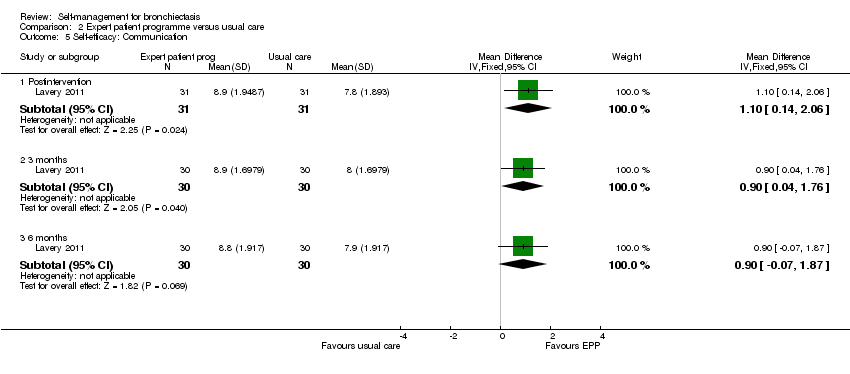
Comparison 2 Expert patient programme versus usual care, Outcome 5 Self‐efficacy: Communication.

Comparison 2 Expert patient programme versus usual care, Outcome 6 Self‐efficacy: Manage disease.

Comparison 2 Expert patient programme versus usual care, Outcome 7 Self‐efficacy: Do chores.
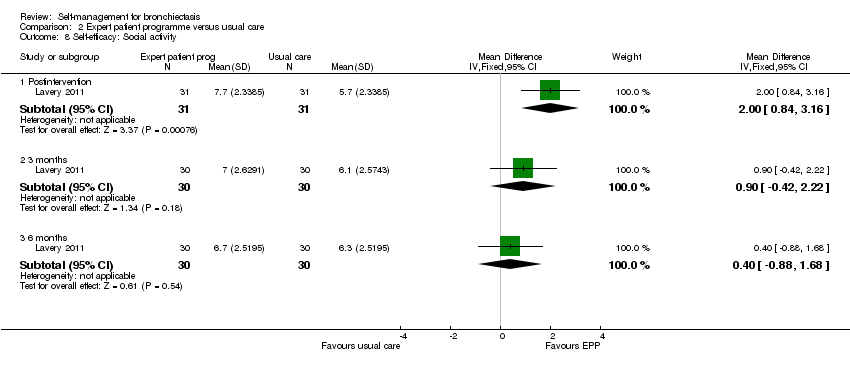
Comparison 2 Expert patient programme versus usual care, Outcome 8 Self‐efficacy: Social activity.

Comparison 2 Expert patient programme versus usual care, Outcome 9 Self‐efficacy: Manage symptoms.
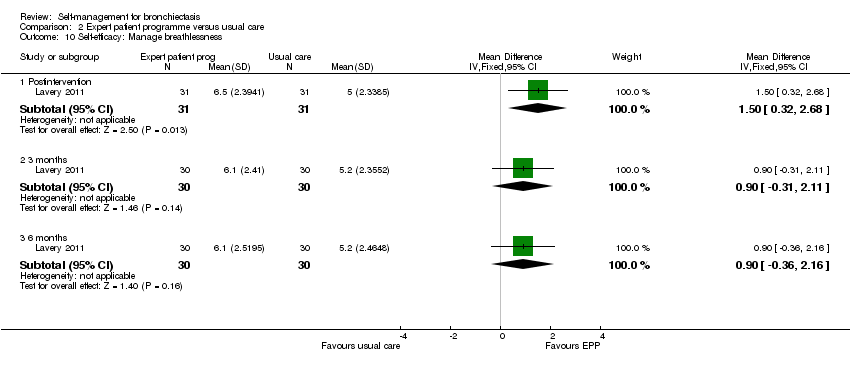
Comparison 2 Expert patient programme versus usual care, Outcome 10 Self‐efficacy: Manage breathlessness.

Comparison 2 Expert patient programme versus usual care, Outcome 11 Self‐efficacy: Manage depression.
| Self‐management compared to usual care for bronchiectasis | ||||||
| Patient or population: people with non‐cystic fibrosis bronchiectasis | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
| Risk with usual care | Risk with self‐management | |||||
| Health‐related quality of life | The mean health‐related quality of life was 56.02 points | MD 10.27 lower | ‐ | 20 | ⊕⊝⊝⊝ | No clear benefit or harm from self‐management (very low‐quality evidence) |
| Health‐related quality of life Follow up: range post‐intervention to 6 months | The mean health‐related quality of life was 44.7 points | MD 3.2 higher | ‐ | 60 | ⊕⊕⊝⊝ | No clear benefit or harm from self‐management |
| Exacerbations requiring antibiotics | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| Serious adverse events: mortality | ‐ | ‐ | not estimable | 20 | ||
| Hospital admissions (number admitted at least once) | ‐ | ‐ | not estimable | 20 | ‐ | |
| Lung function assessed with: FEV1 L | The mean FEV1 was 1.03 L | MD 0.3 higher | ‐ | 20 | ⊕⊝⊝⊝ | No clear benefit or harm from self‐management |
| Self‐efficacy assessed with: CDSS Follow‐up: postintervention to 6 months | ‐ | ‐ | not estimable | 60 | ⊕⊕⊝⊝ | Six out of ten scales showed significant improvements over time with the intervention. We elected not to include all 10 scales in the table but graded the evidence based on overall quality of the study |
| Economic costs | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1One point deducted for the unblinded nature of the comparison. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 SGRQ Total: mean difference Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 6 weeks | 1 | 13 | Mean Difference (IV, Fixed, 95% CI) | ‐12.70 [‐30.39, 4.99] |
| 1.2 3 months | 1 | 12 | Mean Difference (IV, Fixed, 95% CI) | ‐9.15 [‐28.08, 9.78] |
| 1.3 12 months | 1 | 7 | Mean Difference (IV, Fixed, 95% CI) | ‐10.27 [‐45.15, 24.61] |
| 2 FEV1 L: mean difference Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 Discharge | 1 | 17 | Mean Difference (IV, Fixed, 95% CI) | ‐0.13 [‐0.60, 0.34] |
| 2.2 6 weeks | 1 | 14 | Mean Difference (IV, Fixed, 95% CI) | 0.07 [‐0.55, 0.69] |
| 2.3 3 months | 1 | 13 | Mean Difference (IV, Fixed, 95% CI) | 0.15 [‐0.55, 0.85] |
| 2.4 12 months | 1 | 8 | Mean Difference (IV, Fixed, 95% CI) | 0.30 [‐1.11, 1.71] |
| 3 Mortality Show forest plot | 1 | 20 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.0 [0.64, 39.06] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 SGRQ Total: mean difference Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 Post‐intervention | 1 | 62 | Mean Difference (IV, Fixed, 95% CI) | ‐6.5 [‐16.59, 3.59] |
| 1.2 3 months | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐2.60 [‐12.97, 7.77] |
| 1.3 6 months | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 3.20 [‐6.64, 13.04] |
| 2 Self‐efficacy: Exercise Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 Postintervention | 1 | 62 | Mean Difference (IV, Fixed, 95% CI) | 2.10 [0.89, 3.31] |
| 2.2 3 months | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 1.40 [0.14, 2.66] |
| 2.3 6 months | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.80 [‐0.46, 2.06] |
| 3 Self‐efficacy: Disease info Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 Postintervention | 1 | 62 | Mean Difference (IV, Fixed, 95% CI) | 1.5 [0.07, 2.93] |
| 3.2 3 months | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 1.75 [0.38, 3.12] |
| 3.3 6 months | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 1.30 [‐0.13, 2.73] |
| 4 Self‐efficacy: Obtain help Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 Postintervention | 1 | 62 | Mean Difference (IV, Fixed, 95% CI) | 1.10 [0.05, 2.15] |
| 4.2 3 months | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.80 [‐0.34, 1.94] |
| 4.3 6 months | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 1.0 [‐0.19, 2.19] |
| 5 Self‐efficacy: Communication Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.1 Postintervention | 1 | 62 | Mean Difference (IV, Fixed, 95% CI) | 1.10 [0.14, 2.06] |
| 5.2 3 months | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.90 [0.04, 1.76] |
| 5.3 6 months | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.90 [‐0.07, 1.87] |
| 6 Self‐efficacy: Manage disease Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6.1 Postintervention | 1 | 62 | Mean Difference (IV, Fixed, 95% CI) | 1.10 [0.17, 2.03] |
| 6.2 3 months | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 1.10 [0.27, 1.93] |
| 6.3 6 months | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.70 [‐0.27, 1.67] |
| 7 Self‐efficacy: Do chores Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7.1 Postintervention | 1 | 62 | Mean Difference (IV, Fixed, 95% CI) | 2.0 [0.78, 3.22] |
| 7.2 3 months | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 1.20 [‐0.14, 2.54] |
| 7.3 6 months | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 1.20 [‐0.01, 2.41] |
| 8 Self‐efficacy: Social activity Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 8.1 Postintervention | 1 | 62 | Mean Difference (IV, Fixed, 95% CI) | 2.0 [0.84, 3.16] |
| 8.2 3 months | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.90 [‐0.42, 2.22] |
| 8.3 6 months | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.40 [‐0.88, 1.68] |
| 9 Self‐efficacy: Manage symptoms Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 9.1 Postintervention | 1 | 62 | Mean Difference (IV, Fixed, 95% CI) | 1.90 [0.78, 3.02] |
| 9.2 3 months | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 1.20 [0.13, 2.27] |
| 9.3 6 months | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.70 [‐0.41, 1.81] |
| 10 Self‐efficacy: Manage breathlessness Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 10.1 Postintervention | 1 | 62 | Mean Difference (IV, Fixed, 95% CI) | 1.5 [0.32, 2.68] |
| 10.2 3 months | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.90 [‐0.31, 2.11] |
| 10.3 6 months | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.90 [‐0.36, 2.16] |
| 11 Self‐efficacy: Manage depression Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 11.1 Postintervention | 1 | 62 | Mean Difference (IV, Fixed, 95% CI) | 2.0 [0.91, 3.09] |
| 11.2 3 months | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 1.40 [0.19, 2.61] |
| 11.3 6 months | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 1.30 [0.09, 2.51] |

