Intermittent versus continuous systemic therapy as treatment for unresectable metastatic colorectal cancer
Información
- DOI:
- https://doi.org/10.1002/14651858.CD012440Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 21 diciembre 2016see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Protocol
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Colorrectal
- Copyright:
-
- Copyright © 2016 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
MF, AB, EB and EK drafted the protocol. All protocol authors read and approved the final draft.
Declarations of interest
EB has no known conflicts of interest.
AJB has no known conflicts of interest.
YC has no known conflicts of interest.
MAF has no known conflicts of interest.
EK has no known conflicts of interest.
GJL has no known conflicts of interest.
SM has no known conflicts of interest.
CJHV has no known conflicts of interest.
Acknowledgements
We thank Dr. Henning Keinke Andersen, Marija Barbateskovic and the members of the Cochrane Colorectal Cancer Group editorial board for their evaluation and advice when writing this protocol.
Version history
| Published | Title | Stage | Authors | Version |
| 2016 Dec 21 | Intermittent versus continuous systemic therapy as treatment for unresectable metastatic colorectal cancer | Protocol | Martine A. Frouws, Yvette Claassen, Anne J Breugom, Esther Bastiaannet, Simone Mocellin, Cornelis JH van de Velde, Gerrit‐Jan Liefers, Ellen Kapiteijn | |
PICO
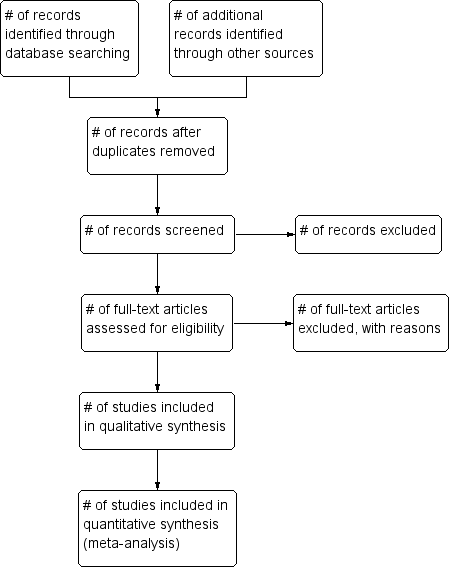
Study flow diagram.
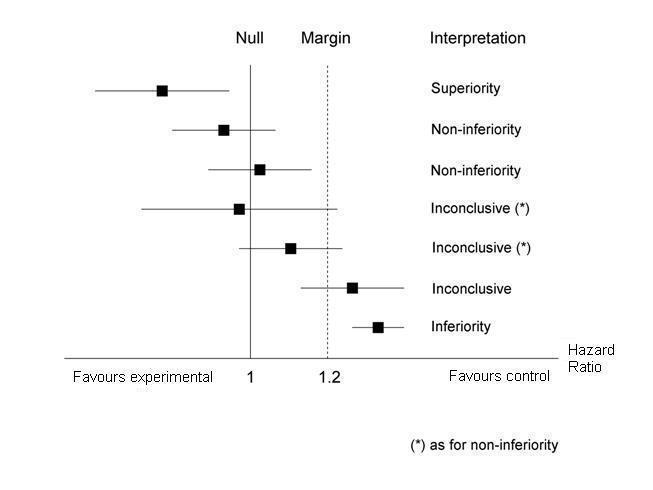
Non‐inferiority interpretation of meta‐analysis findings
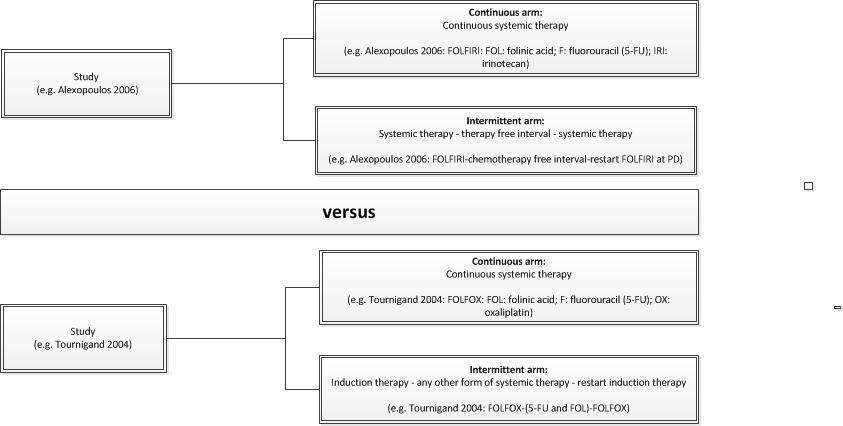
Planned subgroup analysis
| Table 1. Examples of intermittent versus continuous treatment schemes | |
| 1. | Intermittent: FOLFOX17, 12 weeks; sLV5‐FU22, 24 weeks; FOLFOX7, 12 weeks |
| Continuous: FOLFOX4 every 2 weeks until progression of disease (PD) (Tournigand 2004) | |
| 2. | Intermittent: FOLFIRI3, 12 weeks; chemotherapy‐Free‐Interval (CFI); restart FOLFIRI at PD |
| Continuous: FOLFIRI, 24 weeks (Alexopoulos 2006) | |
| 3. | Intermittent: Bevacizumab + capecitabine/oxaliplatin, 18 weeks; CFI; restart Bevacizumab+ capecitabine/oxaliplatin at PD |
| Continuous: Bevacizumab + capecitabine/oxaliplatin, 18 weeks; bevacizumab+capecitabine, restart bevacizumab+capecitabine/oxaliplatin at PD (Simkens 2015) | |
| 1 FOLFOX: FOL: folinic acid; F: fluorouracil (5‐FU); OX: oxaliplatin. | |

