Intervenciones para las exacerbaciones de otoño del asma en niños
Referencias
References to studies included in this review
References to studies excluded from this review
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Study design: randomised, double‐blind, placebo‐controlled trial. Aim: to determine whether montelukast, added to usual asthma therapy, would reduce days with worse asthma symptoms and unscheduled physician visits of children during the September epidemic. Study centres and method of recruitment: recruited through advertising and through clinical practices in Hamilton and Brantford, Canada. Dates of study: 1 September 2005 to 15 October 2005. Run‐in period: no run‐in period. Duration of participation: 45 days. Consent: approved by the research ethics board at St. Joseph's Healthcare Hamilton. Informed consent from parents and assent from appropriately aged children. Power: a 40% reduction was expected in days with worse asthma symptoms in the montelukast group based upon results of a pilot study. Based upon 80% power and a 0.05 significance level, a sample‐size requirement of 88 per group was estimated. A 10% dropout rate was allowed for, so the final sample requirement was 97 per group. Imputation of missing data, i.e. assumptions made for ITT analysis: all randomised children completed the study and were included in analysis. | |
| Participants | Age (mean, range): not reported, 2 to 14 years. Gender: 65.0% male. Asthma severity: not explicitly mentioned, but 90% required inhaled corticosteroids (likely moderate to severe). Diagnostic criteria: physician‐diagnosed asthma. Number recruited: 196 Number randomised (intervention, control): 98, 96 Number completed (intervention, control): 98, 96 Number analysed (intervention, control): 98, 96 Withdrawals: 100% completed, no withdrawals. Inclusion criteria: 2 to 14 years old; physician‐diagnosed asthma needing a rescue inhaler in the last year; missing ≥ 1 day from school because of asthma in the last year or having significant limitation of normal activity; having a history of asthma exacerbations associated with apparent respiratory viral infections; ability to communicate in English. Exclusion criteria: significant cardiorespiratory comorbidity; using an LTRA; using regular OCS medication; asthma exacerbation in the month before study inception. | |
| Interventions | Intervention: montelukast age‐specific dose from 1 September to 15 October. Comparison: matched placebo. Concomitant medication: usual therapy. Excluded medication: already on montelukast. | |
| Outcomes | Primary outcome: percentage of days with worsening asthma symptoms during the intervention period (worsening symptoms defined as symptoms that were worse than usual or needed extra asthma medication, or requiring an unscheduled visit to a doctor or treatment with oral corticosteroids). Secondary outcome: number of unscheduled care visits. Time points measured: daily, then at the end of the study. Secondary outcome results: the montelukast group experienced a 78% reduction in unscheduled physician visits for asthma (4 for montelukast vs 18 for placebo, P = 0.011). Adverse events: minor adverse events occurred in 25 children in the montelukast group and in 35 children in the placebo group. 2 children discontinued study medication due to adverse events, 1 due to a personality change and 1 with change in appetite and increased tiredness; both children were taking placebo. The trial code was not broken, and symptom recording was continued. Another significant event was identified at the follow‐up interview after a child assigned to receive montelukast required emergency treatment for acute behaviour disorder. | |
| Notes | Funding: Merck Frosst Canada Ltd. Subgroups: subgroup analyses were exploratory risk of asthma worsening intervention vs control:
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation schedule. Randomly assigned in blocks of 4 according to gender and age. |
| Allocation concealment (selection bias) | Low risk | Randomisation schedule described as "concealed" and generated by an individual "not otherwise involved in the study". Mechanism of concealment described as based upon identical containers issued by third party (further information supplied by authors). |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blinded. Intervention drug and placebo prepared by Merck Frosst, no reason to suspect parent or child could identify intervention drug from placebo. |
| Blinding of outcome assessment (detection bias) | Low risk | Given the use of a placebo, unlikely that the assessors would have knowledge of participant group. Subjective participant‐reported parent‐assessed symptoms and questionnaire used to assess other outcomes; these could have been affected if blinding inadequate, but no reason to suspect placebo led to incomplete blinding. Physician validated unscheduled care. |
| Incomplete outcome data (attrition bias) | Low risk | Intention‐to‐treat primary analysis, 100% children completed the trial and returned 99.7% diary data. Adherence good in both groups (91.7% intervention vs 93.2% placebo). |
| Selective reporting (reporting bias) | Unclear risk | Unclear whether all prespecified outcomes included in the analysis |
| Other bias | Low risk | No baseline differences between groups, except more lifetime hospitalisations: 37.8% intervention vs 25.0% placebo |
| Methods | Study design: cluster‐randomised controlled trial. Aim: to assess the impact of an NHS‐delivered public health intervention on unscheduled medical contacts in children with asthma during September and to perform a health economic analysis of the intervention. Study centres and method of recruitment: 142 UK general practices. Recruitment predominantly via the Clinical Practice Research Datalink (CPRD). A recruitment pack, including study information and an expression of interest form, was sent by post to the preferred contact at the practice to all 433 practices contributing to CPRD in England and Wales at the time of recruitment. Non‐responding practices were sent a reminder e‐mail, followed by a second reminder e‐mail and then final reminders by e‐mail and post. Some practices were also contacted by telephone, by CPRD or the study team at the Sheffield Clinical Trials Research Unit. Practices returned the completed expression of interest form, confirming or updating as necessary the information about the practice held by CPRD. Responses were tracked by CPRD to ensure practices that had replied were not contacted again. The expressions of interest were then forwarded to the study team to contact practices. Dates of study: 29 July 2013 to 30 September 2014. Run‐in period: none. Duration of participation: intervention commenced the week of 29 July 2013. Unscheduled care outcomes measured: September 2013, September to December 2013, September 2013 to August 2014, September 2014. Health economic outcomes measured: 1 August 2013 to 31 July 2014. Consent: ethics approval for the study was given by South Yorkshire Research Ethics Committee on 25 October 2012 (reference number: 12/YH/04). NHS permissions to conduct the study were obtained for all the primary care trusts in England and health boards in Wales. Power: the study was designed to detect a difference of 5% (30% vs 25%) with 90% power and a 2‐sided significance level of 5%, with an intraclass correlation of 0.03 to account for clustering. Based on this, 70 practices were estimated to be required per arm. It was expected that the sample size of 140 practices would equate to approximately 14,000 school‐aged children with asthma. Imputation of missing data, i.e. assumptions made for ITT analysis: analyses of effectiveness were performed as both ITT and PP, with the ITT being primary. If practices stopped submitting data to the CPRD before the end of a given follow‐up period, they were excluded from all analyses for that time period. The health economic analyses were based on the PP population. ITT analyses included all practices for which data were obtained by study period. The PP analyses were the subset of children in the ITT analyses to whom the intervention was delivered as intended by the protocol (i.e. individuals or practices not receiving a letter were excluded from PP analyses). | |
| Participants | Age (mean, range): 10.5 years, 5 to 16 years. 4‐year‐old children analysed separately. Gender: 60.0% male. Asthma severity: majority most likely mild (severity data not presented). Diagnostic criteria: coded diagnosis of asthma. Eligible participants identified in accordance with pre‐agreed diagnostic codes for asthma by the CPRD. Number recruited: 12,179 Number randomised (intervention, control): 5917, 6262 Number completed (intervention, control): 4411, 4438 Number analysed (intervention, control): 4411, 4438 (Note: figures above are for completing the entire trial until September 2014. ITT analyses of outcomes in September 2013, the primary outcome period, were based on 5305 intervention and 5586 control participants.) Withdrawals: from experimental group: discontinued intervention withdrawal before 30 September 2014: 13 practices, 506 children. From control group: discontinued intervention withdrawal before 30 September 2014: 18 practices, 1824 children. Inclusion criteria: aged between 4 and 16 years on 1 September 2013; coded diagnosis of asthma; prescribed asthma medication March 2012 to March 2013. Exclusion criteria: aged 4 years or under on 1 September 2013 or 16 years or over on 31 August 2013; not considered appropriate for this intervention by GP; not receiving asthma medication; coexisting neoplastic disease. | |
| Interventions | Intervention: NHS‐delivered public health intervention (a letter sent from the GP to parents/carers of school‐aged children with asthma reminding of the importance to take medications and the need to get sufficient medication sent out during the week commencing 29 July 2013). Comparison: no letter, control arm continue with standard care as usual, no other activity required. Concomitant medication: usual therapy. Excluded medication: none. | |
| Outcomes | Primary outcome: proportion of children with unscheduled contacts in September 2013. Secondary outcomes: number/proportion/time to first unscheduled contact; number/proportion/time to first unscheduled contacts for respiratory diagnosis; number/proportion/time to first all medical contacts; proportion scheduled contacts; number collecting prescriptions; QALYs gained; and NHS costs. Time points measured:
Primary outcome result: proportion of children with unscheduled contacts in September intervention vs control: 45.2 vs 43.7; OR 1.09, 95% CI 0.96 to 1.25. Secondary outcome results: intervention vs control multiple outcomes and subgroups assessed, most outcomes no significant difference between groups. Proportion prescriptions August 2013: OR 1.43, 95% CI 1.24 to 1.64; number of scheduled contacts per child August 2013: OR 95% CI 1.13, 0.84 to 1.52. No significant difference in unscheduled contacts September to December 2013, September 2013 to August 2014. Mean cost saving across the base case of GBP 36.07 per child and 96.3% probability that the intervention is cost‐saving. Intervention resulted in a QALY loss in 82.9% of samples and a mean loss of 0.00017 QALYs. Adverse events: not reported. | |
| Notes | Funding: National Institute for Health Research. Subgroups: the primary outcome was similar for 5‐ to 16‐year‐old children who had been prescribed preventative steroids compared to all 5‐ to 16‐year‐old children. Among children aged under 5 years, the differences were larger, and of borderline statistical significance, with the intervention being associated with more unscheduled visits for all subgroups. In all cases, the effect among the PP population was greater than that observed in the ITT population. Post hoc analyses demonstrated that for those who collected a prescription within the last 3 months, there was no difference in unscheduled contacts in September (55.2% vs 54.3% control), whilst for those whose last prescription was collected 3 to 6 months ago, there was an excess of unscheduled contacts in September (42.1% vs 39.7% control). (Data confirmed with study author since they differed between the summary and the main text of the report.) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised by practice, stratified by size (confirmed by communication with author that the study statistician had no information about practices prior to randomisation other than list size). |
| Allocation concealment (selection bias) | Unclear risk | Sequence generated by 1 of 2 trial statisticians, then revealed to study manager and research assistant. Statisticians had no information about practice other than list size. However, characteristics of individual practices influenced whether the intervention was enacted or not. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Study team and participants unblinded; this might have affected coding of contacts. Study team had no influence on data capture. Individual practices could choose not send the letter at all or not to send to selected patients. |
| Blinding of outcome assessment (detection bias) | Low risk | Collected via CPRD. Contacts designated as "scheduled", "unscheduled", and "irrelevant" based on an independent adjudication panel comprised of experienced GPs who were blinded to the treatment group. |
| Incomplete outcome data (attrition bias) | Low risk | Missing outcome data due to change in computer system; presumed to be missing completely at random so no imputation. However, this was at least 25% in each group. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported. |
| Other bias | Low risk | No baseline difference in age, gender, and practice size |
| Methods | Study design: randomised, open study. Aim: to investigate whether pranlukast added to usual asthma therapy in Japanese children during the autumn reduces asthma exacerbations. The effects of age and sex on the efficacy of pranlukast were also evaluated. Study centres and method of recruitment: multiple clinical sites in Chiba, Japan. Study participants were recruited between July 2007 and August 2007 through advertising and from the clinical practices in Chiba, Japan. Dates of study: 15 September 2007 to 14 November 2007. Run‐in period: from recruitment until 15 September 2007. Duration of participation: 60 days in addition to run‐in period. Consent: the investigation was approved by the Research Ethics Board of Chiba Universiy, Chiba (approval number: 631). Written informed consent was obtained from the parents of all participants and child assent when appropriate. Power: no a priori calculation. Imputation of missing data, i.e. assumptions made for ITT analysis: 13.6% of children excluded after randomisation in the pranlukast group (2.8% placebo), but no imputation made. | |
| Participants | Age (mean, range): 5.5 years (not reported but supplied by author), 1 to 14 years (divided into 2 age groups: 1 to 5 years and 6 to 14 years). Gender: 62.8% male. Asthma severity: 54.5% required inhaled corticosteroids. Diagnostic criteria: physician‐diagnosed asthma. Asthma was diagnosed by primary care doctors based on the Japanese paediatric guidelines for the treatment and management of bronchial asthma 2005. Number recruited: 204 Number randomised (intervention, control): 102, 102 Number completed (intervention, control): 59, 72 Number analysed (intervention, control): 51, 70 Withdrawals: 43 from intervention group and 30 from control group excluded before trial due to respiratory symptoms or insufficient diary recording by caregivers, or both, during the observation period. 8 from intervention group and 2 from control group excluded during the study period due to poor compliance or insufficient diary recording by caregivers, or both. Inclusion criteria: age 1 to 14 years old, physician‐diagnosed asthma needing a rescue inhaler in the last year, with a history of asthma exacerbations associated with apparent respiratory viral infections. Children who had been treated with LTRA were included after 14‐day washout period. Exclusion criteria: significant cardiorespiratory comorbidity; using regular oral corticosteroid; or had an asthma exacerbation in the month before treatment with pranlukast started. Children who had respiratory symptoms or problems with diary recording during observation, or both, were excluded from the study. | |
| Interventions | Intervention: regular pranlukast, an LTRA. 7 mg/kg, twice daily, in addition to their usual asthma therapy. Comparison: usual therapy. Concomitant medication: intervention taken in addition to usual asthma therapy. No restriction, but children who had been treated with LTRA were included after a 14‐day washout period. Excluded medication: no restriction, but 14‐day washout of LTRA. | |
| Outcomes | Primary outcome: total asthma score calculated during 8 weeks. Total asthma score was evaluated as follows: a blue sticker (score, 0) was applied on days when a child had no asthma symptoms; a green sticker (score, 1) indicated mild asthma symptoms; a yellow sticker (score, 2) indicated symptoms that were worse than usual or needed extra asthma medication, and an orange sticker (score, 3) was applied if a child's breathing symptoms required an unscheduled visit to a physician or treatment with oral corticosteroids. Secondary outcomes: days with worse asthma symptoms, number of colds, and days with fever. Days with worse asthma symptoms were defined as those with either an orange or a yellow sticker. A fever was defined as a temperature exceeding 38 °C. A “cold” was defined as the presence of more than 2 consecutive purple stickers indicating days with cold symptoms. At least 5 days with no cold symptoms were required before a subsequent new cold was identified. Time points measured: contemporaneous data collection at the end of 60 days. Primary outcome result: there were no significant differences between pranlukast and control group in total asthma score at 8 weeks (5.5 vs 7.8, P = 0.35), and in the days in which a child experienced a worsening of asthma symptoms (1.5 vs 1.8, P = 0.67) (data obtained through correspondence with the author). Secondary outcome results: higher number of colds in the control group compared to the pranlukast group (P = 0.06), and children taking pranlukast experienced fewer days with fever compared to the control group (P = 0.04). Adverse events: no children discontinued study medication due to adverse events. | |
| Notes | Funding: not stated. Subgroups: Boys vs girls. 1 to 5 years vs 6 to 14 years. Boys aged 1 to 5 years had the lower total asthma score at 8 weeks (P = 0.002), and experienced fewer cold episodes (P = 0.007). In boys, pranlukast significantly reduced total asthma score among 1‐ to 5‐year‐olds (P = 0.010), but did not reduce it among 6‐ to 14‐year‐olds. In girls, pranlukast did not affect total asthma score among 1‐ to 5‐year‐olds, but increased total asthma score among 6‐ to 14‐year‐olds (P = 0.027). 60 cold episodes were reported in the pranlukast group and 107 cases in the control group. A significant reduction in the number of cold episodes was observed in 1‐ to 5‐year‐old boys who were treated with pranlukast (P < 0.001). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random assignment to either the pranlukast intervention group or the control group. Randomisation conducted according to sex and within the predefined age groups (1 to 5 years and 6 to 14 years). |
| Allocation concealment (selection bias) | Unclear risk | No description reported. |
| Blinding of participants and personnel (performance bias) | High risk | Study was of open‐label design. The authors recognised this as a limitation of the study. |
| Blinding of outcome assessment (detection bias) | High risk | Symptoms were reported subjectively by study participants. Participants and study observers were not blinded. |
| Incomplete outcome data (attrition bias) | High risk | High rate of exclusions from pranlukast group after randomisation |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported. |
| Other bias | Low risk | No baseline differences between groups. Comparisons of the baseline characteristics of the study groups were conducted using Chi² and Mann‐Whitney U‐tests. |
| Methods | Study design: 3‐arm, randomised, double‐blind, double placebo‐controlled, multicentre clinical trial. Aim: to compare (1) omalizumab with placebo and (2) omalizumab with an ICS boost with regard to autumn exacerbation rates when initiated 4 to 6 weeks before return to school. Study centres and method of recruitment: 8 US urban clinical research centres, no recruitment method information given. Dates of study: October 2011 to November 2013. Run‐in period: 2‐ to 12‐week screening. Duration of participation: from 4 to 6 weeks before school return until 90 days after school return. Consent: approved by all 8 institutional review boards. Consent from guardians and assent according to local guidelines. Power: enrolment of 453 participants (223 in the omalizumab arm, 155 in the inhaled corticosteroid boost arm, and 75 in the placebo arm (52 in steps 2 to 4 and 23 in step 5)) estimated to provide greater than 90% power to compare the omalizumab and placebo arms (11.8% vs 35.9% estimated effect) and 80% power to compare the omalizumab and ICS boost arms (12.9% vs 25.8% estimated effect). Imputation of missing data, i.e. assumptions made for ITT analysis: main analysis was based on modified ITT (children who were randomised, began study treatment, and had 1 or more study contact during the 90‐day outcome period were included in mITT). Supplemental volume included sensitivity analyses of mITT, PP, complete‐case, best‐case, worst‐case, and multiple imputation models. | |
| Participants | Age (mean, range): 10.2 years, 6 to 17 years. Gender: 63.4% male. Asthma severity: National Heart, Lung and Blood Institute Expert Panel Report‐3 based steps 2‐5 (mild‐severe). Diagnostic criteria: asthma diagnosis or symptoms for more than 1 year. Number recruited: 727 Number randomised steps 2‐4 (omalizumab, placebo, steroid boost): 133, 47, 138 Number randomised treatment step 5 (omalizumab, placebo): 145, 50 Number completed treatment: 439 total. Efficacy Number analysed steps 2‐4 (omalizumab, placebo, steroid boost): 121, 43, 130 Number analysed treatment step 5 (omalizumab, placebo): 138, 46 Safety Number analysed steps 2‐4 (steroid boost, placebo): 131, 45 Number analysed treatment steps 2‐5 (omalizumab, placebo): 268, 93 Withdrawals: 585 excluded pre‐enrolment, 214 excluded pre‐randomisation, 59 withdrew consent and were excluded pre‐enrolment, 35 withdrew consent and were excluded pre‐randomisation.
Inclusion criteria:
(Note: children requiring 500 μg of fluticasone or equivalent twice daily for control during the run‐in phase (step 5) were not entered into the ICS boost arm and instead were randomised at a ratio of 3:1 to omalizumab or injected placebo.) Exclusion criteria: not reported distinct from inclusion criteria. | |
| Interventions | Intervention: omalizumab standard dosing based on IgE and weight 4 to 6 weeks before, until 90 days after school start. Comparison: 1) placebo, or 2) ICS boost (doubled dose). Concomitant medication: ongoing guidelines‐based management EPR‐3. Excluded medication: none reported. | |
| Outcomes | Primary outcome: asthma exacerbation in the 90‐day period beginning on the first day of each child’s school year, defined as worsening of asthma control requiring systemic corticosteroids or hospitalisation. Secondary outcome: 11 prespecified, non‐mechanistic secondary outcomes (analysed exacerbation during 90‐day intervention according to subgroups based upon: exacerbation during run‐in, eosinophil count, total IgE, roach IgE, age, fraction FeNO, FEV1, BMI, ethnicity, and gender). IFNα responses to rhinovirus were measured in PBMCs from a subset of participants. Time points measured: 2 to 4 weekly during intervention. Primary outcome result: asthma exacerbation in the 90‐day period beginning on the first day of each child’s school year:
Secondary outcome results: exacerbation during 90‐day intervention according to subgroups. The following results differed significantly according to group: in those with an exacerbation during run‐in omalizumab vs placebo OR 0.12, 95% CI 0.02 to 0.64 (steps 2‐5), omalizumab vs ICS boost OR 0.05, 95% CI 0.002 to 0.98 (step 2‐4); in those with BMI centile ≥ 85 omalizumab vs ICS boost OR 0.13, 95% CI 0.03 to 0.61, (steps 2‐4); in those with BMI percentile < 85 ICS boost vs placebo OR 0.19, 95% CI 0.04 to 0.84 (steps 2‐4); in those with IgE < 255 kU/L omalizumab vs ICS boost OR 0.24, 95% CI 0.06 to 0.93 (steps 2‐4); in those with IgE 255 kU/L ICS boost vs placebo OR 0.24, 95% CI 0.06 to 0.87 (steps 2‐4); IFN‐α responses to rhinovirus were significantly increased in the omalizumab‐treated group (P = 0.03); among the omalizumab‐treated group, children with increases in ex vivo IFN‐α responses to rhinovirus to greater than the median value had a significantly lower rate of exacerbations during the outcome period OR 0.14, 95% CI 0.01 to 0.88. Adverse events: adverse events were reported by 54.5% of children in the omalizumab arm and 54.8% of children in the placebo arm (P > 0.99, steps 2–5) during the intervention phase. 1 or more adverse events were reported by 43.5% of children in the ICS boost arm and 53.3% of children in the placebo arm (P = 0.30, steps 2–4). 3 cases of grade 1 anaphylaxis occurred in the ICS boost, 2 in the placebo, and 3 in the omalizumab arm. Two serious AEs occurred during the intervention period, 1 each in the placebo (seventh nerve palsy) and ICS boost (anaphylaxis) arm. There were no deaths and no non–asthma‐related hospitalisations during the intervention phase. | |
| Notes | Funding: National institute for Allergy and Immune Diseases and an unrestricted grant from Novartis. Omalizumab and matching placebo were donated by Novartis. The ICS boost and matching placebo were donated by GlaxoSmithKline. Both companies had the opportunity to comment on the study design, but they had no role in the trial’s performance, data analysis, manuscript preparation, or decision to submit the manuscript for publication. Adrenaline auto injectors were provided by Mylan. Subgroups: 11 subgroups were based on: exacerbation during run‐in, eosinophil count, total IgE, roach IgE, age, FeNO, FEV1, BMI, ethnicity, and gender. A prespecified subgroup analysis was conducted considering children with an exacerbation during the run‐in phase. Omalizumab was more efficacious than both placebo (6.4% vs 36.3%; OR 0.12, 95% CI 0.02 to 0.64) and ICS boost (2.0% vs 27.8%; OR 0.05, 95% CI 0.002 to 0.98). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Centralised, computer‐based random allocation scheme |
| Allocation concealment (selection bias) | Low risk | Described as centralised. No information on allocation concealment in report, but study authors confirmed that allocation was concealed using a third party and identical containers. |
| Blinding of participants and personnel (performance bias) | Low risk | Placebo, inhalers and injections. No evidence that adverse events differed between placebo and interventions, and no other reasons to suspect participants could identify to which group they had been assigned. Participants and other staff blinded. Unblinded nurses administered injections but not involved in outcome measurement. |
| Blinding of outcome assessment (detection bias) | Low risk | Mix of objective and subjective outcomes, but assessors all blinded. |
| Incomplete outcome data (attrition bias) | Low risk | Primary analysis was modified intention‐to‐treat restricted to children who were randomised, began study treatment, and had more than or equal to 1 study contact during the 90‐day outcome period. There was good retention (94%) and similar dropout rates and reasons between groups. |
| Selective reporting (reporting bias) | Low risk | Secondary outcomes predefined. All reported in online supplement. |
| Other bias | Low risk | Groups balanced according to baseline characteristics. |
| Methods | Study design: randomised, double‐blind, placebo‐controlled, multicentre study Aim: to determine the effectiveness of montelukast therapy in reducing asthma burden in children when initiated prophylactically on school return. Study centres and method of recruitment: 165 allergy and clinical paediatric practices in the United States and Canada. Hospital‐led recruitment. No recruitment information given. Dates of study: 28 June 2006 to 20 November 2006. Run‐in period: 2‐ to 12‐week screening. Duration of participation: 10 weeks. Consent: approved by local institutional review boards or ethical review committees with informed consent obtained from participants and parents or guardians. Power: assuming a treatment difference of 5% and a standard deviation of 24%, 495 evaluable participants in each treatment group was estimated to provide 90% power (2‐sided alpha 0.05) to demonstrate the superiority of montelukast. Imputation of missing data, i.e. assumptions made for ITT analysis: efficacy analysis was based on the analysis set population, which included all children who had received at least 1 dose of study medication and had a valid measurement of the percentage of days with worsening asthma during the study period (derived from at least 7 days of diary data). All randomised children who had received at least 1 dose of study drug were included in the safety analysis. | |
| Participants | Age (mean, range): 9.9 years, 6 to 14 years. Gender: 61.2% male montelukast group, 59.5% male placebo group. Asthma severity: 30% prescribed inhaled corticosteroids at randomisation (likely low/moderate). Diagnostic criteria: history of chronic asthma. Number recruited: 1162 Number randomised (intervention, control): 580, 582 Number completed (intervention, control): 536, 545 Number analysed (intervention, control): efficacy analysis 499, 499; safety analysis 566, 566. Withdrawals:
Inclusion criteria:
Exclusion criteria:
| |
| Interventions | Intervention: montelukast 5 mg from the night before the first day of school for 8 weeks Comparison: matching placebo Concomitant medication: usual medications Excluded medication: none reported beyond exclusion criteria | |
| Outcomes | Primary outcome: percentage of days with worsening asthma symptoms, defined as 1 or more of: increased beta‐agonist use > 70% from baseline and a minimum increase of 2 puffs; increased daytime symptoms score > 50% from baseline; awake 'all night'; increased ICS use ≥ 100% from baseline or OCS rescue for worsening asthma; unanticipated visits to a doctor, emergency department, or hospital for asthma. Secondary outcomes:
Time points measured: 4, 8, and 10 weeks. Primary outcome result: percentage of days with worsening asthma symptoms: montelukast 24.3% vs placebo 27.2%; least squares means difference 3.0, 95% CI 6.21 to 0.29; P = 0.07 (OR for use of OCS obtained from authors and unpublished: OR 0.79, 95% CI 0.59 to 1.06). Secondary outcome results: no significant changes in components of primary outcome, safety outcomes, or interaction terms for subgroup analyses. Adverse events: 4 SAEs in the intervention group, 1 SAE in the placebo group. No SAE thought to be treatment related. The most common AEs were upper respiratory tract infections. | |
| Notes | Funding: Merck & Co. Subgroups: intervention better than control in boys and children 10 to 14 years, but interaction terms for age and gender non‐significant. No difference between groups according to inhaled corticosteroid use at entry, presence of cold symptoms, or according to individual components of the primary outcome.
Additional post hoc subgroup analyses suggested an increased percentage of days with asthma symptoms in the placebo compared to the intervention group at 3 to 4 weeks after school return and near‐significant superiority of intervention if school return is later than 15 August. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated, randomisation schedule generated by study statistician. |
| Allocation concealment (selection bias) | Unclear risk | No description of schedule. Numbered containers, not specified whether identical. |
| Blinding of participants and personnel (performance bias) | Low risk | Identical placebo used. Study double‐blinded including laboratory technicians, monitors, and study site personnel. |
| Blinding of outcome assessment (detection bias) | Low risk | Assessors blinded, outcome systematic but largely subjective participant‐reported. |
| Incomplete outcome data (attrition bias) | Low risk | Primary analysis based on a modified intention‐to‐treat design, including all children who had received at least 1 dose of study medication and had a valid measurement of the percentage of days with worsening asthma during the study period (derived from at least 7 days of diary data). There was no imputation of missing data, but similar dropout rates and reasons between groups. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported. |
| Other bias | Low risk | Generally balanced groups at baseline except inhaled corticosteroids last year intervention 54.1% vs placebo 48.7%. |
AE: adverse event
BMI: body mass index
CI: confidence interval
CPRD: Clinical Practice Research Datalink
EPR‐3: Expert Panel Report 3
GP: general practitioner
ICS: inhaled corticosteroids
IgE: immunoglobulin E
IFNα: interferon alpha
ITT: intention‐to‐treat
FeNO: fractional exhaled nitric oxide
FEV1: forced expiratory volume in the first second of expiration
LABA: long‐acting beta‐agonist
LTRA: leukotriene receptor antagonist
mITT: modified intention‐to‐treat
NHS: National Health Service
OCS: oral corticosteroid
OR: odds ratio
PBMCs: peripheral blood mononuclear cells
PP: per protocol
SAE: serious adverse event
QALY: quality‐adjusted life year
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Not restricted to children (≤ 18 years). The average age of participants was 27.1 years. Also did not specifically address problems associated with school return. | |
| Not restricted to children (≤ 18 years). Sample group selected from adult volunteers. Also relates to the ragweed season rather than specifically addressing school return. | |
| Incorrect seasonal focus. Children participated during influenza season. Study lacks specific purpose of reducing school‐return exacerbations of asthma. | |
| Incorrect methodology. Exacerbations after school return were reported as an outcome, but this was a post hoc analysis. The study was not a randomised controlled trial of an intervention specifically designed to reduce exacerbations after school return. | |
| Does not refer to asthma and incorrect seasonal focus. Study refers to hay fever grass pollen allergy during the summer months between May and July. | |
| Study not restricted to children (≤ 18 years). Mean age for placebo group was 35.1 years. Mean age for nasal beclomethasone dipropionate group was 36.1 years. Also study was designed to reduce asthma and rhinitis symptoms during the autumn pollen season rather than addressing the problem of school return. | |
| No mention of seasonal exacerbations of asthma | |
| Incorrect seasonal focus. Main seasons of symptomatology extended from May to August. | |
| No mention of seasonal exacerbations of asthma. This study examined data from the Preventative Omalizumab or Step‐up Therapy for Severe Fall Exacerbations (PROSE) study reported in Teach 2015a but considered 'colds' as the outcome. | |
| Not limited to children (≤ 18 years). Mean age was 37 years. Also intervention not specifically designed to reduce exacerbations after school return. | |
| Not restricted to children (≤ 18 years). All but one participant older than 30 years. Also intervention not specifically designed to reduce exacerbations after school return. | |
| Incorrect seasonal focus, referred to pollinotic asthma in the height of spring | |
| Incorrect methodology. Purpose was not to compare intervention designed to reduce school‐return exacerbations of asthma with usual care. Randomised controlled cross‐over trial of year‐round hand sanitiser compared to normal hand hygiene | |
| Not restricted to children (≤ 18 years). Aged 12 to 70 years. Also intervention not specifically designed to reduce exacerbations after school return but rather to prevent exacerbations associated with the pollen season. | |
| No mention of seasonal exacerbations of asthma | |
| No mention of seasonal exacerbations of asthma | |
| No mention of seasonal exacerbations of asthma | |
| No mention of seasonal exacerbations of asthma | |
| No mention of seasonal exacerbations of asthma | |
| No mention of seasonal exacerbations of asthma | |
| Purpose was not to compare intervention designed to reduce school‐return exacerbations of asthma with usual care. Compared fluticasone propionate/salmeterol to fluticasone propionate rather than a usual care control. | |
| Purpose was not to compare intervention designed to reduce school‐return exacerbations of asthma with usual care. Compared suplatast tosilate to mequitazine rather than to a usual care control. |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Exacerbations defined according to the review's primary outcome Show forest plot | 1 | Odds Ratio (Random, 95% CI) | Subtotals only | |
| Analysis 1.1 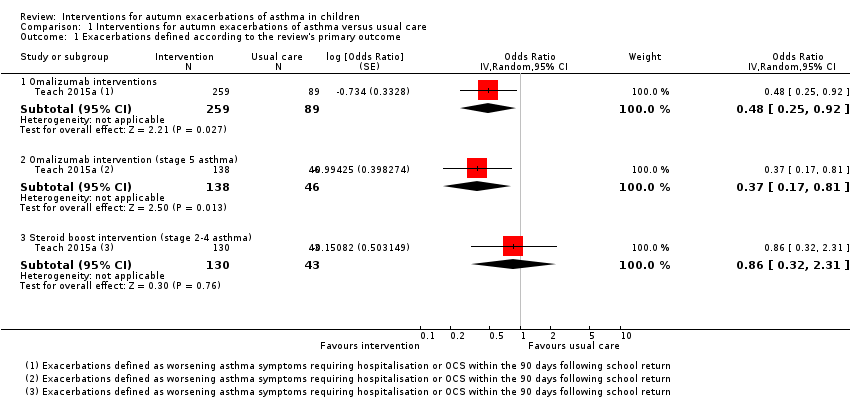 Comparison 1 Interventions for autumn exacerbations of asthma versus usual care, Outcome 1 Exacerbations defined according to the review's primary outcome. | ||||
| 1.1 Omalizumab interventions | 1 | 348 | Odds Ratio (Random, 95% CI) | 0.48 [0.25, 0.92] |
| 1.2 Omalizumab intervention (stage 5 asthma) | 1 | 184 | Odds Ratio (Random, 95% CI) | 0.37 [0.17, 0.81] |
| 1.3 Steroid boost intervention (stage 2‐4 asthma) | 1 | 173 | Odds Ratio (Random, 95% CI) | 0.86 [0.32, 2.31] |
| 2 Exacerbations defined according to study‐specific definitions Show forest plot | 4 | Odds Ratio (Random, 95% CI) | Subtotals only | |
| Analysis 1.2 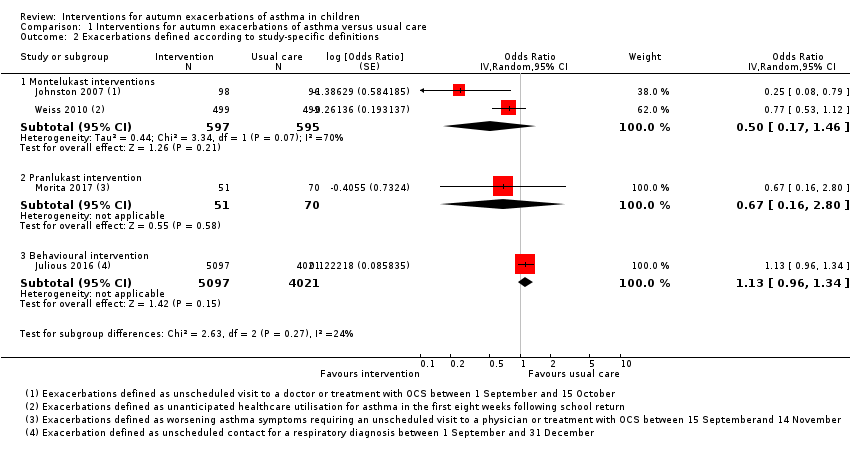 Comparison 1 Interventions for autumn exacerbations of asthma versus usual care, Outcome 2 Exacerbations defined according to study‐specific definitions. | ||||
| 2.1 Montelukast interventions | 2 | 1192 | Odds Ratio (Random, 95% CI) | 0.50 [0.17, 1.46] |
| 2.2 Pranlukast intervention | 1 | 121 | Odds Ratio (Random, 95% CI) | 0.67 [0.16, 2.80] |
| 2.3 Behavioural intervention | 1 | 9118 | Odds Ratio (Random, 95% CI) | 1.13 [0.96, 1.34] |
| 3 Adverse effects Show forest plot | 3 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.3  Comparison 1 Interventions for autumn exacerbations of asthma versus usual care, Outcome 3 Adverse effects. | ||||
| 3.1 Omalizumab intervention (stage 2‐5 asthma) | 1 | 361 | Odds Ratio (M‐H, Random, 95% CI) | 0.99 [0.61, 1.58] |
| 3.2 Steroid boost intervention (stage 2‐4 asthma) | 1 | 176 | Odds Ratio (M‐H, Random, 95% CI) | 0.67 [0.34, 1.33] |
| 3.3 LTRA interventions | 2 | 1326 | Odds Ratio (M‐H, Random, 95% CI) | 0.91 [0.63, 1.32] |

Study flow diagram.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
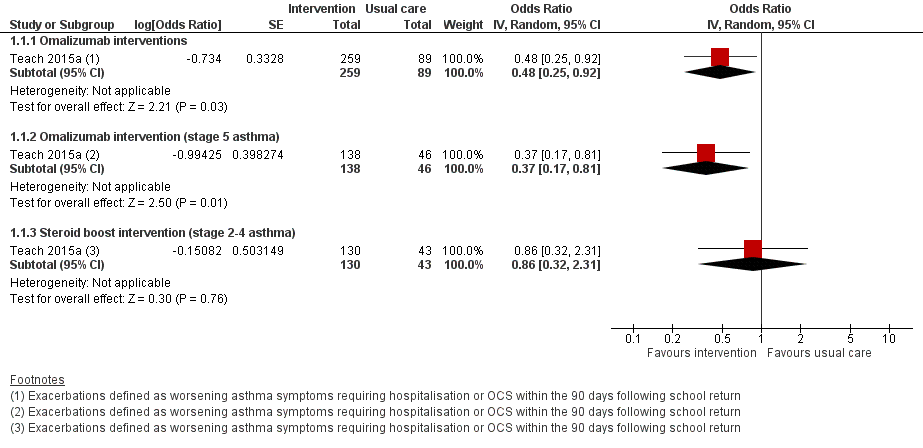
Forest plot of comparison: 1 Interventions for autumn exacerbations of asthma versus usual care, outcome: 1.1 Exacerbations defined according to the review's primary outcome.

Forest plot of comparison: 1 Interventions for autumn exacerbations of asthma versus usual care, outcome: 1.2 Exacerbations defined according to study‐specific definitions.

Forest plot of comparison: 1 Interventions for autumn exacerbations of asthma versus usual care, outcome: 1.3 Adverse effects.

Comparison 1 Interventions for autumn exacerbations of asthma versus usual care, Outcome 1 Exacerbations defined according to the review's primary outcome.

Comparison 1 Interventions for autumn exacerbations of asthma versus usual care, Outcome 2 Exacerbations defined according to study‐specific definitions.

Comparison 1 Interventions for autumn exacerbations of asthma versus usual care, Outcome 3 Adverse effects.
| Omalizumab compared to usual care for autumn asthma exacerbations in children | ||||||
| Patient or population: autumn asthma exacerbations in children Setting: community | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with usual care | Risk with omalizumab | |||||
| Exacerbations | 210 per 1000 | 113 per 1000 (62 to 197) | OR 0.48 (0.25 to 0.92) | 348 (1 RCT) | ⊕⊕⊕⊝ | Absolute effects calculated using control risk of 21.0% from Teach 2015a. |
| Exacerbations | 326 per 1000 | 152 per 1000 | OR 0.37 | 184 | ⊕⊕⊕⊝ | Absolute effects calculated using control risk of 32.6% from Teach 2015a. |
| Exacerbations | 127 per 1000 | 83 per 1000 | OR 0.63 | 164 | ⊕⊕⊕⊝ | Absolute effects calculated using control risk of 12.7% from Teach 2015a. |
| Adverse events | 548 per 1000 | 546 per 1000 | OR 0.99 | 361 | ⊕⊕⊕⊝ | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded once for imprecision because few children studied. | ||||||
| A boost of inhaled corticosteroids compared to usual care for autumn asthma exacerbations in children | ||||||
| Patient or population: autumn asthma exacerbations in children Setting: community | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with usual care | Risk with a boost of inhaled corticosteroids | |||||
| Exacerbations | 127 per 1000 | 111 per 1000 | OR 0.86 | 173 | ⊕⊕⊕⊝ | Absolute effects calculated using control risk of 12.7% from Teach 2015a. |
| Adverse events | 533 per 1000 | 434 per 1000 | OR 0.67 | 176 | ⊕⊕⊕⊝ | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded once for imprecision because few children studied. | ||||||
| Leukotriene receptor antagonist (LTRA) compared to usual care for autumn asthma exacerbations in children | ||||||
| Patient or population: autumn asthma exacerbations in children Setting: community | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with usual care | Risk with montelukast | |||||
| Exacerbations assessed with: oral corticosteroid or hospitalisation | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| Exacerbations | 146 per 1000 | 79 per 1000 | OR 0.50 | 1326 | ⊕⊕⊝⊝ | Absolute effects calculated using control risk of 14.6% from Johnston 2007. |
| Adverse events | 328 per 1000 | 307 per 1000 | OR 0.91 | 1326 | ⊕⊕⊕⊕ | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded once for inconsistency because asthma severity of children differed between included studies, and thresholds for medical contact or oral steroids appeared to differ between studies. | ||||||
| Behavioural intervention compared to usual care for autumn asthma exacerbations in children | ||||||
| Patient or population: autumn asthma exacerbations in children | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with usual care | Risk with behavioural intervention | |||||
| Exacerbations | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| Exacerbations | 167 per 1000 | 185 per 1000 | OR 1.13 | 10,481 | ⊕⊕⊕⊝ | Absolute effects calculated using control rate of 16.7% from Julious 2016. |
| Adverse events | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded once for indirectness because studies contained no data on hospitalisation and need for oral steroids, so unscheduled contacts for a respiratory diagnosis used as a proxy outcome. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Exacerbations defined according to the review's primary outcome Show forest plot | 1 | Odds Ratio (Random, 95% CI) | Subtotals only | |
| 1.1 Omalizumab interventions | 1 | 348 | Odds Ratio (Random, 95% CI) | 0.48 [0.25, 0.92] |
| 1.2 Omalizumab intervention (stage 5 asthma) | 1 | 184 | Odds Ratio (Random, 95% CI) | 0.37 [0.17, 0.81] |
| 1.3 Steroid boost intervention (stage 2‐4 asthma) | 1 | 173 | Odds Ratio (Random, 95% CI) | 0.86 [0.32, 2.31] |
| 2 Exacerbations defined according to study‐specific definitions Show forest plot | 4 | Odds Ratio (Random, 95% CI) | Subtotals only | |
| 2.1 Montelukast interventions | 2 | 1192 | Odds Ratio (Random, 95% CI) | 0.50 [0.17, 1.46] |
| 2.2 Pranlukast intervention | 1 | 121 | Odds Ratio (Random, 95% CI) | 0.67 [0.16, 2.80] |
| 2.3 Behavioural intervention | 1 | 9118 | Odds Ratio (Random, 95% CI) | 1.13 [0.96, 1.34] |
| 3 Adverse effects Show forest plot | 3 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Omalizumab intervention (stage 2‐5 asthma) | 1 | 361 | Odds Ratio (M‐H, Random, 95% CI) | 0.99 [0.61, 1.58] |
| 3.2 Steroid boost intervention (stage 2‐4 asthma) | 1 | 176 | Odds Ratio (M‐H, Random, 95% CI) | 0.67 [0.34, 1.33] |
| 3.3 LTRA interventions | 2 | 1326 | Odds Ratio (M‐H, Random, 95% CI) | 0.91 [0.63, 1.32] |

