Intervenciones para las exacerbaciones de otoño del asma en niños
Información
- DOI:
- https://doi.org/10.1002/14651858.CD012393.pub2Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 08 marzo 2018see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Vías respiratorias
- Copyright:
-
- Copyright © 2018 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
KCP drafted the protocol.
KCP and MA identified studies for inclusion and extracted data from the included studies.
KCP performed the analyses and drafted the final review.
KMH extracted data from the included studies and resolved any disagreements between KCP and MA.
KCP, DK, and MA selected studies for inclusion in the review.
KCP, KMH, DK, and MA reviewed the protocol and the review for accuracy before submission.
Sources of support
Internal sources
-
The National Institute for Health Research (NIHR), through the Comprehensive Clinical Research Network and the NIHR Biomedical Research Centre at Great Ormond Street Hospital for Children NHS Foundation Trust and University College London, UK.
Employment (Katharine Pike)
External sources
-
NIHR CLAHRC North Thames, UK.
Katherine Harris is in receipt of funding from the NIHR CLAHRC North Thames for her PhD. Katherine Harris was supported by the National Institute for Health Research (NIHR) Collaboration for Leadership in Applied Health Research and Care (CLAHRC) North Thames at Bart’s Health NHS Trust. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR, or the Department of Health.
Declarations of interest
KCP: none known.
MA: none known.
DK: none known.
KMH: none known.
Acknowledgements
Our thanks to the NIHR Collaborative Leadership in Applied Health Research and Care (CLAHRC) for their continued support.
The Background and Methods sections of this review are based on a standard template used by Cochrane Airways.
This project was supported by the National Institute for Health Research (NIHR), via Cochrane Infrastructure funding to the Cochrane Airways Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, National Health Service (NHS), or the Department of Health.
Christian Osadnik was the Editor for this review and commented critically on the review.
Version history
| Published | Title | Stage | Authors | Version |
| 2018 Mar 08 | Interventions for autumn exacerbations of asthma in children | Review | Katharine C Pike, Melika Akhbari, Dylan Kneale, Katherine M Harris | |
| 2016 Oct 12 | Interventions for autumn exacerbations of asthma in children | Protocol | Katharine C Pike, Katherine M Harris, Dylan Kneale | |
Differences between protocol and review
Our original intention was to include randomised controlled trials, quasi‐randomised controlled trials, and observational studies. We believed observational trials presenting exacerbation data on a month‐by‐month basis might identify treatments or other potentially modifiable factors associated with a lessening of the autumn peak in asthma exacerbations. After conducting searches, we did not feel we could reliably identify all studies presenting these data since it was difficult to identify search terms to capture studies where seasonal differences were not the main focus. This review was therefore restricted to randomised controlled trials of interventions specifically designed to reduce asthma exacerbations in children after the return to school for the autumn term. The comparator was usual care since there are no established interventions for this problem. In a pragmatic change to our protocol due to the small number of studies returned, we decided not to restrict the review to school‐age children, since the autumn peak is less pronounced but still observed in preschool‐aged children, but does not occur appreciably in adults.
Unfortunately, due to the small number of studies identified and to differences in both interventions and outcomes, it was not possible to conduct subgroup or sensitivity analyses. We were also unable to assess any secondary outcomes except adverse events due to lack of data relating to these outcomes in the included trials. When pooling data from studies using a comparable intervention, we employed a Mantel‐Haenszel random‐effects model for the meta‐analysis of adverse effects, since these data were reported as absolute values in the included studies. We used an inverse variance model for the exacerbation outcome; however, as although odds ratios were reported or obtainable from study authors, the absolute number of children was not appropriate for use in Teach 2015a and Weiss 2010 studies, where the authors had adjusted for covariables in the odds ratio calculation.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
- *Disease Progression;
- *Seasons;
- Acetates [therapeutic use];
- Adrenal Cortex Hormones [*administration & dosage, adverse effects];
- Anti‐Allergic Agents [adverse effects, *therapeutic use];
- Anti‐Asthmatic Agents [adverse effects, *therapeutic use];
- Asthma [epidemiology, *prevention & control];
- Behavior Therapy;
- Chromones [therapeutic use];
- Cyclopropanes;
- Leukotriene Antagonists [adverse effects, *therapeutic use];
- Omalizumab [adverse effects, therapeutic use];
- Quinolines [therapeutic use];
- Randomized Controlled Trials as Topic;
- Sulfides;
Medical Subject Headings Check Words
Child; Humans;
PICO

Study flow diagram.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
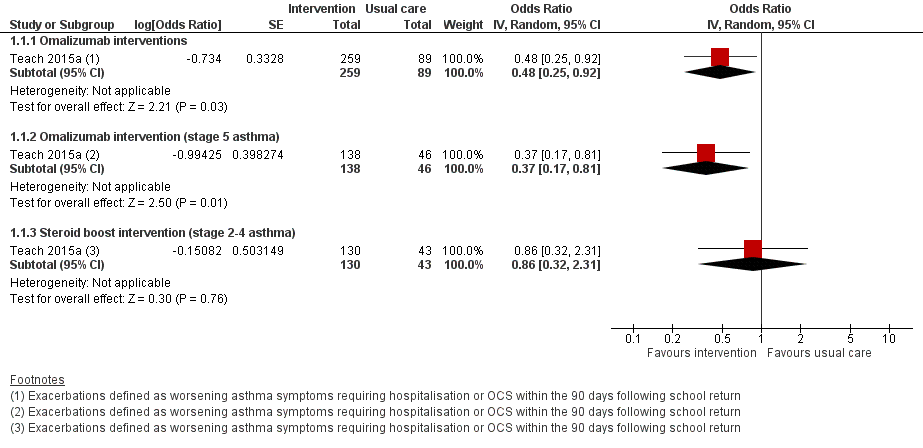
Forest plot of comparison: 1 Interventions for autumn exacerbations of asthma versus usual care, outcome: 1.1 Exacerbations defined according to the review's primary outcome.

Forest plot of comparison: 1 Interventions for autumn exacerbations of asthma versus usual care, outcome: 1.2 Exacerbations defined according to study‐specific definitions.

Forest plot of comparison: 1 Interventions for autumn exacerbations of asthma versus usual care, outcome: 1.3 Adverse effects.

Comparison 1 Interventions for autumn exacerbations of asthma versus usual care, Outcome 1 Exacerbations defined according to the review's primary outcome.

Comparison 1 Interventions for autumn exacerbations of asthma versus usual care, Outcome 2 Exacerbations defined according to study‐specific definitions.

Comparison 1 Interventions for autumn exacerbations of asthma versus usual care, Outcome 3 Adverse effects.
| Omalizumab compared to usual care for autumn asthma exacerbations in children | ||||||
| Patient or population: autumn asthma exacerbations in children Setting: community | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with usual care | Risk with omalizumab | |||||
| Exacerbations | 210 per 1000 | 113 per 1000 (62 to 197) | OR 0.48 (0.25 to 0.92) | 348 (1 RCT) | ⊕⊕⊕⊝ | Absolute effects calculated using control risk of 21.0% from Teach 2015a. |
| Exacerbations | 326 per 1000 | 152 per 1000 | OR 0.37 | 184 | ⊕⊕⊕⊝ | Absolute effects calculated using control risk of 32.6% from Teach 2015a. |
| Exacerbations | 127 per 1000 | 83 per 1000 | OR 0.63 | 164 | ⊕⊕⊕⊝ | Absolute effects calculated using control risk of 12.7% from Teach 2015a. |
| Adverse events | 548 per 1000 | 546 per 1000 | OR 0.99 | 361 | ⊕⊕⊕⊝ | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded once for imprecision because few children studied. | ||||||
| A boost of inhaled corticosteroids compared to usual care for autumn asthma exacerbations in children | ||||||
| Patient or population: autumn asthma exacerbations in children Setting: community | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with usual care | Risk with a boost of inhaled corticosteroids | |||||
| Exacerbations | 127 per 1000 | 111 per 1000 | OR 0.86 | 173 | ⊕⊕⊕⊝ | Absolute effects calculated using control risk of 12.7% from Teach 2015a. |
| Adverse events | 533 per 1000 | 434 per 1000 | OR 0.67 | 176 | ⊕⊕⊕⊝ | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded once for imprecision because few children studied. | ||||||
| Leukotriene receptor antagonist (LTRA) compared to usual care for autumn asthma exacerbations in children | ||||||
| Patient or population: autumn asthma exacerbations in children Setting: community | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with usual care | Risk with montelukast | |||||
| Exacerbations assessed with: oral corticosteroid or hospitalisation | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| Exacerbations | 146 per 1000 | 79 per 1000 | OR 0.50 | 1326 | ⊕⊕⊝⊝ | Absolute effects calculated using control risk of 14.6% from Johnston 2007. |
| Adverse events | 328 per 1000 | 307 per 1000 | OR 0.91 | 1326 | ⊕⊕⊕⊕ | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded once for inconsistency because asthma severity of children differed between included studies, and thresholds for medical contact or oral steroids appeared to differ between studies. | ||||||
| Behavioural intervention compared to usual care for autumn asthma exacerbations in children | ||||||
| Patient or population: autumn asthma exacerbations in children | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with usual care | Risk with behavioural intervention | |||||
| Exacerbations | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| Exacerbations | 167 per 1000 | 185 per 1000 | OR 1.13 | 10,481 | ⊕⊕⊕⊝ | Absolute effects calculated using control rate of 16.7% from Julious 2016. |
| Adverse events | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded once for indirectness because studies contained no data on hospitalisation and need for oral steroids, so unscheduled contacts for a respiratory diagnosis used as a proxy outcome. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Exacerbations defined according to the review's primary outcome Show forest plot | 1 | Odds Ratio (Random, 95% CI) | Subtotals only | |
| 1.1 Omalizumab interventions | 1 | 348 | Odds Ratio (Random, 95% CI) | 0.48 [0.25, 0.92] |
| 1.2 Omalizumab intervention (stage 5 asthma) | 1 | 184 | Odds Ratio (Random, 95% CI) | 0.37 [0.17, 0.81] |
| 1.3 Steroid boost intervention (stage 2‐4 asthma) | 1 | 173 | Odds Ratio (Random, 95% CI) | 0.86 [0.32, 2.31] |
| 2 Exacerbations defined according to study‐specific definitions Show forest plot | 4 | Odds Ratio (Random, 95% CI) | Subtotals only | |
| 2.1 Montelukast interventions | 2 | 1192 | Odds Ratio (Random, 95% CI) | 0.50 [0.17, 1.46] |
| 2.2 Pranlukast intervention | 1 | 121 | Odds Ratio (Random, 95% CI) | 0.67 [0.16, 2.80] |
| 2.3 Behavioural intervention | 1 | 9118 | Odds Ratio (Random, 95% CI) | 1.13 [0.96, 1.34] |
| 3 Adverse effects Show forest plot | 3 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Omalizumab intervention (stage 2‐5 asthma) | 1 | 361 | Odds Ratio (M‐H, Random, 95% CI) | 0.99 [0.61, 1.58] |
| 3.2 Steroid boost intervention (stage 2‐4 asthma) | 1 | 176 | Odds Ratio (M‐H, Random, 95% CI) | 0.67 [0.34, 1.33] |
| 3.3 LTRA interventions | 2 | 1326 | Odds Ratio (M‐H, Random, 95% CI) | 0.91 [0.63, 1.32] |

