Gabapentina para el dolor de la fibromialgia en adultos
Referencias
References to studies included in this review
References to studies awaiting assessment
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Randomised: yes Controlled: placebo controlled Blinding: double‐blind Design: multi centre, parallel groups, LOCF analysis Study dates and duration: September 2003 to January 2006 Participants were seen weekly for the first 2 weeks of treatment; thereafter, study visits were scheduled at 2‐week intervals. Participants then entered a 1‐week tapering phase. | |
| Participants | Inclusion criteria: female and male, 18 years and over, FM patients meeting ACR criteria for FM (1990), score ≥ 4 on BPI scale at screening and randomisation Exclusion Criteria: Other rheumatic or medical disorders that contributed to the symptoms of FM; pain from traumatic injury or structural or regional rheumatic disease; rheumatoid arthritis, inflammatory arthritis, or autoimmune disease; unstable medical or psychiatric illness; lifetime history of psychosis, hypomania or mania, epilepsy, or dementia; substance abuse in the last 6 months; serious risk of suicide; pregnancy or breastfeeding; unacceptable contraception in those of childbearing potential; patients who, in the opinion of the investigator, were treatment refractory; previous treatment with gabapentin or pregabalin; and treatment with an investigational drug within 30 days of screening. Concomitant medication exclusions consisted of medications or herbal agents with CNS effects, with the exception of episodic use of sedating antihistamines (antidepressants required a 14‐day washout period, or 30 days for fluoxetine); analgesics, with the exception of paracetamol or OTC NSAIDs; and unconventional or alternative therapies. N = 150 Gender: F (135) 90%, M (15) 10% Age: intervention: 49.2 ± 10.6 years; control: 47.3 ± 11.8 years Number randomised: 75 intervention, 75 control Number completed: 57 intervention, 62 control Setting: 3 outpatient centres, USA | |
| Interventions | Duration of treatment: 12 weeks + 1 week taper Follow‐up period: unstated Treatment group (n = 75): gabapentin 1200 to 2400 mg/day in 3 doses; titration to limit of tolerability or maximum 2400 mg daily over 6 weeks, then 6 weeks at stable dose (12 weeks in total) “300 mg once a day at bedtime for 1 week, 300 mg twice a day for 1 week, 300 mg twice a day and 600 mg once a day at bedtime for 2 weeks, 600 mg 3 times a day for 2 weeks, and 600 mg twice a day and 1,200 mg once a day at bedtime (2,400 mg/day) for the remainder of the study beginning at week 6. If a patient could not tolerate 2,400 mg/day, the dosage was reduced to a minimum of 1,200 mg/day, administered 3 times a day. The study medication dose was stable for at least the last 4 weeks of the therapy phase. During the tapering phase, the dosage was decreased by 300 mg/day until discontinuation.” Control group (n = 75): placebo Standard treatments to all groups: paracetamol and OTC NSAIDs allowed (no dose limit stated) Co‐interventions: none mentioned | |
| Outcomes | Primary Outcomes
Secondary Outcomes
| |
| Notes | Oxford Quality Score: R = 1, DB = 2, W = 1, Total = 4 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: Method of randomisation not given |
| Allocation concealment (selection bias) | Unclear risk | Comment: Method of allocation concealment not given |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: “matching placebo” |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: “Self‐reported or self‐administered” Comment: No clear mention of who provides and prepares drugs or who monitors the weekly visits, and whether they are blinded or not |
| Incomplete outcome data (attrition bias) | High risk | Comment: LOCF imputation; more adverse event withdrawals with active |
| Selective reporting (reporting bias) | Low risk | Comment: All primary and secondary outcomes were reported upon, with clear data provided. |
| Size | Unclear risk | Comment: 75 participants per treatment arm |
| Other bias | Low risk | None known |
ACR: American College of Rheumatology; BPI: Brief Pain Inventory; CNS: central nervous system; DB: double‐blind; FM: fibromyalgia; LOCF: last observation carried forward; MOS: Medical Outcomes Study; N: number of participants in study; n: number of participants in treatment arm; NSAID: nonsteroidal anti‐inflammatory drug; OTC: over‐the‐counter; R: randomised; W: withdrawals.
Characteristics of studies awaiting assessment [ordered by study ID]
Ir a:
| Methods | Randomised: yes Controlled: active comparator Blinding: not stated Design: multicentre, parallel groups Study dates and duration: January 2008 to May 2011 |
| Participants | Inclusion criteria: patients meeting ACR criteria for the diagnosis of fibromyalgia and presenting to the department Exclusion criteria: patients with other painful disorders N = 68 Age: unknown Gender: unknown Number randomised: unknown Number completed: unknown Setting: Orthepaedic Kythira General Hospital, Potamos and Chios General Hospital, Chios, Greece |
| Interventions | Duration of treatment: 2 months Follow‐up period: 2 years Treatment group (n = unknown): 1200 mg oral gabapentin, daily for 2 months Control group (n = unknown): 60 mg oral duloxetine, daily for 2 months Additional analgesia/standard treatments to all groups: unknown Co‐interventions: unknown |
| Outcomes | Primary Outcomes
Secondary Outcomes
|
| Notes |
ACR: American College of Rheumatology; MOS: Medical Outcomes Study; N: number of participants in study; n: number of participants in treatment arm; VAS: visual analogue scale
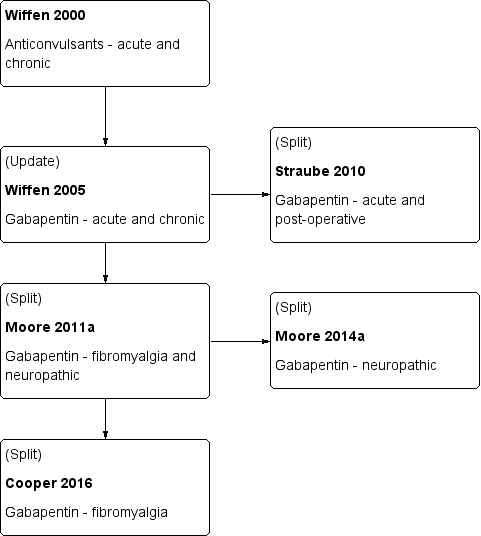
History of Earlier Reviews
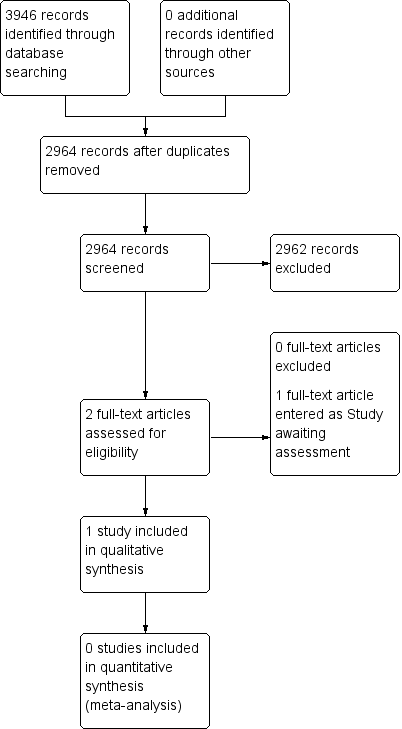
Study flow diagram.
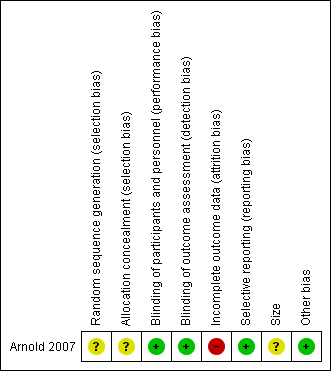
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
| Gabapentin compared with placebo for fibromyalgia | ||||||
| Patient or population: adults with fibromyalgia Settings: community Intervention: gabapentin Comparison: placebo | ||||||
| Outcomes | Assumed risk ‐ probable outcome with intervention | Corresponding risk ‐ probable outcome with control | Relative effect | Number of | Quality of the evidence | Comments |
| gabapentin | placebo | |||||
| 30% pain reduction at 12 weeks | 38/75 | 23/75 | Not calculated | 1 study, 150 participants | very low | One included study of fewer than 200 participants; LOCF imputation. Downgraded three levels because of small numbers and study limitations |
| 50% pain reduction at 12 weeks | No data | No data | ‐ | ‐ | very low | Outcome not reported |
| PGIC ‐ any category of "better" at 12 weeks | 68/75 | 35/75 | Not calculated | 1 study, 150 participants | very low | One included study of fewer than 200 participants; LOCF imputation; non‐standard outcome ‐ usually top two categories of better, not top three, used Downgraded three levels because of small numbers and study limitations |
| Withdrawals due to adverse events | 12/75 | 7/75 | Not calculated | 1 study, 150 participants | very low | One included study of fewer than 200 participants; few events Downgraded three levels because of small numbers |
| Serious adverse events | "No significant group differences" | ‐ | 1 study, 150 participants | very low | ‐ | |
| Deaths | None reported | ‐ | 1 study, 150 participants | very low | ‐ | |
| CI: Confidence interval; LOCF: last observation carried forward; PGIC: Patient Global Impression of Change | ||||||
| Descriptors for levels of evidence (EPOC 2015): † Substantially different: a large enough difference that it might affect a decision. | ||||||

