Gabapentina para el dolor de la fibromialgia en adultos
Información
- DOI:
- https://doi.org/10.1002/14651858.CD012188.pub2Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 03 enero 2017see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Dolor y cuidados paliativos
- Copyright:
-
- Copyright © 2018 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
PW registered the title.
TC, RAM, SD, and PW wrote the protocol.
TC and PW performed screening and data extraction.
All authors were involved in writing the full review.
PW will be responsible for updates in the future.
Sources of support
Internal sources
-
Oxford Pain Relief Trust, UK.
General institutional support
External sources
-
The National Institute for Health Research (NIHR), UK.
NIHR Cochrane Programme Grant: 13/89/29 ‐ Addressing the unmet need of chronic pain: providing the evidence for treatments of pain
Declarations of interest
TC: none known.
SD: none known.
PW: none known.
RAM has received grant support from Grünenthal relating to individual patient level analyses of trial data regarding tapentadol in osteoarthritis and back pain (2015). He has received honoraria for attending boards with Menarini concerning methods of analgesic trial design (2014), with Novartis (2014) about the design of network meta‐analyses, and RB on understanding pharmacokinetics of drug uptake (2015). He has received honoraria from Omega Pharma (2016) and Futura Pharma (2016) for providing advice on trial and data analysis methods.
Acknowledgements
The Oxford Pain Relief Trust provided institutional support.
Cochrane Review Group funding acknowledgement: the National Institute for Health Research (NIHR) is the largest single funder of the Cochrane Pain, Palliative and Supportive Care (PaPaS) Review Group. Disclaimer: the views and opinions expressed therein are those of the authors and do not necessarily reflect those of the NIHR, National Health Service (NHS) or the Department of Health.
The protocol followed the agreed template for fibromyalgia, which we developed in collaboration with the Cochrane Musculoskeletal Group and Cochrane Neuromuscular Diseases (NMD) Group. The Cochrane PaPaS Group managed the editorial process.
We thank the peer reviewer Mike Lunn, Joint Co‐ordinating Editor of the Cochrane NMD Group, for his useful comments on the protocol.
Version history
| Published | Title | Stage | Authors | Version |
| 2017 Jan 03 | Gabapentin for fibromyalgia pain in adults | Review | Tess E Cooper, Sheena Derry, Philip J Wiffen, R Andrew Moore | |
| 2016 May 11 | Gabapentin for fibromyalgia pain in adults | Protocol | Tess E Cooper, R Andrew Moore, Sheena Derry, Philip J Wiffen | |
Differences between protocol and review
We have extended the description of the GRADE assessment for exceptional circumstances to explain possible decisions. We have also removed one secondary outcome (any disability‐related or mental health‐related outcome) because, on reflection, this is not usually reported in trials.
Notes
A restricted search in March 2018 did not identify any potentially relevant studies likely to change the conclusions. The authors and editors are confident that further research will not change the conclusions. Therefore, this review has now been stabilised following discussion with the authors and editors. If appropriate, we will update the review if new evidence likely to change the conclusions is published, or if standards change substantially which necessitate major revisions.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
Medical Subject Headings Check Words
Adult; Female; Humans; Male; Middle Aged;
PICO
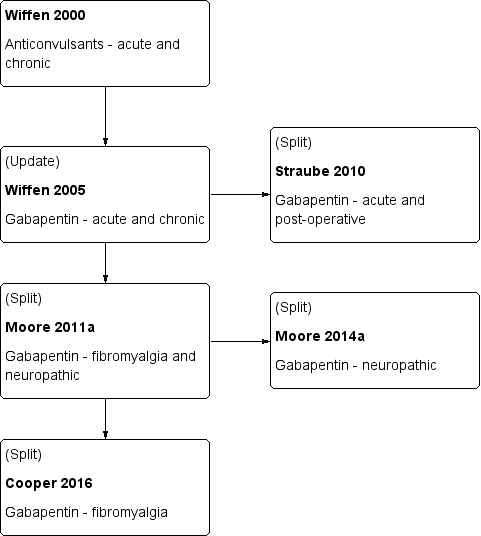
History of Earlier Reviews
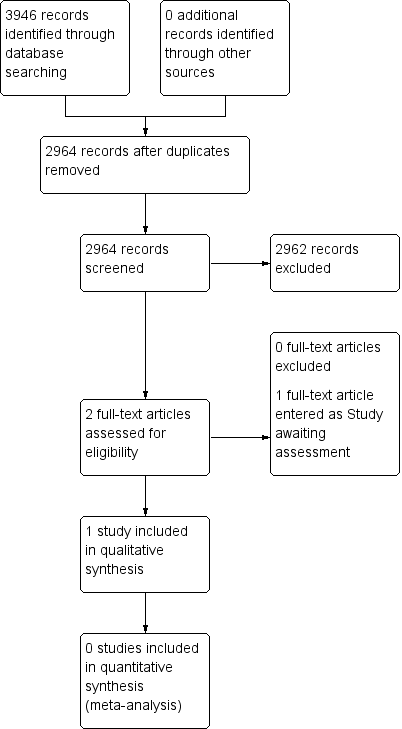
Study flow diagram.
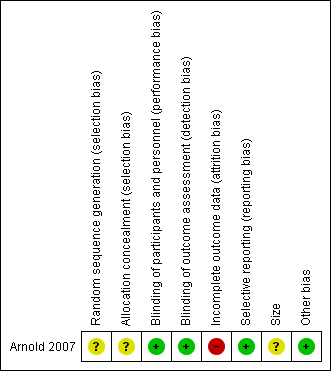
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
| Gabapentin compared with placebo for fibromyalgia | ||||||
| Patient or population: adults with fibromyalgia Settings: community Intervention: gabapentin Comparison: placebo | ||||||
| Outcomes | Assumed risk ‐ probable outcome with intervention | Corresponding risk ‐ probable outcome with control | Relative effect | Number of | Quality of the evidence | Comments |
| gabapentin | placebo | |||||
| 30% pain reduction at 12 weeks | 38/75 | 23/75 | Not calculated | 1 study, 150 participants | very low | One included study of fewer than 200 participants; LOCF imputation. Downgraded three levels because of small numbers and study limitations |
| 50% pain reduction at 12 weeks | No data | No data | ‐ | ‐ | very low | Outcome not reported |
| PGIC ‐ any category of "better" at 12 weeks | 68/75 | 35/75 | Not calculated | 1 study, 150 participants | very low | One included study of fewer than 200 participants; LOCF imputation; non‐standard outcome ‐ usually top two categories of better, not top three, used Downgraded three levels because of small numbers and study limitations |
| Withdrawals due to adverse events | 12/75 | 7/75 | Not calculated | 1 study, 150 participants | very low | One included study of fewer than 200 participants; few events Downgraded three levels because of small numbers |
| Serious adverse events | "No significant group differences" | ‐ | 1 study, 150 participants | very low | ‐ | |
| Deaths | None reported | ‐ | 1 study, 150 participants | very low | ‐ | |
| CI: Confidence interval; LOCF: last observation carried forward; PGIC: Patient Global Impression of Change | ||||||
| Descriptors for levels of evidence (EPOC 2015): † Substantially different: a large enough difference that it might affect a decision. | ||||||

