Gabapentina para el dolor de la fibromialgia en adultos
Resumen
Antecedentes
Esta revisión reemplaza parte de una revisión anterior que evaluó la gabapentina para el dolor neuropático y la fibromialgia, ahora dividida en revisiones separadas para las dos afecciones. Esta revisión sólo considerará el dolor en la fibromialgia.
La fibromialgia se asocia con dolor generalizado, que dura más de tres meses y es acompañado frecuentemente por síntomas como sueño deficiente, fatiga, depresión y menor calidad de vida. La fibromialgia es más frecuente en mujeres.
La gabapentina es un fármaco antiepiléptico autorizado ampliamente para el tratamiento del dolor neuropático. No está autorizado para el tratamiento de la fibromialgia, pero se usa con frecuencia porque la afección puede responder a los mismos medicamentos que el dolor neuropático.
Objetivos
Evaluar la eficacia analgésica de la gabapentina para el dolor de la fibromialgia en adultos y los eventos adversos asociados con su uso en ensayos clínicos.
Métodos de búsqueda
Se hicieron búsquedas en el Registro Cochrane Central de Ensayos Controlados (Cochrane Central Register of Controlled Trials) (CENTRAL) vía the Cochrane Register of Studies Online, MEDLINE vía Ovid y Embase vía Ovid desde su inicio hasta 24 mayo 2016. También se realizaron búsquedas en las listas de referencias de estudios y revisiones recuperados, y se realizaron búsquedas en registros de ensayos clínicos en línea.
Criterios de selección
Ensayos aleatorios, doble ciego, de ocho semanas o más de duración, del tratamiento del dolor de la fibromialgia en adultos, que compararon la gabapentina con placebo o un comparador activo.
Obtención y análisis de los datos
Dos autores de la revisión independientes extrajeron los datos y evaluaron la calidad de los ensayos y el riesgo de sesgo. Se planificó usar datos dicotómicos para calcular el cociente de riesgos y el número necesario a tratar para un evento adicional, con el uso de métodos estándar. La evidencia se evaluó mediante GRADE (Grading of Recommendations Assessment, Development and Evaluation) y se creó una tabla "Resumen de los hallazgos".
Resultados principales
Dos estudios analizaron la gabapentina para el tratamiento del dolor de la fibromialgia. Uno se identificó en versiones anteriores de la revisión y se incluye aquí. Se identificó otro estudio como un resumen de una conferencia, con detalles insuficientes para determinar su elegibilidad para la inclusión;está a la espera de evaluación. El estudio incluido de 150 participantes fue un estudio de grupos paralelos, controlado con placebo, doble ciego, aleatorio y multicéntrico, de 12 semanas, que usó la imputación de la última observación transferida (LOCF, sigla en inglés) para los retiros. La dosis máxima fue de 2400 mg diarios. El riesgo de sesgo general fue bajo, excepto por el sesgo de deserción.
Al final del ensayo, no se informó el resultado de la reducción de un 50% del dolor sobre el valor inicial. El resultado de una reducción de un 30% o mayor del dolor sobre el valor inicial fue logrado por 38/75 pacientes (49%) con gabapentina en comparación con 23/75 (31%) con placebo (muy baja calidad). La impresión global del paciente de cambio en cualquier categoría de "mejor" fue lograda por 68/75 (91%) con gabapentina y 35/75 (47%) con placebo (muy baja calidad).
Diecinueve participantes interrumpieron el estudio debido a los eventos adversos: 12 en el grupo de gabapentina (16%) y siete en el grupo de placebo (9%) (muy baja calidad). No se informó el número de eventos adversos graves ni las muertes (muy baja calidad).
Conclusiones de los autores
Sólo se cuenta con pruebas de calidad muy baja, y no hay mucha certeza acerca de los cálculos de los efectos beneficiosos y perjudiciales debido a la cantidad reducida de datos de un único ensayo. No existen pruebas suficientes para apoyar ni refutar la sugerencia de que la gabapentina alivia el dolor de la fibromialgia.
PICOs
Resumen en términos sencillos
Gabapentina para el dolor en adultos con fibromialgia
Conclusión
No existen pruebas convincentes para apoyar ni contradecir la sugerencia de que la gabapentina en dosis diarias de 1200 a 2400 mg alivia el dolor de la fibromialgia.
Antecedentes
La fibromialgia es un trastorno complejo caracterizado por dolor generalizado, fatiga, sueño deficiente, decaimiento y otros síntomas físicos. En general, los fármacos habituales para aliviar el dolor, como el paracetamol y el ibuprofeno no se consideran efectivos. Los fármacos antiepilépticos se usan comúnmente para tratar la fibromialgia pero hay incertidumbre acerca de su efectividad.
La gabapentina es un medicamento utilizado para tratar el dolor causado por nervios que no funcionan de manera adecuada. La gabapentina cambia la manera en que los nervios envían señales al cerebro. Puede tomarse en forma de comprimido o líquido, con o sin alimentos. La dosis habitual es de 1200 mg a 2400 mg por día. Al comienzo del tratamiento se usan dosis bajas para disminuir los efectos secundarios, y luego se aumenta gradualmente después de unas semanas.
Características de los estudios
En mayo de 2016 se buscaron ensayos clínicos en que la gabapentina se usó para tratar el dolor por fibromialgia en adultos. Se encontró un estudio que cumplía con los requisitos para esta revisión. El estudio examinó la gabapentina en dosis de 1200 a 2400 mg/día en comparación con un placebo, por 12 semanas, en 150 pacientes.
Resultados clave
El estudio no informó el número de pacientes con una reducción del dolor a la mitad al finalizar la semana 12. En ese momento, el dolor se redujo al menos en un tercio en cinco de cada 10 pacientes que tomaban la gabapentina y tres de cada 10 que tomaban el placebo. Nueve de cada 10 pacientes que tomaban gabapentina y cinco de cada 10 que tomaban placebo presentaron un informe de mejoría en cualquier grado.
Cerca de dos de cada 10 pacientes que tomaban la gabapentina dejaron de tomarla por los efectos secundarios, en comparación con uno de cada 10 que tomaban el placebo. El estudio no informó el número de pacientes con efectos secundarios graves, pero informó que no se registraron muertes.
Calidad de la evidencia
Se calificó la calidad de las pruebas como muy baja porque había sólo un único estudio pequeño con limitaciones considerables. Varios factores redujeron la confianza en el resultado. La evidencia de calidad muy baja significa que existe una menor seguridad en los resultados.
Authors' conclusions
Summary of findings
| Gabapentin compared with placebo for fibromyalgia | ||||||
| Patient or population: adults with fibromyalgia Settings: community Intervention: gabapentin Comparison: placebo | ||||||
| Outcomes | Assumed risk ‐ probable outcome with intervention | Corresponding risk ‐ probable outcome with control | Relative effect | Number of | Quality of the evidence | Comments |
| gabapentin | placebo | |||||
| 30% pain reduction at 12 weeks | 38/75 | 23/75 | Not calculated | 1 study, 150 participants | very low | One included study of fewer than 200 participants; LOCF imputation. Downgraded three levels because of small numbers and study limitations |
| 50% pain reduction at 12 weeks | No data | No data | ‐ | ‐ | very low | Outcome not reported |
| PGIC ‐ any category of "better" at 12 weeks | 68/75 | 35/75 | Not calculated | 1 study, 150 participants | very low | One included study of fewer than 200 participants; LOCF imputation; non‐standard outcome ‐ usually top two categories of better, not top three, used Downgraded three levels because of small numbers and study limitations |
| Withdrawals due to adverse events | 12/75 | 7/75 | Not calculated | 1 study, 150 participants | very low | One included study of fewer than 200 participants; few events Downgraded three levels because of small numbers |
| Serious adverse events | "No significant group differences" | ‐ | 1 study, 150 participants | very low | ‐ | |
| Deaths | None reported | ‐ | 1 study, 150 participants | very low | ‐ | |
| CI: Confidence interval; LOCF: last observation carried forward; PGIC: Patient Global Impression of Change | ||||||
| Descriptors for levels of evidence (EPOC 2015): † Substantially different: a large enough difference that it might affect a decision. | ||||||
Background
This Cochrane review is based on a template for reviews of drugs used to relieve fibromyalgia. The aim is for all reviews to use the same methods, based on new criteria for what constitutes reliable evidence in chronic pain (Moore 2010a; Appendix 1).
This Cochrane review has been split from an earlier review on fibromyalgia and neuropathic pain (Moore 2011a), and will consider only fibromyalgia, due to the pathogenesis of the pain being different from neuropathic pain. The most recent version of the review is being amended and updated to focus solely on neuropathic pain (Moore 2014a).
The history of earlier versions of this Cochrane review is available in Figure 1 and Appendix 2.

History of Earlier Reviews
Description of the condition
Fibromyalgia symptoms can be assessed by patient self‐report using the fibromyalgia criteria and severity scales for clinical and epidemiological studies, a modification of the American College of Rheumatology (ACR) Preliminary Diagnostic Criteria for Fibromyalgia (so‐called Fibromyalgia Symptom Questionnaire; Wolfe 2011). Fibromyalgia was previously defined by the ACR 1990 classification criteria as widespread pain lasting for longer than three months with tenderness on palpation at 11 or more of 18 specified tender points (Wolfe 1990). For a clinical diagnosis, the ACR 1990 classification criteria and the ACR 2010 preliminary diagnostic criteria can both be used (Wolfe 1990 and Wolfe 2010 respectively). As there is no specific laboratory test, diagnosis is established by obtaining a history of the key symptoms and the exclusion of somatic diseases sufficiently explaining the key symptoms (Wolfe 2010). The indexing of fibromyalgia within the international classification of diseases is under debate. While some rheumatologists have thought of it as a specific pain disorder and central sensitivity syndrome (Clauw 2014; Yunus 2008), recent research points at small fibre pathology in a subgroup of fibromyalgia patients that may be of pathophysiological importance (Oaklander 2013; Üçeyler 2013a). In psychiatry and psychosomatic medicine, fibromyalgia symptoms are categorised as a functional somatic syndrome, a bodily distress syndrome, a somatic physical symptom disorder, or a somatoform disorder (Häuser 2014).
Fibromyalgia is a heterogenous condition. The definite aetiology (causes) of this syndrome remains unknown. A model of interacting biological and psychosocial variables in the predisposition, triggering, and development of the chronicity of fibromyalgia symptoms has been suggested (Sommer 2012). Depression (Forseth 1999), genetics (Arnold 2013; Lee 2012), obesity combined with physical inactivity (Mork 2010), physical and sexual abuse in childhood (Häuser 2011), sleep problems (Mork 2010), and smoking (Choi 2010) are associated with future development of fibromyalgia. Psychosocial stress (working place and family conflicts) and physical stress (infections, surgery, accidents) might trigger the onset of chronic widespread pain and fatigue (Clauw 2014; Sommer 2012). Depression and post‐traumatic stress disorder worsen fibromyalgia symptoms (Häuser 2013a; Lange 2010).
Several factors are associated with the pathophysiology (functional changes associated with or resulting from disease) of fibromyalgia, but the relationship is unclear. The functional changes include alteration of sensory processing in the brain, reduced reactivity of the hypothalamus‐pituitary‐adrenal axis to stress, increased pro‐inflammatory and reduced anti‐inflammatory cytokine profiles (produced by cells involved in inflammation), and disturbances in neurotransmitters such as dopamine and serotonin (Oaklander 2013; Sommer 2012; Üçeyler 2013a). Prolonged exposure to stress, as outlined above, may contribute to these functional changes in predisposed individuals (Bradley 2009). There are similarities to, and differences from, neuropathic pain (Koroschetz 2011).
Patients often report high disability levels and poor quality of life along with extensive use of medical care (Häuser 2015). Many people with fibromyalgia are significantly disabled, and experience moderate or severe pain for many years (Bennett 2007). Chronic painful conditions comprised five of the 11 top‐ranking conditions for years lived with disability in 2010 (Vos 2012), and are responsible for considerable loss of quality of life, employment, and increased health costs (Moore 2014a).
Fibromyalgia is common. One component of fibromyalgia, chronic widespread pain, is not only associated with other symptoms such as poor sleep, fatigue, and depression (Wolfe 2014), but is also estimated to affect 11% of the general population (Mansfield 2016). Numerous studies have investigated prevalence of fibromyalgia in different settings and countries. One review gives a global mean prevalence of potential cases of fibromyalgia of 2.7% (range 0.4% to 9.3%), with a mean of 3.1% in the Americas, 2.5% in Europe, and 1.7% in Asia (Queiroz 2013). Changes in diagnostic criteria do not appear to have significantly affected estimates of prevalence (Wolfe 2013). A large US survey using a modification of the 2010 ACR criteria found a prevalence of 1.8%, but 73% of these patients were given a different diagnosis by their physician (Walitt 2015). Estimates of prevalence in specific populations vary greatly, but have been reported to be as high as 9% in female textile workers in Turkey and 10% in metalworkers in Brazil (59% in those with repetitive strain injury; Queiroz 2013). When the 1990 ACR criteria are used for clinical surveys, women are more frequently diagnosed with the disorder. Using these criteria, the women to men ratio has ranged from 8:1 to 30:1 in patients who were studied in clinical institutions and surveys. However, with criteria that do not use tender point examination, the sex ratio can be close to equal. The sex ratio has ranged from 4:1 to 1:1 in studies that were conducted in the general population using the research criteria for fibromyalgia (Häuser 2015; Queiroz 2013).
Fibromyalgia pain is known to be difficult to treat effectively, with only a minority of individuals experiencing a clinically relevant benefit from any intervention. A multidisciplinary approach is recommended by recent evidence‐based guidelines, with pharmacological treatment being combined with physical or cognitive training, or both. Interventions aim to reduce the key symptoms of fibromyalgia (pain, sleep problems, fatigue) and the associated symptoms (depression, disability) and to improve daily functioning (Eich 2012; Fitzcharles 2013). Conventional analgesics are usually not effective. Antidepressants such as serotonin and noradrenaline reuptake inhibitors (Häuser 2013b; Lunn 2014), tricyclic agents such as amitriptyline (Moore 2012a), or antiepileptics like gabapentin or pregabalin (Moore 2014b; Üçeyler 2013b; Wiffen 2013) are often offered as treatment. The proportion of patients who achieve worthwhile pain relief (typically at least 50% reduction in pain intensity) is small (Moore 2013b), and generally reaches only 10% to 25% more than with placebo, with number needed to treat for an additional beneficial outcome (NNT) between 9.8 and 14 in fibromyalgia (Wiffen 2013); somewhat higher (worse) than for neuropathic pain (Kalso 2013; Wiffen 2013). Those who do experience good levels of pain relief, however, also benefit from substantial reductions in other symptoms, such as fatigue, depression, and anxiety, with significant improvement in ability to function, sleep, work, and quality of life (Moore 2010c; Straube 2011). Fibromyalgia is not particularly different from other chronic pain with regard to a small proportion of trial participants having a good response to analgesic treatment (Moore 2013b).
Description of the intervention
Gabapentin, whilst licensed for the treatment of neuropathic pain in adults in many parts of the world, is not licensed for the treatment of fibromyalgia in any country. As fibromyalgia can respond to the same medicines as neuropathic pain, it has been used off‐license for the treatment of fibromyalgia. Pregabalin, which is closely related to gabapentin, is licensed for treatment of fibromyalgia in the USA.
Gabapentin is given orally, usually as tablets or capsules, but sometimes as an oral solution (50 mg/mL). Guidance suggests that gabapentin treatment can be started at a dose of 300 mg per day for treating neuropathic pain, which is replicated in its use for treating fibromyalgia pain. Based on individual patient response and tolerability, the dosage may be increased by 300 mg per day until the patient experiences satisfactory pain relief or adverse effects make taking the drug intolerable (EMC 2009). Gabapentin is marketed under various trade names, including NeurontinTM, and is also available as generic products in some parts of the world.
Gabapentin has a half‐life of five to seven hours. It is absorbed through a saturable transport system, so that absorption is not linear, and the transporter is found only in the proximal small bowel. This means that the drug needs to be administered at least three times daily, and may result in plasma trough levels. Two new formulations have attempted to improve the availability of the drug. The first is an extended release, gastro‐retentive formulation, designed to provide continuous delivery at the optimal site of absorption over 8 to 10 hours (Sang 2013). The second uses an extended‐release prodrug (gabapentin encarbil) that is absorbed through a high capacity transport system found throughout the intestine, and then undergoes rapid hydrolysis to gabapentin. It is claimed to provide sustained, dose‐proportional gabapentin exposure (Backonja 2011), and can be administered twice daily.
Gabapentin can also be formulated as an aqueous solution for injection. This formulation is not available commercially.
How the intervention might work
Gabapentin's mechanism of action is primarily attributed to its effect on calcium channels located throughout the peripheral and central nervous systems, which modify the release of neurotransmitters and reduce excitability of nerve cells (Boyle 2014; Chang 2014). This mode of action confers antiepileptic, analgesic, and sedative effects. Research also indicates that gabapentin acts by blocking new synapse formation (Eroglu 2009).
Why it is important to do this review
Gabapentin is used off‐license to treat fibromyalgia. The earlier Cochrane review considered fibromyalgia alongside neuropathic pain (Moore 2011a), but an editorial decision was made to split the review by condition, which necessitated a new review and update of the evidence.
Like the earlier Cochrane review, this new review assesses evidence in ways that make both statistical and clinical sense, and uses developing criteria for what constitutes reliable evidence in chronic pain (Appendix 1; Moore 2010a). We have followed the standards of the Cochrane Pain, Palliative and Supportive Care (PaPaS) Group, as set out in the Cochrane PaPaS Group Author and Referee Guidance for pain studies (PaPaS 2012).
Objectives
To assess the analgesic efficacy of gabapentin for fibromyalgia pain in adults and the adverse events associated with its use in clinical trials.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) with double‐blind assessment of outcomes reporting outcomes after eight weeks of treatment or longer. We required full journal publication, with the exception of extended abstracts with sufficient data for analysis and online clinical trial results summaries of otherwise unpublished clinical trials. We excluded short abstracts (usually meeting reports), non‐randomised studies, studies of experimental pain, case reports, and clinical observations.
Types of participants
Studies included participants aged 18 years and above, with fibromyalgia diagnosed using the 1990 or 2010 criteria (Wolfe 1990; Wolfe 2010), and with initial pain of at least moderate intensity.
Types of interventions
Gabapentin at any dose, by any route, administered for the relief of pain in fibromyalgia, and compared to placebo or any active comparator. We excluded studies that used gabapentin to treat pain resulting from the use of other drugs.
Types of outcome measures
We anticipated that studies would use a variety of outcome measures, with most studies using standard subjective scales (numerical rating scale (NRS) or visual analogue scale (VAS)) for pain intensity or pain relief, or both. We were particularly interested in Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials (IMMPACT) definitions for moderate and substantial benefit in chronic pain studies (Dworkin 2008). These were defined as:
-
at least 30% pain relief over baseline (moderate);
-
at least 50% pain relief over baseline (substantial);
-
much or very much improved on Patient Global Impression of Change (PGIC; moderate);
-
very much improved on PGIC (substantial).
These outcomes differ from those used in most earlier reviews (Wiffen 2005), as they concentrate on dichotomous outcomes where pain ratings do not follow a normal (Gaussian) distribution. People with chronic pain desire high levels of pain relief, ideally more than 50%, and ideally leaving them with no worse than mild pain (Moore 2013a; O'Brien 2010).
Primary outcomes
-
Participant‐reported pain relief of 30% or greater.
-
Participant‐reported pain relief of 50% or greater.
-
PGIC much or very much improved.
-
PGIC very much improved.
Secondary outcomes
-
Any pain‐related outcome indicating some improvement.
-
Withdrawals due to lack of efficacy or adverse events, or for any cause.
-
Any adverse event.
-
Any serious adverse event. Serious adverse events typically include any untoward medical occurrence or effect that results in death, is life‐threatening, requires hospitalisation or prolongation of existing hospitalisation, results in persistent or significant disability or incapacity, is a congenital anomaly or birth defect, is an 'important medical event' that may jeopardise the patient, or that may require an intervention to prevent one of the above characteristics or consequences.
-
Specific adverse events, particularly somnolence and dizziness.
These outcomes are not eligibility criteria for this review, but are outcomes of interest within the studies that meet the inclusion criteria of the review.
Search methods for identification of studies
Two review authors (TC and PW) independently performed literature searches for eligible studies. We resolved any disagreements or uncertainties by discussion with a third review author where necessary.
Electronic searches
We searched the following databases, without language restrictions.
-
Cochrane Central Register of Controlled Trials (CENTRAL; via the Cochrane Register of Studies Online) to 24 May 2016.
-
MEDLINE (via Ovid) from 1946 to 24 May 2016.
-
Embase (via Ovid) from 1974 to 24 May 2016.
The individual search strategies for CENTRAL, MEDLINE, and Embase are shown in Appendix 4.
Searching other resources
We reviewed the bibliographies of all relevant RCTs and review articles, and searched the following clinical trial databases: ClinicalTrials.gov (ClinicalTrials.gov) and the World Health Organization International Clinical Trials Registry Platform (apps.who.int/trialsearch/), to identify additional published or unpublished data. We did not contact study investigators or study sponsors.
Data collection and analysis
Selection of studies
In order to determine study eligibility, two authors (TC and PW) independently read the abstract of each study identified by the search. We eliminated studies that clearly did not satisfy the inclusion criteria, and obtained full‐text copies of the remaining study reports. TC and PW independently read these reports and reached agreement regarding inclusion by discussion. We did not anonymise the studies in any way before assessment. We have included a Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) flow chart (Figure 2).
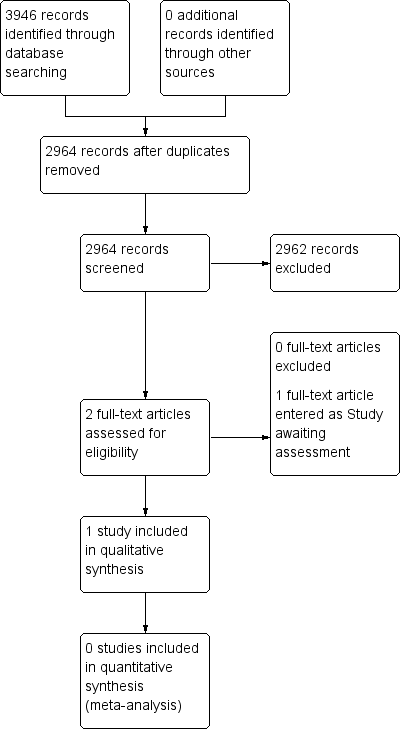
Study flow diagram.
Data extraction and management
Two review authors (TC and PW) independently extracted the data using a standard form and confirmed agreement before entering the data into Review Manager 5 (RevMan 2014), or any other analysis tool. We included information about the pain condition and number of participants treated, drug and dosing regimen, study design (placebo or active control), study duration and follow‐up, analgesic outcome measures and results, withdrawals, and adverse events (participants experiencing any adverse event or serious adverse event).
Assessment of risk of bias in included studies
We used the Oxford Quality Score as the basis for inclusion (Jadad 1996), limiting inclusion to studies that were randomised and double‐blind as a minimum.
Two review authors (TC and PW) independently assessed the risk of bias for each included study, using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), and adapted from those used by the Cochrane Pregnancy and Childbirth Group. We resolved any disagreements by discussion. We assessed the following for each included study.
-
Random sequence generation (checking for possible selection bias): We assessed the method used to generate the allocation sequence as being at either low risk of bias (any truly random process, for example random number table or computer random number generator) or unclear risk of bias (when the method used to generate the sequence is not clearly stated). We excluded studies at high risk of bias that use a non‐random process (for example, odd or even date of birth; hospital or clinic record number).
-
Allocation concealment (checking for possible selection bias): The method used to conceal allocation to interventions prior to assignment determines whether intervention allocation could have been foreseen in advance of, or during, recruitment, or changed after assignment. We assessed the methods as being at either low risk of bias (for example, telephone or central randomisation; consecutively numbered, sealed, opaque envelopes) or unclear risk of bias (when the method is not clearly stated). We excluded studies that did not conceal allocation and were therefore at high risk of bias (for example, an open list).
-
Blinding of participants, personnel, and outcome assessment (checking for possible performance and detection bias): We assessed the methods used to blind study participants and outcome assessors from knowledge of which intervention a participant received. We assessed the methods as being at either low risk of bias (authors state that it was blinded and describe the method used to achieve blinding, for example, identical tablets, matched in appearance and smell) or unclear risk of bias (authors state that it was blinded but do not provide an adequate description of how blinding was achieved). We excluded studies at high risk of bias that were not double‐blind.
-
Incomplete outcome data (checking for possible attrition bias due to the amount, nature, and handling of incomplete outcome data): We assessed the methods used to deal with incomplete data as being at low risk of bias (less than 10% of participants did not complete the study, or authors used 'baseline observation carried forward' analysis, or both), unclear risk of bias (used 'last observation carried forward' (LOCF) analysis), or high risk of bias (used 'completer' analysis).
-
Size of study (checking for possible biases confounded by small size): We assessed studies as being at low risk of bias (200 participants or more per treatment arm), unclear risk of bias (50 to 199 participants per treatment arm), or high risk of bias (fewer than 50 participants per treatment arm).
Measures of treatment effect
We used dichotomous data to calculate the risk ratio (RR) with 95% confidence interval (CIs) using a fixed‐effect model unless we found evidence of significant statistical heterogeneity (see Assessment of heterogeneity).
We calculated NNTs as the reciprocal of the absolute risk reduction (McQuay 1998). For unwanted effects, the NNT becomes the number needed to treat for an additional harmful outcome (NNTH) and is calculated in the same manner. Where the unwanted effect is less common with treatment than control, we used the term Number Needed to Treat to Prevent (NNTp).
We did not use continuous data in analyses because it is inappropriate where there is an underlying skewed distribution, as is usually the case with analgesic response.
Unit of analysis issues
We accepted randomisation to the individual participant only. In the event of a study having more than one active treatment arm in which data were not combined for analysis, we planned to split the control treatment arm between active treatment arms.
Dealing with missing data
We used intention‐to‐treat (ITT) analysis where the ITT population consists of participants who were randomised, took at least one dose of the assigned study medication, and provided at least one post‐baseline assessment. We assigned missing participants zero improvement wherever possible.
Assessment of heterogeneity
We planned to deal with clinical heterogeneity by combining studies that examine similar conditions, and to assess statistical heterogeneity visually (L'Abbé 1987), and with the I² statistic. When the I² statistic value was greater than 50%, we would consider the possible reasons for this.
Assessment of reporting biases
The aim of this Cochrane review was to use dichotomous outcomes of known utility and of value to patients (Hoffman 2010; Moore 2010b; Moore 2010c; Moore 2010d; Moore 2013a). The review does not depend on what the authors of the original studies chose to report or not, though clearly difficulties would arise if included studies failed to report relevant dichotomous results.
We planned to assess publication bias using a method designed to detect the amount of unpublished data with a null effect required to make any result clinically irrelevant (usually taken to mean a NNT of 10 or higher in this condition; Moore 2008). In the event, this was not possible.
Data synthesis
We planned to use a fixed‐effect model for meta‐analysis. We would have used a random‐effects model if there was significant clinical heterogeneity and it was considered appropriate to combine studies.
We planned to analyse data in three tiers, according to outcome and freedom from known sources of bias.
-
The first tier would use data meeting current best standards, where studies report the outcome of at least 50% pain intensity reduction over baseline (or its equivalent), without the use of LOCF analysis or other imputation method for dropouts, report an ITT analysis, last eight or more weeks, have a parallel‐group design, and have at least 200 participants (preferably at least 400) in the comparison (Moore 1998; Moore 2010a; Moore 2012a; Moore 2012b). We planned to report these top‐tier results first.
-
The second tier would use data from at least 200 participants but where one or more of the first‐tier conditions above was not met (for example, reporting at least 30% pain intensity reduction, using LOCF or a completer analysis, or lasting four to eight weeks).
-
The third tier of evidence related to data from fewer than 200 participants, or where there were expected to be significant problems because, for example, of very short duration studies of less than four weeks, where there was major heterogeneity between studies, or where there were shortcomings in allocation concealment, attrition, or incomplete outcome data. For this third tier of evidence, no data synthesis is reasonable and may be misleading, but an indication of beneficial effects might be possible.
Quality of the evidence
We used the GRADE approach to assess the quality of evidence related to each of the key outcomes, and report our judgement on the quality of the evidence in the 'Summary of findings' table (Chapter 12, Appendix 6; Higgins 2011). Two review authors independently rated the quality of evidence for each outcome.
In addition, there may be circumstances where the overall rating for a particular outcome needs to be adjusted as recommended by GRADE guidelines (Guyatt 2013a). For example, if there are so few data that the results are highly susceptible to the random play of chance (Moore 2008b), or if a studies use LOCF imputation in circumstances where there are substantial differences in adverse event withdrawals (Moore 2012b), one would have no confidence in the result, and would need to downgrade the quality of the evidence by 3 levels, to very low quality. In circumstances where there were no data reported for an outcome, we would report the level of evidence as very low quality (Guyatt 2013b).
Summary of findings table
We have included a 'Summary of findings' table as set out in the PaPaS author guide (PaPaS 2012), and recommended in the Cochrane Handbook (Chapter 11, Higgins 2011). The table includes, where possible, outcomes equivalent to moderate or substantial benefit of at least 30% and at least 50% pain intensity reduction, PGIC (possibly at least substantial improvement and at least moderate improvement) (Dworkin 2008), withdrawals due to lack of efficacy, withdrawals due to adverse events, serious adverse events, and death (a particular serious adverse event).
For the 'Summary of findings' table we used the following descriptors for levels of evidence (EPOC 2015):
High: This research provides a very good indication of the likely effect. The likelihood that the effect will be substantially different† is low.
Moderate: This research provides a good indication of the likely effect. The likelihood that the effect will be substantially different† is moderate.
Low: This research provides some indication of the likely effect. However, the likelihood that it will be substantially different† is high.
Very low: This research does not provide a reliable indication of the likely effect. The likelihood that the effect will be substantially different† is very high.
† Substantially different: a large enough difference that it might affect a decision.
Subgroup analysis and investigation of heterogeneity
We did not plan to perform any subgroup analyses since the experience of previous reviews indicated that there would be too few data for any meaningful subgroup analysis.
Sensitivity analysis
We did not plan to perform any sensitivity analyses.
Results
Description of studies
For this review we made no attempt to contact authors or manufacturers of gabapentin, as in previous versions of this review (Moore 2011a; Moore 2014a). Clinical trial reports or synopses from previously unpublished studies had became available as a result of legal proceedings in the USA for the previous update.
Results of the search
Searching identified two studies using gabapentin to treat pain in fibromyalgia. One was completed and could be included (Arnold 2007); this study was identified in a previous version of the review. Another was available only as a conference abstract, with insufficient detail to determine eligibility for inclusion (Mouzopoulos 2014); this study has been put into Studies awaiting classification. A flow diagram of the search results is shown in Figure 2.
Included studies
Arnold 2007 investigated 150 participants in a multicentre, randomised, double‐blind, placebo‐controlled, parallel‐group study. Participants had diagnosis made using ACR (1990) criteria and had a pain score of 4/10 or greater on an NRS (moderate or severe pain) at randomisation; the mean initial pain score was 5.8/10. The median age was 48 years and 90% were women. The dose of gabapentin was titrated over the first six weeks of treatment, kept stable for a further six weeks, then tapered over one week. During the stable treatment phase, participants were required to take between 1200 and 2400 mg daily, administered in three doses. The median dosage was 1800 mg daily at endpoint.
Participants were excluded if they had previously been treated with gabapentin or pregabalin. Results were analysed using LOCF imputation for withdrawals.
See Characteristics of included studies table.
Excluded studies
We did not exclude any studies after reading the full article.
Risk of bias in included studies
We judged the included study to be at low or unclear risk of bias for all domains except for incomplete outcome reporting (attrition), for which we judged the risk of bias to be high. There was an unclear risk of bias for selection (both random sequence generation and allocation concealment). There was a low risk of bias for performance and detection bias (blinding), selective reporting, and size. See Figure 3 and the Characteristics of included studies table for our reasoning.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Effects of interventions
See: Summary of findings for the main comparison Gabapentin compared with placebo for fibromyalgia
Efficacy
There was no first or second tier evidence.
Third tier evidence came from one study with fewer than 200 participants. We did not carry out any data synthesis because any results would potentially be misleading. See summary of findings Table for the main comparison.
Participant‐reported pain relief of 30% or greater
This outcome was reported in 38/75 (49%) of participants with gabapentin and 23/75 (31%) with placebo, using LOCF.
Participant‐reported pain relief of 50% or greater
This outcome was not reported.
PGIC very much improved, or much or very much improved
These outcomes were not reported, but the study did report that 68% of participants described their condition as "better" at the end of treatment with gabapentin, as did 35% with placebo. These results are estimated from a graph, and use LOCF.
Any pain‐related outcomes
The Brief Pain Inventory average pain severity score assesses average pain over the previous 24 hours. After 12 weeks of treatment, the average score was 3.2 (standard deviation (SD) 2.0) with gabapentin and 4.6 (SD 2.6) with placebo, and the estimated difference (by longitudinal analysis) was ‐0.92 (95% CI ‐1.75 to ‐0.71).
Withdrawals
Lack of efficacy
One participant taking gabapentin and two taking placebo withdrew from the study due to lack of efficacy.
Adverse events
Twelve participants taking gabapentin and seven taking placebo withdrew from the study due to adverse events.
Any cause
Eighteen participants withdrew from treatment with gabapentin and 13 with placebo. Reasons for withdrawal other than lack of efficacy or adverse events were loss to follow up, withdrawal of consent, and patient decision (gabapentin 5, placebo 4).
Adverse events
Any adverse event
The number of participants experiencing any adverse event was not reported.
Any serious adverse event
The study reported that there were "no significant group differences in the percentage of serious treatment‐emergent adverse events", but not the number of participants experiencing any serious adverse event.
Specific adverse events
Treatment‐emergent adverse events occurring in at least 5% of participants in the gabapentin group were reported. Dizziness, lightheadedness, and sedation were reported significantly more often with gabapentin than placebo.
Discussion
Summary of main results
Limited evidence from a single trial over 12 weeks suggested that a small number of people with fibromyalgia may have a useful degree of pain relief with gabapentin, at a maximum dose of 2400 mg daily, compared with placebo. Other drugs ‐ pregabalin, duloxetine, milnacipran – have better evidence to support their use, but they also only provide benefits to around 10% or so of people with fibromyalgia (Cording 2015; Lunn 2014; Moore 2009). More and larger studies are obviously required to determine whether gabapentin is effective in fibromyalgia, and just what proportion of people would benefit.
Overall completeness and applicability of evidence
The evidence is very weak. A single study with small numbers of participants and events, and with other possible quality issues, means that no reliance can be placed in the results we have for both efficacy and harm.
Quality of the evidence
We downgraded the evidence to very low because of the sparseness of evidence from this single trial (Guyatt 2013a), and because the use of LOCF imputation in the face of greater adverse event withdrawals for active than placebo can lead to overestimation of treatment effects (Moore 2012b). Very low quality evidence means that we are very uncertain about the results.
Potential biases in the review process
We carried out extensive searches of major databases using broad search criteria, and also searched two large clinical trial registries. We feel that it is unlikely that we have missed significant studies.
Agreements and disagreements with other studies or reviews
Two previous systematic reviews found the same single study for gabapentin in fibromyalgia (Häuser 2009; Tzellos 2010), as did previous versions of this review (Moore 2011a; Moore 2014a). Guidelines for treatment of fibromyalgia recommend a multidisciplinary approach with an emphasis on development of coping strategies and exercise, but pharmacological interventions may be useful for some people; these may include anticonvulsants, but most of the evidence supporting their use relates to pregabalin (Fitzcharles 2013; Macfarlane 2016).

History of Earlier Reviews

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
| Gabapentin compared with placebo for fibromyalgia | ||||||
| Patient or population: adults with fibromyalgia Settings: community Intervention: gabapentin Comparison: placebo | ||||||
| Outcomes | Assumed risk ‐ probable outcome with intervention | Corresponding risk ‐ probable outcome with control | Relative effect | Number of | Quality of the evidence | Comments |
| gabapentin | placebo | |||||
| 30% pain reduction at 12 weeks | 38/75 | 23/75 | Not calculated | 1 study, 150 participants | very low | One included study of fewer than 200 participants; LOCF imputation. Downgraded three levels because of small numbers and study limitations |
| 50% pain reduction at 12 weeks | No data | No data | ‐ | ‐ | very low | Outcome not reported |
| PGIC ‐ any category of "better" at 12 weeks | 68/75 | 35/75 | Not calculated | 1 study, 150 participants | very low | One included study of fewer than 200 participants; LOCF imputation; non‐standard outcome ‐ usually top two categories of better, not top three, used Downgraded three levels because of small numbers and study limitations |
| Withdrawals due to adverse events | 12/75 | 7/75 | Not calculated | 1 study, 150 participants | very low | One included study of fewer than 200 participants; few events Downgraded three levels because of small numbers |
| Serious adverse events | "No significant group differences" | ‐ | 1 study, 150 participants | very low | ‐ | |
| Deaths | None reported | ‐ | 1 study, 150 participants | very low | ‐ | |
| CI: Confidence interval; LOCF: last observation carried forward; PGIC: Patient Global Impression of Change | ||||||
| Descriptors for levels of evidence (EPOC 2015): † Substantially different: a large enough difference that it might affect a decision. | ||||||

