Contenido relacionado
Revisiones y protocolos relacionados
Philip J Wiffen, Sheena Derry, Rae Frances Bell, Andrew SC Rice, Thomas Rudolf Tölle, Tudor Phillips, R Andrew Moore | 9 junio 2017
Tess E Cooper, Lauren C Heathcote, Jacqui Clinch, Jeffrey I. Gold, Richard Howard, Susan M Lord, Neil Schechter, Chantal Wood, Philip J Wiffen | 5 agosto 2017
Tess E Cooper, Philip J Wiffen, Lauren C Heathcote, Jacqui Clinch, Richard Howard, Elliot Krane, Susan M Lord, Navil Sethna, Neil Schechter, Chantal Wood | 5 agosto 2017
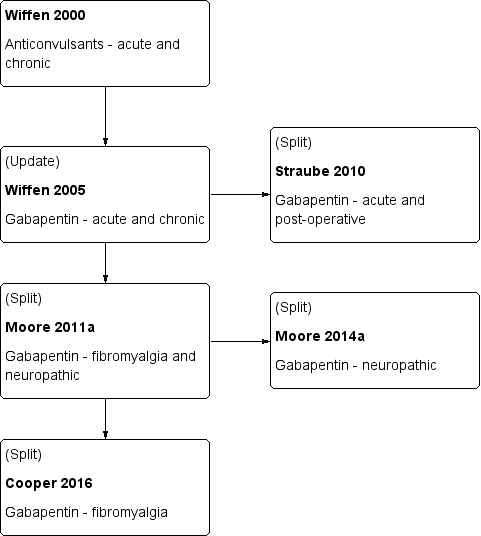
Sheena Derry, Philip J Wiffen, Dominic Aldington, R Andrew Moore | 8 enero 2015
Sebastian Straube, Sheena Derry, R Andrew Moore, Philip J Wiffen, Henry J McQuay | 12 mayo 2010
Xavier Basurto Ona, Dimelza Osorio, Xavier Bonfill Cosp | 15 julio 2015
Mattias Linde, Wim M Mulleners, Edward P Chronicle, Douglas C McCrory | 24 junio 2013
Philip J Wiffen, Henry J McQuay, Jayne Edwards, R Andrew Moore | 16 marzo 2011
Philip J Wiffen, Sheena Derry, R Andrew Moore, Dominic Aldington, Peter Cole, Andrew SC Rice, Michael PT Lunn, Katri Hamunen, Maija Haanpaa, Eija A Kalso | 11 noviembre 2013
Philip J Wiffen, Sally Collins, Henry J McQuay, Dawn Carroll, Alejandro Jadad, R Andrew Moore | 20 enero 2010