Bacilo de Calmette‐Guérin intravesical con interferón‐alfa versus bacilo de Calmette‐Guérin para el tratamiento del cáncer de vejiga sin invasión de la muscular
Referencias
References to studies included in this review
References to studies excluded from this review
Additional references
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Start date, end date of recruitment: May 1991, end date not specified Ethics approval obtained: Not reported | |
| Participants | Setting: Not reported (author affiliated to institution in Italy) Population/inclusion criteria: CIS, Ta, T1, G1‐G3 transitional cell tumours, after complete TURBT or diathermocoagulation Exclusion criteria: Not reported Method of recruitment: Not reported Informed consent obtained: Not reported Total number randomly assigned: 36 participants were enrolled in the 2 relevant trial arms. Baseline imbalances: Not reported Withdrawals or exclusions: Introduction of Bercovich 1995 paper states that there were initially 3 treatment groups (BCG plus IFN‐α, BCG alone, and IFN‐α alone), but the IFN‐α alone arm was discontinued because of "evidence of no effect in blocking disease recurrence". Characteristics (age, race, gender, severity of illness, comorbidities): Age (mean, years):
Stage:
Grade:
| |
| Interventions | Intervention Total number randomised: 18 enrolled Description: IFN‐α2b 10 MU and BCG Pasteur F 75 mg diluted in 0.9% NaCl, once a week for 6 weeks and once a month for 10 months. After participant emptied bladder, drugs were injected along with 50 mL saline. Participants were instructed not to urinate for 1 hour after the injection. Integrity of delivery/compliance: Not reported Comparison Total number randomised: 18 enrolled Description: BCG Pasteur F 150 mg diluted in 0.9% NaCl, once a week for 6 weeks and once a month for 10 months. After participant emptied bladder, drugs were injected along with 50 mL saline. Participants were instructed not to urinate for 1 hour after the injection. Integrity of delivery/compliance: Not reported Other co‐interventions for both groups: After intravesical injection, ketoprofen 200 mg per day for 2 days associated with norfloxacin or cinoxacin 1 g per day for 3 days was administered for the treatment of eventual infections. | |
| Outcomes | Time‐to‐recurrence Time points measured: Follow‐up was planned every 3 months for the first 2 years, then every 6 months. Time points reported: Number of recurrences was reported for both the BCG plus IFN‐α and BCG alone groups (mean follow‐up was 24.11 +/‐ 8.15 months for BCG alone group and 16.72 +/‐ 8.7 months for BCG plus IFN‐α group). Outcome definition: Not reported Person measuring/reporting: Not reported Subgroups: Not reported Time‐to‐progression Not reported Discontinuation of therapy due to adverse events Not reported Disease‐specific survival Not reported Time‐to‐death Not reported Systemic or local adverse events Time points measured: Not reported Time points reported: Not clearly reported; the occurrence of specific adverse events was reported for the BCG alone group only. Outcome definition: Not reported Person measuring/reporting: Not reported Subgroups: Not reported Disease‐specific quality of life Not reported | |
| Funding sources | Not reported | |
| Declarations of interest | Not reported | |
| Notes | Data extraction performed using translated manuscripts of Irianni 1993 and Bercovich 1995; manuscripts provided by study authors. Authors last contacted 3 October 2016 for further information; received response 31 October 2016 (no additional outcome data provided). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "A double arm random study" and "were randomized". No further details provided. |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not applicable (time‐to‐death not reported) |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not applicable (time‐to‐death not reported) |
| Incomplete outcome data (attrition bias) | Unclear risk | Manuscript reports that 18 participants enrolled into the relevant groups (unclear whether this was total randomised). An additional study arm (IFN‐α only) was discontinued due to evidence of no effect in blocking disease recurrence. No further details provided. |
| Incomplete outcome data (attrition bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Unclear risk | Manuscript reports that 18 participants enrolled into the relevant groups (unclear whether this was total randomised). An additional study arm (IFN‐α only) was discontinued due to evidence of no effect in blocking disease recurrence. While the reporting of recurrences assumes no attrition or exclusions, the authors do not clearly report on whether there were any postrandomisation losses to follow‐up, withdrawals, or trial group changes. |
| Incomplete outcome data (attrition bias) | Unclear risk | Not reported |
| Selective reporting (reporting bias) | High risk | No mortality or progression outcomes reported, unlike most other studies in this area. Adverse events incompletely reported for BCG alone group. |
| Other bias | High risk | Limited reporting of baseline characteristics to determine comparability of groups. Interim results presented (in Irianni 1993) after 18 participants enrolled. Additional third study arm (IFN‐α only) was later disregarded/excluded from reporting due to evidence of no effect in blocking disease recurrence. |
| Methods | Start date, end date of recruitment: 1995 to 2003 Ethics approval obtained: Yes | |
| Participants | Setting: Multi‐institutional study; setting not reported (authors affiliated to institution in Singapore) Population/inclusion criteria: People who underwent TUR for NMIBC (urothelial carcinoma) and were at risk for recurrence or progression (CIS, T1, G2, G3 or multiple or recurrent Ta/G1 tumours) and had no prior intravesical therapy Exclusion criteria: Not reported Method of recruitment: Not reported Informed consent obtained: Yes Total number randomly assigned: "All patients (n = 103) had ..." in Chiong 2011; 140, as reported by Vasdev 2009 and Esuvaranathan 2014 Baseline imbalances: None reported for 99 "evaluable patients" in Chiong 2011 Withdrawals or exclusions:Esuvaranathan 2007 reported that of 93 participants enrolled, 80 were "evaluable" (7 participants defaulted follow‐up, 3 died, and 3 underwent cystectomy for muscle‐invasive disease within 3 months of recruitment). Chiong 2011 reported the total number of participants as 103, with 99 "evaluable patients", therefore 4 were lost to follow‐up/excluded. Esuvaranathan 2014 reported that of the 140 participants, “The evaluable study cohort (n = 108) was followed up to 207 months”, therefore 32 were lost to follow‐up/excluded. Characteristics (age, race, gender, severity of illness, comorbidities): Reported for 99 participants in Chiong 2011 for BCG plus IFN‐α (n = 30); BCG 81 mg (n = 50); BCG 27 mg (n = 19) Age (mean, range) in years:
Gender
Race/Ethnicity
Stage
Grade
| |
| Interventions | Intervention Total number randomised: 30 Description: BCG (Connaught strain) 1/3 dose (27 mg) plus 10 MU of IFN‐α2b. The regimen comprised an induction course of 6 weekly instillations, a 6‐week break, and a final booster course of 3 once‐per‐week instillations. Integrity of delivery/compliance: Not reported Comparison 1 (BCG 81 mg) Total number randomised: 50 Description: BCG (Connaught strain) standard dose (81 mg). The regimen comprised an induction course of 6 weekly instillations, a 6‐week break, and a final booster course of 3 once‐per‐week instillations. Integrity of delivery/compliance: Not reported Comparison 2 (BCG 27 mg) Total number randomised: 19 Description: BCG (Connaught strain) 1/3 dose (27 mg). The regimen comprised an induction course of 6 weekly instillations, a 6‐week break, and a final booster course of 3 once‐per‐week instillations. Integrity of delivery/compliance: Not reported Other co‐interventions for both groups: Not reported | |
| Outcomes | Time‐to‐recurrence Time points measured: The participants were followed up by cystoscopy and urinary cytology every 3 months for 3 years and every 6 months thereafter. Bladder biopsy and urinary cytological examinations were performed when indicated. Time points reported: In Esuvaranathan 2007, participants were followed for a mean of 4.5 years (range 6 to 114 months). In Chiong 2011, median follow‐up time was 60 months. Esuvaranathan 2010 reported on recurrence probabilities at 36 and 60 months. Esuvaranathan 2014 reported 207 months' follow‐up and probabilities at 5 years' postrandomisation. Outcome definition: Number of recurrences reported in Chiong 2011. Vesical recurrence was defined as the occurrence of any new focus of NMIBC. Person measuring/reporting: Not reported Subgroups: Not reported Time‐to‐progression Time points measured: Participants were followed up by cystoscopy and urinary cytology every 3 months for 3 years and every 6 months thereafter. Bladder biopsy and urinary cytological examinations were performed when indicated. Time points reported: In Esuvaranathan 2007, participants were followed for a mean of 4.5 years (range 6 to 114 months). In Chiong 2011, median time to progression was 39 months. Esuvaranathan 2014 reported 207 months' follow‐up. Outcome definition: Number of progressions reported in Chiong 2011. Tumour stage progression was defined as muscle invasion (stage T2 or higher). Person measuring/reporting: Not reported Subgroups: Not reported Discontinuation of therapy due to adverse events Not reported Disease‐specific survival Time points measured: Not reported Time points reported: In Esuvaranathan 2007, mean of 24 months (range 12 to 42 months). In Chiong 2011, median time of 60 months Outcome definition: Cancer‐specific mortality was reported in Chiong 2011; no further definition provided. Person measuring/reporting: Not reported Subgroups: Not reported Time‐to‐death Not reported Systemic or local adverse events Time points measured: Not reported Time points reported: From the 3rd to 9th instillation in Vasdev 2009. During the first 6 instillations, and for the booster instillations in Esuvaranathan 2014. Outcome definition: Autoimmune clinical manifestations were reported in Vasdev 2009; "local and systemic symptoms" were reported in Esuvaranathan 2014. Person measuring/reporting: Not reported Subgroups: Not reported Disease‐specific quality of life Not reported | |
| Funding sources | Study was funded by the National Medical Research Council Singapore (NMRC/0085/1995 and NMRC/0457/2000), and the NLAM, National University of Singapore provided research grants. Gan 1999 notes that IFN‐α was "a generous gift from Schering‐Plough, Kenilworth, NJ". | |
| Declarations of interest | None | |
| Notes | Two similar abstracts published in 2014 outline long‐term follow‐up; full manuscripts are yet to be published. Study authors contacted on 25 April 2016 and 3 October 2016 for further information; awaiting response. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomized to receive"; no further details provided. |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | Unclear risk | "Double‐blinded"; no further details provided. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not applicable (time‐to‐death not reported). |
| Blinding of outcome assessment (detection bias) | Unclear risk | As above |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not applicable (time‐to‐death not reported). |
| Incomplete outcome data (attrition bias) | High risk | In Esuvaranathan 2007, of 93 participants, “seven patients defaulted follow‐up, 3 patients died, and 3 patients underwent cystectomy for muscle‐invasive disease, within 3 months of recruitment, and none of these patients were evaluable for outcome analysis" (80 were "evaluable"). In Chiong 2011, of 103 participants, 99 were "evaluable". Esuvaranathan 2014, which has been reported in abstract form only, stated that 108 of the 140 participants formed an “evaluable study cohort”. No details were available from abstract regarding reasons for losses/exclusions and whether these were balanced across groups. |
| Incomplete outcome data (attrition bias) | High risk | As above |
| Incomplete outcome data (attrition bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | High risk | In Esuvaranathan 2007, of 93 participants, “seven patients defaulted follow‐up, 3 patients died, and 3 patients underwent cystectomy for muscle‐invasive disease, within 3 months of recruitment, and none of these patients were evaluable for outcome analysis" (80 were "evaluable"). In Chiong 2011, of 103 participants, 99 were "evaluable". Esuvaranathan 2014, which has been reported in abstract form only, stated that 108 of the 140 participants formed an “evaluable study cohort”. No details were available from abstract regarding reasons for losses/exclusions and whether these were balanced across groups. |
| Incomplete outcome data (attrition bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | High risk | In Esuvaranathan 2007, of 93 participants, “seven patients defaulted follow‐up, 3 patients died, and 3 patients underwent cystectomy for muscle‐invasive disease, within 3 months of recruitment, and none of these patients were evaluable for outcome analysis" (80 were "evaluable"). In Chiong 2011, of 103 participants, 99 were "evaluable". Esuvaranathan 2014, which has been reported in abstract form only, stated that 108 of the 140 participants formed an “evaluable study cohort”. No details were available from abstract regarding reasons for losses/exclusions and whether these were balanced across groups. |
| Incomplete outcome data (attrition bias) | Unclear risk | Not reported |
| Selective reporting (reporting bias) | Unclear risk | Not possible to confidently assess selective reporting from abstracts of the long‐term follow‐up. To date, results have been reported incompletely. There was no access to trial protocol/registration. |
| Other bias | Low risk | Baseline characteristics, as reported in Chiong 2011, were comparable between groups; no other sources of bias identified. |
| Methods | Start date, end date of recruitment: 1992 to 1996 Ethics approval obtained: Not reported | |
| Participants | Setting: 17 urological units participating in the FinnBladder IV study group Population/inclusion criteria: Participants had at least 2 histologically verified Ta or T1, G1–3 tumours during the previous 18 months. People with previous instillation therapy had at least 1 of the recurrences at 6 months after the last instillation. Exclusion criteria: Radiologic and pathologic assessments were used to exclude upper urinary tract tumours when indicated. Carcinoma in situ during the previous 1.5 years Method of recruitment: Not reported Informed consent obtained: Yes Total number randomly assigned: 236 Baseline imbalances: None. Authors acknowledged differences in the timing of the delivery of the co‐intervention (intravesical mitomycin C). Withdrawals or exclusions: 31 participants (16 in the BCG alone group and 15 in the BCG alternating with IFN‐α group) were excluded for reasons including: inadequate histologic evidence of carcinoma, too‐low preceding recurrence rate, CIS, incomplete TURBT, no instillation started (for reasons not related to participant's compliance or general condition), early carcinoma of the renal pelvis without previous urography at randomisation, pT2 tumour, and no data available. Characteristics (age, race, gender, severity of illness, comorbidities): BCG alternating with IFN‐α (n = 103); BCG alone (n = 102)
| |
| Interventions | Intervention Total number randomised: 118 Description: Alternating intravesical instillations of BCG (5 x 108 CFU in 100 mL saline; OncoTICE 5 x 108 CFU) (equivalent to 50 mg wet weight) or IFN‐α2b (50 MU/100 mL saline; Intron A 50 MU) for 2 hours, given monthly over the course of a year Integrity of delivery/compliance: Not reported Comparison Total number randomised: 118 Description: BCG intravesical instillations (5 x 108 CFU in 100 mL saline; OncoTICE 5 x 108 CFU) (equivalent to 50 mg wet weight) for 2 hours, given monthly over the course of a year Integrity of delivery/compliance: Not reported Other co‐interventions for both groups: All participants received perioperative MMC 40 mg/100 mL for 2 hours after eradication of visible tumours (TURBT or biopsy and fulguration), followed by 4 weekly MMC 40 mg/100 mL instillations. | |
| Outcomes | Time‐to‐recurrence Time points measured: Participants were followed with cytology and cystoscopy every 3 months during the first year and according to a clinician’s decision thereafter. Time points reported: Probability of recurrence was reported at 5‐, 10‐, and 15‐year time points. The overall median follow‐up was 10.3 years in the BCG alone group and 8.6 years in the BCG alternating with IFN‐α group; median time‐to‐recurrence was reported for the BCG alternating with IFN‐α group (10 months), but was "not attained" in the BCG alone group. Outcome definition: A biopsy‐confirmed Ta or T1 tumour, CIS, or positive cytology Person measuring/reporting: Not reported Subgroups: Not reported Time‐to‐progression Time points measured: Participants were followed with cytology and cystoscopy every 3 months during the first year and according to a clinician’s decision thereafter. Time points reported: Probability of progression was reported at 5‐, 10‐, and 15‐year time points; the overall median follow‐up was 10.3 years in the BCG alone group and 8.6 years in the BCG alternating with IFN‐α group. Outcome definition: pT2 or higher disease Person measuring/reporting: Not reported Subgroups: Not reported Discontinuation of therapy due to adverse events Time points measured: Not reported Time points reported: Not reported Outcome definition: "We recorded only major side effects, which in most cases resulted in discontinuation of the instilled agent and/or resulted in additional treatment" Person measuring/reporting: Not reported Subgroups: Not reported Disease‐specific survival Time points measured: The follow‐up was calculated from the date of surgery to the date of death or the date of the latest entry of data. Time points reported: Probability of disease‐specific mortality was reported at 5‐, 10‐, and 15‐year time points; the overall median follow‐up was 10.3 years in the BCG alone group and 8.6 years in the BCG alternating with IFN‐α group. Outcome definition: "Disease‐specific mortality"; no further details provided Person measuring/reporting: Not reported Subgroups: Not reported Time‐to‐death Time points measured: The follow‐up was calculated from the date of surgery to the date of death or the date of the latest entry of data. Time points reported: Probability of overall survival was reported at 5‐, 10‐, and 15‐year time points; the median follow‐up time without death was 15.3 years in the BCG alone group and 15.9 years in the BCG alternating with IFN‐α group. Outcome definition: "Overall survival"; no further details provided Person measuring/reporting: Not reported Subgroups: Not reported Systemic or local adverse events Time points measured: Not reported Time points reported: Not reported Outcome definition: "We recorded only major side effects" Person measuring/reporting: Not reported Subgroups: Not reported Disease‐specific quality of life Not reported | |
| Funding sources | Finnish Cancer Foundation, Teknika, Organon, Pharmacia, Roche, and Schering Plough | |
| Declarations of interest | None | |
| Notes | Authors last contacted 3 October 2016 for further information; received response 25 November 2016 (no additional outcome data provided). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Allocation without blocking was based on a computer‐based list in the secretary’s possession and was confirmed by fax." |
| Allocation concealment (selection bias) | Low risk | "Central randomisation was carried out by the FinnBladder secretary." |
| Blinding of participants and personnel (performance bias) | High risk | Due to the nature of the interventions (BCG alone versus BCG alternating with IFN‐α), it was considered unlikely that participants and personnel were blinded to the intervention. |
| Blinding of participants and personnel (performance bias) | High risk | As above |
| Blinding of outcome assessment (detection bias) | High risk | As above |
| Blinding of outcome assessment (detection bias) | Low risk | Overall survival reported, which was considered an objective outcome unlikely to be influenced by absence of blinding. |
| Incomplete outcome data (attrition bias) | Low risk | 31 participants were excluded following randomisation (BCG alternating with IFN‐α: 15/118; BCG alone: 16/118), in similar numbers with similar reasons for exclusion across the 2 treatment groups. Kaasinen 2000 also reported: "Seven patients without recurrence were followed less than 6 months due to death, protocol violation and other intervening disease (2) in 4, while in the remaining 3 further follow up data were not available after 3 months". Intention‐to‐treat analyses were conducted. |
| Incomplete outcome data (attrition bias) | Low risk | As above |
| Incomplete outcome data (attrition bias) | Low risk | As above |
| Incomplete outcome data (attrition bias) | Low risk | As above |
| Incomplete outcome data (attrition bias) | Low risk | As above |
| Incomplete outcome data (attrition bias) | Low risk | As above |
| Incomplete outcome data (attrition bias) | Unclear risk | Not reported |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported. Of note, median time to recurrence "was not attained" in the BCG alone group. |
| Other bias | Unclear risk | Baseline characteristics were largely balanced. It was unclear whether timing of single immediate chemotherapy instillation differed at baseline between groups, however using the multivariable analysis of potential prognostic variables for primary endpoints with adjustment for significant prognostic variables (including timing of first chemotherapy instillation) did not change the conclusions. Regarding additional treatments during follow‐up, there were more treatments in the BCG alternating with IFN‐α group (27 versus 16), which the authors suggest “may have additionally contributed to decreasing the progression rate and the difference between the groups”. |
| Methods | Start date, end date of recruitment: February 2003 to August 2007 Ethics approval obtained: Not reported | |
| Participants | Setting: Not reported (authors affiliated to institution in Belarus) Population/inclusion criteria: Non‐muscle invasive transitional cell carcinoma with intermediate‐ and high‐risk of recurrence and progression; randomised after transurethral resection of all tumours Exclusion criteria: Not reported Method of recruitment: Not reported Informed consent obtained: Not reported Total number randomly assigned: 149 (120 to the relevant trial arms) Baseline imbalances: None Withdrawals or exclusions: Small number of participants in the IFN‐α monotherapy group was due to cessation of recruitment part way through from interim analysis indicating a higher risk of recurrence. Characteristics (age, race, gender, severity of illness, comorbidities): BCG plus IFN‐α (n = 60); BCG alone (n = 60) Median age overall: 65 years, range 29 to 83 years Median age (years):
Gender
Stage
Grade
| |
| Interventions | Intervention Total number randomised: 60 Description: 6 weekly instillations of 125 mg BCG (full dose, Russian strain) plus 6 MU of IFN‐α Integrity of delivery/compliance: Not reported, however it was noted that 108 participants completed the full course of treatment (it was not clear to which groups these participants had been assigned). Comparison Total number randomised: 60 Description: 6 weekly instillations of 125 mg BCG (full dose, Russian strain) Integrity of delivery/compliance: Not reported (see above) Other co‐interventions for both groups: Not reported | |
| Outcomes | Time‐to‐recurrence Time points measured: Response was assessed by cystoscopy every 3 months after treatment in the first 2 years and then 6 monthly. Time points reported: Number of recurrences were reported; follow‐up: median 38.3 months. Outcome definition: Not reported Person measuring/reporting: Not reported Subgroups: Not reported Time‐to‐progression Time points measured: Response was assessed by cystoscopy every 3 months after treatment in the first 2 years and then 6 monthly. Time points reported: Number of progressions were reported, between 3.6 and 29.7 months. Outcome definition: Not reported Person measuring/reporting: Not reported Subgroups: Not reported Discontinuation of therapy due to adverse events Time points measured: Not reported Time points reported: Reported during the course of treatment Outcome definition: Not reported Person measuring/reporting: Not reported Subgroups: Not reported Disease‐specific survival Time points measured: Not reported Time points reported: 1‐, 2‐, and 3‐year survival rates reported only Outcome definition: Not reported Person measuring/reporting: Not reported Subgroups: Not reported Time‐to‐death Not reported Systemic or local adverse events Time points measured: Not reported Time points reported: Reported during the course of treatment (including disorientation/delirium; macroscopic haematuria) Outcome definition: Not reported Person measuring/reporting: Not reported Subgroups: Not reported Disease‐specific quality of life Not reported | |
| Funding sources | Not reported | |
| Declarations of interest | Not reported | |
| Notes | Data extraction performed using translated manuscript of Minich 2009; manuscript provided by author. There was a third arm in this trial: IFN‐α alone, which we have not included in the review. Authors last contacted 3 October 2016 for further information; awaiting response. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “After histological confirmation patients were randomised into three groups”. After an interim analysis indicated a higher risk of recurrence in the third arm (interferon only), recruitment to that arm ceased; impact on randomisation not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not applicable (time‐to‐death not reported). |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not applicable (time‐to‐death not reported). |
| Incomplete outcome data (attrition bias) | Unclear risk | While the reporting of recurrences assumes no attrition or exclusions, the authors do not clearly report on whether there were any postrandomisation losses to follow‐up, withdrawals, or trial group changes. |
| Incomplete outcome data (attrition bias) | Unclear risk | While the reporting of progression assumes no attrition or exclusions, the authors do not clearly report on whether there were any postrandomisation losses to follow‐up, withdrawals, or trial group changes. |
| Incomplete outcome data (attrition bias) | Unclear risk | Discontinuation of therapy due to adverse events was incompletely reported (a P value provided only); the authors do not clearly report on whether there were any postrandomisation losses to follow‐up, withdrawals, or trial group changes. |
| Incomplete outcome data (attrition bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Unclear risk | While the reporting of adverse events assumes no attrition or exclusions, the authors do not clearly report on whether there were any postrandomisation losses to follow‐up, withdrawals, or trial group changes. |
| Incomplete outcome data (attrition bias) | Unclear risk | Not reported |
| Selective reporting (reporting bias) | High risk | Data reported on all outcomes specified in methods section except all‐cause mortality; discontinuation of therapy due to adverse events was reported incompletely. There was no access to trial protocol/registration to further assess selective reporting. |
| Other bias | Low risk | No differences in baseline characteristics; no sources of other bias identified. |
| Methods | Start date, end date of recruitment: 1999 to 2003 Ethics approval obtained: Yes | |
| Participants | Setting: Multicentre study at 75 centres; locations not specified (authors affiliated to institution in United States). Population/inclusion criteria: Histologically confirmed (tumour resection, biopsy, or abnormal cytology) CIS, Ta, or T1 urothelial cancer diagnosed within 8 weeks; no prior BCG treatment for bladder cancer Exclusion criteria: Any muscle‐invasive, upper tract of metastatic urothelial carcinoma, any other active malignancy that might impact 5‐year survival, pregnancy, immunosuppression or Eastern Cooperative Oncology Group performance status greater than 2 Method of recruitment: Not reported Informed consent obtained: Yes Total number randomly assigned: 670 Baseline imbalances: Not reported Withdrawals or exclusions: Not reported Characteristics (age, race, gender, severity of illness, comorbidities): Mean age: 68.4 years; 76% of participants were male. | |
| Interventions | Intervention 1 (BCG plus IFN‐α and RDA vitamins) Total number randomised: 176 Description: BCG (50 mg TICE strain BCG in 50 mL saline) plus 50 MU of IFN‐α2b (Intron A); induction course of 6 weekly intravesical instillations, participants who were rendered bladder cancer‐free were given maintenance courses consisting of 3 consecutive weekly instillations of BCG reduced to 1/3 dose (16.6 mg/50 mL) and IFN‐α2b 50 MU at 4 months after the start of the induction course, and again at 7, 13, 19, 25, and 37 months as long as they remained bladder cancer‐free; if intolerance occurred during the induction or maintenance period, instillation of study agents was discontinued for 2 weeks followed by re‐initiation of treatment at a BCG dose of 1/3 of that of the prior dose, with further sequential reductions by 1/3 of the prior dose permitted if intolerance continued, with no change in IFN‐α2b dosing. Integrity of delivery/compliance: Not reported Comparison 1 (BCG alone and RDA vitamins) Total number randomised: 160 Description: BCG (50 mg TICE strain BCG in 50 mL saline); induction course of 6 weekly intravesical instillations, participants who were rendered bladder cancer‐free were given maintenance courses consisting of 3 consecutive weekly instillations of BCG reduced to 1/3 dose (16.6 mg/50 mL) at 4 months after the start of the induction course, and again at 7, 13, 19, 25, and 37 months as long as they remained bladder cancer‐free; if intolerance occurred during the induction or maintenance period, instillation of study agents was discontinued for 2 weeks followed by re‐initiation of treatment at a BCG dose of 1/3 of that of the prior dose, with further sequential reductions by 1/3 of the prior dose permitted if intolerance continued. Integrity of delivery/compliance: Not reported Other co‐interventions for both groups (Intervention 1 and Comparison 1): Matched blinded vitamins were given in a dose of 2 tablets twice daily throughout the study, starting at the time of group assignment and continuing for the duration of the study. Each RDA tablet contained 25% of the recommended total daily dose. Intervention 2 (BCG plus IFN‐α and megadose vitamins) Total number randomised: 170 Description: As per Intervention 1 Integrity of delivery/compliance: Not reported Comparison 2 (IFN‐α and megadose vitamins) Total number randomised: 164 Description: As per Comparison 1 Integrity of delivery/compliance: Not reported Other co‐interventions for both groups (Intervention 2 and Comparison 2): Matched blinded vitamins were given in a dose of 2 tablets twice daily throughout the study, starting at the time of group assignment and continuing for the duration of the study. Each Oncovite (megadose vitamin preparation) tablet contained vitamins A (9000 IU), B6 (25 mg), C (500 mg), D3 (400 IU), folate (0.4 mg), and E (100 IU) as well as zinc (7.6 mg). | |
| Outcomes | Time‐to‐recurrence Time points measured: Clinical response was assessed by cystoscopy and cytology every 3 months for the first 24 months of the study, every 6 months during years 3 and 4, and annually thereafter. Time points reported: Not reported Outcome definition: Time‐to‐recurrence was the interval from the date of randomisation (or first date free of disease for participants with CIS) to first recurrence confirmed by biopsy or cytology. Any relapse during follow‐up was counted as a failure of therapy. Person measuring/reporting: Not reported Subgroups: Not reported Time‐to‐progression Not reported Discontinuation of therapy due to adverse events Time points measured: Not reported Time points reported: Not reported Outcome definition: Treatment intolerance was defined as dysuria that persisted for 3 or more days, fever higher than 101°F (38.3°C) or other severe systemic reaction. If treatment intolerance occurred during the induction or maintenance period, instillation of study agents was discontinued for 2 weeks followed by re‐initiation of treatment at a BCG dose of 1/3 that of the prior dose. If intolerance continued, further sequential reductions by 1/3 of the prior dose were permitted (i.e. 1/10th to 1/30th to 1/100th dose). The dosing of IFN‐α was not reduced. Person measuring/reporting: Not reported Subgroups: Not reported Disease‐specific survival Not reported Time‐to‐death Not reported Systemic or local adverse events Time points measured: Not reported Time points reported: Not reported Outcome definition: Treatment intolerance was defined as dysuria that persisted for 3 or more days, fever higher than 101°F (38.3°C) or other severe systemic reaction; fever and "constitutional symptoms" were reported. Person measuring/reporting: Not reported Subgroups: Not reported Disease‐specific quality of life Not reported | |
| Funding sources | Supported by Schering‐Plough Corp, Mission Pharmacal | |
| Declarations of interest | Financial interest and/or other relationships with Abbott Laboratories, Alnylam Pharmaceuticals, Viventia, Anadys Pharmaceuticals, Spectrum, Loras, Endo Pharmaceuticals, Medical Enterprises, and Sanofi‐Pasteur | |
| Notes | Immediate postoperative intravesical chemotherapy was used in 53 (8%) of the 670 participants: mitomycin: 36; thiotepa: 9; doxorubicin: 3; valrubicin: 2; other: 3. Authors last contacted 3 October 2016 for further information; awaiting response. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Patients were randomised by central computer to receive to BCG or BCG plus interferon.” "Patients were randomised using a 1:1:1:1 allocation ratio to 4 treatment arms" |
| Allocation concealment (selection bias) | Low risk | "Randomised by central computer" |
| Blinding of participants and personnel (performance bias) | High risk | “Patients and providers were not blinded to the IFN, which could have affected reporting” |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not applicable (time‐to‐death not reported). |
| Blinding of outcome assessment (detection bias) | High risk | “Patients and providers were not blinded to the IFN, which could have affected reporting” |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not applicable (time‐to‐death not reported). |
| Incomplete outcome data (attrition bias) | Unclear risk | While the reporting of recurrence assumes no attrition or exclusions, the authors do not clearly report on whether there were any postrandomisation losses to follow‐up, withdrawals, or trial group changes. |
| Incomplete outcome data (attrition bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Unclear risk | While the reporting of adverse events assumes no attrition or exclusions, the authors do not clearly report on whether there were any postrandomisation losses to follow‐up, withdrawals, or trial group changes. |
| Incomplete outcome data (attrition bias) | Unclear risk | Not reported |
| Selective reporting (reporting bias) | High risk | No mortality outcomes reported, unlike most other studies in this area. Some results relating to recurrence and progression reported incompletely in text. “Overall 231 of 670 patients (34%) had documented recurrence, including muscle invasive disease in 47 (7%) and discovery of metastatic disease in 7 (1%).” It was not clear to which groups these participants had been allocated. Secondary outcome was reported to be "severity of treatment toxicity", however the reported results were likelihood of fever and likelihood of constitutional symptoms only. |
| Other bias | Unclear risk | Baseline characteristics not reported by treatment group. Immediate postoperative intravesical chemotherapy was used in 53 (8%) of participants; it was not reported to which groups these participants had been assigned. Unclear whether conflicts of interest had any impact on the conduct of the trial. |
BCG: Bacillus Calmette‐Guérin
CFU: colony forming units
CIS: carcinoma in situ
G1: grade 1
G2: grade 2
G3: grade 3
IFN‐α: interferon‐alpha
IU: international units
MMC: mitomycin C
MU: million units
NaCl: sodium chloride
NMIBC: non‐muscle‐invasive bladder cancer
pTa: pathological tumour stage a
pT1: pathological tumour stage 1
pT2: pathological tumour stage 2
RDA: recommended daily allowance
Ta: tumour stage a
T1: tumour stage 1
T2: tumour stage 2
TUR: transurethral resection
TURBT: transurethral resection of bladder tumour
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| This was not a randomised controlled trial. | |
| This was not a randomised controlled trial. |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Time‐to‐recurrence Show forest plot | 1 | 670 | Hazard Ratio (Random, 95% CI) | 1.11 [0.86, 1.43] |
| Analysis 1.1  Comparison 1 Intravesically administered BCG combined with IFN‐α versus intravesically administered BCG alone, Outcome 1 Time‐to‐recurrence. | ||||
| 2 Recurrence Show forest plot | 4 | 925 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.44, 1.32] |
| Analysis 1.2 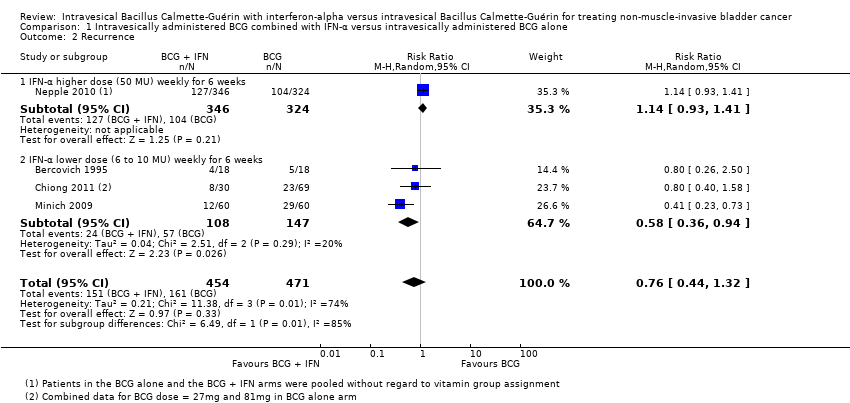 Comparison 1 Intravesically administered BCG combined with IFN‐α versus intravesically administered BCG alone, Outcome 2 Recurrence. | ||||
| 2.1 IFN‐α higher dose (50 MU) weekly for 6 weeks | 1 | 670 | Risk Ratio (M‐H, Random, 95% CI) | 1.14 [0.93, 1.41] |
| 2.2 IFN‐α lower dose (6 to 10 MU) weekly for 6 weeks | 3 | 255 | Risk Ratio (M‐H, Random, 95% CI) | 0.58 [0.36, 0.94] |
| 3 Progression Show forest plot | 2 | 219 | Risk Ratio (M‐H, Random, 95% CI) | 0.26 [0.04, 1.87] |
| Analysis 1.3  Comparison 1 Intravesically administered BCG combined with IFN‐α versus intravesically administered BCG alone, Outcome 3 Progression. | ||||
| 4 Disease‐specific mortality Show forest plot | 1 | 99 | Risk Ratio (M‐H, Random, 95% CI) | 0.38 [0.05, 3.05] |
| Analysis 1.4 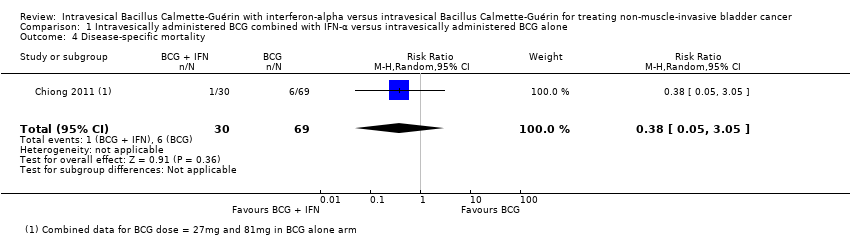 Comparison 1 Intravesically administered BCG combined with IFN‐α versus intravesically administered BCG alone, Outcome 4 Disease‐specific mortality. | ||||
| 5 Systemic or local adverse events Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.5  Comparison 1 Intravesically administered BCG combined with IFN‐α versus intravesically administered BCG alone, Outcome 5 Systemic or local adverse events. | ||||
| 5.1 Any (including disorientation/delirium and macro haematuria) | 1 | 120 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.13, 0.86] |
| 5.2 Fever | 1 | 670 | Risk Ratio (M‐H, Random, 95% CI) | 2.22 [1.27, 3.91] |
| 5.3 Constitutional symptoms | 1 | 670 | Risk Ratio (M‐H, Random, 95% CI) | 1.61 [1.10, 2.36] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Time‐to‐recurrence Show forest plot | 1 | 205 | Hazard Ratio (Random, 95% CI) | 2.86 [1.98, 4.13] |
| Analysis 2.1  Comparison 2 Intravesically administered BCG alternating with IFN‐α versus intravesically administered BCG alone, Outcome 1 Time‐to‐recurrence. | ||||
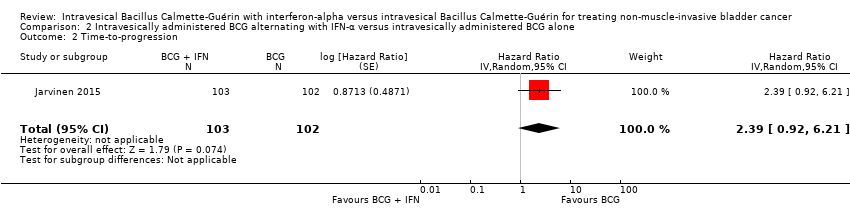
| 2 Time‐to‐progression Show forest plot | 1 | 205 | Hazard Ratio (Random, 95% CI) | 2.39 [0.92, 6.21] |
| Analysis 2.2  Comparison 2 Intravesically administered BCG alternating with IFN‐α versus intravesically administered BCG alone, Outcome 2 Time‐to‐progression. | ||||
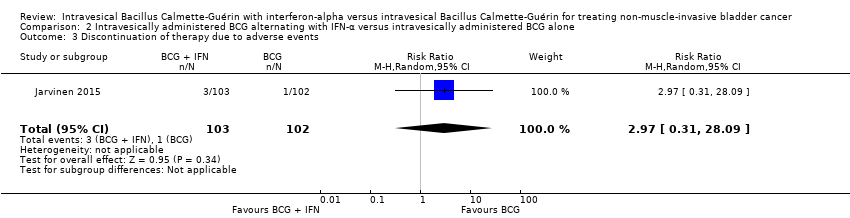
| 3 Discontinuation of therapy due to adverse events Show forest plot | 1 | 205 | Risk Ratio (M‐H, Random, 95% CI) | 2.97 [0.31, 28.09] |
| Analysis 2.3  Comparison 2 Intravesically administered BCG alternating with IFN‐α versus intravesically administered BCG alone, Outcome 3 Discontinuation of therapy due to adverse events. | ||||
| 4 Disease‐specific mortality Show forest plot | 1 | 205 | Hazard Ratio (Random, 95% CI) | 2.74 [0.73, 10.28] |
| Analysis 2.4  Comparison 2 Intravesically administered BCG alternating with IFN‐α versus intravesically administered BCG alone, Outcome 4 Disease‐specific mortality. | ||||
| 5 Overall survival Show forest plot | 1 | 205 | Hazard Ratio (Random, 95% CI) | 1.0 [0.68, 1.47] |
| Analysis 2.5 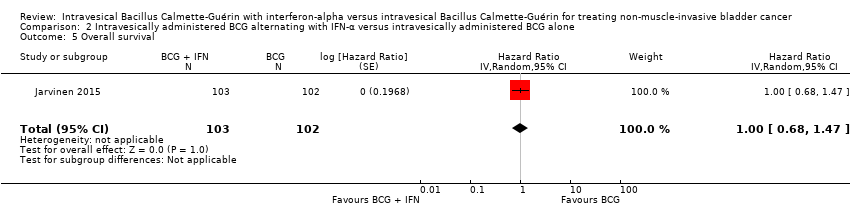 Comparison 2 Intravesically administered BCG alternating with IFN‐α versus intravesically administered BCG alone, Outcome 5 Overall survival. | ||||
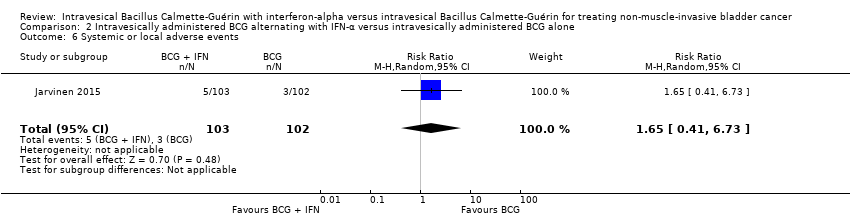
| 6 Systemic or local adverse events Show forest plot | 1 | 205 | Risk Ratio (M‐H, Random, 95% CI) | 1.65 [0.41, 6.73] |
| Analysis 2.6  Comparison 2 Intravesically administered BCG alternating with IFN‐α versus intravesically administered BCG alone, Outcome 6 Systemic or local adverse events. | ||||

Study flow diagram (searched 14 March 2016, updated 25 August 2016).
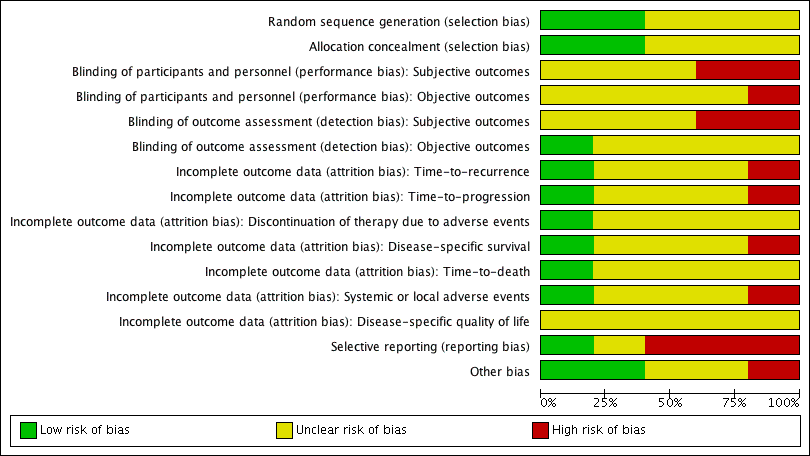
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
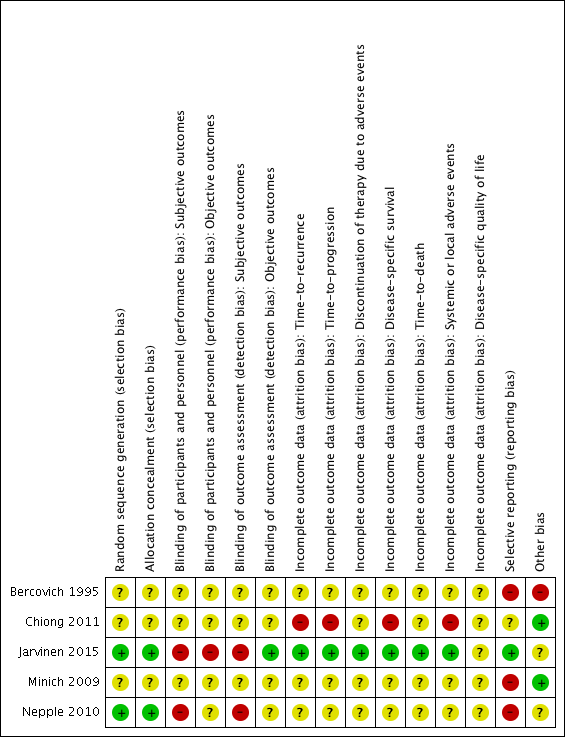
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Intravesically administered BCG combined with IFN‐α versus intravesically administered BCG alone, Outcome 1 Time‐to‐recurrence.

Comparison 1 Intravesically administered BCG combined with IFN‐α versus intravesically administered BCG alone, Outcome 2 Recurrence.
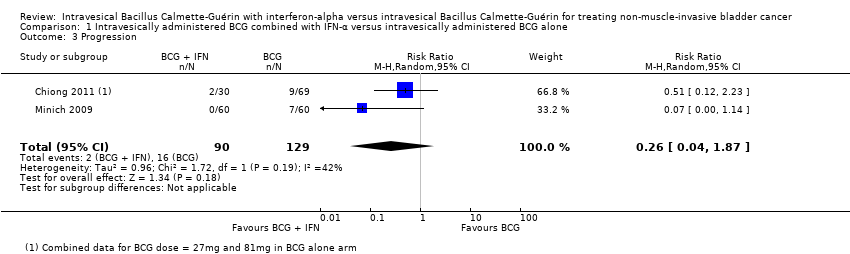
Comparison 1 Intravesically administered BCG combined with IFN‐α versus intravesically administered BCG alone, Outcome 3 Progression.
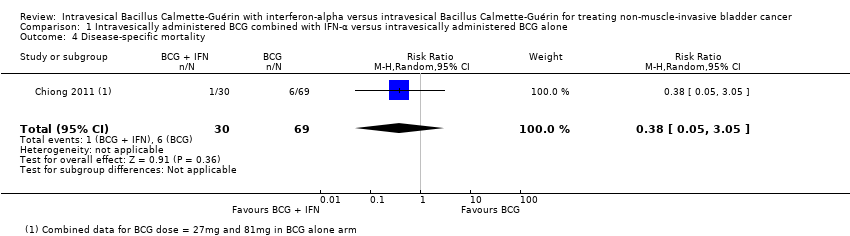
Comparison 1 Intravesically administered BCG combined with IFN‐α versus intravesically administered BCG alone, Outcome 4 Disease‐specific mortality.
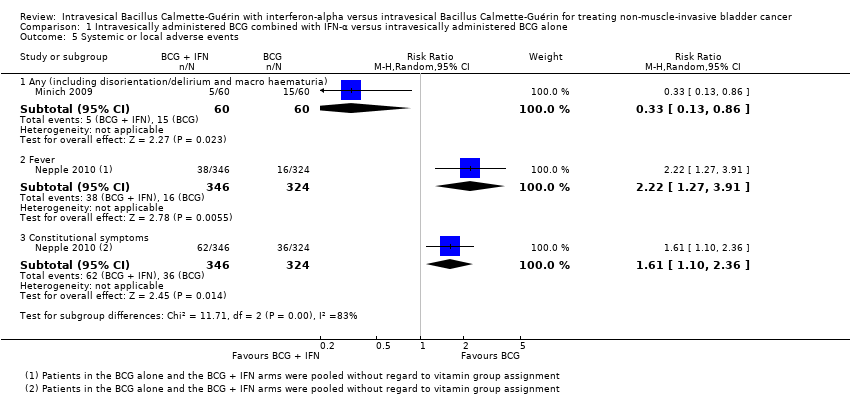
Comparison 1 Intravesically administered BCG combined with IFN‐α versus intravesically administered BCG alone, Outcome 5 Systemic or local adverse events.

Comparison 2 Intravesically administered BCG alternating with IFN‐α versus intravesically administered BCG alone, Outcome 1 Time‐to‐recurrence.
Comparison 2 Intravesically administered BCG alternating with IFN‐α versus intravesically administered BCG alone, Outcome 2 Time‐to‐progression.
Comparison 2 Intravesically administered BCG alternating with IFN‐α versus intravesically administered BCG alone, Outcome 3 Discontinuation of therapy due to adverse events.

Comparison 2 Intravesically administered BCG alternating with IFN‐α versus intravesically administered BCG alone, Outcome 4 Disease‐specific mortality.

Comparison 2 Intravesically administered BCG alternating with IFN‐α versus intravesically administered BCG alone, Outcome 5 Overall survival.
Comparison 2 Intravesically administered BCG alternating with IFN‐α versus intravesically administered BCG alone, Outcome 6 Systemic or local adverse events.
| Intravesically administered BCG combined with IFN‐α compared to intravesically administered BCG alone for treating non‐muscle‐invasive bladder cancer Patient or population: patients with non‐muscle invasive bladder cancer Intervention: BCG combined with IFN‐α Comparison: BCG alone | |||||
| Outcomes | № of participants | Quality of the evidence | Relative effect | Anticipated absolute effects* (95% CI) | |
| Risk with intravesically administered BCG alone | Risk difference with intravesically administered BCG combined with IFN‐α | ||||
| Recurrence Follow‐up: median 38.3 to 60 months | 925 | ⊕⊕⊝⊝ | RR 0.76 | Study population | |
| 342 per 1000 | 82 fewer per 1000 | ||||
| Progression Follow‐up: median 38.3 to 60 months | 219 | ⊕⊕⊝⊝ | RR 0.26 | Study population | |
| 124 per 1000 | 92 fewer per 1000 | ||||
| Discontinuation of therapy due to adverse events ‐ not measured | ‐ | ‐ | ‐ | ‐ | ‐ |
| Disease‐specific mortality Follow‐up: median 60 months | 99 | ⊕⊝⊝⊝ | RR 0.38 | Study population | |
| 87 per 1000 | 54 fewer per 1000 | ||||
| Disease‐specific quality of life ‐ not measured | ‐ | ‐ | ‐ | ‐ | ‐ |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). BCG: Bacillus Calmette‐Guérin; CI: confidence interval; IFN‐α: interferon‐alpha; RCT: randomised controlled trial; RR: risk ratio | |||||
| GRADE Working Group grades of evidence | |||||
| 1Downgraded for study limitations (‐1): high risk of bias: 'blinding of participants and personnel' (Nepple 2010); 'blinding of outcome assessment' (Nepple 2010); 'selective reporting' (Bercovich 1995; Minich 2009; Nepple 2010); 'other bias' (Bercovich 1995). | |||||
| Intravesically administered BCG alternating with IFN‐α compared to intravesically administered BCG alone for treating non‐muscle‐invasive bladder cancer Patient or population: patients with non‐muscle invasive bladder cancer Intervention: BCG alternating with IFN‐α Comparison: BCG alone | |||||
| Outcomes | № of participants | Quality of the evidence | Relative effect | Anticipated absolute effects* (95% CI) | |
| Risk with intravesically administered BCG alone | Risk difference with intravesically administered BCG alternating with IFN‐α | ||||
| Time‐to‐recurrence Follow‐up: median 8.6 to 10.3 years | 205 | ⊕⊕⊕⊝ | HR 2.86 | Study population | |
| 431 per 1000 | 370 more per 1000 | ||||
| Time‐to‐progression Follow‐up: median 8.6 to 10.3 years | 205 | ⊕⊕⊝⊝ | HR 2.39 | Study population | |
| 59 per 1000 | 76 more per 1000 | ||||
| Discontinuation of therapy due to adverse events Follow‐up: median 8.6 to 10.3 years | 205 | ⊕⊕⊝⊝ | RR 2.97 | Study population | |
| 10 per 1000 | 19 more per 1000 | ||||
| Disease‐specific mortality Follow‐up: median 8.6 to 10.3 years | 205 | ⊕⊕⊝⊝ | HR 2.74 | Study population | |
| 29 per 1000 | 49 more per 1000 | ||||
| Disease‐specific quality of life ‐ not measured | ‐ | ‐ | ‐ | ‐ | ‐ |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| 1Downgraded for study limitations (‐1): high risk of bias: 'blinding of participants and personnel', 'blinding of outcome assessment' (Jarvinen 2015). | |||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Time‐to‐recurrence Show forest plot | 1 | 670 | Hazard Ratio (Random, 95% CI) | 1.11 [0.86, 1.43] |
| 2 Recurrence Show forest plot | 4 | 925 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.44, 1.32] |
| 2.1 IFN‐α higher dose (50 MU) weekly for 6 weeks | 1 | 670 | Risk Ratio (M‐H, Random, 95% CI) | 1.14 [0.93, 1.41] |
| 2.2 IFN‐α lower dose (6 to 10 MU) weekly for 6 weeks | 3 | 255 | Risk Ratio (M‐H, Random, 95% CI) | 0.58 [0.36, 0.94] |
| 3 Progression Show forest plot | 2 | 219 | Risk Ratio (M‐H, Random, 95% CI) | 0.26 [0.04, 1.87] |
| 4 Disease‐specific mortality Show forest plot | 1 | 99 | Risk Ratio (M‐H, Random, 95% CI) | 0.38 [0.05, 3.05] |
| 5 Systemic or local adverse events Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 Any (including disorientation/delirium and macro haematuria) | 1 | 120 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.13, 0.86] |
| 5.2 Fever | 1 | 670 | Risk Ratio (M‐H, Random, 95% CI) | 2.22 [1.27, 3.91] |
| 5.3 Constitutional symptoms | 1 | 670 | Risk Ratio (M‐H, Random, 95% CI) | 1.61 [1.10, 2.36] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Time‐to‐recurrence Show forest plot | 1 | 205 | Hazard Ratio (Random, 95% CI) | 2.86 [1.98, 4.13] |
| 2 Time‐to‐progression Show forest plot | 1 | 205 | Hazard Ratio (Random, 95% CI) | 2.39 [0.92, 6.21] |
| 3 Discontinuation of therapy due to adverse events Show forest plot | 1 | 205 | Risk Ratio (M‐H, Random, 95% CI) | 2.97 [0.31, 28.09] |
| 4 Disease‐specific mortality Show forest plot | 1 | 205 | Hazard Ratio (Random, 95% CI) | 2.74 [0.73, 10.28] |
| 5 Overall survival Show forest plot | 1 | 205 | Hazard Ratio (Random, 95% CI) | 1.0 [0.68, 1.47] |
| 6 Systemic or local adverse events Show forest plot | 1 | 205 | Risk Ratio (M‐H, Random, 95% CI) | 1.65 [0.41, 6.73] |

