Diferentes tipos de corticosteroides intranasales para la rinosinusitis crónica
Referencias
Referencias de los estudios incluidos en esta revisión
Referencias de los estudios excluidos de esta revisión
Referencias de los estudios en espera de evaluación
Referencias de los estudios en curso
Referencias adicionales
Referencias de otras versiones publicadas de esta revisión
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | 4‐arm, "double blind", international, multicentre, parallel‐group RCT, with a 4‐month duration of treatment and follow‐up | |
| Participants | Location: 9 countries: Colombia, Guatemala, Honduras, Panama, Peru, Russia, South Africa, Ukraine, United States. No. of sites not presented. Setting of recruitment and treatment: not stated Sample size: 6 to 11 years
12 to 17 years
Participant (baseline) characteristics: 6 to 11 years
Other important effect modifiers:
12 to 17 years
Other important effect modifiers:
Inclusion criteria: children aged 6 to 17 years with nasal polyposis
| |
| Interventions | 6 to 11 years Intervention 1 (n = 18): mometasone furoate nasal spray, 100 µg once per day for 4 months Intervention 2 (n = 18): mometasone furoate nasal spray, 100 µg twice per day for 4 months Comparator group (n = 10): placebo once or twice daily (combined), for 4 months 12 to 17 years Intervention 1 (n = 26): mometasone furoate nasal spray, 200 µg once per day for 4 months Intervention 2 (n = 32): mometasone furoate nasal spray, 200 µg twice per day for 4 months Comparator group (n = 16): placebo once or twice daily (combined) for 4 months Use of additional interventions (common to both treatment arms): inhaled corticosteroids for patients with asthma (up to the equivalent of a moderate dosage regimen according to GINA 2005) | |
| Outcomes | Outcomes of interest in the review: All outcomes were measured at 4 months Primary outcomes: 1. Participants rated signs/symptoms including nasal congestion/obstruction, anterior rhinorrhoea/postnasal drip and loss of sense of smell; rated daily by participants on a 4‐point scale 2. Significant adverse effect: epistaxis Secondary outcomes: 3. Other adverse effects: local irritation (including oral thrush, sore throat) 4. Polyps size, no details on scores used Other outcomes reported by the study:
| |
| Funding sources | "Editorial assistance was provided by Andrew Horgan, PhD, of AdelphiEden Health Communications, New York, NY. This assistance was funded by Merck Sharpe and Dohme Corp." | |
| Declarations of interest | No information provided. (One of the authors of was affiliated with Merck Sharpe and Dome; which was Schering‐Plough in 2008 at the time of the study). | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Subjects were randomly assigned to one of four treatment groups in a 4:4:1:1 ratio... stratified by age" Comment: pg 34, col 1, para 4 |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information about allocation concealment provided |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "received MFNS 200 mcg once daily, MFNS 200 mcg twice daily, placebo once daily, or placebo twice daily" Comment: the abstract mentioned "double‐blind" and a placebo was used. However, instead of using a double‐dummy design, where all participants received the medication twice daily (with a placebo given for those who had once daily treatment), groups either had medication once or twice daily. Therefore, there is no blinding for participants in terms of knowing whether they are on the once daily or twice daily regimen. |
| Blinding of outcome assessment (detection bias) | High risk | Quote: (as above) Comment: most of the outcomes are patient‐reported and therefore blinding of outcome assessment is affected |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: no information about loss to follow‐up or exclusion. However, only 119/127 (93%) of randomised patients were included in their primary endpoint analysis. There were more exclusions/drop‐outs from the 100 μg group compared with the higher‐dose group (6 (12%) versus 1) but no reasons were provided. Adverse effects and symptoms were reported based on 127 participants. Unclear whether there were any imputations. |
| Selective reporting (reporting bias) | High risk | Quote: "No statistical analysis of efficacy end points was pre‐specified in the study protocol, and only descriptive efficacy statistics were collected." Comment: the protocol was identified (NCT00378378) and the purpose as set out in the protocol was "to evaluate the safety and efficacy of Nasonex® (Mometasone Furoate Nasal Spray(MFNS)) in the treatment of nasal polyps in pediatric subjects between the ages of 6 and less than 18 years old. Safety will be the primary focus of this study." The study only reported the change from baseline in points and percentages but not the standard deviations and P values. The values from the treatment groups were very similar to the placebo group for some outcomes (e.g. ‐43% for once daily versus ‐42% for placebo for the outcome of rhinorrhoea). Poor reporting due to lack of beneficial effects cannot be ruled out. |
| Other bias | Unclear risk | Comment: there is no information regarding the validation of the symptom score |
| Methods | 3‐arm, "double‐blind", parallel‐group RCT, with a 12‐week duration of treatment and follow‐up | |
| Participants | Location: Turkey, 1 site Setting of recruitment and treatment: Department of Otorhinolaryngology, Faculty of Medicine, Istanbul University Sample size:
Participant (baseline) characteristics:
Other important effect modifiers:
Inclusion criteria: age 16 years or over with bilateral nasal polyposis | |
| Interventions | Intervention (n = 15): fluticasone proportionate nasal drops, 800 μg/day (400 μg twice daily) for 12 weeks Control (n = 11): fluticasone proportionate nasal drops, 400 μg once daily for 12 weeks Use of additional interventions (common to all treatment arms): some patients underwent polypectomy at the end of trial | |
| Outcomes | Outcomes of interest in the review: Secondary outcomes: 1) Polyps size, by rigid endoscope at 12 weeks. A 4‐point scoring system was used (0 to 3) (definitions: 0 ‐ no polyps, 1 ‐ mild polyposis – small polyp not reaching to upper edge of the inferior turbinate and causing only slight obstruction; 2 – moderate polyposis – medium polyp reaching between the upper and lower inferior turbinate and causing troublesome obstruction; 3 – severe polyposis – large polyp reaching below the lower edge of the inferior turbinate and causing almost/total blockage) Other outcomes reported by the study:
| |
| Funding sources | No information provided | |
| Declarations of interest | No information provided | |
| Notes | One of the arms (fluticasone propionate nasal spray 200 µg per day given in 2 divided doses) is not relevant to this review | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "…randomly divided…" Comment: pg 3, col 1, para 3 No further information provided Baseline age does not appear to be balanced: the mean age of the 400 µg twice daily nasal group was about 17 years younger |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information provided |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "…double‐blind.." Comment: pg 1, col 1, para 2 says that the study was double‐blinded but the interventions were given in a different format (nasal spray versus nasal drops) and at different frequencies (1 versus 2 times per day) so it is difficult to see how either the personnel or participants were blind to the intervention). There was no mention of placebo. |
| Blinding of outcome assessment (detection bias) | High risk | Comment: no mention of placebo used; difficult to see how investigators and/or participants can be blinded to treatment intervention |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: 34 of 39 people randomised completed the trial (87%) but those who did not complete (of which 4/5 were due to worsening of the condition) were not included in the outcomes |
| Selective reporting (reporting bias) | High risk | Comment: numerical information was not well provided; most information for symptoms was presented as figures |
| Other bias | Unclear risk | Comment: no information was provided regarding the validation of the assessment instruments used |
| Methods | Single‐blinded, parallel‐group RCT with 3 months treatment and follow‐up | |
| Participants | Location: Serbia Setting of recruitment and treatment: no information Sample size:
Participant (baseline) characteristics:
Other important effect modifiers, if applicable: all patients have asthma | |
| Interventions | Intervention (n = 62): fluticasone propionate aqueous nasal spray, 200 µg once daily, for 3 months Comparator group (n = 32): mometasone furoate aqueous nasal spray, 200 µg once daily, for 3 months Use of additional interventions (common to both treatment arms): not reported | |
| Outcomes | Outcomes of interest in the review: Primary outcomes: 1. Disease severity symptom score, nasal symptoms score (postnasal drip, anterior rhinorrhoea, obstruction and loss of sense of smell), evaluated daily Secondary outcomes: Other outcomes reported by the study:
| |
| Funding sources | "No information provided" | |
| Declarations of interest | "No information provided" | |
| Notes | Only an abstract was available | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: no information, only published as an abstract. Unclear how randomisation was generated. Ratio does not seem 1:1. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information, only published as an abstract |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "single blind…" Comment: unclear who was blinded and how blinding was maintained |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "single blind…" Comment: unclear who was blinded and how blinding was maintained |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: no information on how many randomised versus completed |
| Selective reporting (reporting bias) | Unclear risk | Comment: no information, only published as an abstract |
| Other bias | Unclear risk | Comment: no mention of any validation of outcome measures. No information to assess whether baseline characteristics were balanced. |
| Methods | 3‐arm, double‐blind, parallel‐group RCT, with a 26‐week duration of treatment and 2 additional weeks of follow‐up | |
| Participants | Location: Sweden, number of sites is unclear Setting of recruitment and treatment: outpatient clinics Sample size:
Participant (baseline) characteristics:
Other important effect modifiers:
Inclusion criteria: bilateral polyposis with a polyp score of 1 or 2 | |
| Interventions | FP group (n = 19): fluticasone propionate, aqueous nasal spray, 2 actuations of 50 μg each to each nostril morning and evening (400 μg/day) for 26 weeks BDP group (n = 18): beclomethasone dipropionate, aqueous nasal spray, 2 actuations of 50 μg each to each nostril morning and evening (400 μg/day) for 26 weeks Placebo group (n = 18): placebo, actuations to each nostril morning and evening containing the same vehicle, as the interventions solutions including benzalkonium chloride as a preservative, for 26 weeks Use of additional interventions (common to all treatment arms): a 4‐week run‐in period during which no treatment for polyposis, except for rescue loratadine, could be used by the patients All patients were supplied with rescue loratadine tablets to use as relief medication, 10 mg loratadine once daily. Any use of rescue medication was documented on the patient's daily record card. | |
| Outcomes | Outcomes of interest in the review: Primary outcomes: 1. Patient‐reported disease severity, measured by daily records of all their nasal symptoms including: nasal blockage; sense of smell; sneezing and rhinorrhoea using a 4‐point rating system (0 = no symptoms; 1 = mild symptoms; 2 = moderate symptoms; 4 = severe symptoms) 2. Physician assessment of symptoms. No details were provided on how these were measured. Measured at 26 weeks. 3. Significant adverse effect: epistaxis Secondary outcomes: 4. Polyp size by endoscopy (0‐ to 4‐point scale) Other outcomes reported by the study: 5. Polyp score 6. Peak nasal inspiratory flow 7. Physician's assessment of change in symptoms | |
| Funding sources | Glaxo Wellcome PLC, England and the Torsten and Ragnar Söderberg Foundation, Sweden | |
| Declarations of interest | No conflicts of interest declared but 2 (of 6) authors had affiliations with Glaxo Wellcome Plc | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomized" Comment: pg 271, col 1, para 3 No further details provided |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information provided in the paper |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "placebo: 2 actuations to each nostril morning and evening containing the same vehicle, as the fluticasone and beclomethasone solutions including benzalkonium chloride as a preservative. The placebo solution was therefore identical to the active treatments but did not contain any active drug." Comment: pg 271, col 1, last para |
| Blinding of outcome assessment (detection bias) | Low risk | Comment: no further information. Should also be low if there is adequate blinding. |
| Incomplete outcome data (attrition bias) | High risk | Comment: 13/54 patients (24%) did not complete trial; 4/19 in fluticasone, 2/18 in beclomethasone, 7/18 (39%) in placebo group. Uneven drop‐out numbers: very high in placebo group. |
| Selective reporting (reporting bias) | High risk | Quote: "The primary efficacy endpoint was the physician's assessments of symptoms and polyp score on all clinic visits" Comment: the methods section described assessment of polyps, and patient‐reported symptom scores. However, "physician assessment of outcomes and polyps score" were reported as primary outcomes in the results section. The results focused on "physician assessment of symptoms" and barely mention the results of the polyps (only "significant" for visit 5 on beclomethasone, not for fluticasone). In addition, there were some outcomes that seemed to have arbitrary, non‐predefined cut‐off points (% of days with symptom score < 2 in results). The denominator for the reported symptom scores outcome measures is not identified. |
| Other bias | High risk | Comment: primary outcome of physician assessment of outcomes was not well described in the paper with little information on the criteria used or any validation/inter‐rater reliability |
| Methods | 3‐arm, "double‐blinded", multicentre, parallel‐group RCT, with a 3‐month duration of treatment and follow‐up | |
| Participants | Location: 4 sites in Denmark, 1 site in Sweden Setting of recruitment and treatment: unclear Sample size:
Participant (baseline) characteristics:
Other important effect modifiers:
Inclusion criteria: clinical diagnosis of eosinophilic nasal polyposis with polyp scores of 2 or less on each side. Eosinophilic polyposis was confirmed by nasal smear and/or biopsy.
| |
| Interventions | Group A (n = unknown): budesonide aqua (Rhinocort Aqua), 50 μg in each nostril x 2, twice daily (400 μg/day), 3 months Group B (n = unknown): budesonide aerosol (Rhinocort Aerosol), 50 μg in each nostril x 2, twice daily (400 μg/day), 3 months Group C (n = unknown): placebo (aqua or aerosol), unclear dose, 3 months Use of additional interventions (common to all treatment arms): unclear ‐ no information was provided | |
| Outcomes | Outcomes of interest in the review: Primary outcomes: 1. Disease severity, measured weekly by patients. Symptoms included were nasal obstruction, sneezing and nasal secretions, recorded for each nasal cavity (scale 0 to 3). 2. Significant adverse effect: epistaxis Secondary outcomes: 3. Other adverse effects: local irritation (including oral thrush, sore throat) 4. Polyp size (assessed using a 0 to 3 scale – definitions provided) Other outcomes reported by the study:
| |
| Funding sources | Astra Danmark A/S and Astra Draco AB, Sweden supported the study financially | |
| Declarations of interest | No information provided | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "...randomised…" Comment: mentioned in abstract but no further mention |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information provided |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "The patients were treated with either budesonide aqua (Rhinocort Aqua) or budesonide aerosol (Rhinocort Aerosol), 50 mcg x 2 in each nostril, twice daily = 400 mcg/day or placebo (aqua) or aerosol)" Comment: whilst there may be adequate blinding for treatment versus placebo, there is no blinding when comparing different dosage forms |
| Blinding of outcome assessment (detection bias) | High risk | Comment: no further information |
| Incomplete outcome data (attrition bias) | Unclear risk | Quote: "Five patients withdrew from the study…" Comment: no reasons given for withdrawals. Not included in any of the outcomes (including safety outcomes). |
| Selective reporting (reporting bias) | Unclear risk | Comment: all outcomes reported in the methods are mentioned in the results section, but numerical information for the results is not provided |
| Other bias | High risk | Comment: no comment on the validation of outcome measurements The paper does not provide clear background characteristics for each group. The number randomised to each group was not provided. |
| Methods | Double‐blind, parallel‐group RCT, with a 12‐week duration of treatment | |
| Participants | Location: UK Setting of recruitment and treatment: tertiary referral centre (Royal National ENT Hospital London) Sample size:
Participant (baseline) characteristics:
Inclusion criteria: Older than 16 years with a diagnosis of bilateral nasal polyposis requiring surgical interventions, meeting one or more of the following criteria:
Exclusion criteria:
| |
| Interventions | Intervention 1 (n = 10): fluticasone propionate aqueous nasal spray 400 µg per day, 2 actuations into each nostril morning and night Intervention 2 (n = 10): beclomethasone dipropionate aqueous nasal spray 400 µg per day, 2 actuations into each nostril morning and night Comparator (n = 9): placebo 2 sprays into each nostril twice a day Use of additional interventions (common to both treatment arms): terfenadine 60 mg as rescue medicine | |
| Outcomes | Outcomes of interest in the review: Primary outcomes:
Secondary outcomes:
Other outcomes reported by the study:
| |
| Funding sources | No information provided | |
| Declarations of interest | No information provided, but 2 of the authors were employed by Glaxo Wellcome and reprint requests were addressed to Glaxo | |
| Notes | Study had a 4‐week run‐in period 34 patients met criteria, 5 withdrew before randomisation (1 AE, 1 required polypectomy, 1 lack of efficacy, 2 did not return) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomly allocated, using a computer‐generated random code and a block size of 6, to receive 1 of 3 treatments" Comment: adequate sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Patients were randomly allocated, using a computer‐generated random code and a block size of 6, to receive 1 of 3 treatments" Comment: method not specified; blocked randomisation, but adequate blinding. Unclear if allocation concealment remained well maintained for this very small study. |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "The placebo was identical to the active formulations with the active ingredient omitted and was indistinguishable from the active treatments, which were themselves identical in appearance, taste, and smell." Comment: there was a 4‐week pre‐treatment period where all patients were exposed to the placebo, but blinding should still be adequate |
| Blinding of outcome assessment (detection bias) | Low risk | Comment: the same investigator did all the clinical assessments for all visits, but an identical placebo was used |
| Incomplete outcome data (attrition bias) | High risk | Quote: "last value carried forward technique" was used Comment: drop‐outs not balanced, 3/10 in fluticasone propionate, 0/10 in beclomethasone and 4/9 in placebo |
| Selective reporting (reporting bias) | High risk | Comment: patient‐reported symptoms were collected (using diaries), but it was not specified how these were planned to be reported. Study only reported percentage of patients with 100% of days without nasal blockage, and the median % of days without nasal symptoms (different criteria). Other outcomes not reported at all. There was also a higher percentage of patients in the fluticasone group (70%) compared to 33% and 30% in the beclomethasone and placebo groups, but details were not reported. Only stated that one of the adverse events in the FP group (throat irritation) was "predictable". |
| Other bias | High risk | Quote: "overall rhinitis symptoms (sneezing, rhinorrhoea, nasal itching)" Comment: symptoms scores (by patients and clinicians) were used but no mention of validation. Some items seems to be single symptom (e.g. nasal blockage), but others seems to encompass a few things (e.g. "overall rhinitis symptoms") Quote: "There was evidence, particularly from the acoustic rhinometric and PNIF data, that the patients randomly allocated to receive BDANS had milder symptoms than those randomly allocated to receive FPANS or placebo, even though all patients had been listed for surgical treatment on an equal basis before the study." Comment: baseline symptoms and other assessment scores were not reported. Unable to judge for other aspects. |
| Methods | 3‐arm, double‐blind, international, multicentre, parallel‐group RCT, with a 12‐week duration of treatment | |
| Participants | Location: 12 centres in Denmark (3 centres), Finland (1 centre) and Sweden (1 centre) Setting of recruitment and treatment: no information provided Sample size:
Participant (baseline) characteristics:
Inclusion criteria: at least 16 years old, bilateral mild or moderate nasal polyposis | |
| Interventions | Intervention A (n = 47): fluticasone propionate nasal drops (FPND), 400 µg twice daily for 12 weeks Intervention B (n = 48): fluticasone propionate nasal drops (FPND), 400 µg once daily for 12 weeks plus placebo drops once daily for 12 weeks Comparator group C (n = 47): placebo nasal drops twice daily for 12 weeks Process: contents were divided between both nostrils (200 µg per nostril) in the head down and forward position Use of additional interventions (common to both treatment arms): all patients underwent a 2‐week run‐in period during which they ceased all medication for polyposis except loratadine tables for relief of troublesome symptoms (10 mg daily maximum) Initial visit: physical and oropharyngeal examinations and details of clinical history Initial and 12‐week visit: blood and urine samples | |
| Outcomes | Outcomes of interest in the review: Primary outcomes: 1. Disease severity, measured by assessing nasal blockage (0 to 3 scale) and overall rhinitis symptoms including sneezing, rhinorrhoea and nasal itching (0 to 3 scale) and sense of smell (0 to 3 scale) at 12 weeks after treatment 2. Significant adverse effect: epistaxis Secondary outcomes: 3. Other adverse effects: local irritation Other outcomes reported by the study: Polyp size, degree of nasal blockage, overall rhinitis, peak nasal inspiratory flow (PNIF), olfactory function, rescue medication usage and adverse events | |
| Funding sources | Funded by Glaxo Wellcome plc, UK | |
| Declarations of interest | No information provided – but one of the authors worked at Glaxo Wellcome Research and Development | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "…double blind randomised treatment...", Figure 1, pg 95 Comment: no further information provided, but this is an "international, multicentre" study in 12 centres across 3 countries with regional monitors. Should have adequate sequence generation procedures. |
| Allocation concealment (selection bias) | Low risk | Quote: "…double blind randomised treatment...", Figure 1, pg 95 Comment: no further information provided. As above, allocation concealment should be adequate. |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "active and placebo nasal drops were provided in identical single‐dose containers …" |
| Blinding of outcome assessment (detection bias) | Low risk | Comment: no further information provided. Should be adequate with use of adequate double‐blinding. |
| Incomplete outcome data (attrition bias) | Unclear risk | Quote: "Sixteen patients were withdrawn during the randomized treatment phase, the majority due to lack of efficacy (five placebo, one FP 400 mg o.d., two FP 800 mg b.i.d.) or adverse events (five placebo, one FP 400 mg o.d., two FP 400 mg b.i.d.). One patient in the placebo group withdrew due to requirement for polypectomy. Two patients withdrew during the open phase, one requiring a polypectomy, the other for unspecified reasons", pg 97, column 2. Comment: 16/142 (11.3%) withdrew; 10/47 placebo, 4/47 400 µg twice daily and 2/48 400 µg once daily did not complete the study. All these patients were included as the ITT population. Percentage in placebo group higher, but still quite small. |
| Selective reporting (reporting bias) | Unclear risk | Comment: all outcome measures in the methods section were discussed in the results section |
| Other bias | Unclear risk | Comment: no mention of validation of the symptom criteria used for the primary outcomes |
| Methods | 3‐arm, double‐blind, multicentre, parallel‐group RCT, with a 4‐month duration of treatment and follow‐up | |
| Participants | Location: 44 medical centres "worldwide" Setting: no information Sample size:
Participant (baseline) characteristics:
Other important effect modifiers:
Inclusion criteria:
Exclusion criteria:
| |
| Interventions | 400 µg group (n = 122): mometasone furoate nasal spray 200 μg twice daily (morning and evening) for 4 months 200 µg group (n = 115): mometasone furoate nasal spray 200 μg once daily (morning, matching placebo used in the evening) for 4 months Placebo group (n = 117): placebo nasal spray twice daily (morning and evening) for 4 months Use of additional interventions (common to both treatment arms): acetaminophen (paracetamol) was encouraged for analgesic purposes; NSAIDs limited to 5 consecutive days if alternative analgesia was required. Antibiotics were administered for bacterial infections at the discretion of the principal investigator. Concomitant medications that would interfere with study evaluations were not permitted, including nasal sodium cromolyn; nasal atropine or ipratropium bromide; corticosteroids (except oral inhaled corticosteroids for asthma or mild‐strength or mid‐strength topical corticosteroids for dermatologic purposes); antihistamines; decongestants; topical, oral or ocular anti‐inflammatory drugs; or topical nasal or oral antifungal agents. | |
| Outcomes | Outcomes of interest in the review: Primary outcomes: 1. Disease severity, patient evaluation of symptoms (congestion/obstruction, loss of sense of smell, anterior rhinorrhoea and postnasal drip) measured daily on a diary card on a 4‐point scale (0 = none, 3 = severe) 2. Significant adverse effect: epistaxis (defined to include a wide range of bleeding episodes, from frank bleeding to bloody nasal discharge to flecks of blood in the mucus) Secondary outcomes: 3. Other adverse effects: local irritation Other outcomes reported by the study:
| |
| Funding sources | Supported by a grant from the Schering‐Plough Research Institute | |
| Declarations of interest | The lead author received research support for POP1998 SAR study, PO1025 Polyps study, PPO2573 Follow up to Polyps study PO2683 Acute rhinosinusitis and PO2692 Acute rhinosinusitis study. The source of the grant was not stated. 2 of the authors were employed by Schering Plough; another author received a research grant from Schering Plough and other pharmaceutical companies. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "…randomised in a 1:1:1 ratio to 3 treatment arms…" Comment: pg 1276, col 1, para 2. No further information. However, this is a relatively recent "international, multicentre" study in 44 centres worldwide. It should therefore have adequate sequence generation procedures. |
| Allocation concealment (selection bias) | Low risk | Comment: no information. However, this is a relatively recent "international, multicentre" study in 44 centres worldwide. It should therefore have adequate sequence generation procedures. |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "double blind, double dummy"; "… matching placebo nasal spray …" Comment: pg 1276, col 1, para 1 and 2. "Matching placebo spray" mentioned and those on the 200 μg/day regimen were also given placebo nasal spray for the evening. |
| Blinding of outcome assessment (detection bias) | Low risk | Comment: no information. Likely to remain well blinded until end of study. |
| Incomplete outcome data (attrition bias) | Unclear risk | Quote: 305/354 patients (86%) patients "completed 4‐month treatment period" Comment: higher % of patients not completing in the placebo group 22/117 (19%); compared to the twice daily or once daily groups 13/122 (11%) and 14/114 (12%), respectively. Study mentioned analyses based on "all randomised subjects" using the "ITT principle" and endpoint was "defined as the last non‐missing reading for the subject" for bilateral polyps score; however, it is unlikely all were analysed as the numbers do not tally exactly with the "meta‐analysis subsequently reported" |
| Selective reporting (reporting bias) | Low risk | Comment: all outcomes reported in the methods section were reported in the results section |
| Other bias | Unclear risk | Comment: no information about the validation of outcome measures |
| Methods | 3‐arm, double‐blind, multicentre, parallel‐group RCT, with a 4‐month duration of treatment and follow‐up | |
| Participants | Location: 24 centres in 17 countries worldwide Setting: study conducted from 25 June 2001 to 20 January 2003 Sample size:
Participant (baseline) characteristics:
Other important effect modifiers:
Inclusion criteria:
Exclusion criteria:
| |
| Interventions | 400 µg group (n = 102): mometasone furoate nasal spray 200 μg twice daily (morning and evening) for 4 months 200 µg group (n = 102): mometasone furoate nasal spray 200 μg once daily (morning, matching placebo used in the evening) for 4 months Placebo group (n = 106): placebo nasal spray twice daily (morning and evening) for 4 months Use of additional interventions (common to both treatment arms): acetaminophen (paracetamol) was encouraged for analgesic purposes; NSAIDs limited to 5 consecutive days if alternative analgesia was required. Antibiotics were administered for bacterial infections at the discretion of the principal investigator. Concomitant medications that would interfere with study evaluations were not permitted, including nasal sodium cromolyn; nasal atropine or ipratropium bromide; corticosteroids (except oral inhaled corticosteroids for asthma or mild‐strength or mid‐strength topical corticosteroids for dermatologic purposes); antihistamines; decongestants; topical, oral, or ocular anti‐inflammatory drugs; or topical nasal or oral antifungal agents. | |
| Outcomes | Outcomes of interest in the review: Primary outcomes: 1. Disease severity, patient evaluation of symptoms (congestion/obstruction, loss of sense of smell, anterior rhinorrhoea and postnasal drip) measured daily on a diary card on a 4‐point scale (0 = none, 3 = severe) 2. Significant adverse effect: epistaxis (defined to include a wide range of bleeding episodes, from frank bleeding to bloody nasal discharge to flecks of blood in the mucus) Secondary outcomes: 3. Other adverse effects: local irritation Other outcomes reported by the study:
| |
| Funding sources | Supported by a grant from the Schering‐Plough Research Institute | |
| Declarations of interest | "Schering Plough (manufacturer) was involved in the design and data analysis of this study and reviewed and approved this article" Dr Stjarne received payment of "approximately $50 000 annually" from the manufacturer for a contribution to the Clarityn website. Dr Mosges was on the advisory board and Drs Staudinger and Danzig were employees of Schering‐Plough. | |
| Notes | The study had a 14‐day, single‐blind run‐in period to exclude placebo responders and identify participants with stable disease. The number of people screened/excluded after the run‐in period is not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was performed in blocks of 3 using random numbers generated by SAS function UNIFORM (SAS Institute, Cary, NC) with seed based on clock time. Randomization was stratified by the presence or absence of concurrent asthma." Comment: computerised randomisation |
| Allocation concealment (selection bias) | Low risk | Comment: although randomisation was blocked, blinding should be adequate |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "double blind"; "…matching placebo nasal spray …" Comment: "Matching placebo spray" mentioned; dosing regimen the same across all groups |
| Blinding of outcome assessment (detection bias) | Low risk | Comment: no information. Likely to remain well blinded until the end of the study. |
| Incomplete outcome data (attrition bias) | Unclear risk | Quote: "More than 85% of subjects completed the 4‐month treatment period, with more than twice as many placebo recipients as active drug recipients discontinuing during the treatment phase (18% vs 8%)." Comment: drop‐out rates not balanced |
| Selective reporting (reporting bias) | Unclear risk | Comment: although all outcomes mentioned in the methods were reported, these were mostly not in sufficient detail (e.g. only P values) |
| Other bias | Unclear risk | Comment: no information about the validation of outcome measures |
AE: adverse event
ASA: acetylsalicylic acid
BDP: beclomethasone dipropionate
CT: computerised tomography
d: day
F: female
FEV1: forced expiratory volume in one second
FP: fluticasone propionate
FPND: fluticasone propionate nasal drops
ITT: intention‐to‐treat
M: male
NSAIDs: non‐steroidal anti‐inflammatory drugs
PNIF: peak nasal inspiratory flow
RCT: randomised controlled trial
SD: standard deviation
UPSIT: University of Pennsylvania Smell Identification Test
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| POPULATION: all patients underwent endoscopic polypectomy at the start of the trial | |
| STUDY DESIGN: not randomised | |
| POPULATION: treatment started 1 week after FESS (continued for 1 year) | |
| DURATION: treatment and follow‐up only 8 weeks | |
| DURATION: treatment and follow‐up only 8 weeks (study compared betamethasone nasal drops (dose unclear) versus 400 µg fluticasone propionate drops) | |
| POPULATION: allergic and non‐allergic rhinitis patients | |
| DURATION: treatment only 8 weeks | |
| DURATION: treatment only 1 month (budesonide: 800 µg versus 400 µg versus placebo) | |
| DURATION: treatment and follow‐up only 4 weeks (budesonide: 400 µg versus 200 µg versus placebo) | |
| OTHER: trial registry entry for a clinical trial of "Beclomethasone aqueous spray and aerosol delivery systems in nasal polyps", registered in 2008. Contact with the study authors identified that this study was not completed and no results were published. The reason for termination was not provided. | |
| DURATION: treatment only 30 days. (Study compared 2 delivery methods for budesonide (mucosal atomisation device versus saline rinse bottle) in patients with CRSwNP) | |
| STUDY DESIGN: not a randomised study Ongoing study evaluating the safety of intranasal administration of 400 μg of fluticasone propionate twice a day using a novel bi‐directional device in participants with chronic rhinosinusitis with or without nasal polyps | |
| POPULATION: this study looked at the impact of fluticasone spray versus budesonide respules on patients who just had FESS | |
| INTERVENTION: comparison of different head positions; treatment only 6 weeks | |
| INTERVENTION: compared different doses (512 µg per day versus 2000 µg per day) and delivery methods of budesonide (nasal spray versus nebulisation). Also had an oral steroids group. DURATION: treatment and follow‐up only 16 days | |
| POPULATION: all patients had sinus surgery | |
| INTERVENTION: beclomethasone dipropionate 400 µg per day delivered as a nasal spray or through a "home‐made insufflator, consisting of a nose‐olive, a plastic tube and a funnel" to inhale powder from Rotacaps capsules meant for asthma treatment | |
| DURATION: treatment and follow‐up only 6 weeks | |
| DURATION: treatment only 1 week |
CRSwNP: chronic rhinosinusitis with nasal polyps
FESS: functional endoscopic sinus surgery
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | — |
| Participants | — |
| Interventions | — |
| Outcomes | — |
| Notes | Conference proceeding: we cannot locate the abstract |
| Methods | — |
| Participants | — |
| Interventions | — |
| Outcomes | — |
| Notes | Conference proceeding: we cannot locate the abstract |
| Methods | — |
| Participants | — |
| Interventions | — |
| Outcomes | — |
| Notes | Conference proceeding: we cannot locate the abstract |
| Methods | — |
| Participants | — |
| Interventions | — |
| Outcomes | — |
| Notes | We cannot locate the abstract |
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | 'Study evaluating the efficacy and safety of intranasal administration of 100, 200, and 400 μg of fluticasone propionate twice a day (bid) using a novel bi directional device in subjects with bilateral nasal polyposis followed by an 8‐week open‐label extension phase to assess safety' |
| Methods | Double‐blind, parallel assignment, randomised controlled trial |
| Participants | Adults with bilateral nasal polyposis |
| Interventions |
For 16 weeks |
| Outcomes |
No secondary outcomes were listed in the trial registry entry |
| Starting date | 2013 |
| Contact information | Optinose US Inc. No further details provided. |
| Notes | Study has been listed as completed on the registry website (October 2015). No results are currently available. |
| Trial name or title | 'Efficacy and safety study of intranasal administration of 100, 200, and 400 μg of fluticasone propionate twice a day (bid) using a novel bi directional device in subjects with bilateral nasal polyposis followed by an 8‐week open‐label extension phase to assess safety' |
| Methods | Double‐blind, parallel assignment, randomised controlled trial |
| Participants | Adults with bilateral nasal polyposis |
| Interventions |
For 16 weeks |
| Outcomes |
No secondary outcomes were listed in the trial registry entry |
| Starting date | 2012 |
| Contact information | Optinose US Inc. No further details provided. |
| Notes | Study has been listed as completed on the registry website (October 2015). No results are currently available. |
| Trial name or title | 'Buparid/PARI SINUS versus Budes® nasal spray in the therapy of chronic rhinosinusitis with polyposis nasi' |
| Methods | Open‐label, parallel assignment randomised controlled trial |
| Participants | Chronic rhinosinusitis with polyposis nasi in adult patients |
| Interventions | Budesonide inhalation versus budesonide spray |
| Outcomes | Change of inflammation of the nasal mucosa and paranasal sinus Magnetic resonance imaging (thickness of mucosa, Lund‐Mackay score) Safety assessment, SNOT‐22 quality of life Nasal obstruction Endoscopic evaluation of nasal polyps |
| Starting date | 2013 |
| Contact information | Stefanie Prante ([email protected]) |
| Notes | Also registered as EUCTR 2013‐002414‐12 on European Registry Study authors were contacted and responded to say that the trial is due to be completed in 2016 |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Disease severity ‐ overall symptoms, measured as average change from baseline at 4 months (range 0 to 3) Show forest plot | 2 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 1.1  Comparison 1 High‐dose versus low‐dose intranasal corticosteroids, Outcome 1 Disease severity ‐ overall symptoms, measured as average change from baseline at 4 months (range 0 to 3). | ||||
| 1.1 Average symptom score (3 domains) | 1 | 237 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.14 [‐0.39, 0.12] |
| 1.2 Average symptom score (2 domains) | 2 | 441 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.21 [‐0.40, ‐0.03] |
| 2 Disease severity ‐ individual symptoms, measured as average change from baseline at 4 months (range 0 to 3) Show forest plot | 2 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 1.2  Comparison 1 High‐dose versus low‐dose intranasal corticosteroids, Outcome 2 Disease severity ‐ individual symptoms, measured as average change from baseline at 4 months (range 0 to 3). | ||||
| 2.1 Nasal blockage | 2 | 441 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.29 [‐0.47, ‐0.10] |
| 2.2 Rhinorrhoea | 2 | 441 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.15 [‐0.34, 0.03] |
| 2.3 Loss of sense of smell | 1 | 237 | Std. Mean Difference (IV, Random, 95% CI) | 0.06 [‐0.20, 0.31] |
| 3 Adverse effects: epistaxis Show forest plot | 4 | 637 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.06 [1.20, 3.54] |
| Analysis 1.3 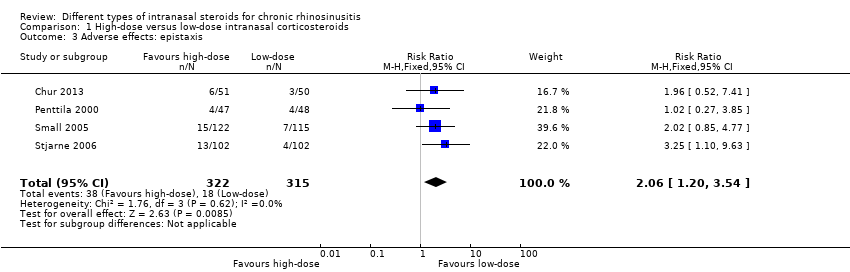 Comparison 1 High‐dose versus low‐dose intranasal corticosteroids, Outcome 3 Adverse effects: epistaxis. | ||||
| 4 Adverse effects: local irritation Show forest plot | 3 | 542 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.28, 3.31] |
| Analysis 1.4 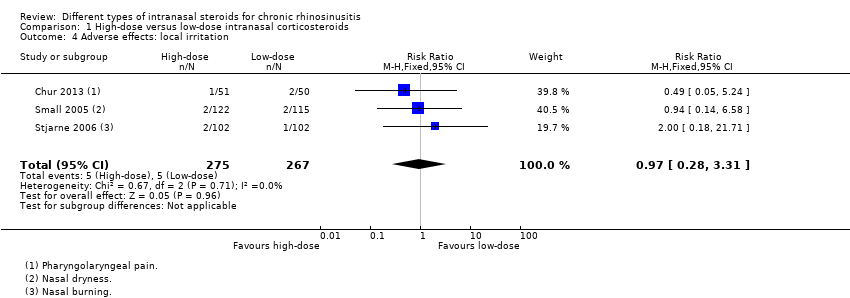 Comparison 1 High‐dose versus low‐dose intranasal corticosteroids, Outcome 4 Adverse effects: local irritation. | ||||
| 5 Nasal polyps size, measured as change from baseline (0 to 3 range scale) at 4 months Show forest plot | 1 | 237 | Mean Difference (IV, Fixed, 95% CI) | 0.19 [‐0.16, 0.54] |
| Analysis 1.5  Comparison 1 High‐dose versus low‐dose intranasal corticosteroids, Outcome 5 Nasal polyps size, measured as change from baseline (0 to 3 range scale) at 4 months. | ||||
| 6 Nasal polyps ‐ proportion with improvement at 12 weeks Show forest plot | 1 | 92 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.71 [0.91, 3.21] |
| Analysis 1.6  Comparison 1 High‐dose versus low‐dose intranasal corticosteroids, Outcome 6 Nasal polyps ‐ proportion with improvement at 12 weeks. | ||||
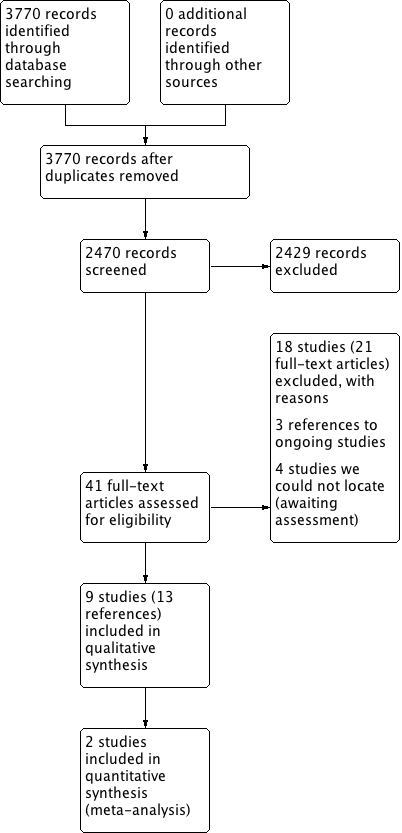
Process for sifting search results and selecting studies for inclusion.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.

Forest plot of comparison: 1 High‐dose versus low‐dose intranasal corticosteroids, outcome: 1.1 Disease severity ‐ overall symptoms, measured as average change from baseline at 4 months (range 0 to 3).
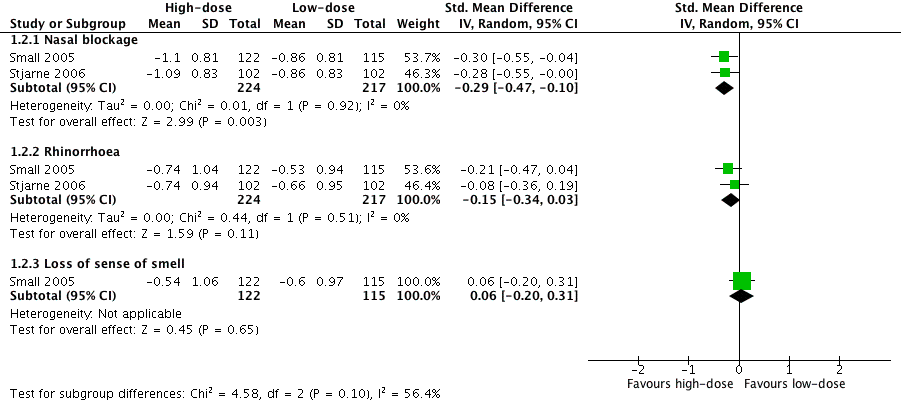
Forest plot of comparison: 1 High‐dose versus low‐dose intranasal corticosteroids, outcome: 1.2 Disease severity ‐ individual symptoms, measured as average change from baseline at 4 months (range 0 to 3).

Comparison 1 High‐dose versus low‐dose intranasal corticosteroids, Outcome 1 Disease severity ‐ overall symptoms, measured as average change from baseline at 4 months (range 0 to 3).

Comparison 1 High‐dose versus low‐dose intranasal corticosteroids, Outcome 2 Disease severity ‐ individual symptoms, measured as average change from baseline at 4 months (range 0 to 3).

Comparison 1 High‐dose versus low‐dose intranasal corticosteroids, Outcome 3 Adverse effects: epistaxis.

Comparison 1 High‐dose versus low‐dose intranasal corticosteroids, Outcome 4 Adverse effects: local irritation.

Comparison 1 High‐dose versus low‐dose intranasal corticosteroids, Outcome 5 Nasal polyps size, measured as change from baseline (0 to 3 range scale) at 4 months.

Comparison 1 High‐dose versus low‐dose intranasal corticosteroids, Outcome 6 Nasal polyps ‐ proportion with improvement at 12 weeks.
| Different types of intranasal corticosteroid molecules for chronic rhinosinusitis | ||||||
| Patient or population: chronic rhinosinusitis (all studies recruited patients with bilateral polyps) | ||||||
| Outcomes № of participants | Relative effect (95%) | Anticipated absolute effects* (95% CI) | Quality | What happens | ||
| Low‐dose intranasal corticosteroids | High‐dose intranasal corticosteroids | Difference | ||||
| Disease‐specific health‐related quality of life | Not measured | Impact unknown | ||||
| Disease severity ‐ overall symptoms
| — |
| ⊕⊝⊝⊝ | No differences observed but evidence was too low quality to draw a conclusion | ||
| Adverse events: epistaxis
| — |
| ⊕⊝⊝⊝ | Unclear whether the risk of epistaxis varies for different types of steroid molecules | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Studies were either very small (n = 20 and n = 26) and had important drop‐outs or were only reported as an abstract with inadequate information available (n = 100). We considered all studies to be at unclear to high risk of selective reporting and attrition bias. The evidence was very low quality due to very serious imprecision and very serious risk of bias concerns. | ||||||
| High‐dose versus low‐dose intranasal corticosteroids for chronic rhinosinusitis | ||||||
| Patient or population: chronic rhinosinusitis (all studies recruited patients with bilateral polyps) | ||||||
| Outcomes № of participants | Relative effect | Anticipated absolute effects* (95% CI) | Quality | What happens | ||
| Low‐dose intranasal corticosteroids | High‐dose intranasal corticosteroids | Difference | ||||
| Disease‐specific health‐related quality of life | Not measured | Impact unknown | ||||
| Disease severity ‐ overall symptoms, measured as average change from baseline at 4 months | ||||||
| All 4 EPOS domains | No information available | |||||
| 3 domains (nasal blockage, rhinorrhoea, loss of sense of smell) Range 0 to 3, lower score = less severe № of participants: 237 | — | The mean disease severity ‐ overall symptoms, measured as average change from baseline at 4 months (range 0 to 3) ‐ average symptom score (3 domains) without high‐dose was ‐0.66 points | — | MD 0.13 points lower (0.37 lower to 0.11 more) than low‐dose group | ⊕⊕⊝⊝ | The average score for 3 types of symptoms seems to be similar between the high‐dose and low‐dose groups. |
| (2 domains: nasal blockage, rhinorrhoea) № of participants: 441 | — | The mean disease severity ‐ overall symptoms, measured as average change from baseline at 4 months (range 0 to 3) ‐ average symptom score (2 domains) without high‐dose was ‐0.73 points | — | MD 0.19 points lower (0.36 lower to 0.02 lower) than low‐dose group | ⊕⊕⊝⊝ | The average score for 2 types of symptoms seems to be slightly lower for the high‐dose group. The clinical significance of this reduction is unclear. |
| Disease severity ‐ measured as average change from baseline at 4 months (range 0 to 3) | ||||||
|
№ of participants: 441 | — | The mean disease severity ‐ individual symptoms, measured as average change from baseline at 4 months (range 0 to 3) ‐ nasal blockage without high‐dose was ‐0.86 points | — | MD 0.24 points lower (0.39 lower to 0.08 lower) than low‐dose group | ⊕⊕⊝⊝ | The nasal blockage score seems to be slightly lower in the high‐dose group. The clinical significance of this reduction is unclear. |
|
№ of participants: 441 (2 RCTs) | — | The mean disease severity ‐ individual symptoms, measured as average change from baseline at 4 months (range 0 to 3) ‐ rhinorrhoea without high‐dose was ‐0.6 points | — | MD 0.15 points lower (0.33 lower to 0.03 higher) than low‐dose group | ⊕⊕⊝⊝ | The average score for rhinorrhoea seems to be similar between the high‐dose and low‐dose groups. |
|
№ of participants: 237 | — | The mean disease severity ‐ individual symptoms, measured as average change from baseline at 4 months (range 0 to 3) ‐ loss of sense of smell without high‐dose was ‐0.6 points | — | MD 0.06 points higher (0.2 lower to 0.32 higher) than low‐dose group | ⊕⊕⊝⊝ | The average score for loss of sense of smell seems to be very similar between the high‐dose and low‐dose groups. |
| Adverse effects: epistaxis № of participants: 637 | RR 2.06 | Study population | ⊕⊕⊕⊝ | The risk of epistaxis is likely to be higher in the higher‐dose groups. However, the studies included very minor nosebleeds, such as blood stains in the mucus, and most of these events are not likely to be severe. | ||
| 57 per 1000 | 118 per 1000 | 61 more per 1000 (11 more to 145 more) | ||||
| Moderate | ||||||
| 60 per 1000 | 124 per 1000 | 64 more per 1000 (12 more to 153 more) | ||||
| Adverse effects: local irritation № of participants: 542 | RR 0.97 | Study population | ⊕⊕⊝⊝ | The risk of local irritation seems to be similar between groups, but the overall risks are underestimated due to the way the data were reported. | ||
| 19 per 1000 | 18 per 1000 | 10 fewer per 1000 (13 fewer to 43 more) | ||||
| Moderate | ||||||
| 17 per 1000 | 17 per 1000 | 10 fewer per 1000 (13 fewer to 40 more) | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Scale validity, particularly discriminant validity (ability to distinguish the differences between groups), was unclear. There was a high risk of reporting bias. Studies tended to report enough information for meta‐analysis only for statistically significant results. One study, which had 101 participants, reported very similar values for both intervention arms for all disease scores but had no information related to SD. 2Small sample size ‐ evidence only from one or two relatively small studies. 3Only data from patients with bilateral nasal polyposis. We considered this to be indirectness of the evidence to patients without polyps but have not further downgraded the evidence. 4One of the studies had inadequate blinding ‐ a double dummy was not used to mask the twice daily (higher) versus once daily (lower) dose; the study had 101 participants. 5Sample size relatively small for a precise estimate of adverse events. We downgraded this outcome once, after taking into consideration the inadequate blinding in one of the studies and the relatively small sample size. 6Studies did not use consistent terminology/methods to report different types of local irritation. For analysis we only selected the most frequent types of local irritation from a list (to avoid double counting). This is a possible underestimation of overall event rates. The relatively low event rates and small sample size contributed to the large confidence intervals. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Disease severity ‐ overall symptoms, measured as average change from baseline at 4 months (range 0 to 3) Show forest plot | 2 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Average symptom score (3 domains) | 1 | 237 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.14 [‐0.39, 0.12] |
| 1.2 Average symptom score (2 domains) | 2 | 441 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.21 [‐0.40, ‐0.03] |
| 2 Disease severity ‐ individual symptoms, measured as average change from baseline at 4 months (range 0 to 3) Show forest plot | 2 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Nasal blockage | 2 | 441 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.29 [‐0.47, ‐0.10] |
| 2.2 Rhinorrhoea | 2 | 441 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.15 [‐0.34, 0.03] |
| 2.3 Loss of sense of smell | 1 | 237 | Std. Mean Difference (IV, Random, 95% CI) | 0.06 [‐0.20, 0.31] |
| 3 Adverse effects: epistaxis Show forest plot | 4 | 637 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.06 [1.20, 3.54] |
| 4 Adverse effects: local irritation Show forest plot | 3 | 542 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.28, 3.31] |
| 5 Nasal polyps size, measured as change from baseline (0 to 3 range scale) at 4 months Show forest plot | 1 | 237 | Mean Difference (IV, Fixed, 95% CI) | 0.19 [‐0.16, 0.54] |
| 6 Nasal polyps ‐ proportion with improvement at 12 weeks Show forest plot | 1 | 92 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.71 [0.91, 3.21] |

