Diferentes tipos de corticosteroides intranasales para la rinosinusitis crónica
Resumen
Antecedentes
Esta revisión es una de seis que examinan las opciones primarias de tratamiento médico para los pacientes con rinosinusitis crónica.
La rinosinusitis crónica es frecuente y se caracteriza por la inflamación del recubrimiento de la nariz y los senos paranasales que da lugar a bloqueo nasal, secreción nasal, presión/dolor facial y pérdida del sentido del olfato. La enfermedad puede presentarse con o sin pólipos nasales. Los corticosteroides tópicos (intranasales) se utilizan con la intención de reducir la inflamación en la mucosa nasosinusal para mejorar los síntomas del paciente.
Objetivos
Evaluar los efectos de diferentes tipos de corticosteroides intranasales en pacientes con rinosinusitis crónica.
Métodos de búsqueda
El Especialista en Información del Grupo Cochrane de Enfermedades de Oído, Nariz y Garganta (Cochrane ENT Group) buscó en el Registro de Ensayos del Grupo; Registro Cochrane Central de Ensayos Controlados (Cochrane Central Register of Controlled Trials) (CENTRAL 2015, número 7); MEDLINE; EMBASE; ClinicalTrials.gov; ICTRP y fuentes adicionales de ensayos publicados y no publicados. La fecha de la búsqueda fue 11 de agosto de 2015.
Criterios de selección
Ensayos controlados aleatorios (ECA) con un período de seguimiento de al menos tres meses que comparaban los corticosteroides intranasales de primera generación (p.ej. dipropionato de beclometasona, acetónido de triamcinolona, flunisolida, budesonida) con corticosteroides intranasales de segunda generación (p.ej. ciclesonida, furoato de fluticasona, propionato de fluticasona, furoato de mometasona, fosfato sódico de betametasona), o sprays versus gotas, o corticosteroides intranasales en dosis bajas versus dosis altas.
Obtención y análisis de los datos
Se utilizaron los procedimientos metodológicos estándar previstos por Cochrane. Los resultados primarios fueron la calidad de vida relacionada con la salud (CVRS) específica de la enfermedad, la gravedad de la enfermedad informada por el paciente y el evento adverso más común ‐ epistaxis (hemorragia nasal). Los resultados secundarios incluyeron la CVRS general, la puntuación endoscópica de los pólipos nasales, la puntuación de la tomografía computadorizada (TC) y el evento adverso de la irritación local. Se utilizó GRADE para evaluar la calidad de las pruebas para cada resultado; esto se indica en cursiva.
Resultados principales
Se incluyeron nueve ECA (911 participantes), incluyendo cuatro comparaciones diferentes. Ninguno de los estudios evaluó la primera medida de resultado primaria, la CVRS específica de la enfermedad.
Propionato de fluticasona versus dipropionato de beclometasona
Se identificaron dos estudios pequeños (56 participantes con pólipos) que evaluaban la gravedad de la enfermedad y consideraban el efecto adverso primario: epistaxis , pero ningún otro resultado. No es posible informar datos numéricos aunque los autores de los estudios informaron que no hubo ninguna diferencia entre los dos corticosteroides. Las pruebas fueron de muy baja calidad.
Propionato de fluticasona versus furoato de mometasona
Se identificó sólo un estudio (100 participantes con pólipos) que evaluaban la gravedad de la enfermedad (puntuaciones de los síntomas nasales), que no informó diferencias (ningún dato numérico disponible). Las pruebas fueron de muy baja calidad.
Corticosteroides en dosis altaversus dosis baja
Se incluyeron cinco estudios (663 participantes con pólipos nasales), tres que administraron furoato de mometasona (400 µg versus 200 µg en adultos y niños mayores, 200 µg versus 100 µg en niños más pequeños) y dos que administraron gotas de propionato de fluticasona (800 µg versus 400 µg). Se encontraron pruebas de baja calidad en relación con la gravedad de la enfermedad y el tamaño de los pólipos nasales; los resultados de los grupos de dosis alta y de dosis baja fueron similares. Aunque todos los estudios informaron una mejoría mayor en la puntuación de los pólipos en el grupo de dosis alta, la importancia de este hecho es incierta debido al tamaño pequeño de las mejorías.
El efecto adverso primario, la epistaxis , fue más común cuando se usaron las dosis mayores (cociente de riesgos [CR] 2,06; intervalo de confianza [IC] del 95%: 1,20 a 3,54; 637 participantes, pruebas de calidad moderada). La mayoría de los estudios que contribuyeron con datos a este resultado usaron una definición amplia de epistaxis, que varió desde hemorragia franca, secreción nasal sanguinolenta hasta manchas de sangre en el moco.
Spray nasal acuoso versus spray en aerosol
Se identificó sólo un estudio informado de manera deficiente (número incierto de participantes para la comparación de interés, 91 en tres brazos de tratamiento), en que hubo diferencias iniciales significativas entre los participantes de los dos grupos. No fue posible extraer conclusiones significativas a partir de los datos.
Conclusiones de los autores
No se hallaron pruebas suficientes para sugerir que un tipo de corticosteroide intranasal es más efectivo que otro en los pacientes con rinosinusitis crónica, o que la efectividad de un spray es diferente a la del aerosol. No se identificaron estudios que compararan gotas con spray.
No está claro si las dosis mayores dan lugar a una mejoría mayor de los síntomas (pruebas de baja calidad), aunque hubo pruebas de calidad moderada de un mayor riesgo de epistaxis como un efecto adverso del tratamiento al administrar dosis mayores. Lo anterior incluyó todos los niveles de gravedad de la epistaxis y es probable que la proporción de eventos que requirieron que los pacientes interrumpieran el uso sea baja debido a los números bajos de retiros atribuidos a la misma. Si la epistaxis está limitada a vetas de sangre en el moco puede ser tolerada por el paciente y puede ser seguro continuar el tratamiento. Sin embargo, puede ser un factor que afecta el cumplimiento.
No hay pruebas suficientes para sugerir que los diferentes tipos de molécula de corticosteroide o spray versus aerosol tienen diferentes efectos. Las dosis inferiores presentan una efectividad similar pero menos efectos secundarios.
Es claro que se necesita más investigación en esta área, con atención específica al diseño de los ensayos, los resultados de la calidad de vida relacionada con la salud específica de la enfermedad y la evaluación de los resultados a más largo plazo y los efectos adversos.
PICOs
Resumen en términos sencillos
Diferentes tipos de corticosteroides intranasales para la rinosinusitis crónica
Pregunta de la revisión
Se examinaron las pruebas sobre los efectos beneficiosos y perjudiciales de diferentes tipos de corticosteroides intranasales (en la nariz) administrados a los pacientes con rinosinusitis crónica.
Antecedentes
La rinosinusitis crónica es una afección frecuente que se define como inflamación de la nariz y los senos paranasales (un grupo de espacios llenos de aire detrás de la nariz, los ojos y las mejillas). Los pacientes con rinosinusitis crónica al menos presentan dos o más de los siguientes síntomas durante al menos 12 semanas: obstrucción nasal, secreción por la nariz o rinorrea, dolor o presión en la cara o disminución del sentido del olfato (hiposmia). Algunos pacientes también presentan pólipos nasales, que son tumefacciones en forma de uva del recubrimiento nasal normal dentro de la vía nasal y los senos. Los corticosteroides tópicos (intranasales) se utilizan con la intención de reducir la inflamación para mejorar los síntomas del paciente.
Características de los estudios
Se incluyeron nueve ensayos controlados aleatorios (ECA) con un total de 910 participantes en esta revisión. Los estudios variaron de tamaño: algunos eran pequeños, con tan sólo 20 pacientes, mientras que otros incluyeron más de 200 participantes. La mayoría de los estudios reclutaron pacientes adultos, pero un estudio incluyó sólo niños. En la mayoría de los estudios en adultos, la mayoría de los participantes eran hombres (72% a 79%). En todos los estudios los participantes presentaban rinosinusitis crónica con pólipos nasales. Los estudios compararon diferentes tipos de corticosteroides (tres estudios), corticosteroides en dosis alta versus dosis baja (cinco estudios), corticosteroides dos veces al día versus una vez al día, o diferentes métodos de administración (spray nasal acuoso versus aerosol ‐ un estudio). Todos los estudios tuvieron un grupo de placebo.
Resultados clave y calidad de las pruebas
Diferentes corticosteroides: propionato de fluticasona versus dipropionato de beclometasona
Dos estudios pequeños (56 participantes, riesgo incierto de sesgo) evaluaron la gravedad de la enfermedad y consideraron el efecto adverso primario, la epistaxis (hemorragia nasal), pero ningún otro resultado. No se encontraron diferencias entre los dos corticosteroides aunque las pruebas se evaluaron como de muy baja calidad.
Diferentes corticosteroides: propionato de fluticasona versus furoato de mometasona
Un estudio (100 participantes, riesgo incierto de sesgo) no encontró ninguna diferencia en la gravedad de la enfermedad (puntuaciones de los síntomas nasales). Estas pruebas se consideraron de muy baja calidad.
Corticosteroides en dosis alta versus dosis baja
Se encontraron cinco estudios (663 participantes, riesgo bajo o incierto de sesgo) que comparaban corticosteroides en dosis alta y en dosis baja, tres que administraron furoato de mometasona (400 µg versus 200 µg en adultos y niños mayores, 200 µg versus 100 µg en niños más pequeños) y dos que administraron gotas de propionato de fluticasona (800 µg versus 400 µg). La efectividad (gravedad de la enfermedad y tamaño de los pólipos nasales) fue similar entre los grupos de dosis alta y de dosis baja(pruebas de baja calidad). Aunque todos los estudios informaron una mejoría mayor en la puntuación de los pólipos en el grupo de dosis alta, la importancia de este hecho es incierta debido a que las mejorías observadas fueron pequeñas.
El efecto adverso primario, la epistaxis, fue más frecuente con la administración de dosis mayores (pruebas de calidad moderada).
Diferentes métodos de administración: spray nasal acuoso versus spray en aerosol
Se identificó sólo un estudio informado de manera deficiente con un alto riesgo de sesgo. No estaba claro cuántos participantes había: se incluyeron 91 en tres brazos. También hubo diferencias significativas entre los participantes de los dos grupos cuando comenzaron el estudio. No fue posible establecer conclusiones significativas a partir de este estudio.
Conclusiones
No se encontraron pruebas de que un tipo de corticosteroide intranasal sea más efectivo que otro en los pacientes con rinosinusitis crónica, ni de que las dosis mayores sean mejores que las más bajas, ni de que la efectividad de un spray sea diferente a la de un aerosol. No se encontró ningún estudio que comparara gotas nasales con spray. Se encontraron pruebas de calidad moderada de un riesgo mayor de epistaxis (hemorragia nasal) como un efecto adverso del tratamiento al administrar dosis mayores.
Más investigación en esta área es claramente necesaria. En el futuro los estudios deben estar diseñados de forma adecuada: deben medir la calidad de vida relacionada con la salud específica de la rinosinusitis crónica y los efectos adversos como resultados, y considerar lo que les sucede a los pacientes que reciben corticosteroides intranasales a más largo plazo.
Conclusiones de los autores
Summary of findings
| Different types of intranasal corticosteroid molecules for chronic rhinosinusitis | ||||||
| Patient or population: chronic rhinosinusitis (all studies recruited patients with bilateral polyps) | ||||||
| Outcomes № of participants | Relative effect (95%) | Anticipated absolute effects* (95% CI) | Quality | What happens | ||
| Low‐dose intranasal corticosteroids | High‐dose intranasal corticosteroids | Difference | ||||
| Disease‐specific health‐related quality of life | Not measured | Impact unknown | ||||
| Disease severity ‐ overall symptoms
| — |
| ⊕⊝⊝⊝ | No differences observed but evidence was too low quality to draw a conclusion | ||
| Adverse events: epistaxis
| — |
| ⊕⊝⊝⊝ | Unclear whether the risk of epistaxis varies for different types of steroid molecules | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Studies were either very small (n = 20 and n = 26) and had important drop‐outs or were only reported as an abstract with inadequate information available (n = 100). We considered all studies to be at unclear to high risk of selective reporting and attrition bias. The evidence was very low quality due to very serious imprecision and very serious risk of bias concerns. | ||||||
| High‐dose versus low‐dose intranasal corticosteroids for chronic rhinosinusitis | ||||||
| Patient or population: chronic rhinosinusitis (all studies recruited patients with bilateral polyps) | ||||||
| Outcomes № of participants | Relative effect | Anticipated absolute effects* (95% CI) | Quality | What happens | ||
| Low‐dose intranasal corticosteroids | High‐dose intranasal corticosteroids | Difference | ||||
| Disease‐specific health‐related quality of life | Not measured | Impact unknown | ||||
| Disease severity ‐ overall symptoms, measured as average change from baseline at 4 months | ||||||
| All 4 EPOS domains | No information available | |||||
| 3 domains (nasal blockage, rhinorrhoea, loss of sense of smell) Range 0 to 3, lower score = less severe № of participants: 237 | — | The mean disease severity ‐ overall symptoms, measured as average change from baseline at 4 months (range 0 to 3) ‐ average symptom score (3 domains) without high‐dose was ‐0.66 points | — | MD 0.13 points lower (0.37 lower to 0.11 more) than low‐dose group | ⊕⊕⊝⊝ | The average score for 3 types of symptoms seems to be similar between the high‐dose and low‐dose groups. |
| (2 domains: nasal blockage, rhinorrhoea) № of participants: 441 | — | The mean disease severity ‐ overall symptoms, measured as average change from baseline at 4 months (range 0 to 3) ‐ average symptom score (2 domains) without high‐dose was ‐0.73 points | — | MD 0.19 points lower (0.36 lower to 0.02 lower) than low‐dose group | ⊕⊕⊝⊝ | The average score for 2 types of symptoms seems to be slightly lower for the high‐dose group. The clinical significance of this reduction is unclear. |
| Disease severity ‐ measured as average change from baseline at 4 months (range 0 to 3) | ||||||
|
№ of participants: 441 | — | The mean disease severity ‐ individual symptoms, measured as average change from baseline at 4 months (range 0 to 3) ‐ nasal blockage without high‐dose was ‐0.86 points | — | MD 0.24 points lower (0.39 lower to 0.08 lower) than low‐dose group | ⊕⊕⊝⊝ | The nasal blockage score seems to be slightly lower in the high‐dose group. The clinical significance of this reduction is unclear. |
|
№ of participants: 441 (2 RCTs) | — | The mean disease severity ‐ individual symptoms, measured as average change from baseline at 4 months (range 0 to 3) ‐ rhinorrhoea without high‐dose was ‐0.6 points | — | MD 0.15 points lower (0.33 lower to 0.03 higher) than low‐dose group | ⊕⊕⊝⊝ | The average score for rhinorrhoea seems to be similar between the high‐dose and low‐dose groups. |
|
№ of participants: 237 | — | The mean disease severity ‐ individual symptoms, measured as average change from baseline at 4 months (range 0 to 3) ‐ loss of sense of smell without high‐dose was ‐0.6 points | — | MD 0.06 points higher (0.2 lower to 0.32 higher) than low‐dose group | ⊕⊕⊝⊝ | The average score for loss of sense of smell seems to be very similar between the high‐dose and low‐dose groups. |
| Adverse effects: epistaxis № of participants: 637 | RR 2.06 | Study population | ⊕⊕⊕⊝ | The risk of epistaxis is likely to be higher in the higher‐dose groups. However, the studies included very minor nosebleeds, such as blood stains in the mucus, and most of these events are not likely to be severe. | ||
| 57 per 1000 | 118 per 1000 | 61 more per 1000 (11 more to 145 more) | ||||
| Moderate | ||||||
| 60 per 1000 | 124 per 1000 | 64 more per 1000 (12 more to 153 more) | ||||
| Adverse effects: local irritation № of participants: 542 | RR 0.97 | Study population | ⊕⊕⊝⊝ | The risk of local irritation seems to be similar between groups, but the overall risks are underestimated due to the way the data were reported. | ||
| 19 per 1000 | 18 per 1000 | 10 fewer per 1000 (13 fewer to 43 more) | ||||
| Moderate | ||||||
| 17 per 1000 | 17 per 1000 | 10 fewer per 1000 (13 fewer to 40 more) | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Scale validity, particularly discriminant validity (ability to distinguish the differences between groups), was unclear. There was a high risk of reporting bias. Studies tended to report enough information for meta‐analysis only for statistically significant results. One study, which had 101 participants, reported very similar values for both intervention arms for all disease scores but had no information related to SD. 2Small sample size ‐ evidence only from one or two relatively small studies. 3Only data from patients with bilateral nasal polyposis. We considered this to be indirectness of the evidence to patients without polyps but have not further downgraded the evidence. 4One of the studies had inadequate blinding ‐ a double dummy was not used to mask the twice daily (higher) versus once daily (lower) dose; the study had 101 participants. 5Sample size relatively small for a precise estimate of adverse events. We downgraded this outcome once, after taking into consideration the inadequate blinding in one of the studies and the relatively small sample size. 6Studies did not use consistent terminology/methods to report different types of local irritation. For analysis we only selected the most frequent types of local irritation from a list (to avoid double counting). This is a possible underestimation of overall event rates. The relatively low event rates and small sample size contributed to the large confidence intervals. | ||||||
Antecedentes
Descripción de la afección
La rinosinusitis crónica se define como la inflamación de la nariz y los senos paranasales caracterizada por dos o más síntomas, uno de los cuales debe ser bloqueo / obstrucción / congestión nasal o secreción nasal (goteo nasal anterior / posterior). Los otros síntomas posibles incluyen dolor / presión facial, disminución o pérdida del sentido del olfato (en los adultos) o tos (en los niños). Los síntomas deben haber continuado durante al menos 12 semanas. Además los pacientes deben presentar cambios en la mucosa dentro del complejo osteomeatal o los senos según lo demostrado en la tomografía computadorizada (TC) o signos endoscópicos de al menos uno de los siguientes: pólipos nasales, secreción mucopurulenta principalmente del meato medio o edema / obstrucción de la mucosa principalmente en el meato medio (EPOS 2012).
La rinosinusitis crónica representa una fuente frecuente de enfermedad; el 11% de los adultos del Reino Unido informaron síntomas de rinosinusitis crónica en un estudio en la población general (Hastan 2011). Los síntomas, que incluyen obstrucción nasal, secreción nasal, dolor facial, anosmia y trastornos del sueño, tienen una repercusión importante sobre la calidad de vida, supuestamente mayor en varios dominios del SF‐36 que la angina o la enfermedad respiratoria crónica (Gliklich 1995). Son frecuentes las exacerbaciones agudas, el control insuficiente de los síntomas y la exacerbación de las enfermedades respiratorias. Las complicaciones son poco frecuentes, pero pueden incluir deficiencia visual e infección intracraneal.
Se han identificado dos fenotipos principales de rinosinusitis crónica según la presencia o ausencia de pólipos nasales al examen. Los pólipos nasales son tumefacciones hiperplásicas similares a tumores de la mucosa nasal, y la mayoría se origina habitualmente desde el interior del complejo osteomeatal (Larsen 2004). La rinosinusitis crónica con pólipos nasales (RSCcPN) se diagnostica cuando se observan pólipos (en el examen directo o endoscópico) bilaterales en el meato medio. Las siglas RSCsPN se utilizan para la afección en la que no hay pólipos presentes.
Aunque la etiología de la rinosinusitis crónica no se comprende completamente, puede incluir anomalías en la respuesta del huésped a los irritantes, microorganismos comensales y patógenos y alérgenos, obstrucción de las vías de drenaje de los senos, anomalías de la función mucociliar normal, pérdida de la barrera de la mucosa normal o infección. Se pueden observar dos perfiles característicos en cuanto a los mediadores inflamatorios; en la rinosinusitis crónica eosinofílica, que se asocia habitualmente con pólipos nasales, es posible encontrar niveles altos de eosinófilos, inmunoglobulina E (IgE) e interleucina (IL)‐5, mientras que en la rinosinusitis crónica neutrofílica, asociada con mayor frecuencia con la rinosinusitis crónica sin pólipos, predominan los neutrófilos, con el interferón (IFN) gamma, la IL‐8 y el factor de necrosis tumoral (FNT) elevados (EPOS 2012).
Aunque las decisiones sobre el tratamiento se deben tomar según una comprensión del fenotipo de la rinosinusitis crónica del paciente y su probable etiología, en la práctica el tratamiento se puede iniciar sin el conocimiento del estado de los pólipos, en particular en la atención primaria. Esta revisión (y la mayoría de las revisiones acompañantes) consideran juntos a los pacientes con y sin pólipos para la evaluación inicial de los efectos del tratamiento. Sin embargo, los análisis de subgrupos exploran las posibles diferencias entre estos pacientes.
Las intervenciones utilizadas con mayor frecuencia para la rinosinusitis crónica se utilizan tópica (en spray nasal) o sistémicamente (por vía oral) e incluyen corticosteroides, antibióticos y solución salina.
Descripción de la intervención
El tratamiento antiinflamatorio tiene una función significativa en el tratamiento de la rinosinusitis crónica. Incluye corticosteroides y macrólidos a dosis baja. Los corticosteroides tópicos se utilizan de manera más amplia que los corticosteroides orales debido a que el tratamiento se puede administrar durante un tiempo mayor sin efectos adversos significativos.
El tratamiento intranasal con corticosteroides se les prescribe a menudo a los pacientes con rinosinusitis crónica, pero existe una variabilidad considerable en el momento, la frecuencia, la dosis, el método de administración tópica y el agente específico utilizado (Benninger 2003; Spector 1998). El método de aplicación tópica afecta de modo significativo la cantidad de esteroides que entran en contacto con la mucosa de los senos paranasales (Grobler 2008; Harvey 2009). Los métodos de administración intranasal más sencillos son las gotas, los sprays, los aerosoles, los nebulizadores y los atomizadores. Dichos métodos contrastan con los que involucran la canalización directa de los senos y la irrigación nasal con frascos exprimibles y recipientes para limpieza nasal, que es probable que proporcionen un mejor acceso a los senos, especialmente en el contexto posterior a la cirugía de los senos (Grobler 2008; Harvey 2009; Thomas 2013).
Las clases de corticosteroides tópicos incluyen los esteroides intranasales de primera generación (dipropionato de beclometasona, acetónido de triamcinolona, flunisolida y budesonida) y preparados más nuevos (propionato de fluticasona, furoato de mometasona, ciclesonida y furoato de fluticasona).
De qué manera podría funcionar la intervención
La administración de corticosteroides tópicos (intranasales) para el tratamiento de la rinosinusitis crónica se ha recomendado ampliamente debido a la creencia de que la inflamación es un componente principal de esta afección(Fokkens 2007; Hamilos 2000; McNally 1997). El mecanismo de acción es una combinación de efectos antinflamatorios (por ejemplo, reducción de la transcripción del gen proinflamatorio, y aumento del antiinflamatorio, y reducción de la infiltración de células inflamatorias en las vías respiratorias) y supresión de la producción de mediadores proinflamatorios, factores quimiotácticos de las células y moléculas de adhesión (Mullol 2009). Los corticosteroides diferentes, en diferentes dosis, administrados en formas diferentes (como sprays versus gotas, por ejemplo) pueden ser distintos en cuanto a su efectividad. Los efectos adversos también pueden ser diferentes.
Por qué es importante realizar esta revisión
Los corticosteroides intranasales son la base y el tratamiento recomendado actualmente para la rinosinusitis crónica. Esta revisión incorpora una actualización de dos revisiones Cochrane anteriores (Kalish 2012; Snidvongs 2011). Esta revisión es importante debido a que considera la pregunta clínica importante de qué tipo, dosis o método de administración de los corticosteroides intranasales es más efectivo o seguro para el tratamiento de la rinosinusitis crónica. A diferencia de la revisión complementaria que procura establecer la efectividad de los corticosteroides intranasales versus placebo (Chong 2016a), esta revisión considera los estudios que proporcionan comparaciones directas de estos factores.
Esta revisión es una de un conjunto de revisiones Cochrane que examinan las opciones habituales de tratamiento para los pacientes con rinosinusitis crónica (Chong 2016a; Chong 2016b; Head 2016a; Head 2016b; Head 2016c), y utiliza las mismas medidas de resultado entre las revisiones. No se incluyeron los estudios diseñados para evaluar las intervenciones en el período periquirúrgico inmediato, que se centran en la evaluación de la repercusión de la intervención sobre el procedimiento quirúrgico o en la modificación de los resultados posquirúrgicos (prevenir la recurrencia).
Objetivos
Evaluar los efectos relativos de diferentes tipos, métodos de administración y dosis de los corticosteroides intranasales.
Métodos
Criterios de inclusión de estudios para esta revisión
Tipos de estudios
Se incluyeron estudios con las siguientes características de diseño:
-
ensayos controlados aleatorios, incluidos los ensayos aleatorios grupales y los ensayos cuasialeatorios (los ensayos cruzados solamente se incluyeron si estaban disponibles los datos de la primera fase); y
-
los pacientes se siguieron durante al menos dos semanas.
Se excluyeronestudios con las siguientes características de diseño:
-
pacientes asignados al azar por lado de la nariz (controlado en el mismo paciente) porque es difícil asegurar que los efectos de cualquiera de las intervenciones consideradas puedan ser localizados; o
-
estudios perioperatorios, en los que el único objetivo del estudio fue investigar el efecto de los corticosteroides intranasales sobre el resultado quirúrgico.
Tipos de participantes
Pacientes con rinosinusitis crónica, con o sin pólipos.
Se excluyeron los estudios que incluyeron una mayoría de pacientes con:
-
fibrosis quística;
-
sinusitis micótica alérgica / micótica eosinofílica / rinosinusitis mucinosa;
-
enfermedades respiratorias exacerbadas por la aspirina;
-
pólipos antrocoanales (pólipos benignos que se originaron de la mucosa del seno maxilar);
-
pólipos malignos;
-
discinesia ciliar primaria
-
antecedentes de cirugía por pólipos nasales en el transcurso de seis semanas del ingreso al estudio.
Tipos de intervenciones
Todos los corticosteroides intranasales; se incluyeron los sprays nasales y las gotas nasales.
Corticosteroides intranasales de primera generación:
-
Dipropionato de beclometasona
-
Acetónido de triamcinolona
-
Flunisolida
-
Budesonida
Corticosteroides intranasales de segunda generación:
-
Ciclesonida
-
Furoato de fluticasona
-
Propionato de fluticasona
-
Furoato de mometasona
-
Fosfato sódico de betametasona
Si se utilizaron otras intervenciones, se deberían haber utilizado en ambos brazos de tratamiento. Las cointervenciones permitidas incluyeron:
-
irrigación nasal con solución salina;
-
antibióticos; y
-
descongestionantes nasales intermitentes.
El principal par de comparación posible fue:
-
cualquier corticosteroide de primera generación versus cualquier corticosteroide de segunda generación.
Otros pares de comparación posibles fueron:
-
corticosteroide intranasal administrado como spray versus corticosteroide intranasal administrado como gotas; y
-
corticosteroide intranasal en dosis baja versus corticosteroide intranasal en dosis alta.
Esta revisión forma parte de una serie mayor de seis revisiones para el tratamiento de la rinosinusitis crónica.
-
Corticosteroides intranasales versus placebo o ninguna intervención para la rinosinusitis crónica (Chong 2016a).
-
Diferentes tipos de corticosteroides intranasales para la rinosinusitis crónica (esta revisión). Esta revisión compara diferentes clases, dosis y métodos de administración de los corticosteroides intranasales para la rinosinusitis crónica.
-
Corticosteroides orales de corta duración solos para la rinosinusitis crónica (Head 2016a). Esta revisión compara los corticosteroides orales de corta duración solos con placebo o ninguna intervención, o versus otras intervenciones farmacológicas como los antibióticos o la irrigación nasal de solución salina.
-
Corticosteroides orales de corta duración como tratamiento complementario para la rinosinusitis crónica (Head 2016b). Esta revisión compara los corticosteroides orales cuando se utilizaron como tratamiento complementario a otros tratamientos para la rinosinusitis crónica (como corticosteroides intranasales, antibióticos o solución salina).
-
Irrigación con solución salina para la rinosinusitis crónica(Chong 2016b). Esta revisión compara la irrigación nasal con solución salina para la rinosinusitis crónica con placebo / ninguna intervención y con corticosteroides intranasales o antibióticos.
-
Antibióticos sistémicos y tópicos para la rinosinusitis crónica (Head 2016c). Esta revisión compara los antibióticos tópicos y sistémicos con placebo / ningún tratamiento, dos antibióticos diferentes entre sí y antibióticos con corticosteroides intranasales.
Tipos de medida de resultado
Se analizaron los siguientes resultados en la revisión, aunque no se utilizaron como base para incluir o excluir los estudios.
Resultados primarios
-
Calidad de vida relacionada con la salud, mediante puntuaciones de calidad de vida relacionada con la salud específica de la enfermedad como la Sino‐Nasal Outcome Test‐22 (SNOT‐22), las Rhinosinusitis Outcome Measures‐31 (RSOM‐31) y la SNOT‐20.
-
Gravedad de la enfermedad, medida según la puntuación de los síntomas informados por el paciente (como el Chronic Sinusitis Survey [CSS] y escalas analógicas visuales). A falta de los datos validados de la puntuación de los síntomas, se informaron las puntuaciones de los síntomas individuales informadas por los pacientes para los siguientes síntomas: obstrucción/bloqueo/congestión nasal, secreción nasal (rinorrea), presión/dolor facial, pérdida del sentido del olfato (adultos) y tos (niños).
-
Efecto adverso significativo: epistaxis.
Resultados secundarios
-
Calidad de vida relacionada con la salud, mediante puntuaciones genéricas de la calidad de vida como SF‐36, EQ‐5D y otros instrumentos correctamente validados.
-
Otros efectos adversos: irritación local (incluida la candidiasis bucal, dolor de garganta y otra irritación nasal local como sequedad, picazón etc.).
-
Otros efectos adversos:
-
en los niños ‐ crecimiento atrofiado (punto temporal mínimo: seis meses de tratamiento y seguimiento);
-
en adultos: osteoporosis.
-
-
Puntuación endoscópica (según la población, o la puntuación del tamaño de los pólipos nasales o la puntuación por endoscopia, p.ej. Lund‐Mackay/Lund‐Kennedy).
-
Puntuación del examen por tomografía computarizada (TC) (p.ej. Lund‐Mackay).
Los resultados se midieron a los tres a seis meses, a los seis a 12 meses y más de 12 meses. Para los eventos adversos, se analizaron los datos de los períodos de tiempo más largos.
Results
Description of studies
Results of the search
The searches retrieved a total of 2470 references after removal of duplicates. We screened titles and abstracts and subsequently removed 2429 references. We assessed 41 full texts for eligibility. We excluded 18 studies (21 references), with reasons. We included nine studies (13 references). We identified three ongoing studies. There are four studies awaiting assessment because we cannot locate the full‐text papers.
A flow chart of study retrieval and selection is provided in Figure 1.
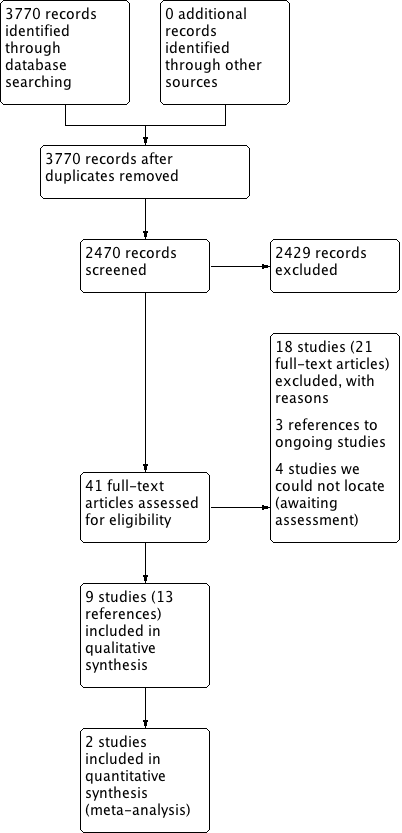
Process for sifting search results and selecting studies for inclusion.
Included studies
Design
All studies included were randomised trials and most were double‐blinded (in two studies blinding was not stated).
Sample sizes
The studies included ranged in size from small studies with as few as 20 patients in the treatment arms of interest (Lund 1998), to much larger studies, which included over 200 participants (Small 2005; Stjarne 2006).
Setting
All studies were conducted in a secondary or tertiary care setting and in various international locations, including three Scandinavian studies. It was notable that there were no studies from the Asian continent.
Participants
The participants in all but one study were adults ranging from 18 to 86 years old; the one paediatric study had an age range of 6 to 17. The adult participants in all but one study were predominantly male (range 72% to 79%), with one study including only 38% male participants. In all studies the participants had chronic rhinosinusitis with nasal polyps with visible polyps on nasal examination. There were no studies including patients with chronic rhinosinusitis without nasal polyps.
Interventions
The details of the interventions are shown in Table 1 under the following headings: comparison of different steroid molecules (three studies), high‐dose versus low‐dose (five studies), twice daily versus once daily and different delivery methods (one study). All studies had a placebo arm, except one (Demirel 2008).
Intranasal steroid formulations included were fluticasone propionate, beclomethasone dipropionate, mometasone furoate and budesonide (see below).
Summary of studies comparing different steroid molecules
| Study ID | Polyps status | Intervention | Comparison | Delivery method | Daily dose | Dosing regime | Treatment time |
| Bilateral polyposis in asthma patients | Fluticasone propionate | Mometasone furoate | Nasal spray | 200 µg | Once daily | 3 months | |
| Bilateral polyps (polyp score 1 or 2) | Fluticasone propionate | Beclomethasone dipropionate | Nasal spray | 400 µg | Twice daily | 26 weeks | |
| Bilateral nasal polyposis requiring surgical intervention | Fluticasone propionate | Beclomethasone dipropionate | Nasal spray | 400 µg | Twice daily | 12 weeks |
Summary of studies comparing high‐dose versus low‐dose steroids
| Study ID | Polyps status | Drug | Delivery method | Daily dose (Intervention) | Regimen | Daily dose (Comparison) | Regime | Duration of treatment |
| Bilateral | Mometasone furoate | Nasal spray | 200 µg (6 to 11 years); 400 µg (12 to 18 years) | Twice daily | 100 µg (6 to 11 years); 200 µg (12 to 18 years) | Once daily | 4 months | |
| Bilateral, clinically significant congestion/obstruction | Mometasone furoate | Nasal spray | 400 µg | Twice daily | 200 µg | Once daily | 4 months | |
| Bilateral, clinically significant congestion/obstruction | Mometasone furoate | Nasal spray | 400 µg | Twice daily | 200 µg | Once daily | 4 months | |
| Bilateral mild or moderate nasal polyposis | Fluticasone propionate | Nasal drops | 800 µg | Twice daily | 400 µg | Once daily | 12 weeks | |
| Bilateral | Fluticasone propionate | Nasal drops | 800 µg | Twice daily | 400 µg | Once daily | 12 weeks |
Summary of studies comparing different delivery methods
| Study ID | Polyps status | Drug | Method | Daily dose | Regime | Drug | Method | Daily dose | Regime | Duration |
| Eosinophilic nasal polyposis with polyp scores of 2 or less on each side | Budesonide | Aqueous nasal spray | 400 µg | Twice daily | Budesonide | Aerosol | 400 µg | Twice daily | 3 months |
Outcomes
Only one study included a disease‐specific health‐related quality of life (HRQL) tool for outcome assessment and only three studies included an assessment of overall disease severity. Nasal obstruction and loss of sense of smell as individual symptoms were assessed in all studies but other chronic rhinosinusitis symptoms were variably and inconsistently checked. No studies included generic HRQL tools. Endoscopic grading of polyps was reported in all studies. Adverse events were reported in all but one study (Demirel 2008). Epistaxis, which is an outcome of interest of this review, was defined to include a wide range of bleeding episodes, from frank bleeding to bloody nasal discharge to flecks of blood in the mucus in two studies (Small 2005; Stjarne 2006). The other studies did not provide a definition of epistaxis, but would have been likely to include non‐severe episodes since very few of the withdrawals were related to epistaxis.
Funding and conflict of interest
All of the studies (except Demirel 2008 and Filipovic 2006, which did not provide any information on funding or conflicts of interest) were either directly funded by pharmaceutical companies that manufacture one or more of the interventions compared, financially supported by industry including the companies (Glaxo Wellcome, Schering Plough, Astra and Merck Sharpe and Dohme), or had authors who were employees or recipients of other types of funding from the companies.
Excluded studies
We excluded 17 papers after reviewing the full text. Further details for the reasons for exclusion can be found in the Characteristics of excluded studies table. Ten of the studies were clinical trials that made a comparison relevant to this review but we excluded them due to the duration of the treatment not meeting the inclusion criterion of 12 weeks. Five of these treated and followed up patients for one month or less (Lildholdt 1995; NCT01405339; Reychler 2015; Toft 1982; Wang 2012), and four treated and followed up patients for between six and eight weeks (Filiaci 2000; Jankowski 2001; Raghavan 2006; Tos 1998). The remaining study compared betamethasone with fluticasone propionate with a treatment duration of eight weeks, although the follow‐up time was 12 weeks (Fowler 2002).
We excluded five studies due to the included population. In four of these papers all patients underwent sinus surgery either immediately before the trial started or during the trial (Bross‐Soriano 2004; Dijkstra 2004; NCT02194062; Singhal 2008). We excluded the other study due to the population: it stated that the participants had allergic or non‐allergic chronic rhinosinusitis, but on closer inspection of the inclusion criteria we thought that it included only people with allergic or non‐allergic rhinitis (Giger 2003).
Of the remaining two studies, one was a clinical trial register record of a study that was going to compare two different delivery methods (aerosol versus spray) but the study authors confirmed that the trial had not been completed or published (NCT00788463). The reason for early termination was not provided. The other was a study looking at the optimal method for delivery of intranasal spray, which studied the distribution of dye at five sinonasal sites (Cannady 2005).
Ongoing studies
We identified three relevant ongoing studies, all of which are in adults with chronic rhinosinusitis with nasal polyps (NCT01622569; NCT01624662; NCT01946711). Two of these are large, multicentre trials each with a planned population of over 300 patients (NCT01622569; NCT01624662). These two trials will make the same comparisons, comparing three different doses of fluticasone proportionate (400 µg bid, 200 µg bid and 100 µg bid) with placebo. All of the arms will use a novel bi‐directional device. The studies were completed in October 2015 but no study data were available at the time of writing. The other trial compares two delivery methods for budesonide (inhalation versus nasal spray) (NCT01946711). We contacted the investigators and they reported that the trial should be completed during 2016.
Risk of bias in included studies
See Figure 2 for the 'Risk of bias' graph (our judgements about each risk of bias item presented as percentages across all included studies) and Figure 3 for the 'Risk of bias' summary (our judgements about each risk of bias item for each included study).
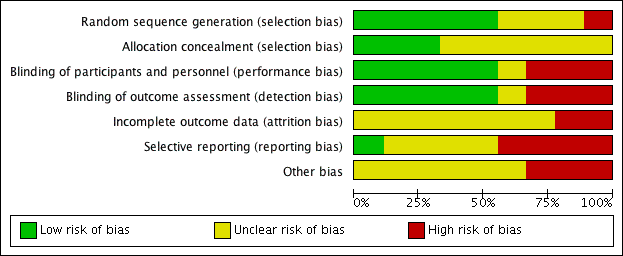
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
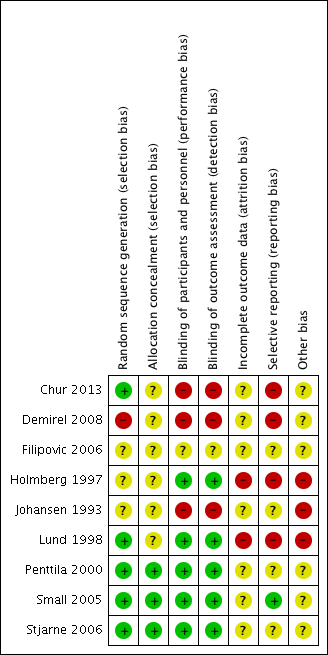
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Sequence generation
Three of the included studies provided a description that suggested that adequate sequence generation was conducted (Chur 2013; Lund 1998; Stjarne 2006). Another three stated that the trials were randomised but did not provide further information, making them at an 'unclear' risk of bias (Filipovic 2006; Holmberg 1997; Johansen 1993). Penttila 2000 and Small 2005 also did not provide details of randomisation. However, these studies were conducted fairly recently as multinational trials, and therefore should have sufficient methodology and resources to ensure that adequate sequence generation procedures were carried out. We rated these as low risk of bias.
Another study stated that patients were "randomly divided" (Demirel 2008). However, we rated this study as high risk of bias because the baseline risks, particularly the age of the participants, were not balanced between the groups. It was also a very small study, with 11 participants randomised to the once daily group and 15 to the twice daily group.
Allocation concealment
None of the studies described how allocation concealment was carried out, so we judged them all as unclear risk of bias. However, Penttila 2000, Small 2005 and Stjarne 2006 are large multinational trials, which should have adequate sequence generation, adequate blinding and no other factors suggesting that allocation concealment could be compromised. We considered these to have low risk of bias. Although Chur 2013 also had adequate sequence generation, it used blocked randomisation with unclear effectiveness of blinding and therefore it is unclear whether allocation concealment was well maintained.
Blinding
The ratings for the risk of performance bias versus detection bias were very well correlated for this review.
Most of the outcomes were assessed by patients and the overall risks of bias were low when both participants and investigators were adequately blinded. We did not find information suggesting that the clinicians could have obtained extra information from blood tests etc. to 'guess' the which treatment the patients were allocated to.
One study was an abstract and stated that it was a single‐blinded study but did not provide information on who was blinded (Filipovic 2006). However, since the study compared different drugs with the same delivery method (nasal spray) and dosing schedule (once daily), we rated this as unclear risk of bias rather than high risk.
All the other eight studies described using a "double blinded" design their report. However, we only considered the risk of both performance and detection bias to be low for five of the studies, with adequate measures to mask the type of treatment given (Holmberg 1997; Lund 1998; Penttila 2000; Small 2005; Stjarne 2006).
We rated blinding as inadequate (high risk of bias) in three studies, despite their being reported as 'double‐blinded' studies (Chur 2013; Demirel 2008; Johansen 1993). The blinding was inadequate in these studies, as there was no placebo or 'dummy' used to account for differences in the number of times treatment was administered or methods of delivery. In Chur 2013, participants "received MFNS 200 mcg once daily, MFNS 200 mcg twice daily, placebo once daily, or placebo twice daily", instead of using a double‐dummy design, where all participants received the medication twice daily (with a placebo given for those who had once daily treatment); groups either had medication once or twice daily. Therefore, there was no blinding of participants in terms of knowing whether they were on the once daily or twice daily regimen.
Similarly, Johansen 1993 stated that "The patients were treated with either budesonide aqua (Rhinocort Aqua) or budesonide aerosol (Rhinocort Aerosol), 50 mcg x 2 in each nostril, twice daily = 400 mcg/day or placebo (aqua) or aerosol)." Whilst there may be adequate blinding for treatment versus placebo, there is no blinding when comparing different dosage forms.
Although Demirel 2008 claimed to be double‐blinded, the interventions were given in a different format (nasal spray versus nasal drops) and at different frequencies (one versus two times per day), so it is difficult to see how either the personnel or participants were blind to the intervention. There was no mention of a placebo.
Incomplete outcome data
The risk of attrition bias was unclear in seven of the included studies (Chur 2013; Demirel 2008; Filipovic 2006; Johansen 1993; Penttila 2000; Small 2005; Stjarne 2006). These studies did not provide enough information to adequately judge the risk. For example, Johansen 1993 reported that 5/91 (5.5%) participants did not complete the study. There is no information on how many were randomised to each group in Johansen 1993, so it is difficult to determine whether this could have affected the results.
In two studies that were three‐arm trials including a placebo group (Small 2005; Stjarne 2006), we considered the overall risk of attrition bias to be high due to imbalances in the proportion of drop‐outs between the active and placebo groups. However, the drop‐out rates for the active intervention groups, which are of interest in this review, were similar and we still considered them acceptable. Therefore we considered these studies as being at an unclear risk of attrition bias for this review, but at a high risk for our accompanying review, which assesses intranasal steroids versus placebo (Chong 2016a).
We rated the risk of attrition bias as high for two studies. Lund 1998 only included 10 participants in each of the fluticasone and beclomethasone groups. Three patients dropped out from the fluticasone group (70%), but none dropped out from the beclomethasone group. This study carried out last observed carried forward observation (LOCF) for the missing outcomes. In Holmberg 1997, the number of participants who dropped out was twice as high in one group (4/19 in the fluticasone propionate group and 2/18 in the beclomethasone propionate group).
Selective reporting
Many of the study reports only presented effectiveness outcomes in graphs and only provided limited, selective information, for example P values or mean values when statistical significance was noted. Since many of the effectiveness outcomes did not show a significant difference between the intervention and comparison groups in this review (i.e. there were no noticeable differences between the different types of corticosteroids, methods of delivery, doses or number of administrations per day), we are uncertain whether this lack of detail in reporting is related to the lack of 'positive' results.
We considered only one study to be at low risk of bias, as all expected outcomes were reported (Small 2005).
We considered the risk of selective reporting bias to be high in four studies (Chur 2013; Demirel 2008; Holmberg 1997; Lund 1998).
Two studies reported the use of diaries for patients to record symptoms (Holmberg 1997; Lund 1998). However, neither study provided information on how the collected data would be analysed and the results were subsequently presented in a variety of ways with different cut‐off points, where it is not clear why they were selected.
The primary endpoint in Chur 2013 was "safety" (cortisol levels) and despite presenting the mean change values for effectiveness outcomes, they did not provide any information on P values or standard deviations. The study authors' rationale for collecting but not fully reporting the data was: "No statistical analysis of efficacy end points was pre‐specified in the study protocol, and only descriptive efficacy statistics were collected." We observed that these values (mean changes) were similar between groups and unlikely to be statistically significant, so poor reporting due to lack of beneficial effects cannot be ruled out. Similarly, Demirel 2008 mainly reported outcomes in graphs and did not provide information on standard deviations and P values, which are necessary for meta‐analysis.
We considered the remaining three studies to be at unclear risk. There was not enough information in the methods and/or protocol and we found it difficult to judge whether there was a risk of reporting bias (Filipovic 2006; Johansen 1993; Penttila 2000).
Other potential sources of bias
Use of validated outcome measures
The lack of use of validated outcome measures is a major concern in terms of bias. If an instrument is insensitive for measuring differences, this biases towards a finding of 'no difference' in the studies and also in this review.
None of the included studies mentioned using validated outcome measures, for either of the primary outcomes of effectiveness (disease‐specific health‐related quality of life and disease severity/symptom scores). Of the studies that attempted to use patient diaries or questionnaires to measure severity, most used a 0 to 3 scale. There is no evidence that this scale, especially when used as a single scale, has the sensitivity to distinguish between groups of patients who improved versus those who did not improve (discriminant validity). None of the studies attempted to assess all of the four symptoms used to define chronic rhinosinusitis that are mentioned in EPOS 2012 (nasal blockage, rhinorrhoea/rhinitis, loss of sense of smell and facial pain (adults)/cough (children)). Facial pain was not measured by most studies.
The scales used to measure nasal polyps were generally well described. However, again it is unclear whether a 0 to 3 scale is has the discriminant validity to detect a difference in these small trials.
Effects of interventions
See: Summary of findings for the main comparison Different types of intranasal corticosteroid molecules for chronic rhinosinusitis; Summary of findings 2 High‐dose versus low‐dose intranasal corticosteroids for chronic rhinosinusitis
Where the range of scales and values for minimal important differences were unclear, we used the standardised mean difference (SMD) to estimate the effect sizes. As suggested in the Cochrane Handbook for Systematic Reviews of Interventions (Handbook 2011), we used standard rules of thumb in the interpretation of effect sizes (SMD, or Cohen's effect size of < 0.41 = small, 0.40 to 0.70 = moderate, > 0.70 = large) (Cohen 1988).
Comparison 1: Different type of corticosteroids: fluticasone propionate versus beclomethasone dipropionate
We found two studies in participants with bilateral polyps (a combined sample size of 56) comparing fluticasone propionate aqueous nasal spray (FPANS) versus beclomethasone dipropionate aqueous nasal spray (BDANS) at a daily dose of 400 µg, delivered using nasal sprays twice a day. However, the results were poorly reported and there was insufficient information to conduct any pooling of data (Holmberg 1997; Lund 1998). The follow‐up was 26 weeks for Holmberg 1997 and 12 weeks for Lund 1998.
Primary outcomes
Health‐related quality of life, using disease‐specific health‐related quality of life scores, such as the Sino‐Nasal Outcome Test‐22 (SNOT‐22), Rhinosinusitis Outcome Measures‐31 (RSOM‐31) and SNOT‐20
Neither Holmberg 1997 nor Lund 1998 mentioned measuring quality of life.
Disease severity, as measured by patient‐reported symptom score (such as the Chronic Sinusitis Survey (CSS) questionnaire and visual analogue scales)
Neither of the studies provided patient‐reported total symptoms score results using an instrument validated in a chronic rhinosinusitis population. Both studies included information about measuring a patient‐reported symptom score in their methods section, but did not report much information at all about these. Instead, some form of physician‐rated scores were reported.
In Holmberg 1997, the methods section described patients recording the following symptoms on daily record cards: nasal blockage on waking in the morning, nasal blockage during the rest of the day, sense of smell and rhinorrhoea. The outcomes were reported on a four‐point scale (0 to 3, 0 = no symptoms, 3 = severe symptoms). These were not well reported in the results. Instead, they reported "physician's assessment of symptoms", which was not mentioned in the methods section or defined anywhere else in the paper.
The methods section of Lund 1998 reported that patients were issued with daily record cards to assess nasal blockage, sense of smell, degree of nasal discomfort (facial pain and headache) and overall rhinitis symptoms (sneezing, rhinorrhoea, nasal itching) on a five‐point rating scale (0 to 4). However, the results section only reported percentage of days with "no nasal blockage during the day" ("...trend for FPANS to be more effective") and percentage of days with "no rhinitis symptoms in the day" (a median value of 89% and 96% for FPANS‐treated and BDANS‐treated groups, respectively, at week 12).
Significant adverse effect: epistaxis
Holmberg 1997 reported that "Adverse events were reported in 14 patients (78%) receiving placebo, 13 patients (68%) receiving fluticasone propionate aqueous nasal spray, and 16 patients (89%) receiving beclomethasone dipropionate aqueous nasal spray". However, they mentioned that "The only predictable adverse event considered drug related was epistaxis". No specific figures were provided.
Lund 1998 reported that "There were more adverse events (7 [70%]) reported in the FPANS‐treated group compared with those (3 [33%]) in the group receiving placebo and in the BDANS‐treated group (3 [30%])." There was no information about whether any of these events were epistaxis.
Secondary outcomes
Health‐related quality of life, using generic quality of life scores, such as the SF‐36, EQ‐5D and other well‐validated instruments
Neither Holmberg 1997 nor Lund 1998 mentioned measuring quality of life.
Other adverse effects: local irritation (including nasal irritation, oral thrush, sore throat)
Lund 1998 reported that there was "1 predictable adverse event ‐ throat irritation ‐ in the FPANS‐treated group" (1 in 10 patients). It is unclear whether any other events reported by the 10 patients with adverse effects (seven in the fluticasone group, three in the budesonide group) were related to other forms of local irritation.
Other adverse effects, such as stunted growth in children and osteoporosis in adults (minimum time point: six months of treatment and follow‐up)
Although Holmberg 1997 followed up patients for six months, this outcome was not reported.
Endoscopic score (depending on population, either nasal polyps size score or endoscopy score, e.g. Lund‐Kennedy/Lund‐Mackay)
Lund 1998 reported that the median total polyps score (range 0 to 6) was 2 in the fluticasone group and 2.5 in the beclomethasone group, with a reported P value of 0.66. However, this included values from patients who had dropped out from the study (3/10 in the fluticasone group) and was imputed using a last observation carried forward (LOCF) method.
Computerised tomography (CT) scan score (e.g. Lund‐Mackay)
A CT scan was conducted at baseline to determine eligibility in Lund 1998 but was not reported as an outcome.
The quality of the evidence is very low (GRADE) for all outcomes in this comparison. See summary of findings Table for the main comparison.
Comparison 2: Different types of corticosteroids: fluticasone propionate versus mometasone furoate
We only found one abstract for a study that compared fluticasone propionate versus mometasone furoate, 200 µg, administered once daily as an aqueous spray (Filipovic 2006).
The abstract only mentioned that "both drugs produced statistically significant reductions" (P value < 0.01) in nasal obstruction, postnasal drip, anterior rhinorrhoea and an improvement, which is presumably compared to baseline. The study also stated that "no statistically significant differences were observed between the two drugs for most evaluated parameters".
The study reported that both drugs were "well tolerated" without providing any further information.
The quality of the evidence is very low (GRADE) for all outcomes in this comparison. See summary of findings Table for the main comparison.
Comparison 3: High‐dose versus low‐dose intranasal steroids
There were five studies, with a total of 663 participants in the intervention arms, which compared a higher dose of intranasal corticosteroids (administered twice a day) versus a lower dose (administered once a day) (Chur 2013; Demirel 2008; Penttila 2000; Small 2005; Stjarne 2006). One of these was in children aged between 6 and 18 years (Chur 2013).
| Study ID | Polyps status | Drug | Delivery method | Daily dose (Intervention) | Regime | Daily dose (Comparison) | Regime | Duration of treatment |
| Bilateral | Mometasone furoate | Nasal spray | 200 µg (6 to 11 years); 400 µg (12 to 18 years) | Twice daily | 100 µg (6 to 11 years) | Once daily | 4 months | |
| Bilateral, clinically significant congestion/obstruction | Mometasone furoate | Nasal spray | 400 µg | Twice daily | 200 µg | Once daily | 4 months | |
| Bilateral, clinically significant congestion/obstruction | Mometasone furoate | Nasal spray | 400 µg | Twice daily | 200 µg | Once daily | 4 months | |
| Bilateral mild or moderate nasal polyposis | Fluticasone propionate | Nasal drops | 800 µg | Twice daily | 400 µg | Once daily | 12 weeks | |
| Bilateral | Fluticasone propionate | Nasal drops | 800 µg | Twice daily | 400 µg | Once daily | 12 weeks |
Primary outcomes
Health‐related quality of life, using disease‐specific health‐related quality of life scores, such as the Sino‐Nasal Outcome Test‐22 (SNOT‐22), Rhinosinusitis Outcome Measures‐31 (RSOM‐31) and SNOT‐20
None of the studies mentioned measuring quality of life.
Disease severity, as measured by patient‐reported symptom score (such as the Chronic Sinusitis Survey (CSS) questionnaire and visual analogue scales)
None of the papers provided results for a patient‐reported total symptoms score using an instrument validated in a chronic rhinosinusitis population. Where available, we combined the results for the individual symptoms into a total score according to the methods set out in Dealing with missing data. In order to be included in the analysis the results had to at least meet the EPOS 2012 diagnostic criteria, which requires at least two symptoms, one of which must be nasal blockage/obstruction/congestion or nasal discharge (anterior/posterior nasal drip) with the other possible symptoms being facial pressure/pain, loss of sense of smell (adults) or cough (children).
Three studies reported results for individual symptoms but the results were presented in different ways making analysis difficult (Chur 2013; Small 2005; Stjarne 2006). The remaining two studies only recorded clinician‐rated symptoms so this information has not been presented (Demirel 2008; Penttila 2000).
Chur 2013 measured and partially reported some data for the individual symptoms of nasal congestion/obstruction, anterior rhinorrhoea/postnasal drip and loss of sense of smell. The symptoms were reported by participants (with the assistance of a parent or guardian if needed) and scored on a 0‐ to 4‐point scale. These results were presented as mean change from baseline at four months. The paper did not present standard deviations or P values for the results, the rationale for which was that the study's primary outcome was safety and they had not specified in the protocol that the effectiveness results would be analysed. However, with a mean difference of change of 0.1 points, it is unlikely that there is an important difference between the groups either clinically or statistically (see results presented below).
Small 2005 and Stjarne 2006 both asked participants to score the symptoms nasal congestion/obstruction, loss of sense of smell and anterior rhinorrhoea on a four‐point scale. The results were presented separately in graphs as the change from baseline values. P values for the between‐group differences were only given for some comparison pairs to denote the level of statistical significance, for example "P < 0.05", "P < 0.01" etc. There was sufficient information to impute standard deviations based on these values for nasal blockage and rhinorrhoea for both studies. However, there was no statistically significant difference between the groups for loss of sense of smell in Stjarne 2006 and no P values were reported.
Overall symptom scores
None of the studies provided enough information to enable the calculation of an overall symptom score for all four groups of symptoms used for the definition of chronic rhinosinusitis in EPOS 2012.
Only one study provided enough information to estimate a total score based on three of the four EPOS domains used for definition of chronic rhinosinusitis in EPOS 2012 (Small 2005). This study provided enough information to calculate the average score for nasal blockage, rhinorrhoea and loss of sense of smell. Although Stjarne 2006 also measured all of the same symptoms, it did not report the P values or standard deviations for loss of sense of smell because the results were not statistically different. Therefore, these results could only be used to measure an average symptom score based on two domains (nasal blockage and rhinorrhoea). The following are the pooled results:
-
Average combined score for three EPOS 2012 domains (nasal blockage, rhinorrhoea, loss of sense of smell): the mean difference (MD) was ‐0.13 (95% confidence interval (CI) ‐0.37 to 0.11; 237 participants; one study) on a 0 to 3 scale. It is a very small effect size and is not likely to be a clinically important difference (Analysis 1.1).
-
Average combined score for two EPOS 2012 domains (nasal blockage, rhinorrhoea): the MD was ‐0.19 (95% CI ‐0.36 to ‐0.02; 441 participants; two studies; I2 = 0%) on a 0 to 3 scale, favouring the high‐dose group. However, it is a very small effect size and this may not be a clinically important difference (Analysis 1.1).
These results have to be interpreted carefully because the studies only appeared to present their results in sufficient detail for further analysis when they showed a statistically significant improvement compared to placebo, therefore biasing the results towards a positive finding.
Individual symptom scores
Chur 2013 analysed the mean change from baseline for 51 participants in the high‐dose group and 50 participants in the low‐dose group. The mean change (recorded on a 0‐ to 4‐point scale) and percentage change compared to baseline values are shown below.
| Symptoms | Mean (%) change from baseline on a 0‐ to 4‐point scale | |
| High‐dose group | Low‐dose group | |
| Nasal congestion | ‐0.99 (‐49%) | ‐0.91 (‐38%) |
| Rhinorrhoea | ‐0.73 (‐38%) | ‐0.70 (‐43%) |
| Loss of sense of smell | ‐0.53 (‐43%) | ‐0.55 (‐49%) |
Small 2005 and Stjarne 2006 presented mean differences (MD) in the change from baseline symptom score between the high‐dose and low‐dose groups at four months, on a 0‐ to 3‐point scale. (We used these values to calculate the overall symptom scores above). Negative values show that there is a greater decrease in severity in the high‐dose (twice daily) group.
-
Nasal congestion: MD ‐0.24 (95% CI ‐0.39 to ‐0.08; 441 participants; two studies; I2 = 0%); there is a slightly larger reduction (small effect size) in nasal blockage in the high‐dose group.
-
Rhinorrhoea: MD ‐0.15 (95% CI ‐0.33 to 0.03; 441 participants; two studies; I2 = 0%); there is similar reduction in rhinorrhoea in both groups.
-
Loss of sense of smell: MD 0.06 (95% CI ‐0.20 to 0.32; 237 participants; one study); there is similar reduction in loss of sense of smell in both groups in Small 2005, but no statistically significant reduction in Stjarne 2006 (‐0.40 versus ‐0.33, MD ‐0.07) (see Analysis 1.2).
The quality of the evidence is very low (GRADE) for the measures of disease severity. See summary of findings Table 2.
Significant adverse effect: epistaxis
There was an increased risk of epistaxis in the high‐dose group (risk ratio (RR) 2.06, 95% CI 1.20 to 3.54; 637 participants; four studies; I2 = 0%) (Analysis 1.3).
Two of the four studies, which had the most weight in the pooled results, defined epistaxis to include a wide range of bleeding episodes, from frank bleeding to bloody nasal discharge to flecks of blood in the mucus (Small 2005; Stjarne 2006). Chur 2013 did not provide a definition but there is a high chance that they also used similar definitions to the other two studies, since this series of studies shared many common points in their protocols. The fourth study also did not provide a definition, but of the eight events reported, only one required a withdrawal (Penttila 2000).
The quality of the evidence is moderate (GRADE) for this comparison. See summary of findings Table 2.
Secondary outcomes
Health‐related quality of life, using generic quality of life scores, such as the SF‐36, EQ‐5D and other well‐validated instruments
None of the studies mentioned measuring quality of life.
Other adverse effects: local irritation (including nasal irritation, oral thrush, sore throat)
Similar numbers of patients experienced local irritation in both groups (RR 0.97, 95% CI 0.28 to 3.31; 542 participants; three studies; I2 = 0%) (Analysis 1.4), in the studies where these results could be analysed (Chur 2013; Small 2005; Stjarne 2006). However, the total number of events we have included in the analysis is an underestimation of the frequency of local irritation; the studies all used different descriptions (such as nasal burning, nasal dryness, nasal irritation and throat irritation) and we could only choose the most frequent type of local irritation for each study in the analysis to prevent double‐counting.
Other adverse effects, such as stunted growth in children and osteoporosis in adults (minimum time point: six months of treatment and follow‐up)
All the studies followed up participants for about four months. This was not long enough to provide a reliable measure of the longer‐term adverse effects and none of the studies reported these.
Endoscopic score (depending on population, either nasal polyps size score or endoscopy score, e.g. Lund‐Kennedy/Lund‐Mackay)
Small 2005 reported the mean change from baseline in nasal polyps score (0 to 3 range). The MD was 0.19 (95% CI ‐0.16 to 0.54; 237 participants) favouring the once daily group (Analysis 1.5). However, this difference is unlikely to be of clinical significance.
Stjarne 2006 did not find a statistically significant difference in polyps size between the low‐dose group and the placebo arms and therefore did not provide any P values to allow for the estimation of standard deviations. The polyps score (0 to 3 range) decreased by 0.96 points in the high‐dose group and 0.78 points in the low‐dose group. A mean difference of 0.18 between the two groups on a four‐point scale has no clinical significance, especially as the correlation between polyp size and symptoms is poor.
Chur 2013 reported that polyps size, measured on a four‐point scale (0 to 3) decreased by 1.1 points (‐34%) compared to baseline in the high‐dose group (n = 51) and by 0.92 points (‐26%) in the low‐dose group (n = 50). Standard deviations and P values were not provided, therefore it is not possible to estimate the statistical significance of this difference. As in Stjarne 2006, a mean difference of 0.18 between the two groups on a four‐point scale has no clinical significance, especially as the correlation between polyp size and symptoms is poor.
Demirel 2008 investigated fluticasone propionate nose drops and reported a decrease of 0.84 points (54%) compared to baseline in the twice daily (800 µg/day) group (n = 13), as opposed to a decrease of 0.9 points (40%) in the once daily (400 µg/day) group (n = 10). This is unlikely to represent a clinically significant reduction, since the baseline scores differed by about 0.7 points on a scale of 0 to 3 and the sample sizes are very small.
Penttila 2000 reported the "percentage of patients showing improvement" (it is unclear how this was defined). The risk ratio for "improvement" was 1.71 (95% CI 0.91 to 3.21; 92 participants) at 12 weeks for patients in the high‐dose group (Analysis 1.6).
Overall, all five studies reported some decrease in polyps score in the high‐dose group, but the clinical significance of this is unclear.
Computerised tomography (CT) scan score (e.g. Lund‐Mackay)
There was no mention that CT scans were conducted at follow‐up in any of the studies.
Comparison 4: Different types of delivery methods: aqueous nasal spray versus aerosol spray
One study compared two methods (aqueous nasal spray versus aerosol spray) of delivering 400 µg of budesonide per day, given as two divided doses (morning and night) for three months in patients who had eosinophilic nasal polyposis with polyp scores of 2 or less on each side (Johansen 1993). This study reported randomising 91 patients into three groups and 86 completed. However, the numbers in each group were not reported.
The study presented the results in graphs and not much further information was provided to allow for analysis. Where possible, we tried to obtain the estimates of mean change from baseline values for the outcomes (the baseline seemed to vary between groups for most outcomes) using a digital graph reader (http://arohatgi.info/WebPlotDigitizer/app/).
Primary outcomes
Health‐related quality of life, using disease‐specific health‐related quality of life scores, such as the Sino‐Nasal Outcome Test‐22 (SNOT‐22), Rhinosinusitis Outcome Measures‐31 (RSOM‐31) and SNOT‐20
The study did not mention measuring quality of life.
Disease severity, as measured by patient‐reported symptom score (such as the Chronic Sinusitis Survey (CSS) questionnaire and visual analogue scales)
The study did not provide results for a patient‐reported total symptoms score using an instrument validated in a chronic rhinosinusitis population. Patients recorded the symptoms of blocked nose (nasal obstruction) and runny nose (rhinorrhoea) for each nasal cavity on a scale of 0 to 3 in a weekly diary and they were asked whether they had experienced any change in smell using a 0 to 3 scale during clinic visits.
We estimated the point estimates for mean change from baseline for individual symptom scores using the digital graph reader:
-
Nasal congestion: the aqueous nasal spray and aerosol groups improved by 0.6 and 0.4 points, respectively.
-
Rhinorrhoea: we estimated the decrease in score from baseline for the aerosol and aqueous nasal spray groups to be about 0.5 points and 0.2 points, respectively.
-
Change in sense of smell: the study reported there was no "statistically significant difference" between the groups.
The significance of these differences is difficult to interpret, since the magnitude is not large and the baseline scores were different. Patients in the aerosol group consistently had less severe symptoms at baseline compared to the spray group (by about 0.3 points).
Significant adverse effect: epistaxis
No details of adverse events were reported. The paper only stated that "Few side effects such as dry nose, headache and epistaxis were reported and with no difference between the treatment groups".
Secondary outcomes
Health‐related quality of life, using generic quality of life scores, such as the SF‐36, EQ‐5D and other well‐validated instruments
The study did not mention measuring quality of life.
Other adverse effects: local irritation (including nasal irritation, oral thrush, sore throat)
No details about adverse events were reported. The paper only stated that "Few side effects such as dry nose, headache and epistaxis were reported and with no difference between the treatment groups".
Other adverse effects, such as stunted growth in children and osteoporosis in adults (minimum time point: six months of treatment and follow‐up)
No details about adverse events were reported. The paper only stated "Few side effects such as dry nose, headache and epistaxis were reported and with no difference between the treatment groups".
Endoscopic score (depending on population, either nasal polyps size score or endoscopy score, e.g. Lund‐Kennedy/Lund‐Mackay)
The study reported that "During the study a statistically significant decrease mean total polyps scores was seen in both groups treated with budesonide. The patients treated with placebo, however, had a mean increase in total polyps score during the treatment period." However, the "increase" in polyps size was only 0.1 points in the placebo group, whereas the decrease in polyps size score was 0.6 in the aerosol group and 1.4 in the aqueous group. As with the symptom score, the patients in the aerosol group had a lower baseline severity score (by about 0.3 points).
Computerised tomography (CT) scan score (e.g. Lund‐Mackay)
There were no indications that CT scans were used.
The quality of the evidence is very low (GRADE) for all outcomes in this comparison, due to very serious methodological concerns and imprecision.
Discusión
Resumen de los resultados principales
Se encontraron nueve estudios que informan sobre cuatro comparaciones diferentes (Chur 2013; Demirel 2008; Filipovic 2006; Holmberg 1997; Johansen 1993; Lund 1998; Penttila 2000; Small 2005; Stjarne 2006). Debido a la elección de las medidas de resultado usadas en estos estudios y la presentación incompleta de los resultados, para la mayoría de las comparaciones no fue posible encontrar muchas pruebas.
El siguiente es un resumen de los hallazgos clave para cada comparación:
Comparación 1: Diferentes tipos de corticosteroides: propionato de fluticasona versus dipropionato de beclometasona
Se incluyeron dos pequeños estudios en la revisión (Holmberg 1997, n = 37; Lund 1998, n = 20). Ambos estudios usaron 400 µg/día de cada fármaco, administrado dos veces al día mediante sprays nasales. Informaron una efectividad muy similar entre los grupos en cuanto a la gravedad de la enfermedad y la epistaxis. Sin embargo, estos estudios son demasiado pequeños para proporcionar certidumbre en cuanto a los resultados (evaluación GRADE: pruebas de muy baja calidad). Los otros resultados no se midieron o se informaron de manera muy deficiente. Ver Resumen de los hallazgos para la comparación principal.
Comparación 2: Diferentes tipos de corticosteroides: propionato de fluticasona versus furoato de mometasona
Se encontró sólo un estudio (Filipovic 2006, n = 100). Este estudio usó una dosis diaria de 200 µg administrada como un spray acuoso y no encontró diferencias en las puntuaciones de los síntomas nasales entre los grupos (evaluación GRADE: pruebas de muy baja calidad). Ver Resumen de los hallazgos para la comparación principal.
Comparación 3: Corticosteroides intranasales en dosis alta versus en dosis baja
Se encontraron cinco estudios para esta comparación. Tres de los mismos utilizaron furoato de mometasona (Chur 2013; Small 2005; Stjarne 2006): una dosis diaria de 400 µg versus 200 µg para los adultos y los niños mayores, 200 µg versus 100 µg en los niños más pequeños (Chur 2013). Demirel 2008 y Penttila 2000 administraron gotas nasales de propionato de fluticasona (una dosis diaria de 800 µg versus 400 µg).
La efectividad (gravedad de la enfermedad y tamaño de los pólipos nasales) fue similar entre los grupos de dosis alta y de dosis baja, excepto por la posibilidad de un beneficio pequeño en cuanto a la obstrucción nasal y la rinorrea al usar una dosis mayor de mometasona. Aunque todos los estudios informaron una mejoría mayor en la puntuación de los pólipos en el grupo de dosis alta, la importancia de este hecho no está clara debido al tamaño pequeño de las mejorías. Sin embargo, el cociente de riesgos (CR) para los eventos adversos fue mayor para la epistaxis (CR 2,06; intervalo de confianza [IC] del 95%: 1,20 a 3,54; 637 participantes; cuatro estudios; I2 = 0%) (evaluación GRADE: pruebas de calidad moderada). Existe menos seguridad en cuanto a si el riesgo de irritación local fue similar debido a los intervalos de confianza amplios y al informe más deficiente (CR 0,97; IC del 95%: 0,28 a 3,31; 542 participantes; cuatro estudios; I2 = 0%) (evaluación GRADE: pruebas de baja calidad). Ver Resumen de los hallazgos 2.
Comparación 4: Diferentes tipos de métodos de administración: spray nasal acuoso versus spray en aerosol
Se encontró sólo un estudio para esta comparación.(Johansen 1993). Este estudio no se informó de manera adecuada y pareció haber diferencias iniciales en el tamaño de los pólipos. Los resultados para la gravedad de la enfermedad parecieron ser similares para las puntuaciones de los síntomas, aunque es difícil interpretar la importancia de la diferencia de 0,5 puntos en el tamaño de los pólipos debido a las diferencias iniciales.
En resumen, a pesar de tener nueve estudios incluidos no había mucha información disponible. Todos los informes indicaron una efectividad similar entre los diferentes tipos de corticosteroides intranasales, las dosis, los métodos de administración y las formulaciones. Sin embargo, existe la posibilidad de un mayor riesgo de efectos adversos, en particular de epistaxis con la dosis mayor de furoato de mometasona (400 µg versus 200 µg por día).
Compleción y aplicabilidad general de las pruebas
Las dosis usadas en los estudios estuvieron de acuerdo con las recomendaciones de los fabricantes y son aplicables a la población estudiada. Es probable que en la población de pacientes con rinosinusitis crónica y pólipos nasales la administración de corticosteroides intranasales se inicie como un tratamiento en ámbitos de atención tanto primaria como secundaria. No hubo ningún estudio que incluyera a pacientes con rinosinusitis crónica sin pólipos nasales para que se pudiesen evaluar, lo cual señala a una deficiencia en las pruebas actualmente disponibles para este subgrupo.
La calidad de vida relacionada con la salud específica de la enfermedad, que es tanto específica para la enfermedad como importante para los pacientes, no se utilizó en los estudios incluidos como una medida de resultado. Por lo tanto no hay información en absoluto sobre si los diferentes tipos de corticosteroides intranasales tienen una repercusión sobre la calidad de vida de los pacientes.
Calidad de la evidencia
La calidad de las pruebas para todos los resultados de estas comparaciones fue baja omuy baja (evaluación de GRADE), debido al número pequeño de participantes disponibles para el análisis (lo cual resultó en intervalos de confianza amplios) y a las limitaciones en los métodos de realización y el informe de los estudios. Hay una inquietud grave en cuanto al sesgo de informe selectivo, en particular para los datos de efectividad, donde los estudios sólo proporcionaron datos numéricos y valores de P (lo cual permitió calcular las desviaciones estándar) cuando hubo una diferencia estadísticamente significativa entre los grupos o en comparación con placebo.
La única excepción a la evaluación de las pruebas de calidad baja/muy baja es el resultado de la epistaxis, donde es posible tener mayor seguridad de que hay un aumento en el riesgo al administrar dosis mayores de corticosteroides intranasales (evaluación GRADE: pruebas de calidad moderada).
Sesgos potenciales en el proceso de revisión
En la mayoría de los casos los estudios no revelaron suficiente información para analizar aún más los resultados. Se han tenido que tomar lecturas de los gráficos mediante a un lector de gráficos digital e imputar las desviaciones estándar basado en los valores de p informados. A menudo sólo se informaron como “valor de p < 0,05” o “valor de p < 0,01” en las comparaciones donde los estudios hallaron significación estadística. Las imputaciones se basan en estos valores (mediante el valor de p = 0,01 o valor de p = 0,05) y por lo tanto la revisión es conservadora en cuanto a la estimación de las desviaciones estándar. Sin embargo, esta falta de información acerca de los resultados no significativos podría haber impedido la extracción de resultados más concluyentes acerca de la ausencia de diferencias entre los grupos.
Para la gravedad de la enfermedad, sólo se procuró incluir resultados medidos con instrumentos validados. Sin embargo, ninguno de los estudios de esta revisión, y en realidad la mayoría de los estudios de la serie de revisiones (Chong 2016a; Chong 2016b; Head 2016a; Head 2016b; Head 2016c)los había utilizado. Por lo tanto, se realizó un acuerdo y se incluyeron resultados mediante escalas no validadas para obtener alguna información.
Acuerdos y desacuerdos con otros estudios o revisiones
Esta revisión forma parte de una serie de revisiones sobre la rinosinusitis crónica (Chong 2016a; Chong 2016b; Head 2016a; Head 2016b; Head 2016c). La finalidad de esta revisión es responder a la pregunta de si hay diferencias entre los diversos tipos, dosificaciones y regímenes de corticosteroides intranasales. Una revisión complementaria considera la efectividad de los corticosteroides intranasales comparados con placebo (Chong 2016a). No se sabe de la existencia de otras revisiones que hayan considerado específicamente la efectividad y la seguridad relativas de diferentes tipos, dosis y métodos o regímenes de administración de corticosteroides intranasales. Aunque Chong 2016a planificó análisis de subgrupos para diferentes tipos, dosis y métodos de administración de corticosteroides, los mismos no se realizaron porque sólo se observó heterogeneidad para un resultado (dolor facial), en que sólo se incluyeron dos estudios y difirieron en la población de pacientes (pólipos versus ningún pólipo), los tipos y las dosis de corticosteroides usados (budesonida 128 µg/día versus fluticasona 800 µg/día) y el método de administración (gotas nasales versus inhalador accionado por la respiración). Dicha revisión encontró un riesgo mayor de epistaxis en los pacientes que recibieron corticosteroides intranasales versus placebo, aunque a pesar de la inclusión de diferentes dosis, tipos de corticosteroides y métodos de administración, no se observó heterogeneidad.
Actualmente se han completado ensayos internacionales recientes que utilizan el dispositivo Optinose (ensayos Navigate I y II) (NCT01622569; NCT01624662). Estos estudios han incluido dosis diferentes dentro de sus protocolos, por lo cual próximamente habrá aún más información sobre las dosis y los dispositivos una vez que se publiquen estos resultados.
Dos revisiones Cochrane anteriores han considerado los corticosteroides tópicos en pacientes con rinosinusitis crónica con pólipos nasales (Kalish 2012) y sin pólipos nasales (Snidvongs 2011), y también incluyeron la comparación de diferentes tipos y dosis de corticosteroides en su alcance. A diferencia de estas revisiones, la presente revisión sólo incluye estudios con una duración mínima de tres meses de tratamiento y seguimiento. Se excluyeron de esta revisión los estudios que investigaron el impacto de los corticosteroides intranasales en los resultados quirúrgicos, ya sea administrados de forma perioperatoria o en las semanas de la intervención quirúrgica para prevenir las recaídas. De estos estudios, se excluyeron cuatro incluidos en Kalish 2012 debido a que la duración del tratamiento y el seguimiento no cumplió con el criterio de inclusión de 12 semanas (rango de cuatro a ocho semanas)(Filiaci 2000; Jankowski 2001; Lildholdt 1995; Tos 1998) y un estudio que sólo incluyó a pacientes después de la cirugía de los senos(Dijkstra 2004). Estas revisiones tampoco encontraron una diferencia entre las dosis o los tipos de corticosteroides intranasales.
El artículo EPOS 2012 divide a la población con rinosinusitis crónica en con y sin pólipos nasales. En los pacientes que presentan rinosinusitis crónica sin pólipos nasales no encontraron pruebas directas sobre las comparaciones entre las clases de corticosteroides intranasales (p.ej. comparación de los métodos de administración, las dosis o los diferentes corticosteroides). Para los pacientes con rinosinusitis crónica con pólipos nasales las pruebas sobre las comparaciones entre las clases de corticosteroides intranasales no se declararon explícitamente como una comparación de interés, aunque se planificaron análisis de subgrupos para el método de administración tópico (spray nasal versus gotas nasales) y el tipo de corticosteroide (moderno versus de primera generación). La comparación de la dosis alta versus dosis baja no se consideró aunque la revisión declaró que se identificaron ocho estudios que informaban esta comparación (Dijkstra 2004; Filiaci 2000; Jankowski 2001; Lildholdt 1995; Penttila 2000; Small 2005; Stjarne 2006; Tos 1998). Los resultados del “corticosteroide intranasal versus placebo” fueron subagrupados según el método de administración y no se encontró ninguna diferencia, aunque debe reconocerse que estas pruebas son indirectas. De igual manera la comparación de corticosteroides intranasales “modernos” versus “de primera generación” se hizo al considerar un análisis de subgrupos indirecto de los ensayos de los corticosteroides intranasales versus placebo sin mención de los tres ensayos que los autores identificaron específicamente como que realizaron esta comparación (Bross‐Soriano 2004; Holmberg 1997; Lund 1998). La conclusión de este análisis fue que los "CSIN modernos no presentan una eficacia clínica mayor (aunque potencialmente menos efectos adversos [sic]) en comparación con los CSIN de primera generación".

Process for sifting search results and selecting studies for inclusion.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
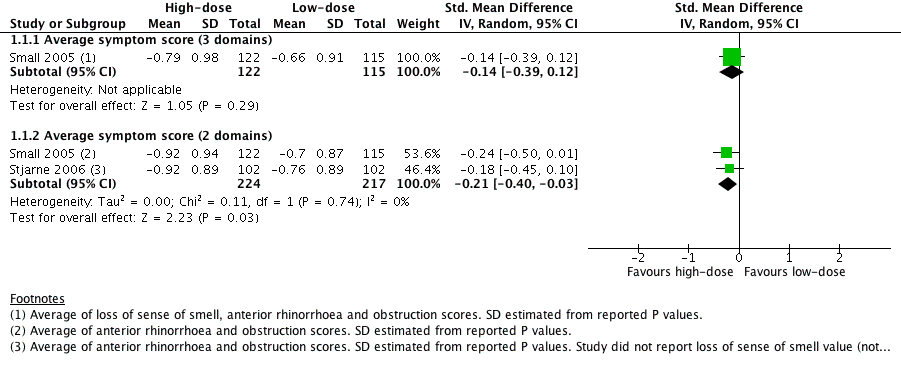
Forest plot of comparison: 1 High‐dose versus low‐dose intranasal corticosteroids, outcome: 1.1 Disease severity ‐ overall symptoms, measured as average change from baseline at 4 months (range 0 to 3).
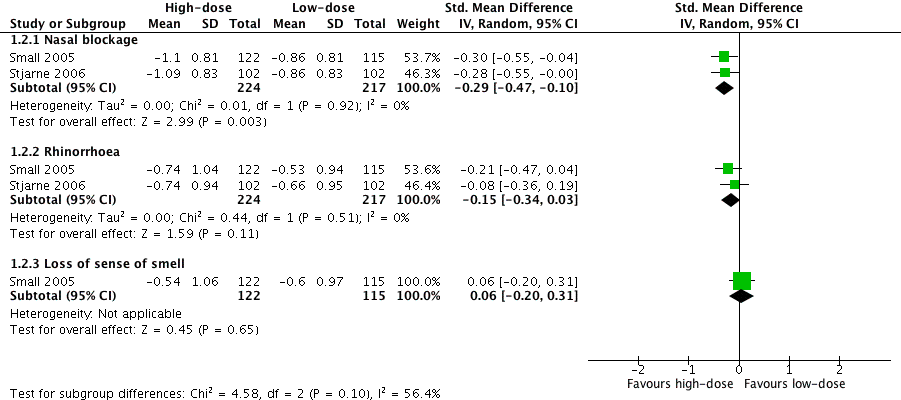
Forest plot of comparison: 1 High‐dose versus low‐dose intranasal corticosteroids, outcome: 1.2 Disease severity ‐ individual symptoms, measured as average change from baseline at 4 months (range 0 to 3).

Comparison 1 High‐dose versus low‐dose intranasal corticosteroids, Outcome 1 Disease severity ‐ overall symptoms, measured as average change from baseline at 4 months (range 0 to 3).

Comparison 1 High‐dose versus low‐dose intranasal corticosteroids, Outcome 2 Disease severity ‐ individual symptoms, measured as average change from baseline at 4 months (range 0 to 3).
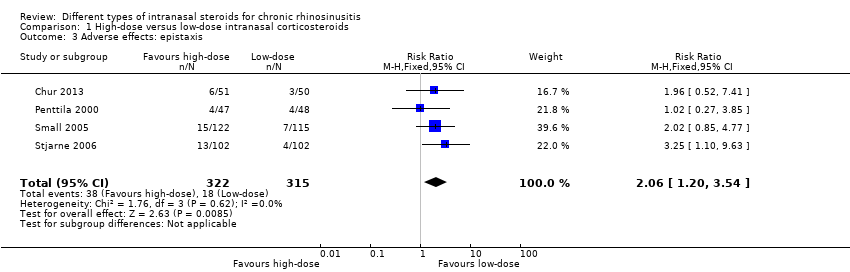
Comparison 1 High‐dose versus low‐dose intranasal corticosteroids, Outcome 3 Adverse effects: epistaxis.
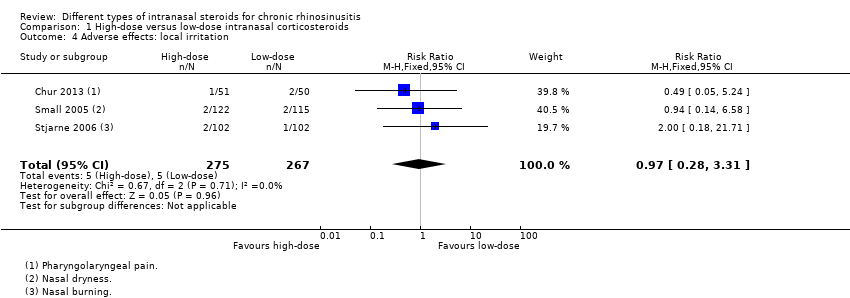
Comparison 1 High‐dose versus low‐dose intranasal corticosteroids, Outcome 4 Adverse effects: local irritation.

Comparison 1 High‐dose versus low‐dose intranasal corticosteroids, Outcome 5 Nasal polyps size, measured as change from baseline (0 to 3 range scale) at 4 months.

Comparison 1 High‐dose versus low‐dose intranasal corticosteroids, Outcome 6 Nasal polyps ‐ proportion with improvement at 12 weeks.
| Different types of intranasal corticosteroid molecules for chronic rhinosinusitis | ||||||
| Patient or population: chronic rhinosinusitis (all studies recruited patients with bilateral polyps) | ||||||
| Outcomes № of participants | Relative effect (95%) | Anticipated absolute effects* (95% CI) | Quality | What happens | ||
| Low‐dose intranasal corticosteroids | High‐dose intranasal corticosteroids | Difference | ||||
| Disease‐specific health‐related quality of life | Not measured | Impact unknown | ||||
| Disease severity ‐ overall symptoms
| — |
| ⊕⊝⊝⊝ | No differences observed but evidence was too low quality to draw a conclusion | ||
| Adverse events: epistaxis
| — |
| ⊕⊝⊝⊝ | Unclear whether the risk of epistaxis varies for different types of steroid molecules | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Studies were either very small (n = 20 and n = 26) and had important drop‐outs or were only reported as an abstract with inadequate information available (n = 100). We considered all studies to be at unclear to high risk of selective reporting and attrition bias. The evidence was very low quality due to very serious imprecision and very serious risk of bias concerns. | ||||||
| High‐dose versus low‐dose intranasal corticosteroids for chronic rhinosinusitis | ||||||
| Patient or population: chronic rhinosinusitis (all studies recruited patients with bilateral polyps) | ||||||
| Outcomes № of participants | Relative effect | Anticipated absolute effects* (95% CI) | Quality | What happens | ||
| Low‐dose intranasal corticosteroids | High‐dose intranasal corticosteroids | Difference | ||||
| Disease‐specific health‐related quality of life | Not measured | Impact unknown | ||||
| Disease severity ‐ overall symptoms, measured as average change from baseline at 4 months | ||||||
| All 4 EPOS domains | No information available | |||||
| 3 domains (nasal blockage, rhinorrhoea, loss of sense of smell) Range 0 to 3, lower score = less severe № of participants: 237 | — | The mean disease severity ‐ overall symptoms, measured as average change from baseline at 4 months (range 0 to 3) ‐ average symptom score (3 domains) without high‐dose was ‐0.66 points | — | MD 0.13 points lower (0.37 lower to 0.11 more) than low‐dose group | ⊕⊕⊝⊝ | The average score for 3 types of symptoms seems to be similar between the high‐dose and low‐dose groups. |
| (2 domains: nasal blockage, rhinorrhoea) № of participants: 441 | — | The mean disease severity ‐ overall symptoms, measured as average change from baseline at 4 months (range 0 to 3) ‐ average symptom score (2 domains) without high‐dose was ‐0.73 points | — | MD 0.19 points lower (0.36 lower to 0.02 lower) than low‐dose group | ⊕⊕⊝⊝ | The average score for 2 types of symptoms seems to be slightly lower for the high‐dose group. The clinical significance of this reduction is unclear. |
| Disease severity ‐ measured as average change from baseline at 4 months (range 0 to 3) | ||||||
|
№ of participants: 441 | — | The mean disease severity ‐ individual symptoms, measured as average change from baseline at 4 months (range 0 to 3) ‐ nasal blockage without high‐dose was ‐0.86 points | — | MD 0.24 points lower (0.39 lower to 0.08 lower) than low‐dose group | ⊕⊕⊝⊝ | The nasal blockage score seems to be slightly lower in the high‐dose group. The clinical significance of this reduction is unclear. |
|
№ of participants: 441 (2 RCTs) | — | The mean disease severity ‐ individual symptoms, measured as average change from baseline at 4 months (range 0 to 3) ‐ rhinorrhoea without high‐dose was ‐0.6 points | — | MD 0.15 points lower (0.33 lower to 0.03 higher) than low‐dose group | ⊕⊕⊝⊝ | The average score for rhinorrhoea seems to be similar between the high‐dose and low‐dose groups. |
|
№ of participants: 237 | — | The mean disease severity ‐ individual symptoms, measured as average change from baseline at 4 months (range 0 to 3) ‐ loss of sense of smell without high‐dose was ‐0.6 points | — | MD 0.06 points higher (0.2 lower to 0.32 higher) than low‐dose group | ⊕⊕⊝⊝ | The average score for loss of sense of smell seems to be very similar between the high‐dose and low‐dose groups. |
| Adverse effects: epistaxis № of participants: 637 | RR 2.06 | Study population | ⊕⊕⊕⊝ | The risk of epistaxis is likely to be higher in the higher‐dose groups. However, the studies included very minor nosebleeds, such as blood stains in the mucus, and most of these events are not likely to be severe. | ||
| 57 per 1000 | 118 per 1000 | 61 more per 1000 (11 more to 145 more) | ||||
| Moderate | ||||||
| 60 per 1000 | 124 per 1000 | 64 more per 1000 (12 more to 153 more) | ||||
| Adverse effects: local irritation № of participants: 542 | RR 0.97 | Study population | ⊕⊕⊝⊝ | The risk of local irritation seems to be similar between groups, but the overall risks are underestimated due to the way the data were reported. | ||
| 19 per 1000 | 18 per 1000 | 10 fewer per 1000 (13 fewer to 43 more) | ||||
| Moderate | ||||||
| 17 per 1000 | 17 per 1000 | 10 fewer per 1000 (13 fewer to 40 more) | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Scale validity, particularly discriminant validity (ability to distinguish the differences between groups), was unclear. There was a high risk of reporting bias. Studies tended to report enough information for meta‐analysis only for statistically significant results. One study, which had 101 participants, reported very similar values for both intervention arms for all disease scores but had no information related to SD. 2Small sample size ‐ evidence only from one or two relatively small studies. 3Only data from patients with bilateral nasal polyposis. We considered this to be indirectness of the evidence to patients without polyps but have not further downgraded the evidence. 4One of the studies had inadequate blinding ‐ a double dummy was not used to mask the twice daily (higher) versus once daily (lower) dose; the study had 101 participants. 5Sample size relatively small for a precise estimate of adverse events. We downgraded this outcome once, after taking into consideration the inadequate blinding in one of the studies and the relatively small sample size. 6Studies did not use consistent terminology/methods to report different types of local irritation. For analysis we only selected the most frequent types of local irritation from a list (to avoid double counting). This is a possible underestimation of overall event rates. The relatively low event rates and small sample size contributed to the large confidence intervals. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Disease severity ‐ overall symptoms, measured as average change from baseline at 4 months (range 0 to 3) Show forest plot | 2 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Average symptom score (3 domains) | 1 | 237 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.14 [‐0.39, 0.12] |
| 1.2 Average symptom score (2 domains) | 2 | 441 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.21 [‐0.40, ‐0.03] |
| 2 Disease severity ‐ individual symptoms, measured as average change from baseline at 4 months (range 0 to 3) Show forest plot | 2 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Nasal blockage | 2 | 441 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.29 [‐0.47, ‐0.10] |
| 2.2 Rhinorrhoea | 2 | 441 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.15 [‐0.34, 0.03] |
| 2.3 Loss of sense of smell | 1 | 237 | Std. Mean Difference (IV, Random, 95% CI) | 0.06 [‐0.20, 0.31] |
| 3 Adverse effects: epistaxis Show forest plot | 4 | 637 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.06 [1.20, 3.54] |
| 4 Adverse effects: local irritation Show forest plot | 3 | 542 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.28, 3.31] |
| 5 Nasal polyps size, measured as change from baseline (0 to 3 range scale) at 4 months Show forest plot | 1 | 237 | Mean Difference (IV, Fixed, 95% CI) | 0.19 [‐0.16, 0.54] |
| 6 Nasal polyps ‐ proportion with improvement at 12 weeks Show forest plot | 1 | 92 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.71 [0.91, 3.21] |

