Pruebas de orina para el cribado del síndrome de Down
Referencias
References to studies included in this review
References to studies excluded from this review
Additional references
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Clinical features and settings | High‐risk referral for invasive testing. | |
| Participants | 511 participants. USA. August 1996 to January 1997. Singleton pregnancies. Pregnant women. Mean age 37.1 years (SD 2.8 years). 15‐24 weeks' gestation. | |
| Study design | Prospective cohort study. | |
| Target condition and reference standard(s) | Down's syndrome: 18 cases. Reference standard: amniocentesis. | |
| Index and comparator tests | Mid‐trimester urine ß‐core fragment testing (monoclonal antibody B210 assay, 2‐step sandwich method, standardised for creatinine). | |
| Follow‐up | 100% karyotyping. | |
| Aim of study | To ascertain the screening efficiency of a new mid‐trimester Down’s syndrome detection protocol that combines maternal urine testing and ultrasonographic examination. | |
| Notes | Amniocentesis was being conducted on the basis of maternal age. Women who have amniocentesis just due to abnormal screening results were excluded from the study. | |
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Selective testing of high‐risk women as done in practice. |
| Acceptable reference standard? | Yes | Amniocentesis. |
| Partial verification avoided? | Yes | All women received a reference standard. |
| Differential verification avoided? | Yes | All women underwent the same reference standard. |
| Incorporation avoided? | Yes | Reference standard was independent of the index test. |
| Reference standard results blinded? | No | Reference standard interpreted with knowledge of index test results. |
| Index test results blinded? | Yes | Urine testing was conducted blind from the results of amniocentesis. |
| Relevant clinical information? | Yes | Information available as would be in standard clinical practice. |
| Uninterpretable results reported? | No | No details given for test failures/uninterpretable measurements. |
| Withdrawals explained? | No | No details of withdrawals given. |
| Clinical features and settings | High‐risk referral for invasive testing. | |
| Participants | 356 participants: 10 cases and 346 controls. USA. Dates not reported. Singleton pregnancies. Pregnant women. 14‐24 weeks' gestation. | |
| Study design | Case‐control study. | |
| Target condition and reference standard(s) | Down's syndrome: 10 cases. Reference standard: amniocentesis. | |
| Index and comparator tests | Seond trimester urine ß‐core fragment testing (monoclonal antibody B210 assay, 2‐step sandwich method, standardised for creatinine). Second trimester serum AFP. Risk cut points of 1/10, 1/20, 1/30, 1/58, 1/270, 1/526. | |
| Follow‐up | 100% karyotyping. | |
| Aim of study | To determine Down's syndrome screening efficiency of a new protocol that combines maternal serum AFP and beta core fragment/total oestriol ratio. | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Selective testing of high‐risk women as done in practice. |
| Acceptable reference standard? | Yes | Amniocentesis. |
| Partial verification avoided? | Yes | All women underwent a reference standard. |
| Differential verification avoided? | Yes | All women underwent the same reference standard. |
| Incorporation avoided? | Yes | Reference standard was independent of the index test. |
| Reference standard results blinded? | No | Reference standard interpreted with knowledge of index test results. |
| Index test results blinded? | Yes | Index test interpreted without knowledge of reference standard results. |
| Relevant clinical information? | Yes | Information available as would be in standard clinical practice. |
| Uninterpretable results reported? | No | No details given for test failures/uninterpretable measurements. |
| Withdrawals explained? | No | No details of withdrawals given. |
| Clinical features and settings | High‐risk referral for invasive testing. | |
| Participants | 457 participants. USA. August 1996 ‐ June 1997. Pregnant women. Mean age 37.1 years. Singleton pregnancies. 15‐24 weeks' gestation. | |
| Study design | Prospective cohort study. | |
| Target condition and reference standard(s) | Down's syndrome: 13 cases. Reference standard: amniocentesis. | |
| Index and comparator tests | Maternal age. Urinary ß core fragment (monoclonal antibody B210 assay, 2‐step sandwich method, standardised for creatinine). Urinary beta core fragment/total urinary oestriol ratio. | |
| Follow‐up | 100% karyotyping. | |
| Aim of study | To evaluate Down's syndrome screening efficiency of a new algorithm of multiple urinary biochemical and ultrasound markers. | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Selective testing of high‐risk women as done in practice. |
| Acceptable reference standard? | Yes | Amniocentesis. |
| Partial verification avoided? | Yes | All women received a reference standard. |
| Differential verification avoided? | Yes | All women received the same reference standard. |
| Incorporation avoided? | Yes | Reference standard was independent of the index test. |
| Reference standard results blinded? | No | Reference standard interpreted with knowledge of index test results. |
| Index test results blinded? | Yes | Index test interpreted without knowledge of reference standard results. |
| Relevant clinical information? | Yes | Information available as would be in standard clinical practice. |
| Uninterpretable results reported? | No | No details given for test failures/uninterpretable measurements. |
| Withdrawals explained? | No | No details of withdrawals given. |
| Clinical features and settings | High‐risk referral for invasive testing. | |
| Participants | 926 participants. USA. November 1995 ‐ March 1999. Pregnant women. Singleton pregnancies. 15‐24 weeks' gestation. Euploid/Down's karyotype only. | |
| Study design | Prospective cohort study. | |
| Target condition and reference standard(s) | Down's syndrome: 21 cases. Reference standard: amniocentesis. | |
| Index and comparator tests | Maternal age. Second trimester urinary ß core fragment (Spot specimens of urine ‐ 2‐step sandwich assay B120 monoclonal antibody). Second trimester serum AFP. Frozen serum samples tested for second trimester uE3 and free ßhCG (details of serum testing methods not given). | |
| Follow‐up | 100% karyotyping. | |
| Aim of study | To compare Down's syndrome screening efficiency of elevated maternal urine level of beta core fragment with that of a traditional serum triple test. | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Selective testing of high‐risk women as done in practice. |
| Acceptable reference standard? | Yes | Amniocentesis. |
| Partial verification avoided? | Yes | All women received a reference standard. |
| Differential verification avoided? | Yes | All women had the same reference standard. |
| Incorporation avoided? | Yes | Reference standard was independent of the index test. |
| Reference standard results blinded? | No | Reference standard interpreted with knowledge of index test results. |
| Index test results blinded? | Yes | Index test interpreted without knowledge of reference standard results. |
| Relevant clinical information? | Yes | Information available as would be in standard clinical practice. |
| Uninterpretable results reported? | No | No details given for test failures/uninterpretable measurements. |
| Withdrawals explained? | No | No details of withdrawals given. |
| Clinical features and settings | High‐risk referral for invasive testing. | |
| Participants | 1016 participants. USA. May 1995 ‐ June 1998. Singleton pregnancies. Pregnant women. Mean age 37.1 years (19.3‐46 years). 14‐24 weeks' gestation. Euploid or Down's pregnancies only. | |
| Study design | Prospective cohort study. | |
| Target condition and reference standard(s) | Down's syndrome: 23 cases. Reference standard: amniocentesis. | |
| Index and comparator tests | Second trimester urinary hyperglycosylated hCG (Specific monoclonal antibody developed. 2‐step enzyme immunometric assay standardised for creatinine levels). | |
| Follow‐up | 100% karyotyping. | |
| Aim of study | To evaluate the measurement of levels of urine hyperglycosylated hCG in conjunction with ultrasound biometry for Down's syndrome risk prediction in an at risk group. | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Selective testing of high‐risk women as done in practice. |
| Acceptable reference standard? | Yes | Amniocentesis. |
| Partial verification avoided? | Yes | All women had a reference standard. |
| Differential verification avoided? | Yes | All women had the same reference standard. |
| Incorporation avoided? | Yes | Reference standard was independent of the index test. |
| Reference standard results blinded? | No | Reference standard interpreted with knowledge of index test results. |
| Index test results blinded? | Yes | Index test interpreted without knowledge of reference standard results. |
| Relevant clinical information? | Yes | Information available as would be in standard clinical practice. |
| Uninterpretable results reported? | No | No details given for test failures/uninterpretable measurements. |
| Withdrawals explained? | No | No details of withdrawals given. |
| Clinical features and settings | High‐risk referral for invasive testing. | |
| Participants | 524 participants. USA ‐ single hospital. August 1995 ‐ April 1999. Singleton pregnancies. Pregnant women. Mean age 36.6 years (SD 5.3 years) in those with Down’s detected and 37.0 years (SD 3.4 years) in those with euploid pregnancies. 14‐22 weeks' gestation. | |
| Study design | Prospective cohort study. | |
| Target condition and reference standard(s) | Down's syndrome: 24 cases. Reference standard: amniocentesis. | |
| Index and comparator tests | Maternal age. Second trimester serum hCG (IMX total β‐hCG kit, Abbott Laboratories), uE3 (DSL‐1400 Ultra‐sensitive unconjugated Estriol Radioimmunoassay kit) and AFP (IMX AFP kit, Abbott Laboratories). Second trimester urinary beta core fragment (Spot specimens of urine ‐ 2‐step sandwich assay B120 monoclonal antibody). Frozen samples tested for second trimester urinary hyperglycosylated hCG (Specific monoclonal antibody developed. 2‐step enzyme immunometric assay standardised for creatinine levels). | |
| Follow‐up | 100% karyotyping. | |
| Aim of study | To compare the concentration of hyperglycosylated human chorionic gonadotropin with serum triple screen for second trimester Down’s syndrome detection. | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Selective testing of high‐risk women as done in practice. |
| Acceptable reference standard? | Yes | Amniocentesis. |
| Partial verification avoided? | Yes | All women had a reference standard. |
| Differential verification avoided? | Yes | All women had the same reference standard. |
| Incorporation avoided? | Yes | Reference standard was independent of the index test. |
| Reference standard results blinded? | Yes | Reference standard interpreted without knowledge of index test results. |
| Index test results blinded? | Unclear | Unclear if index test interpreted with knowledge of reference standard results. |
| Relevant clinical information? | Yes | Information available as would be in standard clinical practice. |
| Uninterpretable results reported? | No | No details given for test failures/uninterpretable measurements. |
| Withdrawals explained? | No | No details of withdrawals given. |
| Clinical features and settings | Referral for termination of pregnancy, amniocentesis or routine examination. | |
| Participants | 105 participants: 14 cases and 91 controls. USA. Dates not reported. Singleton pregnancies. Pregnant women. 15‐21 weeks' gestation. | |
| Study design | Case‐control study. | |
| Target condition and reference standard(s) | Down's syndrome: 14 cases. Reference standard: karyotyping on termination of pregnancy or amniocentesis. | |
| Index and comparator tests | Maternal age. Frozen samples tested for: second trimester urinary gonadotropin peptide (Triton UGP EIA assay, Alameda); second trimester serum hCG (MAIAclone hCG assay, Serono‐Baker Diagnostics, Allentown). | |
| Follow‐up | No details given for any follow‐up to birth. Reported that the fetal karyotype of control samples was not always known but assumed that none were aneuploid pregnancies. | |
| Aim of study | To assess whether urinary gonadotropin peptide is better than serum hCG as a second trimester screening marker. | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Routine screening and selective testing of high‐risk women as done in practice. |
| Acceptable reference standard? | Yes | Karyotyping or follow‐up to birth. |
| Partial verification avoided? | No | Not all women received a reference standard. |
| Differential verification avoided? | No | Women had different reference standards. |
| Incorporation avoided? | Yes | Reference standard was independent of the index test. |
| Reference standard results blinded? | Yes | Reference standard interpreted without knowledge of index test results. |
| Index test results blinded? | Unclear | Unclear if index test interpreted without knowledge of reference standard results. |
| Relevant clinical information? | Yes | Information available as would be in standard clinical practice. |
| Uninterpretable results reported? | No | No details given for test failures/uninterpretable measurements |
| Withdrawals explained? | No | No details of withdrawals given. |
| Clinical features and settings | High‐risk referral for invasive testing. | |
| Participants | 722 participants. USA – single hospital. August 1995 ‐ May 1996. Pregnant women. Singleton pregnancy. 12‐24 weeks' gestation. | |
| Study design | Cohort study. | |
| Target condition and reference standard(s) | Down's syndrome: 13 cases. Reference standard: amniocentesis. | |
| Index and comparator tests | Second trimester urinary hCG free beta subunit (Immunoenzymometric assay with autoantibody FBT11). | |
| Follow‐up | 100% karyotyping. | |
| Aim of study | To evaluate use of second trimester urinary free beta‐subunit for Down's syndrome screening. | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Selective testing of high‐risk women as done in practice. |
| Acceptable reference standard? | Yes | Amniocentesis. |
| Partial verification avoided? | Yes | All women had a reference standard. |
| Differential verification avoided? | Yes | All women had the same reference standard. |
| Incorporation avoided? | Yes | Reference standard was independent of the index test. |
| Reference standard results blinded? | Yes | Reference standard interpreted without knowledge of index test results. |
| Index test results blinded? | Yes | Index test interpreted without knowledge of reference standard results. |
| Relevant clinical information? | Yes | Information available as would be in standard clinical practice. |
| Uninterpretable results reported? | No | No details given for test failures/uninterpretable measurements. |
| Withdrawals explained? | No | No details of withdrawals given. |
| Clinical features and settings | High‐risk referral for invasive testing. | |
| Participants | 492 participants. USA – single hospital. August 1995 ‐ May 1996. Pregnant women. Singleton pregnancy. 12‐24 weeks' gestation. | |
| Study design | Prospective cohort study. | |
| Target condition and reference standard(s) | Down's syndrome: 12 cases. Reference standard: amniocentesis. | |
| Index and comparator tests | Second trimester urinary hCG free beta subunit (B210 2‐step sandwich assay). Second trimester urinary total oestriol (radioimmunoassay, kit from Diagnostics Products Corporation, Los Angeles). | |
| Follow‐up | 100% karyotyping. | |
| Aim of study | To evaluate use of urinary free beta core fragment combined with urinary total oestriol for Down's syndrome screening. | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Routine screening of typical pregnant population. |
| Acceptable reference standard? | Yes | Amniocentesis. |
| Partial verification avoided? | Yes | All women had a reference standard. |
| Differential verification avoided? | Yes | All women had the same reference standard. |
| Incorporation avoided? | Yes | Reference standard was independent of the index test. |
| Reference standard results blinded? | Yes | Reference standard interpreted without knowledge of index test results. |
| Index test results blinded? | Unclear | Unclear if index test interpreted without knowledge of reference standard results. |
| Relevant clinical information? | Yes | Information available as would be in standard clinical practice. |
| Uninterpretable results reported? | No | No details given for test failures/uninterpretable measurements. |
| Withdrawals explained? | No | No details of withdrawals given. |
| Clinical features and settings | High‐risk referral for invasive testing. | |
| Participants | 1157 participants. USA – 3 hospitals. May 1995 ‐ March 1998. Pregnant women. Singleton pregnancy. 11‐22 weeks' gestation. | |
| Study design | Prospective cohort study. | |
| Target condition and reference standard(s) | Down's syndrome: 23 cases. Reference standards: amniocentesis or CVS. | |
| Index and comparator tests | Urinary hCG beta‐core subunit (B210 2‐step sandwich assay). Urinary total oestriol (radioimmunoassay, kit by Diagnostic Products Corporation, Los Angeles). | |
| Follow‐up | 100% karyotyping. | |
| Aim of study | To evaluate use of urinary free beta‐subunit for Down's syndrome screening. | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Selective testing of high‐risk women as done in practice. |
| Acceptable reference standard? | Yes | Amniocentesis or CVS. |
| Partial verification avoided? | Yes | All women had a reference standard. |
| Differential verification avoided? | No | Women had CVS or amniocentesis depending on their stage of pregnancy. |
| Incorporation avoided? | Yes | Reference standard was independent of the index test. |
| Reference standard results blinded? | Yes | Reference standard interpreted without knowledge of index test results. |
| Index test results blinded? | Yes | Index test interpreted without knowledge of reference standard results. |
| Relevant clinical information? | Yes | Information available as would be in standard clinical practice. |
| Uninterpretable results reported? | No | No details given for test failures/uninterpretable measurements. |
| Withdrawals explained? | No | No details of withdrawals given. |
| Clinical features and settings | High‐risk referral for invasive testing and testing for bacterial analysis. | |
| Participants | 315 participants. UK. Dates not specified. Pregnant women: 24 cases undergoing invasive testing and 294 controls undergoing testing for bacterial analysis 11‐23 weeks' gestation. | |
| Study design | Case‐control study. | |
| Target condition and reference standard(s) | Down's syndrome: 24 cases. Reference standards: amniocentesis or CVS for cases and follow‐up for controls. | |
| Index and comparator tests | Urinary beta core fragment (Modified radioimmunoassay method). Urinary total oestrogen (continuous flow reaction based on the Kuber method). | |
| Follow‐up | No details given of methods of follow‐up. | |
| Aim of study | To evaluate the use of multiple urinary markers rather than serum in order to screen for Down's syndrome. | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Selective testing of high‐risk women as done in practice. |
| Acceptable reference standard? | Yes | Karyotyping or follow‐up to birth. |
| Partial verification avoided? | Yes | All women had a reference standard. |
| Differential verification avoided? | No | Women had different reference standards. |
| Incorporation avoided? | Yes | Reference standard was independent of the index test. |
| Reference standard results blinded? | Yes | Reference standard interpreted without knowledge of index test results. |
| Index test results blinded? | Yes | Index test interpreted without knowledge of reference standard results. |
| Relevant clinical information? | Yes | Information available as would be in standard clinical practice. |
| Uninterpretable results reported? | No | No details given for test failures/uninterpretable measurements. |
| Withdrawals explained? | No | No details of withdrawals given. |
| Clinical features and settings | High‐risk referral for invasive testing and routine screening. | |
| Participants | 349 participants: 45 cases and 304 controls. UK. Dates not specified. Pregnant women. 14‐19 weeks' gestation. | |
| Study design | Retrospective case‐control study. | |
| Target condition and reference standard(s) | Down's syndrome: 45 cases. Reference standard: amniocentesis, CVS or follow‐up to birth. | |
| Index and comparator tests | Frozen samples tested for urinary hyperglycosylated hCG (Immunoassays by 'Cole' method corrected for creatinine levels using Jaffes method). | |
| Follow‐up | Details of follow‐up not reported. | |
| Aim of study | To determine the distribution of hyperglycosylated hCG levels in pregnancies with Down's syndrome. | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Routine screening of typical pregnant population. |
| Acceptable reference standard? | Yes | Amniocentesis, CVS or follow‐up. |
| Partial verification avoided? | Yes | All women had a reference standard. |
| Differential verification avoided? | No | Women had different reference standards. |
| Incorporation avoided? | Yes | Index tests did not form part of the reference standard. |
| Reference standard results blinded? | Yes | Reference standard conducted before the index test. |
| Index test results blinded? | Unclear | Index test conducted after the reference standard and no evidence of blinding. |
| Relevant clinical information? | Yes | Information available as would be in standard clinical practice. |
| Uninterpretable results reported? | No | No details given for test failures/uninterpretable measurements. |
| Withdrawals explained? | No | No details of withdrawals given. |
| Clinical features and settings | High‐risk referral for invasive testing and routine screening. | |
| Participants | 6730 participants. USA, UK and other European countries –multicentre study. Dates not reported. Pregnant women. 14‐19 weeks' gestation. | |
| Study design | Prospective cohort study. | |
| Target condition and reference standard(s) | Down's syndrome: 39 cases. Reference standard: amniocentesis, CVS or postnatal examination. | |
| Index and comparator tests | Maternal urine beta core hCG (Chiron manual assay). | |
| Follow‐up | Methods of follow‐up not reported. | |
| Aim of study | A prospective evaluation of urine beta core hCG for Down's syndrome. | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Selective testing of high‐risk women as done in practice. |
| Acceptable reference standard? | Yes | Karyotyping or follow‐up to birth. |
| Partial verification avoided? | Unclear | Unclear if all women received a reference standard. |
| Differential verification avoided? | No | Women had different reference standards. |
| Incorporation avoided? | Yes | Index tests did not form part of the reference standard. |
| Reference standard results blinded? | No | Reference standard interpreted with knowledge of index test results. |
| Index test results blinded? | Yes | Index test conducted without knowledge of the reference standard. |
| Relevant clinical information? | Yes | Information available as would be in standard clinical practice. |
| Uninterpretable results reported? | No | No details given for test failures/uninterpretable measurements. |
| Withdrawals explained? | No | No details of withdrawals given. |
| Clinical features and settings | High‐risk referral for invasive testing. | |
| Participants | 474 participants: 69 cases and 405 controls. Taiwan and UK. Dates not specified. Pregnant women. Median age cases 36.0 years (21‐44 years), controls 34.5 years (23‐43 years). 14‐26 weeks' gestation. | |
| Study design | Retrospective case‐control study. | |
| Target condition and reference standard(s) | Down's syndrome: 69 cases. Reference standard: amniocentesis. | |
| Index and comparator tests | Maternal age. Urinary beta core fragment (UGP) (UGF‐EIA Toa kit). Urinary free beta hCG (CIS immunoradiometric assay). Urinary total oestriol (Orthoclinical diagnostics oestriol (total) II radioimmunoassay kit). All adjusted for creatinine concentration. Modelled to standardised population for England and Wales 1991‐1994. Cases from Taiwan. | |
| Follow‐up | 100% karyotyping. | |
| Aim of study | To investigate levels of urinary beta core fragment, free beta hCG and total oestriol in a new large set of Down's syndrome pregnancies. | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Selective testing of high‐risk women as done in practice. |
| Acceptable reference standard? | Yes | Amniocentesis. |
| Partial verification avoided? | Yes | All women had a reference standard. |
| Differential verification avoided? | Yes | All women had the same reference standard. |
| Incorporation avoided? | Yes | Reference standard was independent of the index test. |
| Reference standard results blinded? | Yes | Reference standard interpreted without knowledge of index test results. |
| Index test results blinded? | Unclear | Unclear if index test interpreted without knowledge of reference standard results. |
| Relevant clinical information? | Yes | Information available as would be in standard clinical practice. |
| Uninterpretable results reported? | No | No details given for test failures/uninterpretable measurements. |
| Withdrawals explained? | No | No details of withdrawals given. |
| Clinical features and settings | High‐risk referral for invasive testing. | |
| Participants | 726 participants. USA ‐ single centre. August 1995 – May 1996. Pregnant women. Mean age 35.4 years (SD 4.0 years) in mothers of Down’s syndrome babies and 37 years (SD 4.3 years) in mothers of healthy babies. Singleton pregnancies. 12‐24 weeks' gestation. | |
| Study design | Prospective cohort study. | |
| Target condition and reference standard(s) | Down's syndrome: 13 cases. Reference standard: amniocentesis. | |
| Index and comparator tests | Urinary beta core fragment (B210 monoclonal antibody, 2‐step sandwich assay). | |
| Follow‐up | 100% karyotyping. | |
| Aim of study | To present data for prospectively collected samples of urinary beta core fragment for Down’s syndrome screening. | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Selective testing of high‐risk women as done in practice. |
| Acceptable reference standard? | Yes | Amniocentesis. |
| Partial verification avoided? | Yes | All women had a reference standard. |
| Differential verification avoided? | Yes | All women had the same reference standard. |
| Incorporation avoided? | Yes | Reference standard was independent of the index test. |
| Reference standard results blinded? | Yes | Reference standard interpreted without knowledge of index test results. |
| Index test results blinded? | Yes | Index test interpreted without knowledge of reference standard results. |
| Relevant clinical information? | Yes | Information available as would be in standard clinical practice. |
| Uninterpretable results reported? | No | No details given for test failures/uninterpretable measurements. |
| Withdrawals explained? | No | No details of withdrawals given. |
| Clinical features and settings | High‐risk referral for invasive testing. | |
| Participants | 2,055 participants. USA – multicentre study. January 2001 ‐ January 2003. Pregnant women with mean age 38.9 years. 15‐20 weeks' gestation. | |
| Study design | Prospective cohort study. | |
| Target condition and reference standard(s) | Down's syndrome: 28 cases. Reference standard: amniocentesis. | |
| Index and comparator tests | Urinary invasive trophoblastic antigen (ITA) (B207 (detection) and B152 (capture) anti‐hCG monoclonal antibodies). | |
| Follow‐up | 100% karyotyping. | |
| Aim of study | To evaluate ITA as a potential marker for Down's syndrome in the second trimester of pregnancy. | |
| Notes | Clean catch of random urine provided. Sent same day at 4 degrees Celcius on an ice pack. Aliquoted into 1 mL plastic tubes. 1 urine aliquot shipped to lab for testing. Rest stored at ‐70 degrees Celcius. Most samples assayed within 24 hours of reaching lab and all within 48 hours. Anti‐ITA antibody produced. Sample corrected for creatinine levels. | |
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Selective testing of high‐risk population as done in practice. |
| Acceptable reference standard? | Yes | Amniocentesis. |
| Partial verification avoided? | Yes | All women had a reference standard. |
| Differential verification avoided? | Yes | All women had the same reference standard. |
| Incorporation avoided? | Yes | Reference standard was independent of the index test. |
| Reference standard results blinded? | Yes | Reference standard interpreted without knowledge of index test results. |
| Index test results blinded? | Yes | Index test interpreted without knowledge of reference standard results. |
| Relevant clinical information? | Yes | Information available as would be in standard clinical practice. |
| Uninterpretable results reported? | No | No details given for test failures/uninterpretable measurements. |
| Withdrawals explained? | No | No details of withdrawals given. |
| Clinical features and settings | High‐risk referral for invasive testing. | |
| Participants | 429 participants: 29 cases and 400 controls. UK. Date not specified. Pregnant women. Singleton pregnancies. 14‐24 (cases) and 9‐22 (controls) weeks' gestation. | |
| Study design | Case‐control study. | |
| Target condition and reference standard(s) | Down's syndrome: 29 cases. Reference standards: amniocentesis or CVS. | |
| Index and comparator tests | Urine free beta hCG (CIS immunoradiometric assay). Urinary beta core fragment (Ciba Corning diagnostics UGP enzyme immunoassay). | |
| Follow‐up | 100% karyotyping. | |
| Aim of study | To evaluate whether free beta hCG is elevated in the urine of pregnancies affected by Down's syndrome and investigate whether urine free beta hCG may be used as possible screening markers. | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Selective testing of high‐risk women as done in practice. |
| Acceptable reference standard? | Yes | Amniocentesis or CVS. |
| Partial verification avoided? | Yes | All women had a reference standard. |
| Differential verification avoided? | No | Women had different reference standards. |
| Incorporation avoided? | Yes | Reference standard was independent of the index test. |
| Reference standard results blinded? | Yes | Reference standard interpreted without knowledge of index test results. |
| Index test results blinded? | Yes | Index test interpreted without knowledge of reference standard results. |
| Relevant clinical information? | Yes | Information available as would be in standard clinical practice. |
| Uninterpretable results reported? | No | No details given for test failures/uninterpretable measurements. |
| Withdrawals explained? | No | No details of withdrawals given. |
| Clinical features and settings | Routine screening. | |
| Participants | 606 participants: 101 cases, 505 controls matched for gestation, duration of storage and centre. UK and Austria ‐ multicentre trial. September 1996 ‐ April 2000. Pregnant women. 9‐13 and 14‐20 weeks' gestation. | |
| Study design | Case‐control study. | |
| Target condition and reference standard(s) | Down's syndrome: 101 cases. Reference standards: invasive testing (following second trimester screening) or follow‐up to birth. | |
| Index and comparator tests | First trimester NT (midsagittal section, optimal magnification of thickness of translucent space between inner skin surface and fascia covering cervical spine (white black interface (outer) ‐ black white interface (inner), 41 models of ultrasound machine, 20 minutes allotted scanning time). First and second trimester serum AFP, hCG, uE3, PAPP‐A, free beta hCG (time resolved fluoroimmunoassay, AutoDELFIA). First and second trimester inhibin A (Sandwich enzyme‐linked immunosorbent assay, Oxford Bio‐innovation). First and second trimester urinary beta core fragment, total hCG, ITA and free beta hCG (ITA and beta core fragment, Quest diagnostics USA). | |
| Follow‐up | Follow‐up by: 1) Staff at local hospitals completed a study outcome form at, or just after. delivery, 2) Study records of CVS, amniocentesis or karyotype at birth linked to information from cytogenic laboratories, 3) Study records linked to records of cases of Down's syndrome from the National Down's Syndrome Cytogenetic Register, 4) Information obtained from local obstetrical outcome records, 5) Forms sent to all women with a request to return details of the outcome of their pregnancy, 6) Individual searches in respect of women whose outcomes of pregnancy had not been obtained by any of the previous methods. 4% of women in the total cohort did not have a documented outcome of pregnancy. Unclear if any of these women were included in this nested case‐control study. | |
| Aim of study | To identify the most effective, safe and cost‐effective strategy for antenatal screening for Down's syndrome using NT, maternal serum and urine markers in the first and second trimesters of pregnancy and maternal age in various combinations. | |
| Notes | Performance of screening assessed at 17 weeks' gestation. Study tried to be non‐interventional in the first trimester ‐ second trimester testing was aimed to be used as the basis for any referral for invasive testing. | |
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Routine screening of typical pregnant population. |
| Acceptable reference standard? | Yes | Karyotyping or follow‐up to birth. |
| Partial verification avoided? | Unclear | Unclear if all women received a reference standard. |
| Differential verification avoided? | No | Women received different reference standards. |
| Incorporation avoided? | Yes | Reference standard was independent of the index test. |
| Reference standard results blinded? | No | Reference standard interpreted with knowledge of index test results. |
| Index test results blinded? | Unclear | Serum testing conducted after reference standard and unclear if interpreted without knowledge of reference standard results. |
| Relevant clinical information? | Yes | Information available as would be in standard clinical practice. |
| Uninterpretable results reported? | Yes | Rates of NT failure on average 9%. Pre‐10 weeks' gestation, > 33% failure rate, declined to 7% at 12 weeks. |
| Withdrawals explained? | No | No details of withdrawals given. |
| Clinical features and settings | High‐risk referral for invasive testing. | |
| Participants | 63 participants: 8 cases and 55 controls matched for gestational and maternal age, maternal weight, duration of storage and smoking history. The Netherlands – single hospital. October 1997 to May 1999. Pregnant women. 10‐11 weeks' gestation. | |
| Study design | Case‐control study. | |
| Target condition and reference standard(s) | Down's syndrome: 8 cases. Reference standard: CVS. | |
| Index and comparator tests | Urinary hyperglycosylated hCG, (procedures previously described in Cole 1999a). | |
| Follow‐up | 100% karyotyping. | |
| Aim of study | To investigate the value of H‐hCG measurements in very early pregnancy (prior to 12 weeks' gestation). | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? | Yes | Selective testing of high‐risk women as done in practice. |
| Acceptable reference standard? | Yes | CVS. |
| Partial verification avoided? | Yes | All women had a reference standard. |
| Differential verification avoided? | Yes | All women had the same reference standard. |
| Incorporation avoided? | Yes | Reference standard was independent of the index test. |
| Reference standard results blinded? | Yes | Reference standard interpreted without knowledge of index test results. |
| Index test results blinded? | Yes | Index test interpreted without knowledge of reference standard results. |
| Relevant clinical information? | Yes | Information available as would be in standard clinical practice. |
| Uninterpretable results reported? | No | No details given for test failures/uninterpretable measurements. |
| Withdrawals explained? | No | No details of withdrawals given. |
AFP: alpha‐fetoprotein
ßhCG: beta human chorionic gonadotrophin
CVS: chorionic villus sampling
hCG: human chorionic gonadotrophin
ITA: invasive trophoblast antigen
NT: nuchal translucency
PAPP‐A: Pregnancy‐associated plasma protein A
SD: standard deviation
uE3: unconjugated oestriol
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Unable to extract useful data. | |
| No Down's syndrome pregnancies. | |
| Unable to extract useful data. | |
| Unable to extract useful information. | |
| Unable to extract useful data. | |
| Fewer than 80% of pregnancies had gestational age confirmed by USS. | |
| Fewer than 80% of pregnancies had gestational age confirmed by USS. | |
| Less than 5 Down's syndrome pregnancies. | |
| Likely fewer than 80% of pregnancies dated by USS. | |
| Women screened at greater than 24 weeks' gestation. | |
| Unable to extract useful data. | |
| Review article. | |
| Data were not relevant to this review ‐ this study was not looking at urine tests for Down's syndrome screening | |
| Unable to extract useful data. | |
| Unable to extract useful data. | |
| Unable to obtain paper. | |
| USS markers greater than 14 weeks' gestation. | |
| USS markers greater than 14 weeks' gestation. | |
| USS markers greater than 14 weeks' gestation. | |
| USS markers greater than 14 weeks' gestation. | |
| Review article. | |
| No Down's pregnancies in study population. | |
| No Down's pregnancies in study population. | |
| No Down's pregnancies in study population. | |
| Unable to extract useful data. | |
| No Down's pregnancies in study population. | |
| Second trimester ultrasound study. | |
| Unclear method of confirmation of gestational age. | |
| Male versus female fetuses. | |
| No Down's syndrome pregnancies in study. | |
| Less than 80% follow‐up. | |
| Less than 80% follow‐up. | |
| No Down's pregnancies in study population. | |
| Less than 80% follow‐up. | |
| Statistical modelling (computer simulation). | |
| Modelled data. | |
| Less than 80% of pregnancies dated by USS. | |
| Editorial. | |
| No Down's pregnancies included. | |
| Mathematical model. | |
| Less than 80% of pregnancies USS dated. | |
| Less than 80% of pregnancies USS dated. | |
| Gestational age not USS estimated. | |
| Unable to extract useful data. | |
| No Down's syndrome pregnancies in study population. | |
| Unable to extract useful data. | |
| No Down's syndrome pregnancies in study population. | |
| No Down's syndrome pregnancies in study population. | |
| Cost‐effectiveness analysis. | |
| Review article. | |
| Unable to extract useful data. | |
| Population risk factor calculations. | |
| Unable to extract useful data. | |
| Review article. | |
| Screen‐negative population gestations not confirmed by ultrasound. | |
| Review article. | |
| USS screening inclusive of women greater than 14 weeks' gestation. | |
| Review article. | |
| Unable to extract useful data. | |
| Unable to extract useful data. | |
| Second trimester ultrasound. | |
| Unable to extract useful data. | |
| Unable to extract useful data. | |
| Unable to extract useful data. | |
| Unable to extract useful data. | |
| No Down's syndrome pregnancies in study population. | |
| Second trimester ultrasound. | |
| Second trimester ultrasound. | |
| Review article. | |
| No Down's syndrome pregnancies in study population. | |
| Less than 5 Down's cases in study population. | |
| Unable to extract useful data. | |
| Likely that fewer than 80% of gestational age confirmed by USS. | |
| Case series. No Down's syndrome pregnancies in study population. | |
| No Down's syndrome pregnancies in study population. | |
| No Down's syndrome pregnancies in study population. | |
| Less than 5 Down's cases in study population. | |
| Unable to extract useful data. | |
| Unable to extract useful data. | |
| Less than 5 Down's syndrome pregnancies in study population. | |
| Unable to obtain translation. | |
| Review article. | |
| USS at greater than 14 weeks. | |
| USS at greater than 14 weeks. | |
| USS at greater than 14 weeks. | |
| Unable to extract useful data. | |
| Review article. | |
| Less than 80% of pregnancies had gestational age confirmation by ultrasound. | |
| Less than 80% of pregnancies had gestational age confirmation by ultrasound. | |
| No Down's syndrome pregnancies in study population. | |
| Adjustment factors for smokers. | |
| Gestational age not confirmed by USS. | |
| Gestational age not confirmed by USS. | |
| No gestational age limits given. | |
| Paper presenting adjustment factors. | |
| Data modelled on 4 meta‐analysed studies. | |
| Unable to extract useful data. | |
| Review article. | |
| Abnormal scans only in study population. | |
| Less than 5 Down's syndrome pregnancies in study population. | |
| Second trimester USS. | |
| No Down's syndrome pregnancies in study population. | |
| Unable to extract useful information. | |
| Unable to ascertain whether overlapping populations between several papers ‐ attempted to contact author with no response. | |
| Unable to ascertain whether overlapping populations between several papers ‐ attempted to contact author with no response. | |
| Unable to extract useful data. | |
| Modelled data. | |
| Second trimester ultrasound. | |
| Comment. | |
| Gestational age by USS only in screen‐positive population. | |
| Ultrasound confirmation of gestational age performed in screen‐positive women only. | |
| Second trimester ultrasound. | |
| Unable to extract useful data. | |
| Fewer than 5 Down's syndrome pregnancies in population. | |
| Review article. | |
| Unable to extract useful data. | |
| No Down's syndrome pregnancies in population. | |
| No Down's syndrome pregnancies in population. | |
| Unable to extract useful data. | |
| Unable to extract useful data. | |
| Audit. | |
| No Down's syndrome pregnancies in population. | |
| Unable to extract useful data. | |
| Comparison of male versus female fetuses. | |
| Fewer than 80% of study population had gestational age confirmed by USS. | |
| Greater than 14 weeks USS screening. | |
| Likely that fewer than 80% of pregnancies had gestational age estimated by USS. | |
| Unable to extract useful data. | |
| Less than 80% follow‐up. | |
| Less than 80% follow‐up. | |
| Unaffected pregnancies only. | |
| Unable to extract useful data. | |
| No Down's syndrome pregnancies in population. | |
| No Down's syndrome pregnancies in population | |
| Less than 80% of pregnancies had gestational age confirmed by ultrasound scan. | |
| Less than 5 Down's pregnancies in study population. | |
| Gestational age greater than 24 weeks. | |
| Less than 80% of pregnancies had gestational age confirmed by USS. | |
| Editorial. | |
| Unable to extract useful data. | |
| Less than 5 Down's pregnancies in study population. | |
| Fewer than 80% of pregnancies had gestational age confirmed by USS. | |
| No Down's syndrome pregnancies in population. | |
| No Down's syndrome pregnancies in study population. | |
| Correlation between markers, not evaluation of screening tests. | |
| Unable to extract useful data. | |
| Gestation unclear. | |
| Gestation based on LMP. | |
| Unable to extract useful data. | |
| Unclear method of determination of gestational age. Unable to extract useful data. | |
| CME. | |
| Second trimester USS. | |
| Unable to obtain translation. | |
| No Down's syndrome pregnancies in study population. | |
| Adjustment factors. | |
| No Down's syndrome pregnancies in study population. | |
| No Down's pregnancies. | |
| No Down's syndrome pregnancies in study population. | |
| Study of cardiac function in pregnancies with normal and abnormal NT results. | |
| No Down's syndrome pregnancies in population. | |
| No Down's syndrome pregnancies in population. | |
| Editorial/commentary. | |
| Modelling. | |
| Unable to extract useful data. | |
| No Down's syndrome pregnancies in population. | |
| Less than 5 Down's pregnancies in population. | |
| Review. | |
| Unable to extract useful data. | |
| Review article. | |
| Gestatiojnal age estimated by USS in fewer than 80% of cases. | |
| Normal pregnancies only. | |
| Gestation greater than 14 weeks for USS. | |
| No Down's syndrome pregnancies in study population. | |
| Less than 5 Down's syndrome pregnancies in study population. | |
| Screen‐positive pregnancies only. | |
| Fewer than 80% pregnancies had gestational age estimated by USS. | |
| Summary article. | |
| Less than 5 Down's syndrome pregnancies in population. | |
| Less than 5 Down's syndrome pregnancies in population. | |
| Less than 80% follow‐up. Unable to ascertain proportion of population with gestational age confirmed by USS. | |
| Assumption of normal karyotype without reference standard in significant proportion of control pregnancies. | |
| Review article. | |
| Validation of a specific assay. | |
| Less than 80% of pregnancies had gestational age confirmed by USS. | |
| Review article. | |
| Less than 5 Down's syndrome pregnancies in population. | |
| Unable to extract useful information. | |
| No Down's syndrome pregnancies in study population. | |
| Modelled data. | |
| Adjustment factor. | |
| No Down's cases in population. | |
| Modelled population. | |
| No Down's syndrome pregnancies in study population. | |
| Unable to extract useful data. | |
| Simulation. | |
| Unable to extract useful data. | |
| Fewer than 80% pregnancies had gestational age estimated by USS. | |
| No Down's syndrome pregnancies in population. | |
| Unable to extract useful data. | |
| Study of women's decisions about screening. | |
| Male versus female fetuses. | |
| Fewer than 80% of pregnancies USS dated. | |
| Unable to extract useful data. | |
| Down's syndrome pregnancies only. | |
| Unable to separate twins from singletons therefore unable to extract useful data. | |
| Appears to be a review article (French). | |
| Unable to obtain translation. | |
| Unable to obtain translation. | |
| Unable to obtain translation. | |
| Unable to extract useful data. | |
| Second trimester ultrasound. | |
| Editorial. | |
| Unable to obtain translation. | |
| Gestational age by LMP only. | |
| Fewer than 80% of gestational ages estimated by USS. | |
| Unable to extract useful data. | |
| Unable to extract useful data. | |
| Likely fewer than 80% evaluated for gestational age by ultrasound examination. | |
| Likely fewer than 80% evaluated for gestational age by ultrasound examination. | |
| Review article. | |
| Review article. | |
| Abnormal screening results only. | |
| No Down's syndrome pregnancies in study population. | |
| No normal test results included therefore unable to extract meaningful data. | |
| No Down's syndrome pregnancies in study population. | |
| No Down's syndrome pregnancies in study population. | |
| Modelled data. | |
| USS dating on screen‐positive women only. | |
| Observed versus expected cases of Down's syndrome in a population. | |
| Gestational age not confirmed by USS. | |
| Editorial. | |
| Normal pregnancies only. | |
| Unable to extract useful data. | |
| No Down's syndrome pregnancies in study population. | |
| Unable to extract useful data. | |
| Fewer than 80% gestational age estimated by USS. | |
| Fewer than 80% gestational age estimated by USS. | |
| Gestational age greater than specified limits. | |
| Unable to extract useful data. | |
| Unable to extract useful data. | |
| Less than 5 Down's syndrome pregnancies. | |
| Review article. | |
| Greater than 24 weeks' gestation. | |
| No Down's syndrome pregnancies in study population. | |
| Unable to extract useful data. | |
| Unable to extract useful data. | |
| Getstional age greater than 24 weeks. | |
| Unable to extract meaningful data ‐ unable to separate double and triple test data. | |
| No Down's syndrome pregnancies in study population. | |
| Unable to extract useful data. | |
| Unable to extract useful data. | |
| Fewer than 80% USS dated. | |
| Unable to extract useful data. | |
| No Down's syndrome pregnancies in population. | |
| Unable to extract useful data. | |
| Unable to extract useful data. | |
| Study of outcomes of abnormal NT results. | |
| Review article. | |
| Review article. | |
| Unable to obtain translation ‐ appears to be a review article. | |
| Unable to obtain translation ‐ appears to be a review article. | |
| Unable to obtain translation ‐ appears to be a review article. | |
| Unable to obtain translation ‐ appears to be a review article. | |
| Unable to obtain translation ‐ appears to be a review article. | |
| Review article. | |
| No Down's pregnancies in study population. | |
| No Down's syndrome pregnancies in population. | |
| No Down's syndrome pregnancies in population. | |
| Unable to extract useful data. | |
| Less than 80% of gestational ages confirmed by USS. | |
| Unable to extract useful data. | |
| No Down's syndrome pregnancies in population. | |
| No Down's syndrome pregnancies in population. | |
| Gestational age greater than 14 weeks in USS population. | |
| Unable to extract useful data. | |
| Unable to extract useful data. | |
| Unable to extract useful data. | |
| Unable to extract useful data. | |
| Less than 80% follow‐up. | |
| No Down's syndrome pregnancies in study population. | |
| Twin data used in calculation of the median. | |
| Fewer than 80% USS dated. | |
| No Down's syndrome pregnancies in population. | |
| No Down's syndrome pregnancies in population. | |
| Meta‐analysis. | |
| Unable to extract meaningful data. | |
| Less than 5 Down's syndrome pregnancies in population. | |
| Study of outcomes of abnormal NT results. | |
| Review article. | |
| Unable to extract useful data. | |
| Unable to extract useful data. | |
| No Down's syndrome pregnancies in study population. | |
| No Down's syndrome pregnancies in population. | |
| Smokers versus non smokers. | |
| Unable to extract useful data. | |
| Likely fewer than 80% USS dated. | |
| Gestational age confirmed by USS in less than 80% of population. | |
| Gestational age confirmed by USS in less than 80% of population. | |
| Women screened prior to recruitment. | |
| Unable to extract useful data. | |
| Abnormal results only. | |
| Comparison of prevalence and prediction. | |
| Comparison of a marker in women of different ethnic origins. | |
| Unable to extract useful data. | |
| Unable to obtain translation. | |
| No Down's syndrome pregnancies in population. | |
| Unable to extract useful data. | |
| Review article. | |
| Method of ascertainment of gestational age unclear. Twin gestations included in general population. | |
| Second trimester USS. | |
| Fewer than 5 Down's cases. | |
| Explanation of mathematical techniques. | |
| Unable to extract useful data. | |
| No Down's syndrome pregnancies in study population. | |
| Down's syndrome pregnancies excluded from study. | |
| Unable to extract useful data. | |
| No Down's syndrome pregnancies in study population. | |
| Editorial. | |
| No Down's pregnancies. | |
| Editorial ‐ summary of FASTER trial results. | |
| Review article. | |
| Review article. | |
| Unable to extract useful data. | |
| No Down's syndrome pregnancies in study population. | |
| USS greater than 14 weeks' gestation. | |
| No Down's syndrome pregnancies in population. | |
| Unable to determine method of confirmation of gestational age. | |
| High‐risk results only included (i.e. no screen‐negative group for comparison). | |
| No Down's pregnancies in population. | |
| Unable to ascertain how numbers calculated and from which populations. | |
| Unable to extract useful data. | |
| Down's syndrome secondary to Robertsonian translocation only. No controls. | |
| No Down's syndrome pregnancies in population. | |
| Fewer than 80% had gestational age estimated by USS. | |
| Gestation greater than 14 weeks for nuchal scanning. | |
| Down's syndrome and Edward's syndrome affected pregnancies only. | |
| Unable to extract useful data. | |
| Unable to extract useful data. | |
| No Down's pregnancies in study population. | |
| Less than 5 Down's syndrome pregnancies in study population. | |
| Review article. | |
| No Down's syndrome pregnancies in study population. | |
| No Down's syndrome pregnancies. | |
| Unable to extract useful data. | |
| No Down's syndrome pregnancies in population. | |
| Review article. | |
| Gestational age confirmed by USS in less than 80% of population. | |
| Analysis of screen‐positive results. | |
| Review/meta‐analysis. | |
| Unable to extract useful data. | |
| Meta‐analysis of second trimester ultrasound markers. | |
| Population study, not examining DTA. | |
| Study of prevalence, not screening. | |
| Study of prevalence, not screening. | |
| Less than 80% follow‐up. | |
| Observation of Down's prevalence stratified by age. | |
| Editorial. | |
| Fewer than 80% USS dated. | |
| Likely fewer than 80% USS dated. | |
| Unable to extract useful data. | |
| Unable to extract useful data. | |
| Fewer than 80% USS dated. | |
| No Down's pregnancies in study population. | |
| Unable to extract useful data. | |
| Fewer than 80% of pregnancies had gestational age confirmed by USS. | |
| Unable to extract useful data. | |
| No Down's pregnancies in population. | |
| Fewer than 80% of pregnancies had gestational age confirmed by USS. | |
| Statistical modelling, aneuploid pregnancies only in study population. | |
| No Down's pregnancies in population. | |
| Unable to extract useful data. | |
| Review. | |
| Statistical methods paper. | |
| Examination of median shifts rather than an evaluation of screening. | |
| No Down's syndrome pregnancies in population. | |
| No Down's syndrome pregnancies in population. | |
| No Down's cases. | |
| Male versus female fetuses. | |
| No Down's cases in population. | |
| No Down's pregnancies in population. | |
| No Down's pregnancies in population. | |
| Comparsison of fetal sex. | |
| No Down's syndrome pregnancies in population. | |
| Unable to extract useful data. | |
| Unable to extract useful data. | |
| Unable to extract useful data. | |
| No Down's syndrome pregnancies in population. | |
| No Down's pregnancies. | |
| Risk validation study. | |
| No Down's syndrome pregnancies in population. | |
| Demonstration of median changes with time, rather than evaluation of screening. | |
| No Down's pregnancies in population. | |
| No Down's pregnancies in population. | |
| Calculation of weight correction factor. | |
| Fewer than 5 Down's syndrome pregnancies. | |
| Calculation of smoking correction factor. | |
| No Down's pregnancies. | |
| No Down's pregnancies. | |
| Comparison of 2 different assays ‐ not actual screening evaluation. | |
| Comparison of male and female fetuses. | |
| Literature review. | |
| Review article. | |
| Unable to extract useful data. | |
| Review article. | |
| Unable to ascertain method of confirmation of gestational age. | |
| Fewer than 80% had gestational age estimated by USS. | |
| No Down's syndrome pregnancies in study population. | |
| Examination of inter‐observer variation in NT scanning. | |
| Unable to extract useful data. | |
| Unable to extract useful data. | |
| Geststional age not confirmed by USS. | |
| Information on screen‐positive pregnancies only. | |
| No Down's syndrome pregnancies in study population. | |
| Editorial. | |
| No Down's syndrome pregnancies in population. | |
| Unable to extract useful data. | |
| Less than 5 Down's syndrome pregnancies in study population. | |
| Fewer than 80% pregnancies had gestational age estimated by USS. | |
| Unable to extract useful data. | |
| No Down's syndrome pregnancies in study population. Software comparison study. | |
| Unable to extract useful data. | |
| Unable to extract useful data. | |
| Unable to extract useful data. | |
| Unable to extract useful data. | |
| Unable to extract useful data. | |
| Second trimester USS. | |
| Less than 80% had gestational age confirmed by ultrasound. | |
| Gestational age not confirmed by USS. | |
| No Down's pregnancies in study. | |
| Less than 80% had gestational age confirmed by ultrasound. | |
| No Down's pregnancies in study. | |
| No Down's pregnancies in study. | |
| Fewer than 80% had gestational age estimated by USS | |
| No Down's syndrome pregnancies in population. | |
| Review article. | |
| No Down's pregnancies. | |
| Fewer than 80% had gestational age estimated by USS | |
| No Down's syndrome pregnancies in population. | |
| Gestational age greater than 24 weeks. | |
| Data modelled on 3 separate populations of women. | |
| Unable to extract useful data. | |
| Unable to extract useful data. | |
| Gestational age not confirmed by USS. | |
| No Down's syndrome pregnancies. | |
| Modelled on several studies, some of which have no USS dating. | |
| No cases. | |
| Less than 80% had gestational age confirmed by USS. | |
| Modelled on SURRUS data. | |
| Unable to extract useful data. | |
| No Down's syndrome pregnancies in study population. | |
| Review article. | |
| No Downs syndrome pregnancies in study population. | |
| No Down's syndrome pregnancies in study population. | |
| Unable to extract useful data. | |
| Study of women's views on screening. | |
| Abnormal results only (cystic hygroma). | |
| Less than 80% of pregnancies had gestational age confirmed by USS. | |
| Adjustment factors. | |
| Less than 80% of pregnancies had gestational age confirmed by USS. | |
| Unable to extract useful data. | |
| Unable to extract useful data. | |
| Unable to extract useful data. | |
| Fewer than 80% had gestational age estimated by USS. | |
| Review. | |
| No Down's syndrome pregnancies in study population. | |
| Less than 5 Down's syndrome pregnancies in population. | |
| Mathematical model. | |
| Second trimester USS. | |
| Unable to extract useful data. | |
| Method of determination of gestational age unclear. | |
| Unable to extract useful data. | |
| Male versus female fetuses. | |
| Unable to obtain translation. | |
| Fewer than 80% pregnancies had gestational age estimated by USS. | |
| Only aneuploid pregnancies included in study. | |
| No Down's cases in population. | |
| Unable to obtain translation. | |
| Inappropriate study design. |
Data
Presented below are all the data for all of the tests entered into the review.
| Test | No. of studies | No. of participants |
| 1 Betacore, 1st trimester urine test, 5% FPR Show forest plot | 1 | 516 |
| Test 1 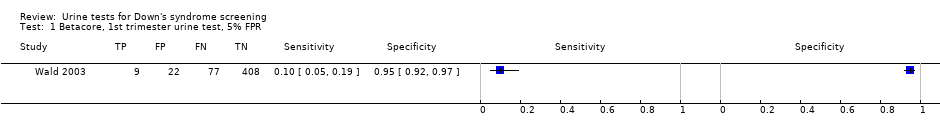 Betacore, 1st trimester urine test, 5% FPR. | ||
| 2 Betacore, 2nd trimester urine test, 5% FPR Show forest plot | 6 | 9613 |
| Test 2  Betacore, 2nd trimester urine test, 5% FPR. | ||
| 3 Betacore, 2nd trimester urine test, cutpoint mixed Show forest plot | 7 | 10124 |
| Test 3  Betacore, 2nd trimester urine test, cutpoint mixed. | ||
| 4 Gonadotropin, 2nd trimester urine test, risk 1:100 Show forest plot | 1 | 105 |
| Test 4  Gonadotropin, 2nd trimester urine test, risk 1:100. | ||
| 5 Gonadotropin, 2nd trimester urine test, risk 1:384 Show forest plot | 1 | 105 |
| Test 5  Gonadotropin, 2nd trimester urine test, risk 1:384. | ||
| 6 Gonadotropin, 2nd trimester urine test, 95% percentile Show forest plot | 1 | 105 |
| Test 6  Gonadotropin, 2nd trimester urine test, 95% percentile. | ||
| 7 ITA, 1st trimester urine test, 5% FPR Show forest plot | 2 | 579 |
| Test 7 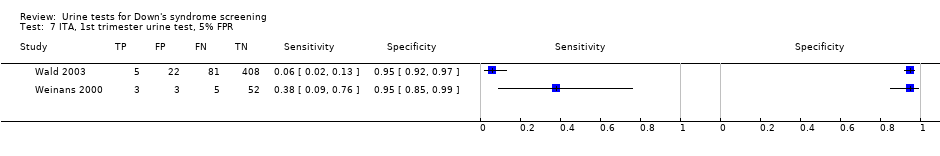 ITA, 1st trimester urine test, 5% FPR. | ||
| 8 ITA, 2nd trimester urine test, 3.74MoM Show forest plot | 1 | 2051 |
| Test 8  ITA, 2nd trimester urine test, 3.74MoM. | ||
| 9 ITA, 2nd trimester urine test, 5% FPR Show forest plot | 3 | 2748 |
| Test 9 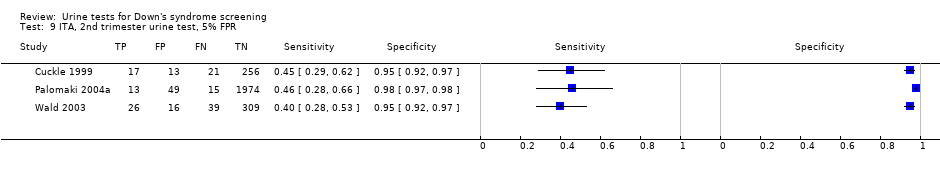 ITA, 2nd trimester urine test, 5% FPR. | ||
| 10 Total hCG, 1st trimester urine test, 5% FPR Show forest plot | 1 | 516 |
| Test 10  Total hCG, 1st trimester urine test, 5% FPR. | ||
| 11 Total hCG, 2nd trimester urine test, 5% FPR Show forest plot | 1 | 390 |
| Test 11 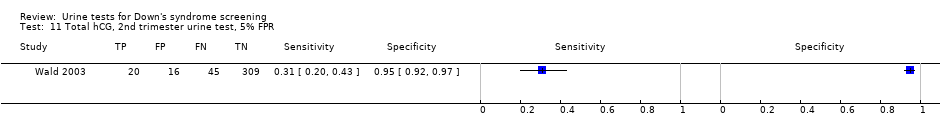 Total hCG, 2nd trimester urine test, 5% FPR. | ||
| 12 Free ßhCG, 1st trimester urine test, 5% FPR Show forest plot | 1 | 516 |
| Test 12  Free ßhCG, 1st trimester urine test, 5% FPR. | ||
| 13 Free ßhCG, 2nd trimester urine test, 5% FPR Show forest plot | 3 | 1517 |
| Test 13  Free ßhCG, 2nd trimester urine test, 5% FPR. | ||
| 14 Oestriol, 2nd trimester urine test, 5% FPR Show forest plot | 2 | 1472 |
| Test 14 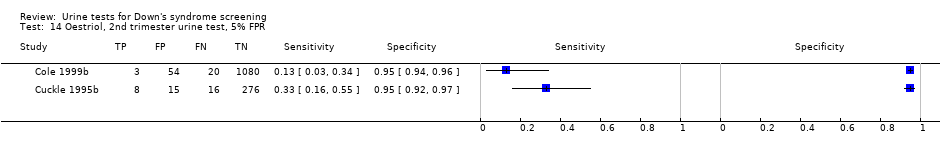 Oestriol, 2nd trimester urine test, 5% FPR. | ||
| 15 Betacore to oestriol ratio, 2nd trimester urine test, 5% FPR Show forest plot | 2 | 1649 |
| Test 15 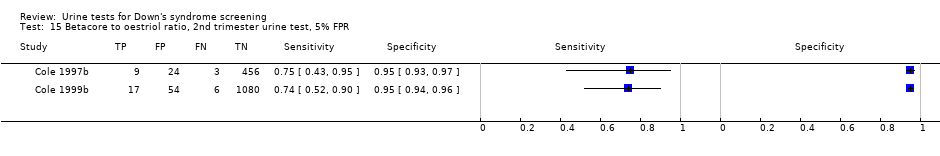 Betacore to oestriol ratio, 2nd trimester urine test, 5% FPR. | ||
| 16 Betacore and oestriol, 2nd trimester 5% FPR Show forest plot | 1 | 315 |
| Test 16  Betacore and oestriol, 2nd trimester 5% FPR. | ||
| 17 AFP and ITA, 2nd trimester urine test, 3% FPR Show forest plot | 1 | 524 |
| Test 17  AFP and ITA, 2nd trimester urine test, 3% FPR. | ||
| 18 AFP and ITA, 2nd trimester urine test, 5% FPR Show forest plot | 1 | 524 |
| Test 18  AFP and ITA, 2nd trimester urine test, 5% FPR. | ||
| 19 AFP and ITA, 2nd trimester urine test,10% FPR Show forest plot | 1 | 524 |
| Test 19  AFP and ITA, 2nd trimester urine test,10% FPR. | ||
| 20 AFP and ITA, 2nd trimester urine test, 15% FPR Show forest plot | 1 | 524 |
| Test 20  AFP and ITA, 2nd trimester urine test, 15% FPR. | ||
| 21 AFP, uE3 and ITA, 2nd trimester urine test, 3% FPR Show forest plot | 1 | 524 |
| Test 21  AFP, uE3 and ITA, 2nd trimester urine test, 3% FPR. | ||
| 22 AFP, uE3 and ITA, 2nd trimester urine test, 5% FPR Show forest plot | 1 | 524 |
| Test 22  AFP, uE3 and ITA, 2nd trimester urine test, 5% FPR. | ||
| 23 AFP, uE3 and ITA, 2nd trimester urine test, 10% FPR Show forest plot | 1 | 524 |
| Test 23  AFP, uE3 and ITA, 2nd trimester urine test, 10% FPR. | ||
| 24 AFP, uE3 and ITA, 2nd trimester urine test, 15% FPR Show forest plot | 1 | 524 |
| Test 24  AFP, uE3 and ITA, 2nd trimester urine test, 15% FPR. | ||
| 25 Age, betacore, 2nd trimester urine test, 1% FPR Show forest plot | 2 | 2083 |
| Test 25  Age, betacore, 2nd trimester urine test, 1% FPR. | ||
| 26 Age, betacore, 2nd trimester urine test, 3% FPR Show forest plot | 2 | 2083 |
| Test 26 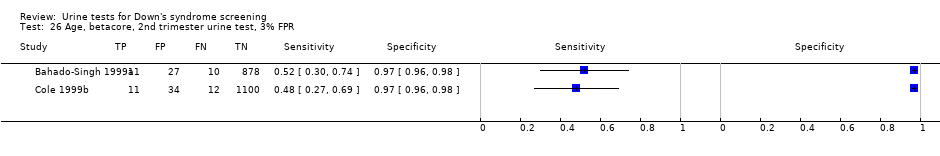 Age, betacore, 2nd trimester urine test, 3% FPR. | ||
| 27 Age, betacore, 2nd trimester urine test, 5% FPR Show forest plot | 5 | 3419 |
| Test 27  Age, betacore, 2nd trimester urine test, 5% FPR. | ||
| 28 Age, betacore, 2nd trimester urine test, 10% FPR Show forest plot | 1 | 926 |
| Test 28  Age, betacore, 2nd trimester urine test, 10% FPR. | ||
| 29 Age, betacore, 2nd trimester urine test, 15% FPR Show forest plot | 1 | 953 |
| Test 29  Age, betacore, 2nd trimester urine test, 15% FPR. | ||
| 30 Age, betacore, 2nd trimester urine test, 20% FPR Show forest plot | 1 | 926 |
| Test 30  Age, betacore, 2nd trimester urine test, 20% FPR. | ||
| 31 Age, ITA, 2nd trimester urine test, 5% FPR Show forest plot | 1 | 1016 |
| Test 31  Age, ITA, 2nd trimester urine test, 5% FPR. | ||
| 32 Age, oestriol, 2nd trimester urine test, 5% FPR Show forest plot | 1 | 474 |
| Test 32  Age, oestriol, 2nd trimester urine test, 5% FPR. | ||
| 33 Age, free ßhCG, 2nd trimester urine test, 5% FPR Show forest plot | 2 | 879 |
| Test 33  Age, free ßhCG, 2nd trimester urine test, 5% FPR. | ||
| 34 Age, betacore to oestriol ratio, 2nd trimester urine test, 1% FPR Show forest plot | 1 | 1157 |
| Test 34  Age, betacore to oestriol ratio, 2nd trimester urine test, 1% FPR. | ||
| 35 Age, betacore to oestriol ratio, 2nd trimester urine test, 3% FPR Show forest plot | 1 | 1157 |
| Test 35  Age, betacore to oestriol ratio, 2nd trimester urine test, 3% FPR. | ||
| 36 Age, betacore to oestriol ratio, 2nd trimester urine test, 5% FPR Show forest plot | 3 | 2088 |
| Test 36 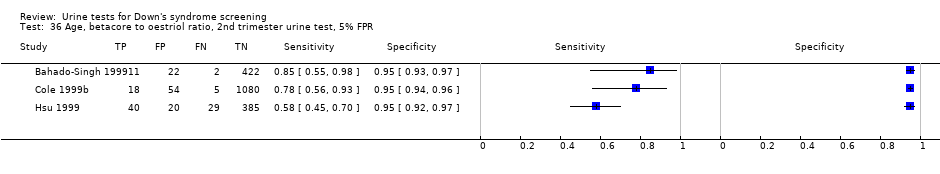 Age, betacore to oestriol ratio, 2nd trimester urine test, 5% FPR. | ||
| 37 Age, free ßhCG to oestriol ratio, 2nd trimester urine test, 5% FPR Show forest plot | 1 | 474 |
| Test 37  Age, free ßhCG to oestriol ratio, 2nd trimester urine test, 5% FPR. | ||
| 38 Age, oestriol and free ßhCG, 2nd trimester, 5% FPR Show forest plot | 1 | 474 |
| Test 38  Age, oestriol and free ßhCG, 2nd trimester, 5% FPR. | ||
| 39 Age, betacore to free ßhCG ratio, 2nd trimester, 5% FPR Show forest plot | 1 | 474 |
| Test 39  Age, betacore to free ßhCG ratio, 2nd trimester, 5% FPR. | ||
| 40 Age, betacore and oestriol, 2nd trimester 1% FPR Show forest plot | 1 | 1157 |
| Test 40  Age, betacore and oestriol, 2nd trimester 1% FPR. | ||
| 41 Age, betacore and oestriol, 2nd trimester, 3% FPR Show forest plot | 1 | 1157 |
| Test 41  Age, betacore and oestriol, 2nd trimester, 3% FPR. | ||
| 42 Age, betacore and oestriol, 2nd trimester, 5% FPR Show forest plot | 2 | 1631 |
| Test 42  Age, betacore and oestriol, 2nd trimester, 5% FPR. | ||
| 43 Age, AFP and betacore to oestriol ratio, 2nd trimester, risk 1:10 Show forest plot | 1 | 356 |
| Test 43  Age, AFP and betacore to oestriol ratio, 2nd trimester, risk 1:10. | ||
| 44 Age, AFP and betacore to oestriol ratio, 2nd trimester, risk 1:20 Show forest plot | 1 | 356 |
| Test 44 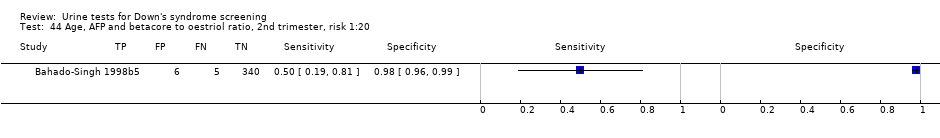 Age, AFP and betacore to oestriol ratio, 2nd trimester, risk 1:20. | ||
| 45 Age, AFP and betacore to oestriol ratio, 2nd trimester, risk 1:30 Show forest plot | 1 | 356 |
| Test 45  Age, AFP and betacore to oestriol ratio, 2nd trimester, risk 1:30. | ||
| 46 Age, AFP and betacore to oestriol ratio, 2nd trimester, risk 1:58 Show forest plot | 1 | 356 |
| Test 46  Age, AFP and betacore to oestriol ratio, 2nd trimester, risk 1:58. | ||
| 47 Age, AFP and betacore to oestriol ratio, 2nd trimester, risk 1:270 Show forest plot | 1 | 356 |
| Test 47  Age, AFP and betacore to oestriol ratio, 2nd trimester, risk 1:270. | ||
| 48 Age, AFP and betacore to oestriol ratio, 2nd trimester, risk 1:526 Show forest plot | 1 | 356 |
| Test 48  Age, AFP and betacore to oestriol ratio, 2nd trimester, risk 1:526. | ||

Methodological quality summary: review authors' judgements about each methodological quality item for each included study.

Detection rates (% sensitivity) at a 5% false positive rate for the five most evaluated or best performing test strategies. The estimates are shown with 95% confidence intervals. The test strategies are ordered on the plot according to decreasing detection rate. The number of studies, cases and women included for each test strategy are shown on the horizontal axis.

Betacore, 1st trimester urine test, 5% FPR.

Betacore, 2nd trimester urine test, 5% FPR.
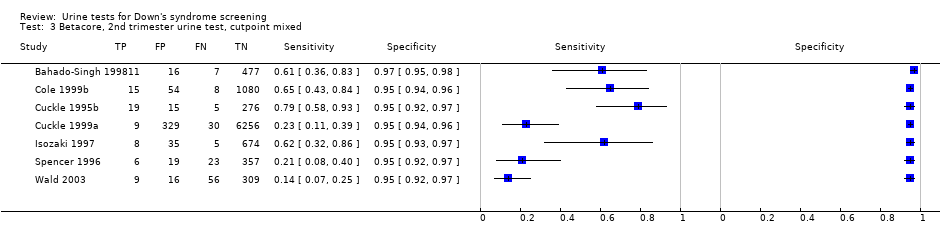
Betacore, 2nd trimester urine test, cutpoint mixed.

Gonadotropin, 2nd trimester urine test, risk 1:100.

Gonadotropin, 2nd trimester urine test, risk 1:384.

Gonadotropin, 2nd trimester urine test, 95% percentile.

ITA, 1st trimester urine test, 5% FPR.

ITA, 2nd trimester urine test, 3.74MoM.
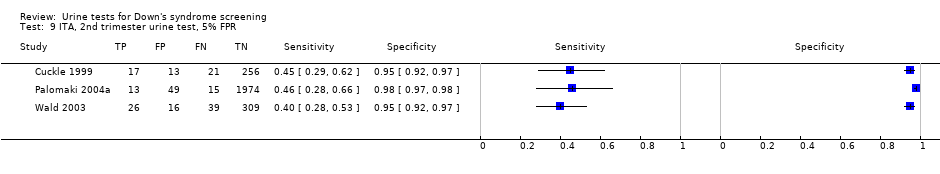
ITA, 2nd trimester urine test, 5% FPR.

Total hCG, 1st trimester urine test, 5% FPR.
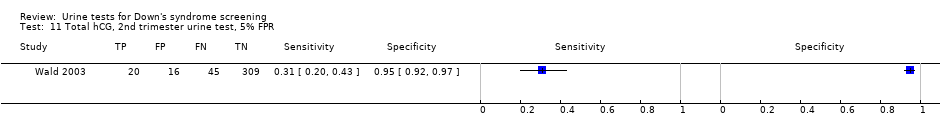
Total hCG, 2nd trimester urine test, 5% FPR.

Free ßhCG, 1st trimester urine test, 5% FPR.

Free ßhCG, 2nd trimester urine test, 5% FPR.

Oestriol, 2nd trimester urine test, 5% FPR.
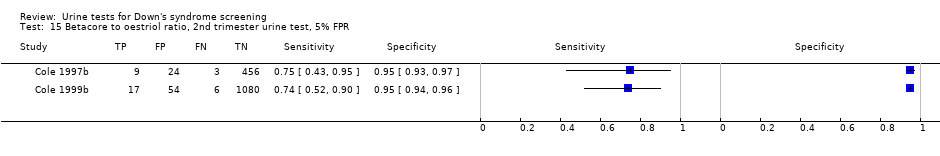
Betacore to oestriol ratio, 2nd trimester urine test, 5% FPR.

Betacore and oestriol, 2nd trimester 5% FPR.

AFP and ITA, 2nd trimester urine test, 3% FPR.

AFP and ITA, 2nd trimester urine test, 5% FPR.

AFP and ITA, 2nd trimester urine test,10% FPR.

AFP and ITA, 2nd trimester urine test, 15% FPR.

AFP, uE3 and ITA, 2nd trimester urine test, 3% FPR.

AFP, uE3 and ITA, 2nd trimester urine test, 5% FPR.

AFP, uE3 and ITA, 2nd trimester urine test, 10% FPR.

AFP, uE3 and ITA, 2nd trimester urine test, 15% FPR.

Age, betacore, 2nd trimester urine test, 1% FPR.

Age, betacore, 2nd trimester urine test, 3% FPR.
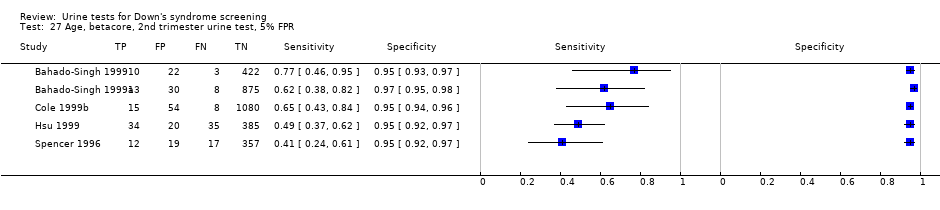
Age, betacore, 2nd trimester urine test, 5% FPR.

Age, betacore, 2nd trimester urine test, 10% FPR.

Age, betacore, 2nd trimester urine test, 15% FPR.

Age, betacore, 2nd trimester urine test, 20% FPR.

Age, ITA, 2nd trimester urine test, 5% FPR.

Age, oestriol, 2nd trimester urine test, 5% FPR.

Age, free ßhCG, 2nd trimester urine test, 5% FPR.
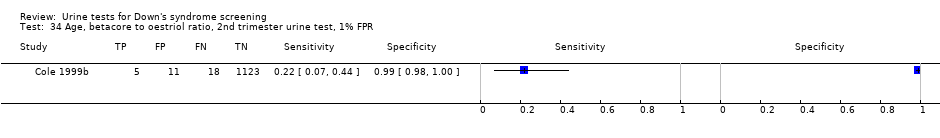
Age, betacore to oestriol ratio, 2nd trimester urine test, 1% FPR.

Age, betacore to oestriol ratio, 2nd trimester urine test, 3% FPR.

Age, betacore to oestriol ratio, 2nd trimester urine test, 5% FPR.

Age, free ßhCG to oestriol ratio, 2nd trimester urine test, 5% FPR.

Age, oestriol and free ßhCG, 2nd trimester, 5% FPR.

Age, betacore to free ßhCG ratio, 2nd trimester, 5% FPR.

Age, betacore and oestriol, 2nd trimester 1% FPR.

Age, betacore and oestriol, 2nd trimester, 3% FPR.
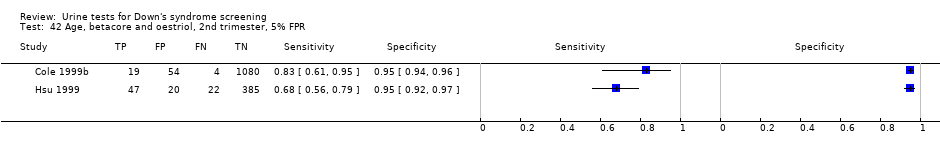
Age, betacore and oestriol, 2nd trimester, 5% FPR.
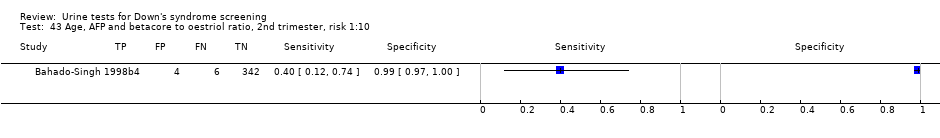
Age, AFP and betacore to oestriol ratio, 2nd trimester, risk 1:10.
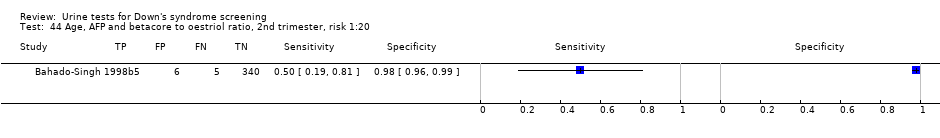
Age, AFP and betacore to oestriol ratio, 2nd trimester, risk 1:20.

Age, AFP and betacore to oestriol ratio, 2nd trimester, risk 1:30.

Age, AFP and betacore to oestriol ratio, 2nd trimester, risk 1:58.

Age, AFP and betacore to oestriol ratio, 2nd trimester, risk 1:270.

Age, AFP and betacore to oestriol ratio, 2nd trimester, risk 1:526.
| Review Question | What is the accuracy of urine based markers for screening for Down's syndrome? | ||||
| Population | Pregnant women at less than 24 weeks' gestation confirmed by ultrasound, who had not undergone previous testing for Down’s syndrome. Most studies were undertaken in women identified to be high risk based on maternal age | ||||
| Settings | All settings | ||||
| Numbers of studies, pregnancies and Down's syndrome cases | 19 studies (reported in 29 publications) involving 18,013 pregnancies of which 527 were Down's syndrome pregnancies. | ||||
| Index tests | Risk scores computed using maternal age and first and second trimester urine markers for AFP; ITA; ß‐core fragment; free ßhCG; total hCG; oestriol (also termed as uE3); gonadotropin peptide. | ||||
| Reference standards | Chromosomal verification (amniocentesis and CVS undertaken during pregnancy, and postnatal karyotyping) and postnatal macroscopic inspection. | ||||
| Study limitations | Seven studies only used selective chromosomal verification during pregnancy, and were at risk of under‐ascertainment of Down's syndrome cases due loss of the pregnancy to miscarriage between the serum test and the reference standard. | ||||
| Test | Studies | Women (Cases) | Sensitivity* (95% CI) | Specificity* (95% CI) | Threshold |
| Test without maternal age | |||||
| Single tests | |||||
| First trimester free ßhCG | 1 | 516 (86) | 5 (1 to 11) | 95 (92 to 97) | 5% FPR |
| First trimester ß‐core fragment | 1 | 516 (86) | 10 (5 to 19) | 95 (92 to 97) | 5% FPR |
| First trimester ITA | 2 | 579 (94) | 15 (2 to 62) | 95 | 5% FPR |
| First trimester total hCG | 1 | 516 (86) | 17 (10 to 27) | 95 (92 to 97) | 5% FPR |
| Second trimester oestriol | 2 | 1472 (47) | 23 (8 to 49) | 95 | 5% FPR |
| Second trimester total hCG | 1 | 390 (65) | 31 (20 to 43) | 95 (92 to 97) | 5% FPR |
| Second trimester free ßhCG | 3 | 1517 (107) | 32 (12 to 63) | 95 | 5% FPR |
| Second trimester ß‐core fragment | 6 | 9613 (193) | 41 (20 to 66) | 95 | 5% FPR |
| Second trimester ITA | 3 | 2748 (131) | 43 (35 to 51) | 95 | 5% FPR |
| Second trimester ß‐core fragment to oestriol ratio | 2 | 1649 (35) | 74 (58 to 86) | 95 | 5% FPR |
| Second trimester gonadotropin test | 1 | 105 (14) | 93 (66 to 100) | 95 (88 to 98) | 1:384 risk |
| Double tests | |||||
| Second trimester AFP and ITA | 1 | 524 (24) | 79 (58 to 93) | 95 (93 to 97) | 5% FPR |
| Second trimester ß‐core fragment and oestriol | 1 | 315 (24) | 83 (63 to 95) | 95 (92 to 97) | 5% FPR |
| Triple tests | |||||
| Second trimester AFP, uE3 and ITA | 1 | 524 (24) | 79 (58 to 93) | 95 (93 to 97) | 5% FPR |
| Test with maternal age | |||||
| Single tests | |||||
| Second trimester oestriol | 1 | 474 (69) | 49 (37 to 62) | 95 (92 to 97) | 5% FPR |
| Second trimester ß‐core fragment | 5 | 3419 (155) | 56 (45 to 66) | 95 | 5% FPR |
| Second trimester free ßhCG | 2 | 879 (98) | 57 (47 to 67) | 95 | 5% FPR |
| Second trimester free ßhCG to oestriol ratio | 1 | 474 (69) | 64 (51 to 75) | 95 (92 to 97) | 5% FPR |
| Second trimester ß‐core fragment to free ßhCG | 1 | 474 (69) | 67 (54 to 78) | 95 (92 to 97) | 5% FPR |
| Second trimester ITA | 1 | 1016 (23) | 70 (47 to 87) | 95 (93 to 96) | 5% FPR |
| Second trimester ß‐core fragment to oestriol ratio | 3 | 2088 (105) | 71 (51 to 86) | 95 | 5% FPR |
| Double tests | |||||
| Second trimester oestriol and free ßhCG | 1 | 474 (69) | 68 (56 to 79) | 95 (92 to 97) | 5% FPR |
| Second trimester ß‐core fragment and oestriol | 2 | 1631 (92) | 73 (57 to 85) | 95 | 5% FPR |
| Second trimester AFP and ß‐core fragment to oestriol ratio | 1 | 356 (10) | 90 (55 to 100) | 95 (93 to 97) | 1:58 risk |
| *Tests evaluated by at least one study are presented in the table. Where two studies reported the same threshold, estimates of summary sensitivity and summary specificity were obtained by using univariate fixed effects logistic regression models to pool sensitivities and specificities separately. if the threshold used was a 5% FPR, then only the sensitivities were pooled. AFP: alpha‐fetoprotein; ßhCG: beta human chorionic gonadotrophin;CI: confidence interval; CVS: chorionic villus sampling; FPR: false positive rate; hCG: beta human chorionic gonadotrophin;ITA: invasive trophoblast antigen; uE3: unconjugated oestriol | |||||
| Ratio of DORs (95% CI); P values (studies) | Second trimester AFP and ß‐core fragment to oestriol ratio, risk 1:58 | Second trimester ß‐core fragment and oestriol, 5% FPR | Second trimester ITA, 5% FPR | Second trimester ß‐core fragment to oestriol ratio, 5% FPR |
| Second trimester ß‐core fragment and oestriol, 5% FPR | – | |||
| Second trimester ITA, 5% FPR | – | – | ||
| Second trimester ß‐core fragment to oestriol ratio, 5% FPR | – | 1.5 (0.7 to 3.0); P = 0.27 (K = 2) | ||
| Second trimester ß‐core fragment, 5% FPR | – | 2.2 (1.1 to 4.5); P = 0.02 (K = 2) | – | 1.5 (0.8 to 2.8); P = 0.21 (K = 3) |
| Direct comparisons were made using only data from studies that compared each pair of tests in the same population. Ratio of diagnostic odds ratios (DOR)s were computed by division of the DOR for the test in the column by the DOR for the test in the row. If the ratio of DORs is greater than one, then the diagnostic accuracy of the test in the column is higher than that of the test in the row; if the ratio is less than one, the diagnostic accuracy of the test in the row is higher than that of the test in the column. AFP: alpha‐fetoprotein; CI: confidence interval; DORs: diagnostic odds ratio; FPR: false positive rate; ITA: invasive trophoblast antigen | ||||
| Ratio of DOR (95% CI); P value | Second trimester AFP and ß‐core fragment to oestriol ratio, risk 1:58 | Second trimester ß‐core fragment and oestriol, 5% FPR | Second trimester ITA, 5% FPR | Second trimester ß‐core fragment to oestriol ratio, 5% FPR | ||
| Studies | 1 | 2 | 1 | 3 | ||
| Studies | DOR (95% CI) | 186 (22, 1560) | 50 (30 to 84) | 43 (17 to 110) | 38 (24 to 59) | |
| Second trimester ß‐core fragment and oestriol, 5% FPR | 2 | 50 (30 to 84) | 3.7 (0.4 to 33.0); P = 0.24 | |||
| Second trimester ITA, 5% FPR | 1 | 43 (17 to 110) | 4.3 (0.4 to 44.0); P = 0.22 | 1.2 (0.4 to 3.4); P = 0.78 | ||
| Second trimester ß‐core fragment to oestriol ratio, 5% FPR | 3 | 38 (24 to 59) | 4.9 (0.6 to 43.4); P = 0.15 | 1.3 (0.7 to 2.6); P = 0.41 | 1.1 (0.4 to 3.2); P = 0.80 | |
| Second trimester ß‐core fragment, 5% FPR | 5 | 25 (18 to 36) | 7.3 (0.8 to 63.1); P = 0.07 | 2.0 (1.1 to 3.7); P = 0.03 | 1.7 (0.6 to 4.6); P = 0.30 | 1.5 (0.8 to 2.6); P = 0.18 |
| Indirect comparisons were made using all available data. Ratio of diagnostic odds ratios (DOR)s were computed by division of the DOR for the test in the column by the DOR for the test in the row. If the ratio of DORs is greater than one, then the diagnostic accuracy of the test in the column is higher than that of the test in the row; if the ratio is less than one, the diagnostic accuracy of the test in the row is higher than that of the test in the column. AFP: alpha‐fetoprotein; CI: confidence interval; DORs: diagnostic odds ratio; FPR: false positive rate; ITA: invasive trophoblast antigen | ||||||
| Test | No. of studies | No. of participants |
| 1 Betacore, 1st trimester urine test, 5% FPR Show forest plot | 1 | 516 |
| 2 Betacore, 2nd trimester urine test, 5% FPR Show forest plot | 6 | 9613 |
| 3 Betacore, 2nd trimester urine test, cutpoint mixed Show forest plot | 7 | 10124 |
| 4 Gonadotropin, 2nd trimester urine test, risk 1:100 Show forest plot | 1 | 105 |
| 5 Gonadotropin, 2nd trimester urine test, risk 1:384 Show forest plot | 1 | 105 |
| 6 Gonadotropin, 2nd trimester urine test, 95% percentile Show forest plot | 1 | 105 |
| 7 ITA, 1st trimester urine test, 5% FPR Show forest plot | 2 | 579 |
| 8 ITA, 2nd trimester urine test, 3.74MoM Show forest plot | 1 | 2051 |
| 9 ITA, 2nd trimester urine test, 5% FPR Show forest plot | 3 | 2748 |
| 10 Total hCG, 1st trimester urine test, 5% FPR Show forest plot | 1 | 516 |
| 11 Total hCG, 2nd trimester urine test, 5% FPR Show forest plot | 1 | 390 |
| 12 Free ßhCG, 1st trimester urine test, 5% FPR Show forest plot | 1 | 516 |
| 13 Free ßhCG, 2nd trimester urine test, 5% FPR Show forest plot | 3 | 1517 |
| 14 Oestriol, 2nd trimester urine test, 5% FPR Show forest plot | 2 | 1472 |
| 15 Betacore to oestriol ratio, 2nd trimester urine test, 5% FPR Show forest plot | 2 | 1649 |
| 16 Betacore and oestriol, 2nd trimester 5% FPR Show forest plot | 1 | 315 |
| 17 AFP and ITA, 2nd trimester urine test, 3% FPR Show forest plot | 1 | 524 |
| 18 AFP and ITA, 2nd trimester urine test, 5% FPR Show forest plot | 1 | 524 |
| 19 AFP and ITA, 2nd trimester urine test,10% FPR Show forest plot | 1 | 524 |
| 20 AFP and ITA, 2nd trimester urine test, 15% FPR Show forest plot | 1 | 524 |
| 21 AFP, uE3 and ITA, 2nd trimester urine test, 3% FPR Show forest plot | 1 | 524 |
| 22 AFP, uE3 and ITA, 2nd trimester urine test, 5% FPR Show forest plot | 1 | 524 |
| 23 AFP, uE3 and ITA, 2nd trimester urine test, 10% FPR Show forest plot | 1 | 524 |
| 24 AFP, uE3 and ITA, 2nd trimester urine test, 15% FPR Show forest plot | 1 | 524 |
| 25 Age, betacore, 2nd trimester urine test, 1% FPR Show forest plot | 2 | 2083 |
| 26 Age, betacore, 2nd trimester urine test, 3% FPR Show forest plot | 2 | 2083 |
| 27 Age, betacore, 2nd trimester urine test, 5% FPR Show forest plot | 5 | 3419 |
| 28 Age, betacore, 2nd trimester urine test, 10% FPR Show forest plot | 1 | 926 |
| 29 Age, betacore, 2nd trimester urine test, 15% FPR Show forest plot | 1 | 953 |
| 30 Age, betacore, 2nd trimester urine test, 20% FPR Show forest plot | 1 | 926 |
| 31 Age, ITA, 2nd trimester urine test, 5% FPR Show forest plot | 1 | 1016 |
| 32 Age, oestriol, 2nd trimester urine test, 5% FPR Show forest plot | 1 | 474 |
| 33 Age, free ßhCG, 2nd trimester urine test, 5% FPR Show forest plot | 2 | 879 |
| 34 Age, betacore to oestriol ratio, 2nd trimester urine test, 1% FPR Show forest plot | 1 | 1157 |
| 35 Age, betacore to oestriol ratio, 2nd trimester urine test, 3% FPR Show forest plot | 1 | 1157 |
| 36 Age, betacore to oestriol ratio, 2nd trimester urine test, 5% FPR Show forest plot | 3 | 2088 |
| 37 Age, free ßhCG to oestriol ratio, 2nd trimester urine test, 5% FPR Show forest plot | 1 | 474 |
| 38 Age, oestriol and free ßhCG, 2nd trimester, 5% FPR Show forest plot | 1 | 474 |
| 39 Age, betacore to free ßhCG ratio, 2nd trimester, 5% FPR Show forest plot | 1 | 474 |
| 40 Age, betacore and oestriol, 2nd trimester 1% FPR Show forest plot | 1 | 1157 |
| 41 Age, betacore and oestriol, 2nd trimester, 3% FPR Show forest plot | 1 | 1157 |
| 42 Age, betacore and oestriol, 2nd trimester, 5% FPR Show forest plot | 2 | 1631 |
| 43 Age, AFP and betacore to oestriol ratio, 2nd trimester, risk 1:10 Show forest plot | 1 | 356 |
| 44 Age, AFP and betacore to oestriol ratio, 2nd trimester, risk 1:20 Show forest plot | 1 | 356 |
| 45 Age, AFP and betacore to oestriol ratio, 2nd trimester, risk 1:30 Show forest plot | 1 | 356 |
| 46 Age, AFP and betacore to oestriol ratio, 2nd trimester, risk 1:58 Show forest plot | 1 | 356 |
| 47 Age, AFP and betacore to oestriol ratio, 2nd trimester, risk 1:270 Show forest plot | 1 | 356 |
| 48 Age, AFP and betacore to oestriol ratio, 2nd trimester, risk 1:526 Show forest plot | 1 | 356 |

