Uso de transfusiones plaquetarias antes de las punciones lumbares o la anestesia epidural para la prevención de las complicaciones en los pacientes con trombocitopenia
Resumen
Antecedentes
Los pacientes con un recuento plaquetario bajo (trombocitopenia) a menudo requieren punciones lumbares o un anestésico epidural. Las punciones lumbares pueden ser diagnósticas (neoplasias malignas hematológicas, hematoma subaracnoideo, meningitis) o terapéuticas (anestesia espinal, administración de quimioterapia). Para la administración de la anestesia epidural, se colocan catéteres epidurales. La práctica actual en muchos países es corregir la trombocitopenia con transfusiones plaquetarias antes de las punciones lumbares y la anestesia epidural para mitigar el riesgo de hemorragia grave relacionada con el procedimiento. Sin embargo, el umbral del recuento plaquetario recomendado antes de estos procedimientos varía significativamente de un país a otro. Esto indica que existe una gran incertidumbre entre los médicos en cuanto al tratamiento correcto de estos pacientes. El riesgo de hemorragia parece ser bajo, aunque si se presenta puede ser muy grave (hematoma espinal). Por lo tanto, los pacientes pueden estar expuestos a los riesgos de una transfusión plaquetaria sin efectos clínicos beneficiosos obvios.
Ésta es una actualización de una revisión Cochrane publicada por primera vez en 2016.
Objetivos
Evaluar los efectos de diferentes umbrales de transfusión plaquetaria antes de una punción lumbar o anestesia epidural en los pacientes con trombocitopenia (recuento plaquetario bajo).
Métodos de búsqueda
Se buscaron ensayos controlados aleatorizados (ECA), ensayos controlados no aleatorizados (ECN), estudios controlados del tipo antes y después (ECAD), estudios de series de tiempo interrumpido (STI) y estudios de cohortes en CENTRAL (la Cochrane Library 2018, Número 1), MEDLINE (desde 1946), Embase (desde 1974), la Transfusion Evidence Library (desde 1950) y en bases de datos de ensayos en curso hasta el 13 de febrero de 2018.
Criterios de selección
Se incluyeron ECA, ECNA, ECAD, STI, y estudios de cohortes que incluían transfusiones de concentrados de plaquetas, preparados a partir de unidades individuales de sangre entera o por aféresis, y administrados para prevenir la hemorragia en pacientes de cualquier edad con trombocitopenia que requirieran la inserción de una aguja de punción lumbar o un catéter epidural.
La revisión original sólo incluía ECA.
Obtención y análisis de los datos
Se utilizaron los procedimientos metodológicos estándar previstos por Cochrane para incluir los ECA, ECNA, ECAD y STI. Dos autores de la revisión evaluaron de forma independiente la elegibilidad y el riesgo de sesgo de los estudios y extrajeron los datos. Los resultados sólo se expresaron de forma narrativa.
Resultados principales
No se identificó ningún ECA, ECNA, ECAD y STI completo o en curso. Ningún estudio incluyó a pacientes sometidos a un procedimiento epidural. Ningún estudio comparó los diferentes umbrales de recuento de plaquetas antes de un procedimiento.
En esta actualización, se identificaron tres estudios de cohorte retrospectivos que contenían participantes que recibieron y no recibieron transfusiones de plaquetas antes de los procedimientos de punción lumbar. Los tres estudios se llevaron a cabo en pacientes con cáncer, la mayoría de las cuales tenían una malignidad hematológica. Dos estudios fueron en niños y uno en adultos.
En un estudio, no se informó del número de participantes que recibieron transfusiones de plaquetas antes de los procedimientos de punción lumbar. Por lo tanto, sólo se resumieron de forma narrativa los resultados pertinentes de dos estudios (150 participantes; 129 niños y 21 adultos), en los que se suministró el número de participantes que recibieron la transfusión.
Se consideró el riesgo general de sesgo de todos los resultados informados para ambos estudios como "graves", en base a la herramienta ROBINS‐I.
No se produjo ninguna hemorragia importante relacionada con el procedimiento en los dos estudios que informaron sobre este resultado (dos estudios, 150 participantes, ningún caso, evidencia de muy baja calidad).
No hubo evidencia de una diferencia en el riesgo de hemorragia leve (punción traumática) en los participantes que recibieron transfusiones de plaquetas antes de una punción lumbar y los que no recibieron una transfusión de plaquetas antes del procedimiento (dos estudios, 150 participantes, evidencia de muy baja calidad). Uno de los 14 adultos que recibieron una transfusión de plaquetas sufrió una hemorragia leve (punción traumática; definida como un mínimo de 500 x 106/L de glóbulos rojos en el líquido cefalorraquídeo); ninguno de los siete adultos que no recibieron una transfusión de plaquetas sufrió este evento. Diez niños sufrieron hemorragias leves (punciones traumáticas; definidas como por lo menos 100 x 106/L de glóbulos rojos en el líquido cefalorraquídeo), seis de los 57 niños que recibieron una transfusión de plaquetas y cuatro de los 72 niños que no recibieron una transfusión de plaquetas.
No se produjeron eventos adversos graves en el único estudio que informó de este resultado (un estudio, 21 participantes, evidencia de muy baja calidad).
No se encontraron estudios que evaluaran la mortalidad por todas las causas dentro de los 30 días posteriores al procedimiento de punción lumbar, la duración de la estancia hospitalaria, la proporción de participantes que recibieron transfusiones de plaquetas o la calidad de vida.
Conclusiones de los autores
No se encontró evidencia de ECA o estudios no aleatorizados en los que basar una evaluación del umbral correcto de transfusión de plaquetas antes de la inserción de una aguja de punción lumbar o un catéter epidural. No hay ECA registrados en curso que evalúen los efectos de diferentes umbrales de transfusión plaquetaria antes de la inserción de una punción lumbar o de la anestesia epidural en pacientes con trombocitopenia. Cualquier estudio futuro tendría que ser muy grande para detectar una diferencia en el riesgo de hemorragia. Se necesitaría diseñar un estudio con al menos 47 030 participantes para poder detectar un aumento en el número de pacientes que tenían hemorragias importantes relacionadas con el procedimiento, de 1 de cada 1000 a 2 de cada 1000. Es probable que la utilización de un registro central de recopilación de datos o de registros electrónicos recopilados de forma rutinaria (grandes datos) sea el único método para reunir sistemáticamente datos pertinentes a esta población.
PICO
Resumen en términos sencillos
Uso de transfusiones de plaquetas antes de una punción lumbar o anestesia epidural en pacientes con un bajo recuento de plaquetas
Pregunta de la revisión
Se evaluó la evidencia para determinar si los pacientes con un recuento plaquetario bajo (aumenta el riesgo de hemorragia) requieren una transfusión de plaquetas antes de la inserción de una aguja de punción lumbar o un catéter epidural, y si es así, cuál es el nivel de recuento plaquetario en el que se requiere una transfusión de plaquetas.
Antecedentes
Las plaquetas se encuentran en la sangre y son una parte esencial de los coágulos sanguíneos. Un recuento plaquetario bajo aumenta el riesgo de hemorragia. Los pacientes con un recuento plaquetario bajo requieren a menudo una punción lumbar o anestesia epidural para la administración del tratamiento o para ayudar en el diagnóstico.
La punción lumbar se realiza generalmente mediante la inserción de una aguja entre los huesos (vértebras) de la parte baja de la columna vertebral en el líquido que rodea la médula espinal (el grupo de nervios que recorre la columna vertebral y conecta el cerebro con el cuerpo). Las punciones lumbares se realizan para obtener una muestra de dicho líquido o para administrar el tratamiento en el líquido (quimioterapia o un anestésico). La aguja de punción lumbar se retira inmediatamente después de que se hayan tomado muestras de líquido o se haya administrado un tratamiento.
La epidural consiste en insertar una aguja de mayor diámetro que la de punción lumbar. La aguja epidural pasa a través de los mismos tejidos que la aguja de punción lumbar, pero no llega a penetrar en el saco de líquido que rodea la médula espinal. En su lugar, cualquier tratamiento se inyecta en el espacio justo fuera del saco de líquido (llamado espacio epidural). A menudo se pasa un tubo pequeño (un catéter epidural) a través de la aguja epidural y se deja en posición para poder administrar medicación anestésica local adicional.
La práctica actual en muchos países consiste en aumentar el recuento de plaquetas por encima de un nivel preestablecido mediante transfusiones de plaquetas (plaquetas inyectadas en una vena) para prevenir hemorragias graves debidas a la punción lumbar o a la anestesia epidural. Aunque el riesgo de hemorragia parece ser bajo, si se presenta puede ser muy grave. Debido a la falta de evidencia, el nivel recomendado de recuento plaquetario antes de la punción lumbar o la anestesia epidural varía significativamente de un país a otro. Lo anterior significa que los médicos no están seguros acerca de cuál es el nivel correcto del recuento plaquetario o de si se requiere una transfusión plaquetaria. Por consiguiente, los pacientes pueden estar expuestos a los riesgos de una transfusión de plaquetas (por ejemplo, reacción alérgica, infección) sin ningún beneficio clínico evidente.
Características de los estudios
Se efectuaron búsquedas en bases de datos científicas para obtener estudios clínicos en pacientes de cualquier edad con recuentos plaquetarios bajos que requerían una punción lumbar o anestesia epidural. La evidencia está actualizada hasta el 13 de febrero de 2018. En esta revisión, se encontraron sólo tres estudios de cohorte. Sólo dos de estos estudios informaron resultados pertinentes a esta revisión. Ambos estudios incluyeron a pacientes con bajos recuentos de plaquetas y cáncer sanguíneo; uno incluyó a 21 adultos y el otro a 129 niños. Ambos estudios compararon a pacientes que habían recibido y no habían recibido transfusiones de plaquetas antes de la inserción de una aguja de punción lumbar. Ningún estudio evaluó el uso de transfusiones de plaquetas antes de la inserción de un catéter epidural o diferentes umbrales de recuento de plaquetas para la administración de transfusiones de plaquetas antes de un procedimiento.
Resultados clave
En ninguno de los dos estudios hubo complicaciones de hemorragia relacionadas con el procedimiento. En el único estudio (21 participantes) que informó sobre este resultado no se produjeron eventos adversos graves.
Hubo poca o ninguna diferencia en el número de complicaciones hemorrágicas menores, tanto en adultos como en niños que recibieron o no transfusiones de plaquetas.
Ninguno de los estudios informó sobre la muerte, el número de transfusiones de plaquetas administradas después del procedimiento, la duración de la estancia en el hospital o la calidad de vida.
Calidad de la evidencia
La calidad de la evidencia de los estudios incluidos era muy pobre.
No se encontró evidencia de ensayos controlados aleatorizados para responder a la pregunta de la revisión.
Sería necesario diseñar un estudio con al menos 47 030 participantes para poder detectar un aumento en el número de pacientes que tuvieron hemorragias después de la punción lumbar o la anestesia epidural de 1 de cada 1000 a 2 de cada 1000. Es probable que un estudio que utilice registros médicos electrónicos recopilados de forma rutinaria (grandes datos) sea el único diseño de estudio que podría responder a nuestra pregunta de revisión.
Authors' conclusions
Summary of findings
| Platelet transfusion compared with no platelet transfusion for lumbar puncture procedures | ||||||
| Patient or population: People with thrombocytopenia Intervention: Platelet transfusion Comparison: No platelet transfusion | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| No platelet transfusions | Platelet transfusion | |||||
| Major procedure‐related bleeding within 24 hours of the procedure | No cases of major bleeding | Not estimated | 150 (2 studies) | ⊕⊝⊝⊝ Very low 1, 2 | ||
| All‐cause mortality up to 30 days after the procedure | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| Transfusion‐related complications within 24 hours of the procedure | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| Procedure‐related (lumbar puncture or epidural anaesthetic) complications within 7 days of the procedure | No serious adverse events | Not estimated | 21 (1 study) | ⊕⊝⊝⊝ Very low1, 2 | ||
| Quality of life (as defined by the individual studies) | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded one level due to imprecision (low event rate). No events occurred. | ||||||
Background
Please see Published notes for a glossary of technical terms.
Description of the condition
Thrombocytopenia
Thrombocytopenia is defined as a platelet count less than 150 x 109/L (BCSH 2003), and severe thrombocytopenia as a platelet count less than 50 x 109/L. Thrombocytopenia can occur due to reduced platelet production in the bone marrow as a result of chemotherapy or a haematological malignancy (blood cancer) (Leguit 2010; Weinzierl 2013); increased platelet consumption, for example due to bleeding or disseminated intravascular coagulation (Levi 2009); or increased platelet destruction, for example due to immune thrombocytopenia or neonatal alloimmune thrombocytopenia (Neunert 2013; Pacheco 2011; Provan 2010). Platelets are an essential component in the formation of a blood clot (BCSH 2003). A low platelet count can lead to a range of bleeding symptoms such as bruising, nosebleeds, and, rarely, life‐threatening or fatal bleeding.
A platelet count less than 150 x 109/L occurs commonly in pregnancy (7% to 12% of pregnancies), but severe thrombocytopenia (platelet count less than 50 x 109/L) is much less common (0.05% to 1% of pregnancies) (Burrows 1990; Nisha 2012; Sainio 2000). A platelet count less than 150 x 109/L is very common in people with chronic liver disease (up to 76%) (Afdhal 2008), people who are critically ill (up to 68%) (Hui 2011), and people with haematological malignancies (Leguit 2010; Weinzierl 2013).
Lumbar puncture
Diagnostic
A diagnostic lumbar puncture (LP) is an invasive procedure to obtain samples of cerebrospinal fluid (Doherty 2014). Cerebrospinal fluid is the fluid that bathes and protects the brain and spinal cord. An LP is usually performed by inserting a needle into the lower back (underneath the spinal L4 bony process) (Williams 2008). The cerebrospinal fluid obtained can then be used in the investigation of haematological malignancies (Vavricka 2003), subarachnoid haemorrhages, meningitis (Riordan 2002), or neurological disorders. Lumbar punctures are performed by doctors or specially trained nurses.
Therapeutic
Therapeutic LPs administer drugs into the cerebrospinal fluid. This can be for the administration of therapeutics such as intrathecal chemotherapy or antibiotics, or the administration of local anaesthetic to the nerves of the lower spine (spinal anaesthetic) (Doherty 2014). This usually involves insertion of a fine needle into the lower back, administration of the therapeutic agent, and then removal of the needle (Ng 2004).
Diagnostic or therapeutic LPs are relatively common hospital procedures in people with haematological disorders who are thrombocytopenic (up to 10% of all procedures) (Estcourt 2012).
Epidural anaesthesia
The most common indication for epidural anaesthesia is in pregnant women to aid in pain relief during labour (Venn 2015). However, epidural anaesthesia can also be used in postoperative pain management, especially for people with lower limb ischaemia (Venn 2015), and people undergoing thoracic surgery (Mendola 2009), as alternatives to general anaesthesia. Epidural anaesthesia typically involves inserting a needle that is larger in diameter than a spinal needle. The epidural needle passes through the same tissues as a spinal needle but stops short of penetrating the dura (tissue sac that contains cerebrospinal fluid). An epidural catheter is often passed through the needle and left in position so that additional local anaesthetic medications can be administered (Ng 2004).
Spinal haematoma
The risk of a spinal haematoma in the general population is very low (1 in 200,000 epidural anaesthetic procedures during labour to 1 in 3600 epidural anaesthetic procedures in older women having knee surgery) (Li 2010; Moen 2004; Ruppen 2006; Vandermeulen 1994). Risk factors for major bleeding are multifactorial and include: increasing age (the procedure is more difficult in older people due to changes to the spine that occur with age), low platelet count, abnormal coagulation (including anticoagulant medication), and traumatic needle or catheter insertion (Erbay 2014; Li 2010; Moen 2004; Vandermeulen 1994). Performing an LP or administration of epidural anaesthesia is a relative contraindication in people with thrombocytopenia due to this perceived higher risk of complications (van Veen 2010). However, there are no current reliable estimates of the risks of adverse effects such as spinal haematomas in people with thrombocytopenia overall (van Veen 2010).
Description of the intervention
Current practice in many countries is to correct thrombocytopenia with platelet transfusions prior to an LP or epidural anaesthesia in order to mitigate the risk of serious peri‐ or postprocedural bleeding. Up to 4% of all platelet components issued in the UK prior to a procedure are given to people with thrombocytopenia who need an LP (Qureshi 2007). The safe platelet count threshold recommended prior to an LP or epidural anaesthesia varies significantly from country to country.
For example, the platelet count threshold for LP in the US is 50 x 109/L (Kaufman 2015); in the UK it is 50 x 109/L in adults (BCSH 2003), but 20 to 40 x 109/L in children (BCSH 2004); and in Germany it is 20 x 109/L unless it is an urgent procedure (e.g. diagnosing bacterial meningitis), when an LP should be performed irrespective of the platelet count (GMA 2009).
The platelet count threshold for epidural anaesthesia also varies. In Italy and the UK, a platelet count of at least 50 x 109/L is recommended (BCSH 2003; Liumbruno 2011), while the recommendation in France is a platelet count of at least 80 x 109/L (Samama 2005).
As there is currently no consensus on the standard platelet count threshold prior to an LP or epidural anaesthesia, we compared the most commonly recommended platelet count threshold in national guidelines (50 x 109/L) against other recommended thresholds (10 x 109/L, 20 x 109/L, 30 x 109/L, 40 x 109/L, 80 x 109/L).
If guidelines recommend a platelet count threshold higher than is necessary to perform an LP or epidural anaesthesia safely, this will mean that people are exposed to the risks of a platelet transfusion unnecessarily. In 2014, 34% of all transfusion‐related adverse events reported to the UK national reporting system (Serious Hazards of Transfusion (SHOT)) were due to platelet components. The most common adverse events due to platelet components were febrile and allergic reactions (Birchall 2015). While most of these reactions are not life‐threatening, they can be extremely distressing for the person. Rarer, but more serious sequelae include anaphylaxis (life‐threatening allergic reaction), transfusion‐transmitted infections, and transfusion‐related acute lung injury (Blumberg 2010; Chapman 2015; Kaufman 2015; Slichter 2007; Vlaar 2013).
If guidelines recommend a platelet count threshold higher than is necessary to perform an LP safely, it may delay the start of lifesaving treatments, which can be time‐critical in conditions such as bacterial meningitis or subarachnoid haemorrhage.
Epidural anaesthesia allows for a safer and more controlled, localised anaesthesia to be administered, reducing the complications associated with general anaesthesia and reducing patient time in hospital. If guidelines recommend a platelet count threshold higher than is necessary to administer an epidural anaesthetic, it may mean that a person is not offered an epidural anaesthetic and instead receives a general anaesthetic.
If guidelines recommend a platelet count threshold lower than is necessary to perform an LP or epidural anaesthesia safely, then people with thrombocytopenia are put at a higher risk of serious or life‐threatening bleeding such as a spinal haematoma.
How the intervention might work
Platelet transfusions are given to people with low platelet counts to increase the platelet count and, therefore, reduce the risk of bleeding during invasive procedures.
However, the risk of bleeding during or after an LP may be low in people with a low platelet count. One systematic review of platelet transfusion indications showed that bleeding events were rare in people who had thrombocytopenia undergoing diagnostic LPs; however, the quality of the evidence was low (Kumar 2015). In the review, there were five case series in children who needed an LP, nearly all of whom had acute lymphocytic leukaemia. In three of these studies, children were grouped by platelet count: 243 LPs were performed at a count less than 20 x 109/L and 817 at a platelet count between 21 x 109/L and 50 x 109/L, and no bleeding complications occurred (van Veen 2010). People may therefore be exposed to the risks of a platelet transfusion without any obvious clinical benefit.
Why it is important to do this review
The platelet count threshold recommended prior to an LP or epidural anaesthesia varies significantly from country to country (BCSH 2003; BCSH 2004; GMA 2009; Kaufman 2015). This indicates significant uncertainty in clinicians of the correct management for safely performing an LP or administering an epidural anaesthetic.
Avoiding the need for unnecessary platelet transfusions in people with thrombocytopenia will have significant logistical and financial implications for national health services as well as decreasing people's exposure to the risks of transfusion. These factors are perhaps even more important in the development of platelet transfusion strategies in low‐income countries, where access to blood components is much more limited than in high‐income countries (Verma 2009).
Objectives
To assess the effects of different platelet transfusion thresholds prior to a lumbar puncture or epidural anaesthesia in people with thrombocytopenia (low platelet count).
Methods
Criteria for considering studies for this review
Types of studies
We included the following study designs, irrespective of language or publication status.
-
randomised controlled trials (RCTs)
-
non‐randomised controlled trials (non‐RCTs)
-
controlled before‐after studies (CBAs)
-
interrupted time series studies (ITSs)
-
cohort studies with a concurrent control.
We excluded cross‐sectional studies, case‐control studies, case series, and case reports.
Types of participants
We included people of any age with thrombocytopenia (as defined by the studies) requiring an LP or epidural anaesthesia. We excluded people who were experiencing clinically significant bleeding at the time of the procedure because such people are routinely given platelet transfusions to treat the bleeding.
Types of interventions
We planned to include studies comparing the following two types of procedure: LP needle insertion or epidural catheter insertion; however, we included only studies involving people undergoing LP needle insertion in the review.
We compared platelet transfusion prior to the procedure versus no platelet transfusion.
We planned to compare platelet transfusion prior to the procedure when the platelet count was less than 50 x 109/L versus platelet transfusion prior to the procedure when:
-
platelet count was less than 10 x 109/L;
-
platelet count was less than 20 x 109/L;
-
platelet count was less than 30 x 109/L;
-
platelet count was less than 40 x 109/L;
-
platelet count was less than 80 x 109/L.
However, no studies compared different platelet count thresholds.
Had we identified relevant studies, we planned to report each analysis separately, as subgroups within the main comparisons.
Types of outcome measures
Primary outcomes
-
Major procedure‐related bleeding within 24 hours of the procedure, e.g. spinal haematoma; intraventricular, intracerebral, or subarachnoid haemorrhage; or major bleeding (not further defined) as reported by individual studies.
-
All‐cause mortality up to 30 days after the procedure.
-
Serious adverse events:
-
transfusion‐related complications within 24 hours of the procedure (including transfusion‐related acute lung injury, transfusion‐transmitted infection, transfusion‐associated circulatory overload, transfusion‐associated dyspnoea, acute transfusion reactions);
-
LP‐related or epidural anaesthetic‐related complications within seven days of the procedure (infection, headache, cerebral herniation, neurological symptoms such as radicular pain or numbness, back pain).
-
Secondary outcomes
-
Minor LP‐related or epidural anaesthetic‐related bleeding within 24 hours of the procedure (defined as prolonged bleeding at the insertion site that only required treatment with a pressure bandage) or minor bleeding (not further defined) as reported by individual studies.
-
Duration of hospital stay (total number of days in hospital).
-
Proportion of people receiving platelet transfusions.
-
Quality of life, as defined by individual studies
Search methods for identification of studies
The Systematic Review Initiative's Information Specialist (CD) formulated the search strategies in collaboration with the Cochrane Haematological Malignancies Group.
Electronic searches
We searched the following databases:
-
Cochrane Central Register of Controlled Trials (CENTRAL 2018, Issue 1) (13 February 2018) (Appendix 1).
-
MEDLINE (1946 to 13 February 2018) (Appendix 2).
-
Embase (1974 to 13 February 2018) (Appendix 3).
-
PubMed (e‐publications only) (Appendix 4).
-
Transfusion Evidence Library (www.transfusionevidencelibrary.com) (1950 to 13 February 2018); this includes a search of grey literature (Appendix 5).
-
World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) (www.who.int/ictrp/en/) (13 February 2018) (Appendix 6).
-
ClinicalTrials.gov (clinicaltrials.gov) (13 February 2018) (Appendix 7).
We combined searches in MEDLINE and Embase with the recommended Cochrane RCT search filters and with systematic review and observational studies filters based on those of the Scottish Intercollegiate Guidelines Network (SIGN) (sign search filters) (Lefebvre 2011). We did not limit searches by language, year of publication, or publication type.
We performed a new search for this update of the review.
Searching other resources
We handsearched reference lists of included studies and reviews in order to identify further relevant studies. We found four additional studies via handsearching other sources. We contacted lead authors of the included studies in order to identify any unpublished material, missing data, or information regarding ongoing studies.
Data collection and analysis
Selection of studies
We selected studies according to the recommendations described in Chapter 7 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a). The Systematic Review Initiative's Information Specialist (CD) initially screened all search hits for relevance against the eligibility criteria and discarded all those that were clearly irrelevant. Thereafter, two review authors (RM, LE) independently screened all the remaining references for relevance against the full eligibility criteria using Covidence. We retrieved full‐text articles for all references for which a decision on eligibility could not be made based on title and abstract alone. We requested additional information from study authors as necessary to assess eligibility for inclusion of individual studies. The two review authors discussed the results of study selection and resolved any discrepancies between themselves without the need for a third review author (JVV). We reported the results of study selection using a PRISMA flow diagram (Moher 2009).
Data extraction and management
As recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a), two review authors (RM, LE) independently extracted data onto standardised forms using Covidence.
For all studies, we extracted the following information.
-
Source: study identity (ID), report ID, review author ID, date of extraction, ID of author checking extracted data, citation of paper, contact authors details.
-
General study information: publication type, study objectives, funding source, conflict of interest declared, other relevant study publication reviewed.
-
Study details and methods: location, country, setting, number of centres, total study duration, recruitment dates, length of follow‐up, power calculation, primary analysis (and definition), stopping rules, method of sequence generation, allocation concealment, blinding (of clinicians, participants, and outcome assessors), and any concerns regarding bias.
-
Characteristics of interventions: number of study arms, description of experimental arm, description of control arm, type of platelet component (e.g. apheresis or pooled), dose of platelet component, type of LP needle used, and any co‐interventions.
-
Characteristics of participants: age, gender, primary diagnosis, type of procedure (diagnostic LP, therapeutic LP, epidural anaesthesia), platelet count, coagulation abnormalities, anticoagulant medications, antiplatelet medications.
-
Participant flow: total number screened for inclusion, total number recruited, total number excluded, total number allocated to each study arm, total number analysed (for review outcomes), number of allocated participants who received planned treatment, number of dropouts with reasons (percentage in each arm), protocol violations, missing data.
-
Outcomes: major procedure‐related bleeding within 24 hours of the procedure, minor procedure‐related (LP or epidural anaesthetic) bleeding within 24 hours of the procedure, transfusion‐related complications within 24 hours of the procedure, procedure‐related complications within seven days of the procedure, duration of hospital stay, proportion of participants receiving platelet transfusions within 24 hours of the procedure, all‐cause mortality up to 30 days from the procedure, quality of life (as defined by the individual studies).
For studies that were not RCTs, we also extracted the following information.
-
Study design.
-
Confounding factors.
-
Comparability of groups on confounding factors.
-
Method of assigning the intervention(s).
-
Method of data analysis: methods used to control for confounding and on multiple effect estimates (both unadjusted and adjusted estimates) as recommended in Chapter 13 of theCochrane Handbook of Systematic Reviews of Interventions (Reeves 2011).
Assessment of risk of bias in included studies
Randomised controlled trials
We planned to perform an assessment of all RCTs using the Cochrane 'Risk of bias' tool according to Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b). However, we included no completed RCTs in the review.
If in future updates RCTs are included in the review, two review authors will independently assess each domain of potential bias listed below as ’high’, ’low’, or ’unclear’ risk of bias. We will provide a brief description of support for our judgements of potential bias in the ’Characteristics of included studies’ table. We will ensure that a consensus on the degree of risk of bias is met through comparison of the review authors’ statements and where necessary, through consultation with a third review author. We will use Cochrane’s tool for assessing risk of bias, which includes the following domains.
-
Selection bias: random sequence generation and allocation concealment.
-
Performance bias: blinding of participants and personnel.
-
Detection bias: blinding of outcome assessment.
-
Attrition bias: incomplete outcome data.
-
Reporting bias: selective reporting.
-
Other bias.
Non‐randomised studies
We used the ROBINS‐I tool (formerly known as ACROBAT‐NRSI) to rate the quality of non‐randomised studies (non‐RCTs, CBAs, ITSs, cohort studies) (Sterne 2016). This tool is based on the Cochrane 'Risk of bias' tool for rating the quality of RCTs (Higgins 2011c).
The tool covers seven domains: two occur in the pre‐intervention phase (bias of confounding and bias in the selection of participants into the study); one domain covers the intervention phase (bias in the classification of the interventions); and the final four domains cover the postintervention phase (bias due to deviations from intended interventions, bias due to missing data, bias in measuring the outcomes and selective reporting).
We judged the quality of evidence for each domain in the tool as 'low risk of bias', 'moderate risk of bias', 'serious risk of bias', or 'critical risk of bias or no information'. The response options for the list of the signalling questions for each domain were 'yes', 'probably yes', 'no', 'probably no', and 'no information'.
We then assigned an overall risk of bias judgement, mapping all the seven domains, for each outcome reported in the review.
-
For 'low risk of bias' the study is judged to be at low risk of bias in all seven domains.
-
For 'moderate risk of bias' the study is judged to be at low to moderate risk of bias in all seven domains.
-
For 'serious risk of bias ' the study is judged to be at serious risk of bias in at least one of the seven domains.
-
For 'critical risk of bias' the study is judged to be at critical risk of bias in at lease one of the seven domains.
-
For 'no information on bias' when information in one or more key risk of bias domains is lacking.
We prespecified the following main potential confounding factors that could influence the intervention.
-
Primary diagnosis of participants (e.g. pregnancy, immune thrombocytopenia, acute lymphoblastic leukaemia)
-
Age: variability in the age of included participants (e.g. paediatric (less than 16 years) versus adult (> 16 years) versus older adult (> 60 years))
-
Gender: male‐to‐female ratio
-
History of previous severe bleeding (e.g. World Health Organization (WHO) Grade 3 or 4 or equivalent)
-
Anticoagulants
-
Antiplatelet agents
-
Haemostasis
-
Type of platelet component
-
Dose of platelet component
We prespecified the following co‐interventions.
-
Plasma transfusions
-
Red cell transfusions
-
Haemostatic agents
Measures of treatment effect
Randomised controlled trials
We did not perform any of the planned analyses because the search identified no RCTs. In future updates of this review we will perform the following.
-
For continuous outcomes, we will record the mean, standard deviation, and total number of participants in both the treatment and control groups.
-
For dichotomous outcomes, we will record the number of events and total number of participants in both the treatment and control groups.
We planned the following analyses for this review, which we will perform in future updates of this review.
-
For continuous outcomes using the same scale, we will perform analyses using the mean difference (MD) with 95% confidence intervals (CIs).
-
For continuous outcomes measured with different scales, we will perform analyses using the standardised mean difference (SMD).
-
We will extract and report hazard ratios (HRs) for time‐to‐event data (mortality or time in hospital) if this information is available. If HRs are not available, we will make every effort to estimate as accurately as possible the HR using the available data and a purpose‐built method based on the Parmar and Tierney approach (Parmar 1998; Tierney 2007). If sufficient studies provide HRs, we will use HRs in favour of risk ratios (RRs) or MDs in a meta‐analysis, but for completeness, we will also perform a separate meta‐analysis of data from studies providing only RRs or MDs for the same outcome.
-
For dichotomous outcomes, we will report the pooled RR with a 95% CI (Deeks 2011). Where the number of observed events is small (less than 5% of sample per group), and where treatment groups in the trials were balanced, we planned to report the Peto odds ratio (OR) with 95% CI (Deeks 2011).
-
For cluster‐randomised trials, we will extract and report direct estimates of the effect measure (e.g. RR with a 95% CI) from an analysis that accounts for the clustered design. We will obtain statistical advice to ensure the analysis is appropriate. If appropriate analyses are not available, we will make every effort to approximate the analysis following the recommendations in Chapter 16 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011c).
Non‐randomised studies
For dichotomous outcomes, we planned to extract and report the RR with a 95% CI from statistical analyses adjusting for baseline differences (such as Poisson regressions or logistic regressions) or the ratio of RRs (i.e. the RR postintervention/RR pre‐intervention) if this information was available. None of the studies reported adjusted analyses.
For continuous variables, we planned to extract and report the absolute change from a statistical analysis adjusting for baseline differences (such as regression models, mixed models, or hierarchical models) or the relative change adjusted for baseline differences in the outcome measures (i.e. the absolute postintervention difference between the intervention and control groups, as well as the absolute pre‐intervention difference between the intervention and control groups/the postintervention level in the control group) if this information was available (EPOC 2017). However, none of the studies reported continuous variables.
All studies
If data allowed, we planned to undertake quantitative assessments using Review Manager 5 (RevMan 2014). However, we were unable to perform a quantitative assessment.
Where appropriate, we planned to report the number needed to treat for an additional beneficial outcome (NNTB) and the number needed to treat for an additional harmful outcome (NNTH) with CIs.
As we could not report the available data in any of the formats described above, we presented a narrative report, but due to the paucity of data we did not need to present the data in tables.
Unit of analysis issues
We planned to treat any unit of analysis issues in accordance with the recommendations in Chapter 16 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011c). However, no unit of analysis issues arose.
If in future updates of this review any unit of analysis issues arise, we will treat them in accordance with the recommendations in Chapter 16 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011c). If participants are randomised more than once, we will contact the authors of the study to obtain data associated with the initial randomisation. For studies with multiple treatment groups, two review authors (RM and LE) will exclude subgroups that are considered irrelevant to the analysis. We will tabulate all subgroups in the 'Characteristics of included studies’ table. When appropriate, we will combine groups to create a single pair‐wise comparison. If this is not possible, we will select the most appropriate pair of interventions and exclude the others (Higgins 2011c).
Dealing with missing data
We tried to contact the primary authors of two of the included studies in this review to obtain participants' individual data across these studies, as we were unable to record the subgroup of participants who had platelet counts less than 80 x 109/L and also the subgroup of participants who received platelet transfusion before undergoing the LP procedure (Howard 2000; van Veen 2004). van Veen 2004 responded to our request, but the data were no longer available.
Assessment of heterogeneity
We did not perform any of the planned analyses. We planned to analyse the data in RCTs, non‐RCTs, CBAs, ITSs, and cohort studies separately. However, we included only cohort studies in the review.
In future updates of this review we will:
-
combine the data to perform a meta‐analysis, if the clinical and methodological characteristics of individual studies are sufficiently homogeneous;
-
evaluate the extent of heterogeneity by visual inspection of forest plots as well as by utilising statistical methods;
-
assess statistical heterogeneity of treatment effects between studies using a Chi2 test with a significance level at P < 0.1. We will use the I2 statistic to quantify the degree of potential heterogeneity, classifying it as low if the I2 is less than or equal to 50%, moderate if the I2 is 50% to 80%, or considerable if the I2 is greater than 80%. We will use the random‐effects model for low to moderate heterogeneity;
-
if statistical heterogeneity is considerable, and we cannot identify an explanation for the heterogeneity, we will not report the overall summary statistic. We will assess potential causes of heterogeneity by sensitivity and subgroup analyses (Deeks 2011).
Assessment of reporting biases
We did not perform a formal assessment of potential publication bias (small‐trial bias) by generating a funnel plot and statistically test using a linear regression test because no meta‐analyses were performed in this review (Sterne 2011). We identified no other unpublished studies for inclusion after searching the clinical trial registries.
Data synthesis
We planned to perform analyses according to the recommendations in Chapter 9 of the Cochrane Handbook for Systematic Reviews of Interventions, using aggregated data for analysis (Deeks 2011). We did not perform any of the planned analyses because the search identified only two cohort studies that reported any of the outcomes of this review. None of the outcomes reported in these two studies were reported as adjusted effect estimates.
In future updates of this review we will perform the following.
If studies are sufficiently homogenous in their study design, we will conduct a meta‐analysis according to the recommendations in Chapter 9 of the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2011). We will not conduct meta‐analyses that involve both RCTs and non‐RCTs. We will conduct separate meta‐analyses for each comparison. We will group different thresholds within the same comparisons together only if they are considered to be clinically similar.
Randomised controlled trials
If meta‐analysis is feasible for RCTs, we will use the random‐effects model for pooling data. For binary outcomes, we will base the estimation of the between‐study variance using the Mantel‐Haenszel method. We will use the inverse‐variance method for continuous outcomes; outcomes that include data from cluster‐RCTs; or outcomes where HRs are available. If heterogeneity is found to be above 80%, and an explanation for the heterogeneity is identified, we will explore this with subgroup analyses. If we cannot find an explanation for the heterogeneity, then we will not perform a meta‐analysis, but comment on the results narratively with the results from all studies presented in tables.
Non‐randomised studies
If meta‐analysis is feasible for non‐RCTs, CBAs, ITSs, and cohort studies, we will analyse the different types of studies separately. We will only analyse outcomes with adjusted effect estimates if these are adjusted for the same factors using the inverse‐variance method as recommended in Chapter 13 of the Cochrane Handbook for Systematic Reviews of Interventions (Reeves 2011).
All studies
We will use the random‐effects model for all analyses, as we anticipate that true effects will be related but will not be the same for included studies. If we cannot perform a meta‐analysis, we will comment on the results narratively with the results from all studies presented in tables.
'Summary of findings' table
We used the GRADE approach to create a 'Summary of findings' table, as suggested in Chapters 11 and 12 of the Cochrane Handbook for Systematic Reviews of Interventions (Schünemann 2011a; Schünemann 2011b). We used the GRADE approach to rate the quality of the evidence as 'high', 'moderate', 'low', or 'very low', employing the five GRADE considerations. We only included three observational studies in this review, therefore the quality of evidence started as 'low' and was downgraded to 'very low'.
-
Risk of bias: serious or very serious.
-
Inconsistency: serious or very serious.
-
Indirectness: serious or very serious.
-
Imprecision: serious or very serious.
-
Publication bias: likely or very likely.
We planned to report separate 'Summary of findings' tables for LPs and epidural anaesthesia, but we only included studies involving participants undergoing LPs in this review. We planned to report the subgroup for each comparison that contained the largest number of studies, however we only identified studies comparing platelet transfusions versus no platelet transfusions.
We included the following outcomes.
-
Major procedure‐related bleeding within 24 hours of the procedure.
-
All‐cause mortality up to 30 days after the procedure.
-
Transfusion‐related complications within 24 hours of the procedure.
-
Procedure‐related (LP or epidural anaesthetic) complications within seven days of the procedure.
-
Quality of life (as defined by the individual studies).
Subgroup analysis and investigation of heterogeneity
We planned to perform subgroup analyses for each of the following outcomes to assess the effect on heterogeneity.
-
Type of procedure (diagnostic LP, therapeutic LP, epidural anaesthesia).
-
Type of participant (intensive care, liver disease, obstetric, leukaemia, other).
-
Age of participant (neonate, child (aged one to 15 years), adult (aged 16 years or older)).
-
Whether participants had associated clotting abnormalities, including disseminated intravascular coagulation, or concomitant use of anticoagulant or antiplatelet agents.
If appropriate, we also planned to investigate heterogeneity between studies as follows.
-
Type of platelet component.
-
Dose of platelet component.
However, we could not perform any subgroup analyses due to insufficient data.
Sensitivity analysis
We planned to assess the robustness of our findings by performing the following sensitivity analyses where appropriate.
-
Including only studies with a low risk of bias (e.g. RCTs with methods assessed as low risk for random sequence generation and concealment of treatment allocation).
-
Including only studies with less than a 20% dropout rate.
-
Including only studies that were published in full.
However, we could not perform any sensitivity analyses due to insufficient data.
Results
Description of studies
See Characteristics of excluded studies.
Results of the search
The original search (conducted on 3 March 2016) identified 1060 potentially relevant records, of which 596 records remained after removal of duplicates. We excluded 592 records based on the abstract, and four records based on the full text (Foerster 2015; Howard 2000; Ning 2016; van Veen 2004.
In this update of the review we performed a new search using the review's original search strategy, with no language restriction. The search (conducted on 13 February 2018) retrieved a total of 2101 references, and handsearching other sources identified four additional studies. Two review authors (LE and RM) independently screened the titles and abstracts of the 999 records left after removal of duplicate records. We excluded 947 records based on the abstract. We checked 52 full‐text articles for eligibility, excluding 47 studies within 48 records. We identified three completed studies for inclusion in this update (Howard 2000; Ning 2016; van Veen 2004). No registered, ongoing, or completed but not yet published studies were identified for inclusion in this update. (See the PRISMA flowchart for study selection in Figure 1.)
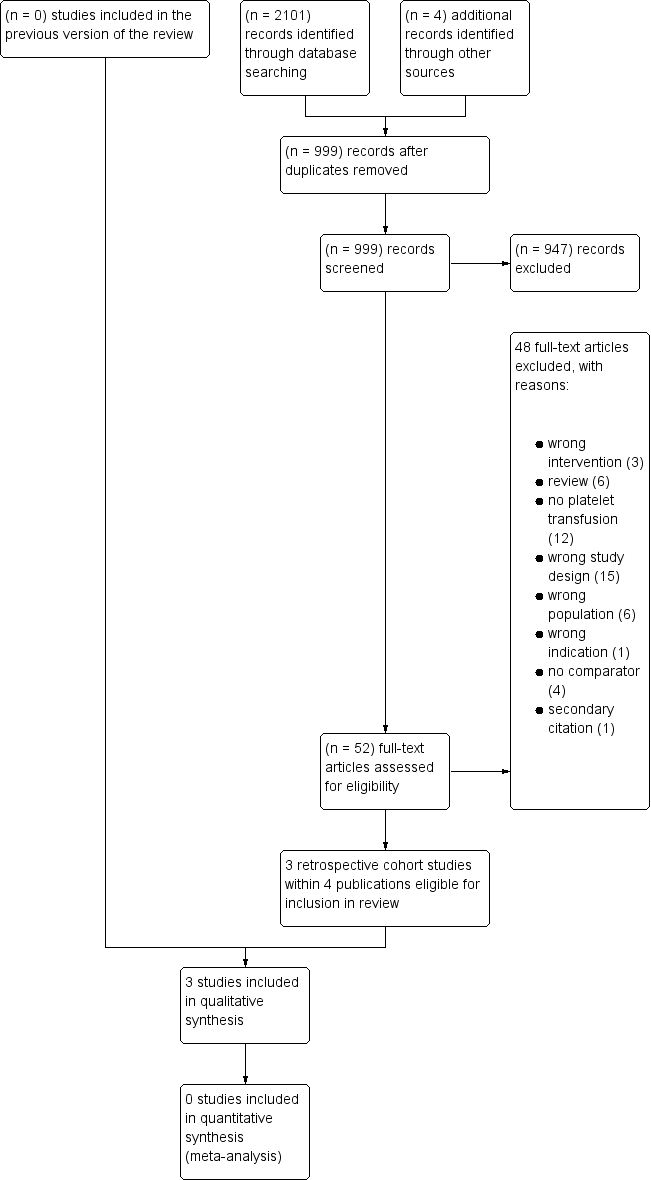
Study flow diagram.
Included studies
See Characteristics of included studies.
Study setting
The three included studies were published in English between 2000 and 2016 (Howard 2000; Ning 2016; Van Veen 2004). One study was based in Canada (Ning 2016), one in the UK (Van Veen 2004), and one in the USA (Howard 2000). Howard 2000 reviewed health records from 1984 to 1998; van Veen 2004 reviewed health records from 1989 to 2003; and Ning 2016 reviewed health records from January 2013 to December 2014.
Study design
All three included studies were retrospective, single‐centre cohort studies.
Participants
Two studies assessed the safety of lumbar puncture procedures (LPs) in children with a low platelet count (Howard 2000; Van Veen 2004). Overall, 1184 children with acute lymphocytic leukaemia (aged up to 18 years old at diagnosis) underwent 5571 LPs (Howard 2000; Van Veen 2004). Of these, 1070 LPs were performed when the platelet count was 50 x 109/L or less.
The third study assessed the safety of LPs in adults with thrombocytopenia (Ning 2016). Overall, 135 adults with cancer and a platelet count below 150 x 109/L were included in the study. Twenty‐nine LPs were performed (21 adults) when the platelet count was 50 x 109/L or less. Coagulopathy was an exclusion criterion.
Platelet transfusion before lumbar puncture
In Howard 2000, despite the fact that platelet transfusions were recorded during induction and consolidation therapy, no further information was available in the published paper.
Two studies administered platelet transfusions to a proportion of participants with a platelet count of 50 x 109/L or less (Ning 2016; Van Veen 2004). These two studies contained 150 participants with a platelet count of 50 x 109/L or less.
Study outcomes
In Howard 2000 the primary outcomes were serious complications defined as as the presence of any neurological, infectious, or haemorrhagic complications after performing LPs.
Participants in Ning 2016 were followed up to one week after undergoing the LP procedure. All haemorrhagic complications, including spinal, subdural, and epidural haematoma and traumatic taps (defined as red blood cells in the cerebral spinal fluid equal to or greater than 500 x 106/L) were monitored.
Van Veen 2004 reported on the occurrence of traumatic tap.
Excluded studies
We excluded 47 studies within 48 full‐text papers. See Characteristics of excluded studies for further details.
-
Three studies compared the wrong intervention (NCT01972529; NCT01976104; Hasegawa 2012).
-
Six studies were reviews (Choi 2009; Feusner 2004; Hua 2014; Mitchell 2012; Valent 2011; Wolfe 2016).
-
Fifteen studies were the wrong study design; the majority were case reports (Breuer 1982; Dresner 2010; Kandemir 2016; Kasama 1997; Kimura 2001; Kotelko 1989; Kotera 2010; Kuczkowski 2006; Lee 2007; Liu 2014; Mayumi 1983; Moller 2015; Pivalizza 2005; Steer 1993; Wirtz 2000).
-
Twelve studies involved no platelet transfusions (Beilin 1997; Bernstein 2016; Foerster 2015; McLure 2003; Noris 2014; Palit 2008; Ramanathan 1988; Rolbin 1988; Ruell 2006; Totadri 2014; Waldman 1987; Welter 2008).
-
Six studies involved the wrong population (Eriksson 2007; Lecompte 2003; McLendon 2011; Moeschler 2016; Self 2007; Wong 1989).
-
Four studies had no comparator (Frenk 2005; Osmanagaoglu 2006; Vavricka 2003; Webert 2003).
-
One study involved the wrong indication (Meneses 2009).
Risk of bias in included studies
We did not perform a 'Risk of bias' assessment for RCTs using the Cochrane 'Risk of bias' tool because no completed RCTs were eligible for inclusion in the review.
We evaluated the risk of bias of the non‐randomised studies included in this review using the ROBINS‐I tool (formerly known as ACROBAT‐NRSI). Only two included studies reported outcomes relevant to this review (Ning 2016; van Veen 2004), therefore we only evaluated the ROBINS‐I assessment for these two studies. Only three outcomes could be assessed using ROBINS‐I (Figure 2).

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
-
Major LP‐related or epidural anaesthetic‐related bleeding within 24 hours of the procedure (reported by Ning 2016 and van Veen 2004).
-
Serious adverse events (reported by Ning 2016).
-
Minor LP‐related or epidural anaesthetic‐related bleeding within 24 hours of the procedure (reported by Ning 2016 and van Veen 2004).
Bias due to confounding
We judged the risk of bias due to confounding factors as 'serious' for all reported outcomes in both studies (Ning 2016; van Veen 2004). Only counts were reported.
In Ning 2016, a list of potential confounding factors was provided including the age of participants in the cohort, gender distribution, primary diagnosis, anticoagulants, antiplatelet agents, coagulopathy status, number of participants with end‐stage renal disease and congenital bleeding. However, no information was provided to suggest that data analysis was adjusted for these factors. In van Veen 2004, information relevant to potential confounding factors before the intervention (at baseline) and the method of data analysis were missing.
Allocation
We judged the risk of bias of selection of participants as 'low' for all reported outcomes in both studies (Ning 2016; van Veen 2004). Selection of participants was based on the pre‐procedure platelet count and that a lumbar puncture had been performed.
Bias in classification of interventions
We judged the risk of bias in classification of the intervention (platelet transfusion or receiving no platelet transfusion) as 'low' for both studies (Ning 2016; van Veen 2004). Presence or absence of a platelet transfusion was clearly defined and would have been recorded in medical records at the time of transfusion.
Bias due to deviations from intended interventions
We judged the risk of bias due to deviations from intended interventions as 'no information' for both studies. No information was available as to whether any deviations from intended intervention had occurred that were beyond those expected in usual practice.
Incomplete outcome data
We judged the risk of bias due to missing data as 'low' for all reported outcomes for both studies. There was no evidence of missing data for the reported outcomes.
Bias in measurement of outcomes
We judged the risk of bias in measurement of outcomes as 'serious' for both studies. The outcome assessors were aware of the participants' intervention status in both studies. Neither study reported methods of assessing bleeding.
Selective reporting
We judged the risk of bias due to selective reporting as 'low' for both studies, as results for all reported outcomes were equally reported between the groups.
Overall bias
Our overall judgement of risk of bias for all reported outcomes in both studies was 'serious'. For each outcome we considered more than one domain to be at 'serious' risk of bias and none to be at 'critical' risk of bias.
Effects of interventions
We have narratively described the outcomes reported in the two studies that reported outcomes separately for participants who did and did not receive platelet transfusions (Ning 2016; van Veen 2004). Both studies provided information on outcomes when the participants' platelet count was 50 x 109/L or below.
Primary outcomes
Major procedure‐related bleeding within 24 hours of the procedure
No cases of major procedure‐related bleeding occurred (2 studies, 150 participants, no events, very low‐quality evidence).
All‐cause mortality up to 30 days after the procedure
Neither study reported mortality during 30 days from the LP procedure.
Serious adverse events
Ning 2016 reported serious adverse events. No transfusion‐related or LP‐related serious adverse events occurred (1 study, 21 participants, no events, very low‐quality evidence).
Secondary outcomes
Minor LP‐related or epidural anaesthetic‐related bleeding within 24 hours of the procedure
Ning 2016 and van Veen 2004 reported the proportion of LPs with minor bleeding (traumatic taps) (2 studies, 150 participants, very low‐quality evidence).
In Ning 2016, one traumatic tap (defined as at least 500 x 106/L red blood cells in the cerebrospinal fluid) occurred in the platelet transfusion group (14 participants), and no traumatic taps occurred in the no‐platelet transfusion group (7 participants).
In van Veen 2004, two traumatic taps (more than 1000 x 106/L red blood cells in the cerebrospinal fluid) occurred in the platelet transfusion group (57 participants), and one traumatic tap occurred in the no‐platelet transfusion group (72 participants). Also, four traumatic taps (100 to 1000 x 106/L red blood cells in the cerebrospinal fluid) occurred in the platelet transfusion group (57 participants), and three traumatic taps occurred in the no‐platelet transfusion group (72 participants).
Duration of hospital stay (total number of days in hospital)
No studies reported on the duration of hospital stay.
Proportion of participants receiving platelet transfusions
No studies reported on whether platelet transfusions were given after the procedure.
Quality of life, as defined by individual studies
No studies measured quality of life of the participants.
Discussion
Summary of main results
We found no completed or ongoing RCTs, nRCTs, CBAs, or ITSs that were relevant to this review.
No studies assessed the use of platelet transfusions prior to the insertion of an epidural catheter.
No studies compared different platelet count thresholds prior to an LP procedure.
In this update of the review we identified three retrospective cohort studies that compared giving platelet transfusions versus not giving platelet transfusions when the platelet count was 50 x 109/L or below prior to an LP procedure. Two studies were conducted in children (Howard 2000; van Veen 2004), and one in adults with haematological malignancies (Ning 2016). The number of children who received platelet transfusions before the LP procedure was not specified in Howard 2000, therefore we reported the findings from the two studies that reported outcomes separately for participants who did and did not receive platelet transfusions (Ning 2016; van Veen 2004).
The only available evidence for the use of platelet transfusions prior to LPs was from two retrospective cohort studies that involved only 150 participants (129 children and 21 adults) with thrombocytopenia and haematological malignancy:
-
no procedure‐related major bleeding occurred in the two studies that reported this outcome (2 studies, 150 participants);
-
there was no evidence of a difference in the risk of minor bleeding (traumatic tap) in participants who received platelet transfusions before an LP and those who did not receive a platelet transfusion before the procedure (2 studies, 150 participants);
-
no serious adverse events occurred in the one study that reported this outcome (21 participants);
-
we found no studies that evaluated all‐cause mortality within 30 days from the procedure; length of hospital stay; proportion of participants who received platelet transfusions; or quality of life.
Overall completeness and applicability of evidence
We are unable to answer our review question using evidence from RCTs, and there is insufficient information to answer this question using the available data from non‐randomised studies.
It is unlikely that any RCTs will be performed in the future with a primary outcome of major bleeding because the event is rare, and if major bleeding does occur it can cause significant neurological impairment.
Any future non‐randomised study would need to be very large to detect a difference in the risk of bleeding. For example, if it was assumed that major bleeding occurred in 1 out of 1000 people who had an LP when their platelet count was raised to 50 x 109/L or above, and that the risk of major bleeding doubled to 2 out of 1000 when their platelet count was only raised to 20 x 109/L or above, we would need to design a study with at least 47,030 participants to be able to detect this difference with 80% power and 5% significance (calculated using a power calculator at Sealed Envelope).
Quality of the evidence
We identified no completed RCTs, nRCTs, CBAs, or ITSs that were relevant to this review. We included three retrospective cohort studies, of which only two studies reported outcomes relevant to this review. Both studies suffered from serious methodological limitations due to their retrospective design, small sample size, the absence of analytic method, and inappropriate reporting of the results. The validity of the results from both studies is therefore questionable.
Overall we rated the quality of the evidence for the two reported outcomes as very low according to GRADE methodology (summary of findings Table for the main comparison) because all data were from non‐randomised studies (low‐quality evidence), and the quality of the evidence was downgraded for imprecision. We were unable to further downgrade the quality of the evidence because it was already classified as very low, but the evidence was at serious risk of bias.
We could assess only three outcomes reported in two studies for risk of bias using the ROBINS‐I tool: major LP‐related or epidural anaesthetic‐related bleeding within 24 hours of the procedure (reported in Ning 2016 and van Veen 2004); serious adverse events (reported in Ning 2016); and minor LP‐related or epidural anaesthetic‐related bleeding within 24 hours of the procedure (reported in Ning 2016 and van Veen 2004).
All outcomes in both studies were at serious risk of bias due to:
-
confounding, as factors known to increase the risk of bleeding were not clearly documented and adjusted for. Potential haemostatic confounding factors such as the coagulopathy status and the intake of anticoagulants were only reported in Ning 2016 as part of this population's baseline characteristics, but it was unclear if the analysis adjusted for these cofounders;
-
lack of blinding, as all studies were open‐label.
There were also limitations due to lack of information reported in both studies with regard to the number of attempts at placing an LP needle and detailed list of medications at baseline. The optimal indication to determine platelet transfusions as well as to perform LP was platelet counts prior to LPs in both studies. The methodology and the reporting of both studies were lacking details. Both studies had a similar design, and data were collected by reviewing medical records of a small sample size.
Potential biases in the review process
To our knowledge, our review process was free from bias. We conducted a comprehensive search, searching data sources (including multiple databases and clinical trial registries) to ensure that we would capture all relevant studies, including any ongoing studies. We carefully assessed the relevance of each paper identified and performed all screening in duplicate. We prespecified all outcomes and subgroups prior to analysis. We could not perform a formal assessment of publication bias as we conducted no meta‐analyses.
Agreements and disagreements with other studies or reviews
We know of two systematic reviews that were relevant to this review (Kumar 2015;van Veen 2010).
The review by Kumar 2015 assessed the evidence for the use of platelet transfusions prior to LPs but did not assess the evidence prior to epidural anaesthesia. Like our review, Kumar 2015 found no RCTs that were relevant to this review's question. The Kumar 2015 review also assessed evidence from non‐randomised studies. They identified seven observational, retrospective, single‐centre studies (1536 participants; 6440 LPs) with varying platelet count thresholds. The studies did not report outcomes separately for those participants who received prophylactic platelet transfusions and those who did not. In addition, the studies did not describe the eligibility criteria or the criteria for transfusion. However, although of poor quality, the studies seemed to indicate a lack of severe bleeding associated with the insertion of an LP needle. There were no serious bleeding complications in five case series of 1450 children with thrombocytopenia. There were two cases of spinal haematoma (86 participants) in two case series of adults with thrombocytopenia. The van Veen 2010 review identified the same seven non‐randomised studies because Kumar 2015 used the search performed by van Veen 2010 that was first published online in September 2009. The van Veen 2010 review also assessed the evidence for the use of platelet transfusions prior to epidural anaesthetics. There were no RCTs; six observational studies included only participants receiving an epidural anaesthetic, and no spinal haematomas occurred. Only one of the included studies included participants who were not pregnant.
Both of these reviews concluded that there is a scarcity of evidence supporting prophylactic platelet transfusions prior to the insertion of a spinal needle for an LP or for the delivery of anaesthetic (Kumar 2015; van Veen 2010).
The most recently published platelet transfusion guidelines from the American Association of Blood Banks used this non‐randomised evidence (Kaufman 2015; Kumar 2015). Another recently published guideline based its recommendations on expert opinion (NICE 2015).

Study flow diagram.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
| Platelet transfusion compared with no platelet transfusion for lumbar puncture procedures | ||||||
| Patient or population: People with thrombocytopenia Intervention: Platelet transfusion Comparison: No platelet transfusion | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| No platelet transfusions | Platelet transfusion | |||||
| Major procedure‐related bleeding within 24 hours of the procedure | No cases of major bleeding | Not estimated | 150 (2 studies) | ⊕⊝⊝⊝ Very low 1, 2 | ||
| All‐cause mortality up to 30 days after the procedure | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| Transfusion‐related complications within 24 hours of the procedure | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| Procedure‐related (lumbar puncture or epidural anaesthetic) complications within 7 days of the procedure | No serious adverse events | Not estimated | 21 (1 study) | ⊕⊝⊝⊝ Very low1, 2 | ||
| Quality of life (as defined by the individual studies) | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded one level due to imprecision (low event rate). No events occurred. | ||||||

