| 1 Service utilisation: 1. Hospital readmission Show forest plot | 1 | 29 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.19 [0.01, 3.60] |
|
| 1.1 short term | 1 | 29 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.19 [0.01, 3.60] |
| 2 Adverse effects: 1a. General ‐ needing antiparkinsonian medication Show forest plot | 2 | 74 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.51, 0.95] |
|
| 2.1 short term | 1 | 33 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.46, 1.46] |
| 2.2 medium term | 1 | 41 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.43, 0.91] |
| 3 Adverse effects: 1b. General ‐ need to reduce antipsychotic dose due to side effects Show forest plot | 1 | | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
|
| 3.1 medium term | 1 | 33 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.26, 2.06] |
| 4 Adverse effects: 2a. Specific ‐ extrapyramidal events (moderate or severe) ‐ short term Show forest plot | 1 | | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
|
| 4.1 akatisia | 1 | 33 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 dyskinesia | 1 | 33 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.3 dystonia | 1 | 33 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.4 rigidity | 1 | 33 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.15 [0.01, 2.90] |
| 4.5 tremor | 1 | 33 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Adverse effects: 2b. Specific ‐ extrapyramidal events (moderate or severe) ‐ medium term Show forest plot | 3 | | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
|
| 5.1 akathisia | 2 | 85 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.19 [0.04, 1.06] |
| 5.2 dyskinesia | 2 | 85 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.14, 6.52] |
| 5.3 dystonia | 1 | 29 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.06, 13.54] |
| 5.4 muscle spasm | 1 | 56 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 70.64] |
| 5.5 rigidity | 2 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.22 [0.04, 1.20] |
| 5.6 tremor | 2 | 85 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.14, 3.43] |
| 6 Adverse effects: 2c. Specific ‐ anticholinergic (moderate or severe) ‐ short term Show forest plot | 1 | | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
|
| 6.1 constipation | 1 | 33 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.42 [0.60, 32.71] |
| 6.2 dry mouth | 1 | 33 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.25 [0.10, 51.46] |
| 6.3 increased salivation | 1 | 33 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.01, 5.72] |
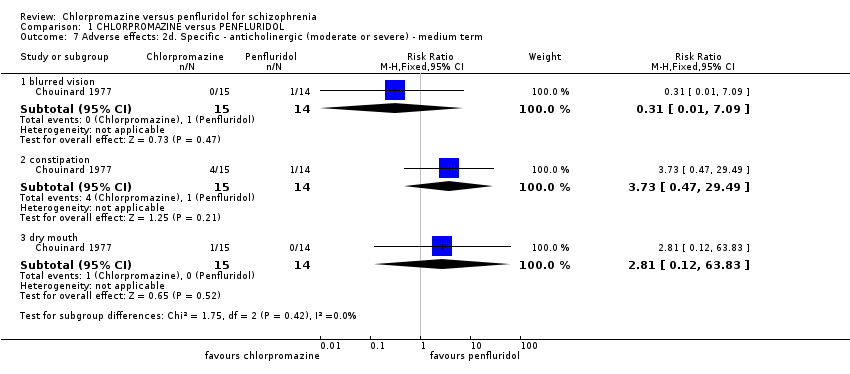
| 7 Adverse effects: 2d. Specific ‐ anticholinergic (moderate or severe) ‐ medium term Show forest plot | 1 | | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
|
| 7.1 blurred vision | 1 | 29 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.31 [0.01, 7.09] |
| 7.2 constipation | 1 | 29 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.73 [0.47, 29.49] |
| 7.3 dry mouth | 1 | 29 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.81 [0.12, 63.83] |
| 8 Adverse effects: 2e. Specific ‐ central nervous system (moderate or severe) ‐ short term Show forest plot | 1 | | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
|
| 8.1 agitation | 1 | 33 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.25 [0.10, 51.46] |
| 8.2 drowsiness | 1 | 33 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.25 [0.49, 137.94] |
| 8.3 dizziness | 1 | 33 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.03, 2.12] |
| 8.4 insomnia | 1 | 33 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.25 [0.10, 51.46] |
| 9 Adverse effects: 2f. Specific ‐ central nervous system (moderate or severe) ‐ medium term Show forest plot | 2 | | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
|
| 9.1 drowsiness | 2 | 85 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.54, 2.05] |
| 9.2 dizziness | 1 | 29 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.05, 4.60] |
| 9.3 excitement | 1 | 29 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.81 [0.12, 63.83] |
| 9.4 faintness | 1 | 29 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.15, 5.76] |
| 9.5 insomnia | 2 | 85 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.17 [0.03, 0.93] |
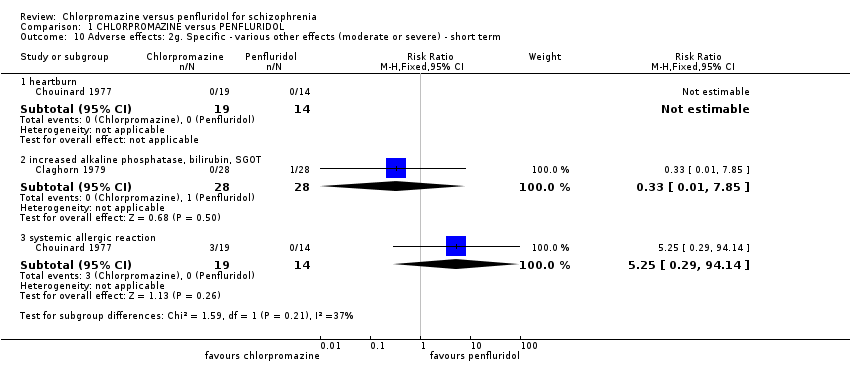
| 10 Adverse effects: 2g. Specific ‐ various other effects (moderate or severe) ‐ short term Show forest plot | 2 | | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
|
| 10.1 heartburn | 1 | 33 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10.2 increased alkaline phosphatase, bilirubin, SGOT | 1 | 56 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.85] |
| 10.3 systemic allergic reaction | 1 | 33 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.25 [0.29, 94.14] |
| 11 Adverse effects: 2h. Specific ‐ various other effects (moderate or severe) ‐ medium term Show forest plot | 1 | | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
|
| 11.1 depression | 1 | 29 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.19 [0.01, 3.60] |
| 11.2 decreased sexual drive | 1 | 29 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.05, 4.60] |
| 11.3 impotence | 1 | 29 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.81 [0.12, 63.83] |
| 11.4 photosensitivity | 1 | 29 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.81 [0.12, 63.83] |
| 11.5 poor appetite | 1 | 29 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.06, 13.54] |
| 12 Leaving the study early: 1a. Any reason Show forest plot | 3 | | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
|
| 12.1 medium term | 3 | 130 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.83, 1.77] |
| 13 Leaving the study early: 1b. Due to adverse events Show forest plot | 2 | | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
|
| 13.1 short term | 1 | 33 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.75 [0.39, 116.00] |
| 13.2 medium term | 2 | 74 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.53 [0.72, 3.28] |