Clorpromazina versus penfluridol para la esquizofrenia
Referencias
References to studies included in this review
References to studies excluded from this review
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Allocation: randomised. Blindness: double‐blind. Location: inpatient and outpatient. Duration: 13 weeks (3 weeks inpatient, 10 weeks outpatient). | |
| Participants | Diagnosis: schizophrenia (DSM‐II). N = 33. Age: 19‐60 years ( mean 35.7 ± 12.6). Sex: 15M and 14F* . History: 15 participants had been hospitalised for less than a year. | |
| Interventions | 1. Chlorpromazine: oral ‐ 300 mg/day the first week, 600 mg/day second week, and 900 mg/day third week. N = 19.** 2. Penfluridol: oral ‐ 40 mg/day the first week, 80 mg/day second week, and 120 mg/day third week. N = 14.** | |
| Outcomes | Adverse events: general (needing antiparkinson medication, reduction in antipsychotic dose due to side effects, specific (extrapyramidal and various other effects). Leaving the study early. Unable to use ‐ Mental state: average score/change BPRS and IMPS (no SD). | |
| Notes | * 4 participants left early during inpatient phase‐not included in follow‐up analyses at 13 weeks. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Subjects were assigned to one of two evaluating psychiatrists in such a way that each psychiatrist evaluated a group that included both male and female patients, permitting the examination of a possible sex effect. Individuals in each group were randomly assigned to one of the two drug treatments, penfluridol or chlorpromazine, which were administered under double‐blind conditions". |
| Allocation concealment (selection bias) | Unclear risk | No description regarding allocation concealment. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Described as double blind‐no further details reported. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No details about blinding of outcome assessment given "Assessment of symptoms was based on clinical interviews conducted by one of the two psychiatrists, the same psychiatrist always evaluating the same group of patients". |
| Incomplete outcome data (attrition bias) | Low risk | Attrition low and clearly described. |
| Selective reporting (reporting bias) | Unclear risk | All outcomes reported, but poor reporting of scale data. |
| Other bias | Unclear risk | Source of funding not reported. |
| Methods | Allocation: randomised. Blindness: double‐blind. Location: inpatient. Duration: 52 weeks. | |
| Participants | Diagnosis: schizophrenia. N = 56. Age: mean ˜37 years. Sex: 32M, 24F. History: duration ill ˜10 years, duration of present episode ˜8 years. | |
| Interventions | 1. Chlorpromazine: oral‐maximum 380 mg/day‐initially , maximum 760 mg/day thereafter; final mean 300mglday. N = 28. 2. Penfluridol: oral‐ maximum 80 mg/week‐initially, maximum 160 mg/week thereafter; final mean 74 mg/week. N = 28. Both groups received antiparkinsonian medication, chloral hydrate as required. | |
| Outcomes | Adverse events: any, central nervous system, hepatic‐"increased alkaline phosphatase, bilirubin, SGOT". Leaving the study early. Unable to use ‐ Global state: CGI (> 50% loss to follow‐up). Mental state: BPRS (> 50% loss to follow‐up). | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | ..."randomly assigned in blocks of 8 or 10". |
| Allocation concealment (selection bias) | Unclear risk | No description regarding allocation concealment. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Described as "double blind"‐placebo tablet given to penfluridol group to cover non‐drug days. No further details reported. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No details about blinding of outcome assessment. |
| Incomplete outcome data (attrition bias) | Unclear risk | Attrition high but clearly described. |
| Selective reporting (reporting bias) | High risk | Adverse effects mostly reported if frequent (unclear how frequent), poor reporting of scale data. |
| Other bias | Unclear risk | Source of funding not reported. |
| Methods | Allocation: randomised. Blindness: double blind. Location: inpatient. Duration: 20 weeks. | |
| Participants | Diagnosis: schizophrenia. N = 41 (double‐blind phase).* Age: 20‐60 years. Sex: all male. History: chronic schizophrenia for at least 18 months prior to the start of the study. Excluded: all participants were stabilised on penfluridol before the trial, participants who did not complete dose titration were excluded before randomisation. | |
| Interventions | 1. Chlorpromazine: oral (150 mg/day to 1050 mg/day). N = 20. 1. Penfluridol: oral (20 mg to 140 mg once a week). N = 21. | |
| Outcomes | Adverse events: use of antiparkinson medication, extrapyramidal. Leaving the study early. Unable to use ‐ Use of additional antipsychotic: not outcome prespecified in protocol. | |
| Notes | Trial had a dose titration phase where all participants stabilised on penfluridol and a double‐blind phase where participants randomised to continue penfluridol or switch to chlorpromazine, we only used data from the double‐blind phase. It is indicated in the paper that there were no statistical differences in scale‐derived data, and that patients on penfluridol had more akathisia than patients on chlorpromazine, but no data presented. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "When the patients were stable, they were randomly assigned to a penfluridol or a chlorpromazine group". |
| Allocation concealment (selection bias) | Unclear risk | No details regarding allocation concealment given. |
| Blinding of participants and personnel (performance bias) | Low risk | "All patients received identical appearing capsules on a special "blister pack" medication card allowing the patient to take the medication on a twice‐a‐day schedule". |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not clear if outcome assessor was blind‐"The rating scales for evaluating efficacy ...... were completed by the psychiatrist investigator". |
| Incomplete outcome data (attrition bias) | Low risk | Attrition low and clearly described. |
| Selective reporting (reporting bias) | Unclear risk | All outcomes reported, but poor reporting of scale data. |
| Other bias | Unclear risk | No further details. |
BPRS‐Brief Psychiatric Rating Scale
CGI‐Clinical Global Impression
ESF‐Evaluation of Social Functioning
IMPS: Inpatient Multidimensional Psychiatric Scale
NOSIE‐Nurse's Observation Scale for Inpatient Evaluation
SD: standard deviation
SGOT: serum glutamic oxaloacetic transaminase
Characteristics of excluded studies [ordered by study ID]
Ir a:
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Service utilisation: 1. Hospital readmission Show forest plot | 1 | 29 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.19 [0.01, 3.60] |
| Analysis 1.1  Comparison 1 CHLORPROMAZINE versus PENFLURIDOL, Outcome 1 Service utilisation: 1. Hospital readmission. | ||||
| 1.1 short term | 1 | 29 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.19 [0.01, 3.60] |
| 2 Adverse effects: 1a. General ‐ needing antiparkinsonian medication Show forest plot | 2 | 74 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.51, 0.95] |
| Analysis 1.2  Comparison 1 CHLORPROMAZINE versus PENFLURIDOL, Outcome 2 Adverse effects: 1a. General ‐ needing antiparkinsonian medication. | ||||
| 2.1 short term | 1 | 33 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.46, 1.46] |
| 2.2 medium term | 1 | 41 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.43, 0.91] |
| 3 Adverse effects: 1b. General ‐ need to reduce antipsychotic dose due to side effects Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.3  Comparison 1 CHLORPROMAZINE versus PENFLURIDOL, Outcome 3 Adverse effects: 1b. General ‐ need to reduce antipsychotic dose due to side effects. | ||||
| 3.1 medium term | 1 | 33 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.26, 2.06] |
| 4 Adverse effects: 2a. Specific ‐ extrapyramidal events (moderate or severe) ‐ short term Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.4  Comparison 1 CHLORPROMAZINE versus PENFLURIDOL, Outcome 4 Adverse effects: 2a. Specific ‐ extrapyramidal events (moderate or severe) ‐ short term. | ||||
| 4.1 akatisia | 1 | 33 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 dyskinesia | 1 | 33 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.3 dystonia | 1 | 33 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.4 rigidity | 1 | 33 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.15 [0.01, 2.90] |
| 4.5 tremor | 1 | 33 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Adverse effects: 2b. Specific ‐ extrapyramidal events (moderate or severe) ‐ medium term Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.5  Comparison 1 CHLORPROMAZINE versus PENFLURIDOL, Outcome 5 Adverse effects: 2b. Specific ‐ extrapyramidal events (moderate or severe) ‐ medium term. | ||||
| 5.1 akathisia | 2 | 85 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.19 [0.04, 1.06] |
| 5.2 dyskinesia | 2 | 85 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.14, 6.52] |
| 5.3 dystonia | 1 | 29 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.06, 13.54] |
| 5.4 muscle spasm | 1 | 56 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 70.64] |
| 5.5 rigidity | 2 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.22 [0.04, 1.20] |
| 5.6 tremor | 2 | 85 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.14, 3.43] |
| 6 Adverse effects: 2c. Specific ‐ anticholinergic (moderate or severe) ‐ short term Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.6 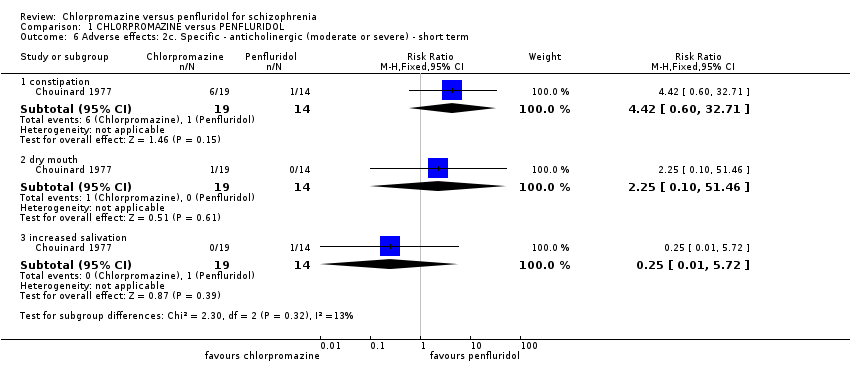 Comparison 1 CHLORPROMAZINE versus PENFLURIDOL, Outcome 6 Adverse effects: 2c. Specific ‐ anticholinergic (moderate or severe) ‐ short term. | ||||
| 6.1 constipation | 1 | 33 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.42 [0.60, 32.71] |
| 6.2 dry mouth | 1 | 33 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.25 [0.10, 51.46] |
| 6.3 increased salivation | 1 | 33 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.01, 5.72] |
| 7 Adverse effects: 2d. Specific ‐ anticholinergic (moderate or severe) ‐ medium term Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.7  Comparison 1 CHLORPROMAZINE versus PENFLURIDOL, Outcome 7 Adverse effects: 2d. Specific ‐ anticholinergic (moderate or severe) ‐ medium term. | ||||
| 7.1 blurred vision | 1 | 29 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.31 [0.01, 7.09] |
| 7.2 constipation | 1 | 29 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.73 [0.47, 29.49] |
| 7.3 dry mouth | 1 | 29 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.81 [0.12, 63.83] |
| 8 Adverse effects: 2e. Specific ‐ central nervous system (moderate or severe) ‐ short term Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.8  Comparison 1 CHLORPROMAZINE versus PENFLURIDOL, Outcome 8 Adverse effects: 2e. Specific ‐ central nervous system (moderate or severe) ‐ short term. | ||||
| 8.1 agitation | 1 | 33 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.25 [0.10, 51.46] |
| 8.2 drowsiness | 1 | 33 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.25 [0.49, 137.94] |
| 8.3 dizziness | 1 | 33 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.03, 2.12] |
| 8.4 insomnia | 1 | 33 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.25 [0.10, 51.46] |
| 9 Adverse effects: 2f. Specific ‐ central nervous system (moderate or severe) ‐ medium term Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.9  Comparison 1 CHLORPROMAZINE versus PENFLURIDOL, Outcome 9 Adverse effects: 2f. Specific ‐ central nervous system (moderate or severe) ‐ medium term. | ||||
| 9.1 drowsiness | 2 | 85 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.54, 2.05] |
| 9.2 dizziness | 1 | 29 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.05, 4.60] |
| 9.3 excitement | 1 | 29 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.81 [0.12, 63.83] |
| 9.4 faintness | 1 | 29 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.15, 5.76] |
| 9.5 insomnia | 2 | 85 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.17 [0.03, 0.93] |
| 10 Adverse effects: 2g. Specific ‐ various other effects (moderate or severe) ‐ short term Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.10 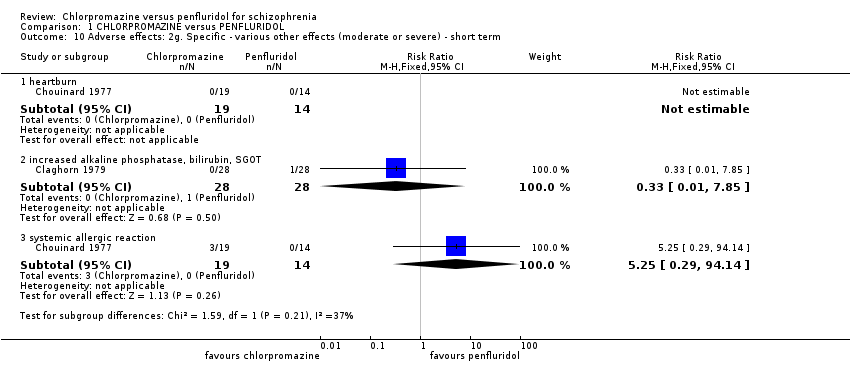 Comparison 1 CHLORPROMAZINE versus PENFLURIDOL, Outcome 10 Adverse effects: 2g. Specific ‐ various other effects (moderate or severe) ‐ short term. | ||||
| 10.1 heartburn | 1 | 33 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10.2 increased alkaline phosphatase, bilirubin, SGOT | 1 | 56 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.85] |
| 10.3 systemic allergic reaction | 1 | 33 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.25 [0.29, 94.14] |
| 11 Adverse effects: 2h. Specific ‐ various other effects (moderate or severe) ‐ medium term Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.11 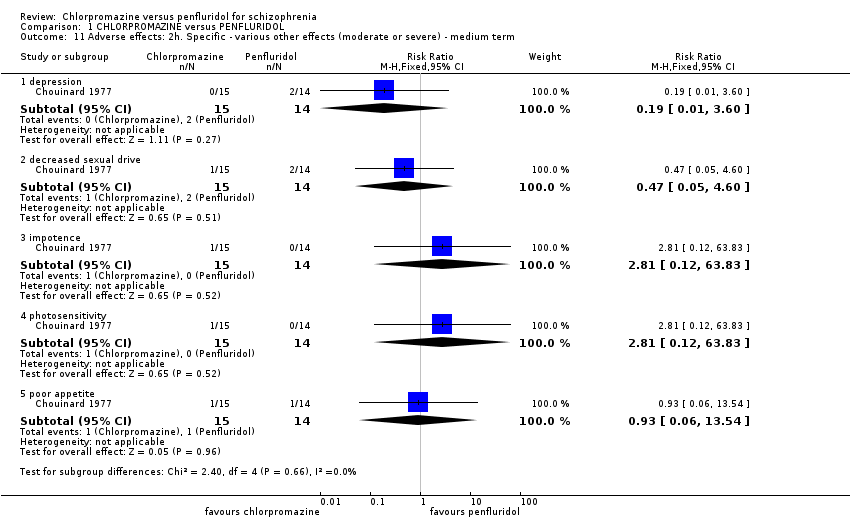 Comparison 1 CHLORPROMAZINE versus PENFLURIDOL, Outcome 11 Adverse effects: 2h. Specific ‐ various other effects (moderate or severe) ‐ medium term. | ||||
| 11.1 depression | 1 | 29 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.19 [0.01, 3.60] |
| 11.2 decreased sexual drive | 1 | 29 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.05, 4.60] |
| 11.3 impotence | 1 | 29 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.81 [0.12, 63.83] |
| 11.4 photosensitivity | 1 | 29 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.81 [0.12, 63.83] |
| 11.5 poor appetite | 1 | 29 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.06, 13.54] |
| 12 Leaving the study early: 1a. Any reason Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.12  Comparison 1 CHLORPROMAZINE versus PENFLURIDOL, Outcome 12 Leaving the study early: 1a. Any reason. | ||||
| 12.1 medium term | 3 | 130 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.83, 1.77] |
| 13 Leaving the study early: 1b. Due to adverse events Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.13  Comparison 1 CHLORPROMAZINE versus PENFLURIDOL, Outcome 13 Leaving the study early: 1b. Due to adverse events. | ||||
| 13.1 short term | 1 | 33 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.75 [0.39, 116.00] |
| 13.2 medium term | 2 | 74 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.53 [0.72, 3.28] |

Chlorpromazine structure
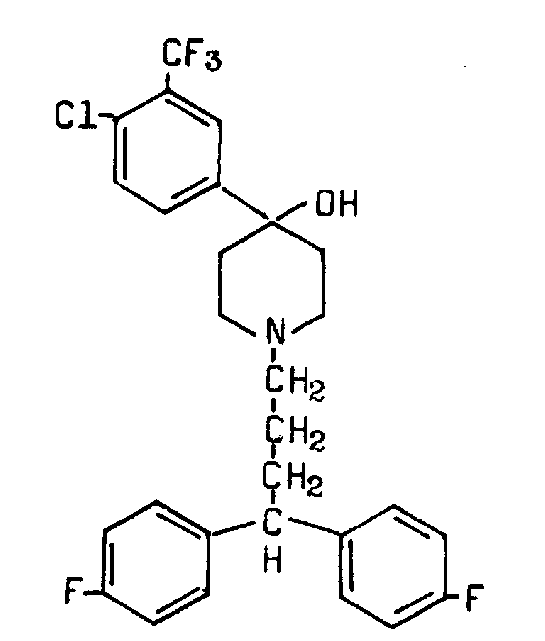
Penfluridol structure

Study flow diagram.
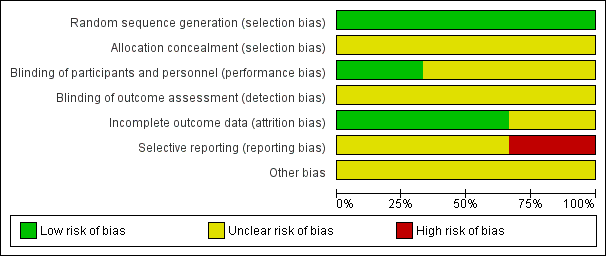
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
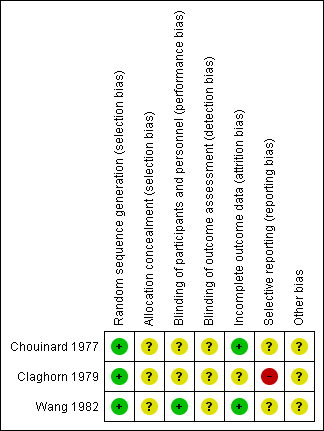
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 CHLORPROMAZINE versus PENFLURIDOL, Outcome 1 Service utilisation: 1. Hospital readmission.

Comparison 1 CHLORPROMAZINE versus PENFLURIDOL, Outcome 2 Adverse effects: 1a. General ‐ needing antiparkinsonian medication.

Comparison 1 CHLORPROMAZINE versus PENFLURIDOL, Outcome 3 Adverse effects: 1b. General ‐ need to reduce antipsychotic dose due to side effects.

Comparison 1 CHLORPROMAZINE versus PENFLURIDOL, Outcome 4 Adverse effects: 2a. Specific ‐ extrapyramidal events (moderate or severe) ‐ short term.

Comparison 1 CHLORPROMAZINE versus PENFLURIDOL, Outcome 5 Adverse effects: 2b. Specific ‐ extrapyramidal events (moderate or severe) ‐ medium term.

Comparison 1 CHLORPROMAZINE versus PENFLURIDOL, Outcome 6 Adverse effects: 2c. Specific ‐ anticholinergic (moderate or severe) ‐ short term.
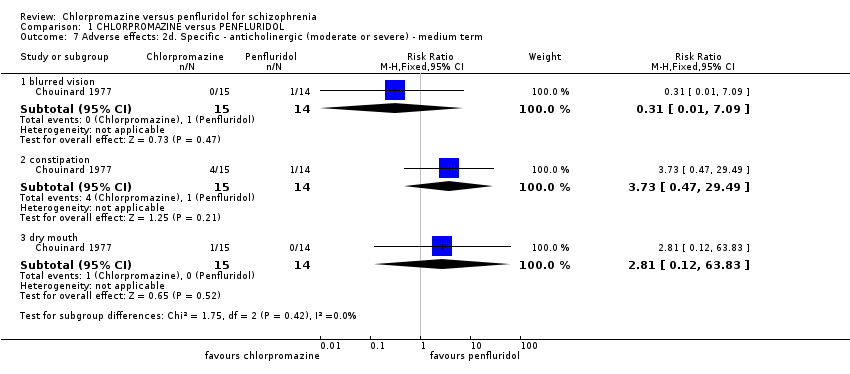
Comparison 1 CHLORPROMAZINE versus PENFLURIDOL, Outcome 7 Adverse effects: 2d. Specific ‐ anticholinergic (moderate or severe) ‐ medium term.

Comparison 1 CHLORPROMAZINE versus PENFLURIDOL, Outcome 8 Adverse effects: 2e. Specific ‐ central nervous system (moderate or severe) ‐ short term.

Comparison 1 CHLORPROMAZINE versus PENFLURIDOL, Outcome 9 Adverse effects: 2f. Specific ‐ central nervous system (moderate or severe) ‐ medium term.
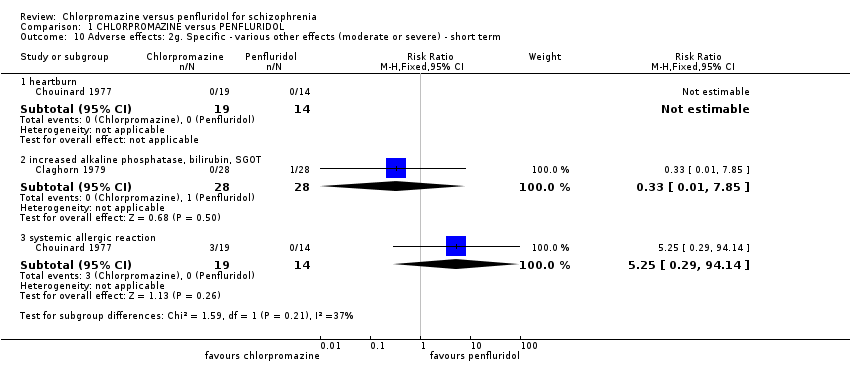
Comparison 1 CHLORPROMAZINE versus PENFLURIDOL, Outcome 10 Adverse effects: 2g. Specific ‐ various other effects (moderate or severe) ‐ short term.

Comparison 1 CHLORPROMAZINE versus PENFLURIDOL, Outcome 11 Adverse effects: 2h. Specific ‐ various other effects (moderate or severe) ‐ medium term.

Comparison 1 CHLORPROMAZINE versus PENFLURIDOL, Outcome 12 Leaving the study early: 1a. Any reason.

Comparison 1 CHLORPROMAZINE versus PENFLURIDOL, Outcome 13 Leaving the study early: 1b. Due to adverse events.
| Methods | Allocation: randomised, fully explicit description of methods of randomisation and allocation concealment. |
| Participants | Diagnosis: schizophrenia. |
| Interventions | 1. Chlorpromazine: oral‐maximum around 400 mg/day. N = 150. 2. Penfluridol: oral‐maximum around 80 mg/week. N = 150. Both groups could receive antiparkinsonian medication as required. |
| Outcomes | Service utilisation: Hospital admission, time to admission. Global state: Clinically significant response in global state, relapse. Mental state: Clinically significant response in mental state. Adverse effects: Clinically significant extrapyramidal side effects, death. Leaving the study early. Functioning: Employed, days working, in supportive relationship, healthy days. Economic outcomes. |
| Notes | * Powered to be able to identify a difference of ˜ 20% between groups for primary outcome with adequate degree of certainty. |
| Chlorpromazine versus Penfluridol for schizophrenia | ||||||
| Patient or population: patients with schizophrenia | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk3 | Corresponding risk | |||||
| Penfluridol | Chlorpromazine | |||||
| Service utilisation: hospital admission (short term) | 150 per 1000 | 28 per 1000 | RR 0.19 (0.01 to 3.60) | 29 | ⊕⊕⊝⊝ low 1,2 | |
| Global state: clinically important change in global state | See comment | Not estimable | 0 | See comment | No studies reported 'clinically important change in global state'. Change in global state was measure using global state scales but all data were presented without SD. | |
| Global state: relapse | See comment | Not estimable | 0 | See comment | No studies reported this outcome. | |
| Mental state: clinically important change in mental state | See comment | Not estimable | 0 | See comment | No studies reported 'clinically important change in mental state'. Change in mental state was measure using mental state scales but all data were presented without SD. | |
| Adverse effect/event: clinically important extrapyramidal adverse events ‐ akathisia (medium term) | 200 per 1000 | 38 per 1000 | RR 0.19 (0.04 to 1.06) | 85 | ⊕⊕⊝⊝ low 1,2 | The same studies reported data for other extrapyramidal adverse events such as rigidity, tremor, dystonia and dyskinesia. There was no observable difference between chlorpromazine and penfluridol regarding any of these adverse effects. |
| Adverse effect/event: death | See comment | Not estimated | 2 RCTs (0) | See comment | No deaths reported. | |
| Leaving the study early: any reason (medium term) | 400 per 1000 | 484 per 1000 | RR 1.21 (0.83 to 1.77) | 130 | ⊕⊕⊝⊝ low 1,2 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Serious risk of bias: downgraded by one level ‐ study had an unclear risk of bias for random sequence generation and blinding of assessors. | ||||||
| Review title | Reference |
| Acetophenazine versus chlorpromazine | |
| Chlorpromazine dose for people with schizophrenia | |
| Cessation of medication for people with schizophrenia already stable on chlorpromazine | |
| Chlorpromazine versus atypical antipsychotic drugs for schizophrenia | |
| Chlorpromazine versus clotiapine for schizophrenia | Developing protocol |
| Chlorpromazine versus haloperidol for schizophrenia | |
| Chlorpromazine versus metiapine | |
| Chlorpromazine versus penfluridol for schizophrenia | Current review |
| Chlorpromazine versus piperacetazine | |
| Chlorpromazine versus placebo for schizophrenia | |
| Chlorpromazine for psychosis induced aggression or agitation |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Service utilisation: 1. Hospital readmission Show forest plot | 1 | 29 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.19 [0.01, 3.60] |
| 1.1 short term | 1 | 29 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.19 [0.01, 3.60] |
| 2 Adverse effects: 1a. General ‐ needing antiparkinsonian medication Show forest plot | 2 | 74 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.51, 0.95] |
| 2.1 short term | 1 | 33 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.46, 1.46] |
| 2.2 medium term | 1 | 41 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.43, 0.91] |
| 3 Adverse effects: 1b. General ‐ need to reduce antipsychotic dose due to side effects Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 medium term | 1 | 33 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.26, 2.06] |
| 4 Adverse effects: 2a. Specific ‐ extrapyramidal events (moderate or severe) ‐ short term Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 akatisia | 1 | 33 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 dyskinesia | 1 | 33 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.3 dystonia | 1 | 33 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.4 rigidity | 1 | 33 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.15 [0.01, 2.90] |
| 4.5 tremor | 1 | 33 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Adverse effects: 2b. Specific ‐ extrapyramidal events (moderate or severe) ‐ medium term Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 akathisia | 2 | 85 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.19 [0.04, 1.06] |
| 5.2 dyskinesia | 2 | 85 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.14, 6.52] |
| 5.3 dystonia | 1 | 29 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.06, 13.54] |
| 5.4 muscle spasm | 1 | 56 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 70.64] |
| 5.5 rigidity | 2 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.22 [0.04, 1.20] |
| 5.6 tremor | 2 | 85 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.14, 3.43] |
| 6 Adverse effects: 2c. Specific ‐ anticholinergic (moderate or severe) ‐ short term Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 constipation | 1 | 33 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.42 [0.60, 32.71] |
| 6.2 dry mouth | 1 | 33 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.25 [0.10, 51.46] |
| 6.3 increased salivation | 1 | 33 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.01, 5.72] |
| 7 Adverse effects: 2d. Specific ‐ anticholinergic (moderate or severe) ‐ medium term Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 blurred vision | 1 | 29 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.31 [0.01, 7.09] |
| 7.2 constipation | 1 | 29 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.73 [0.47, 29.49] |
| 7.3 dry mouth | 1 | 29 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.81 [0.12, 63.83] |
| 8 Adverse effects: 2e. Specific ‐ central nervous system (moderate or severe) ‐ short term Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 agitation | 1 | 33 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.25 [0.10, 51.46] |
| 8.2 drowsiness | 1 | 33 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.25 [0.49, 137.94] |
| 8.3 dizziness | 1 | 33 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.03, 2.12] |
| 8.4 insomnia | 1 | 33 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.25 [0.10, 51.46] |
| 9 Adverse effects: 2f. Specific ‐ central nervous system (moderate or severe) ‐ medium term Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9.1 drowsiness | 2 | 85 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.54, 2.05] |
| 9.2 dizziness | 1 | 29 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.05, 4.60] |
| 9.3 excitement | 1 | 29 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.81 [0.12, 63.83] |
| 9.4 faintness | 1 | 29 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.15, 5.76] |
| 9.5 insomnia | 2 | 85 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.17 [0.03, 0.93] |
| 10 Adverse effects: 2g. Specific ‐ various other effects (moderate or severe) ‐ short term Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 10.1 heartburn | 1 | 33 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10.2 increased alkaline phosphatase, bilirubin, SGOT | 1 | 56 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.85] |
| 10.3 systemic allergic reaction | 1 | 33 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.25 [0.29, 94.14] |
| 11 Adverse effects: 2h. Specific ‐ various other effects (moderate or severe) ‐ medium term Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 11.1 depression | 1 | 29 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.19 [0.01, 3.60] |
| 11.2 decreased sexual drive | 1 | 29 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.05, 4.60] |
| 11.3 impotence | 1 | 29 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.81 [0.12, 63.83] |
| 11.4 photosensitivity | 1 | 29 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.81 [0.12, 63.83] |
| 11.5 poor appetite | 1 | 29 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.06, 13.54] |
| 12 Leaving the study early: 1a. Any reason Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 12.1 medium term | 3 | 130 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.83, 1.77] |
| 13 Leaving the study early: 1b. Due to adverse events Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 13.1 short term | 1 | 33 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.75 [0.39, 116.00] |
| 13.2 medium term | 2 | 74 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.53 [0.72, 3.28] |

