Enseñanza virtual para los profesionales de la salud
Referencias
References to studies included in this review
References to studies excluded from this review
References to studies awaiting assessment
Additional references
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Study type: randomised trial Study arms: 3 | |
| Participants | Participants type: childcare health consultants Number randomised (e‐learning/control): 17/16 Lost to follow‐up: not reported | |
| Interventions | E‐learning type: web training using photographs, quizzes and interactive multiple choice questions E‐learning interactivity: high E‐learning blending: alone E‐learning duration: short; completion within 3 weeks (mean time spent on training 120 minutes) Control type: face‐to‐face training Control duration: 3 hours Follow‐up (from the end of the intervention to the last outcome assessment): short ‐ 0 weeks (immediately after) CanMEDS framework area: medical expertise Regulation: not stated Setting: community setting | |
| Outcomes | Primary: knowledge (by an non‐validated test) Secondary: time spent on training Times the outcomes were assessed after the intervention: 1 | |
| Notes | Study dates: August 2005‐June 2006 Funding source: Centers for Disease Control and Prevention (CDC), North Carolina Division of Public Health, Child Care Bureau Declaration of interest: none declared Country: USA Topic: childhood overweight management | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Was the allocation sequence adequately generated? | Unclear risk | No information reported |
| Was the allocation adequately concealed? | Low risk | Sealed envelopes with a randomisation sequence developed by the study biostatistician |
| Were baseline outcome measurements similar? | Low risk | No important differences across study groups |
| Were baseline characteristics similar? | Unclear risk | No information reported |
| Were incomplete outcome data adequately addressed? | Unclear risk | No information reported |
| Was knowledge of the allocated interventions adequately prevented during the study? | Unclear risk | No information reported |
| Was the study adequately protected against contamination? | Unclear risk | No information reported |
| Was the study free from selective outcome reporting? | Low risk | No evidence of selective reporting of outcomes |
| Was the study free from other risks of bias (e.g. conflicts of interest)? | Low risk | No evidence of other risk of bias |
| OVERALL RISK OF BIAS | Unclear risk | Risk of selection bias: low Risk of attrition bias: unclear Risk of detection bias: unclear |
| Methods | Study type: randomised trial Study arms: 2 | |
| Participants | Participants type: nurses Number randomised (e‐learning/control): 23/21 Lost to follow‐up (number(%); (e‐learning/control)): 13(56.5%)/13(61.9%) | |
| Interventions | E‐learning type: patient cases, photos and schematic illustration E‐learning interactivity: low E‐learning blending: alone E‐learning duration: not reported Control type: traditional classroom lecture Control duration: 45 minutes Follow‐up (time from the end of the intervention to the last outcome assessment): 0 weeks (immediately after) and three months later CanMEDS framework area: medical expertise Regulation: not specified Setting: secondary (hospital) care | |
| Outcomes | Primary: skills Secondary: none Times the outcomes were assessed after the intervention: 2 | |
| Notes | Study dates: May 2012‐December 2012 Funding source: Oslo University Hospital, Norwegian Nurses Organisation, University of Oslo and Sophies Minde Ortopedi AS Declaration of interest: no competing interest Country: Norway Topic: pressure ulcer risk assessment and classification Other: authors provided unpublished data regarding pressure ulcer classification (Brendsen 2016 [pers comm]) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Was the allocation sequence adequately generated? | Low risk | Envelope shuffling |
| Was the allocation adequately concealed? | Low risk | Envelope shuffling |
| Were baseline outcome measurements similar? | Unclear risk | No information reported |
| Were baseline characteristics similar? | Low risk | Chi2/Fisher's Exact test not significant between the 2 groups |
| Were incomplete outcome data adequately addressed? | Low risk | No incomplete data at post‐test immediately after the training |
| Was knowledge of the allocated interventions adequately prevented during the study? | Low risk | Outcome is not likely to be influenced by lack of blinding in this study |
| Was the study adequately protected against contamination? | Unclear risk | Contamination is unlikely |
| Was the study free from selective outcome reporting? | Low risk | The published report includes all expected outcomes |
| Was the study free from other risks of bias (e.g. conflicts of interest)? | High risk | Private sponsor Sophies Minde Ortopedi AS |
| OVERALL RISK OF BIAS | Low risk | Risk of selection bias: low Risk of attrition bias: low Risk of detection bias: low |
| Methods | Study type: randomised trial Study arms: 3 | |
| Participants | Participants type: primary care physicians Number randomised (e‐learning/control): 52/51 Lost to follow‐up (number(%); (e‐learning/control)): 8(15.4%)/2(3.9%) | |
| Interventions | E‐learning type: online lecture, interactive cases with feedback, enabling tools, supporting resources, access to expert advice E‐learning interactivity: high E‐learning blending: core and essential E‐learning duration: short ‐ at participants convenience during a 2‐week period (mean time spent on training 1.4 hours for 3 session) Control type: live lecture interactive cases with feedback, enabling tools, supporting resources, access to expert advice Control duration: 1.5‐2 hours Follow‐up (time from the end of the intervention to the last outcome assessment): 12 weeks CanMEDS framework area: medical expertise Regulation: formally accredited Setting: primary care | |
| Outcomes | Primary: knowledge (by a validated test), behaviours (appropriate screening and treatment for dyslipidaemia) Secondary: time spent on training, satisfaction Times the outcomes were assessed after the intervention: 2 | |
| Notes | Study dates: August 2001‐July 2002 Funding source: AstraZeneca Pharmaceuticals Declaration of interest: grant support from AstraZeneca and other pharmaceutical companies Country: USA Topic: cholesterol management Other: authors provided single participants data about knowledge as requested (Jason 2015 [pers comm]) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Was the allocation sequence adequately generated? | Low risk | Random number generator |
| Was the allocation adequately concealed? | Low risk | Centralised randomisation scheme |
| Were baseline outcome measurements similar? | Low risk | No important differences across study groups |
| Were baseline characteristics similar? | Low risk | No important differences across study groups |
| Were incomplete outcome data adequately addressed? | High risk | Major imbalance in missing data between groups: 15.4% in the e‐learning group and 5.8% in the control group |
| Was knowledge of the allocated interventions adequately prevented during the study? | Low risk | Data analyst blinded to the identification of participants |
| Was the study adequately protected against contamination? | Unclear risk | No information reported |
| Was the study free from selective outcome reporting? | Low risk | No evidence of selective reporting of outcomes |
| Was the study free from other risks of bias (e.g. conflicts of interest)? | High risk | Study supported by a grant from AstraZeneca Pharmaceuticals. |
| OVERALL RISK OF BIAS | High risk | Risk of selection bias: low Risk of attrition bias: high Risk of detection bias: low |
| Methods | Study type: randomised trial Study arms: 3 | |
| Participants | Participants type: primary care physicians Number randomised (e‐learning/control): 49/50 Lost to follow‐up (number(%); (e‐learning/control)): 19(38.8%)/18(36.0%) | |
| Interventions | E‐learning type: on‐line lectures E‐learning interactivity: low E‐learning blending: alone E‐learning duration: short ‐ 4 hours Control type: live lecture Control duration: 4 hours Follow‐up (time from the end of the intervention to the last outcome assessment): long ‐ 12 weeks CanMEDS framework area: medical expertise Regulation: formally accredited Setting: primary care | |
| Outcomes | Primary: knowledge (by a validated test) Secondary: time spent on training, satisfaction Times the outcomes were assessed after the intervention: 2 | |
| Notes | Study dates: September 2005 Funding source: Small Business Innovation and Research (SBIR) grant Declaration of interest: none declared Country: USA Topic: chronic pain Other: we decided to include this study after discussion about the outcome measure used. The know pain 50 assesses a mix of knowledge, attitudes and beliefs but at the end we considered that the most of the items regard knowledge. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Was the allocation sequence adequately generated? | Low risk | Blind name draw |
| Was the allocation adequately concealed? | Low risk | Centralised randomisation scheme |
| Were baseline outcome measurements similar? | Unclear risk | No information reported |
| Were baseline characteristics similar? | Unclear risk | No information reported |
| Were incomplete outcome data adequately addressed? | High risk | Missing data 38.8% in the e‐learning group and 36.0% in the control group |
| Was knowledge of the allocated interventions adequately prevented during the study? | Unclear risk | No information reported |
| Was the study adequately protected against contamination? | Low risk | The authors controlled the participants' room change |
| Was the study free from selective outcome reporting? | Low risk | No evidence of selective reporting of outcomes |
| Was the study free from other risks of bias (e.g. conflicts of interest)? | High risk | The development of the online CME programme and the research study were supported by Small Business Innovation and Research (SBIR) grants |
| OVERALL RISK OF BIAS | High risk | Risk of selection bias: low Risk of attrition bias: high Risk of detection bias: unclear |
| Methods | Study type: randomised trial Study arms: 2 | |
| Participants | Participants type: nurses Number randomised (e‐learning/control): 45/48 Lost to follow‐up (number(%); (e‐learning/control)): 8(17.8%)/15(31.2%) | |
| Interventions | E‐learning type: four 30‐minute online classes E‐learning interactivity: low E‐learning bending: alone E‐learning duration: short ‐ 120 minutes Control type: four 90‐minute evening lectures Control duration: 360 minutes Follow‐up (time from the end of the intervention to the last outcome assessment): long ‐ 4 weeks CanMEDS framework area: medical expertise Regulation: not specified Setting: secondary (hospital) care | |
| Outcomes | Primary: knowledge (by an non‐validated test) Secondary: satisfaction Times the outcomes were assessed after the intervention: 1 | |
| Notes | Study dates: August 2005‐November 2006 Funding source: Japanese Ministry of Education Scientific Research Grant Declaration of interest: none declared Country: Japan Topic: evidence‐based medicine | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Was the allocation sequence adequately generated? | Low risk | Computerised random number generator |
| Was the allocation adequately concealed? | Low risk | Centralised randomisation scheme and sealed opaque envelopes |
| Were baseline outcome measurements similar? | Low risk | No important differences across study groups |
| Were baseline characteristics similar? | High risk | Several imbalance between group in the demographics of participants |
| Were incomplete outcome data adequately addressed? | High risk | Major imbalance in missing data between groups: 17.8% in the e‐learning group and 31.2% in the control group |
| Was knowledge of the allocated interventions adequately prevented during the study? | Unclear risk | No information reported |
| Was the study adequately protected against contamination? | Unclear risk | No information reported |
| Was the study free from selective outcome reporting? | High risk | Inconsistencies between outcomes declared in the Methods and outcomes reported in the Results |
| Was the study free from other risks of bias (e.g. conflicts of interest)? | Low risk | No evidence of other risk of bias |
| OVERALL RISK OF BIAS | High risk | Risk of selection bias: low Risk of attrition bias: high Risk of detection bias: unclear |
| Methods | Study type: randomised trial Study arms: 2 | |
| Participants | Participants type: occupational physicians Number randomised (e‐learning/control): 37/35 Lost to follow‐up (number(%); (e‐learning/control)): 0/2(5.4%) | |
| Interventions | E‐learning type: individual e‐learning E‐learning interactivity: low E‐learning blending: alone E‐learning duration: short ‐ 30 minutes Control type: live lecture Control duration: 30 minutes Follow‐up (time from the end of the intervention to the last outcome assessment): short ‐ 0 weeks (immediately after) CanMEDS framework area: medical expertise Regulation: formally accredited Setting: occupational medicine | |
| Outcomes | Primary: knowledge (by an non‐validated test) Secondary: none Times the outcomes were assessed after the intervention: 1 | |
| Notes | Study dates: December 2006 Funding source: none declared Declaration of interest: none declared Country: Netherlands Topic: Mental health | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Was the allocation sequence adequately generated? | Unclear risk | No information reported |
| Was the allocation adequately concealed? | Unclear risk | No information reported |
| Were baseline outcome measurements similar? | Low risk | No important differences across study groups |
| Were baseline characteristics similar? | Unclear risk | No information reported |
| Were incomplete outcome data adequately addressed? | Low risk | The proportion of missing data was unlikely to overturn the study result: 0% in the e‐learning group and 5.4% in the control group |
| Was knowledge of the allocated interventions adequately prevented during the study? | Unclear risk | No information reported |
| Was the study adequately protected against contamination? | Low risk | It is unlikely that communication between intervention and control groups could have occurred |
| Was the study free from selective outcome reporting? | Low risk | No evidence of selective reporting of outcomes |
| Was the study free from other risks of bias (e.g. conflicts of interest)? | Low risk | No evidence of other risk of bias |
| OVERALL RISK OF BIAS | Unclear risk | Risk of selection bias: unclear Risk of attrition bias: low Risk of detection bias: unclear |
| Methods | Study type: randomised trial Study arms: 2 | |
| Participants | Participants type: nurses Number randomised (e‐learning/control): 70/70 Lost to follow‐up: not reported | |
| Interventions | E‐learning type: 1 week educational material access, chat room, emailing and telephone availability for answering questions E‐learning interactivity: high E‐learning blending: alone E‐learning duration: long ‐ 1 week Control type: face‐to‐face interactive lecture Control duration: 3 hours Follow‐up (time from the end of the intervention to the last outcome assessment): short ‐ 0 weeks (immediately after) CanMEDS framework area: medical expertise Regulation: not specified Setting: secondary (hospital) care | |
| Outcomes | Primary: knowledge (by a validated test) Secondary: none Times the outcomes were assessed after the intervention: 1 | |
| Notes | Study dates: winter 2007 Funding source: none declared Declaration of interest: no competing interest declared Country: Iran Topic: AIDS | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Was the allocation sequence adequately generated? | Unclear risk | No information reported |
| Was the allocation adequately concealed? | Unclear risk | No information reported |
| Were baseline outcome measurements similar? | Low risk | No important differences across study groups |
| Were baseline characteristics similar? | Low risk | No important differences across study groups |
| Were incomplete outcome data adequately addressed? | Unclear risk | No information reported |
| Was knowledge of the allocated interventions adequately prevented during the study? | Unclear risk | No information reported |
| Was the study adequately protected against contamination? | Unclear risk | No information reported |
| Was the study free from selective outcome reporting? | Low risk | No evidence of selective reporting of outcomes |
| Was the study free from other risks of bias (e.g. conflicts of interest)? | Low risk | No evidence of other risk of bias |
| OVERALL RISK OF BIAS | Unclear risk | Risk of selection bias: unclear Risk of attrition bias: unclear Risk of detection bias: unclear |
| Methods | Study type: randomised trial Study arms: 2 | |
| Participants | Participants type: paediatricians Number randomised (e‐learning/control): 15/9 | |
| Interventions | E‐learning type: 2 teleconferences, access to a website with 6 interactive multimedia earning modules and a CD‐ROM with the same learning modules E‐learning interactivity: high E‐learning blending: core and essential E‐learning duration: long ‐ 6 weeks to complete the modules Control type: guidelines dissemination ‐ authors reply on 15 July 2015 (Cabana 2015 [pers comm]) Control duration: 0 weeks Follow‐up (time from the end of the intervention to the last outcome assessment): 32 weeks CanMEDS framework area: medical expertise Regulation: formally accredited Setting: primary care | |
| Outcomes | Primary: satisfaction Secondary: knowledge (by an non‐validated test), attitudes, self‐reported prescription, self‐reported guidelines familiarity Times the outcomes were assessed after the intervention: 2 | |
| Notes | Study dates: February 2007‐March 2008 Funding source: none declared Declaration of interest: no competing interest declared Country: USA Topic: asthma | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Was the allocation sequence adequately generated? | High risk | Authors matched participants from the same practice into pairs: within each pair, they randomised one participant to the control group and the other to the intervention group |
| Was the allocation adequately concealed? | Low risk | Unit of allocation was by institution, team or professional and allocation performed on all units at the start of the study |
| Were baseline outcome measurements similar? | Unclear risk | No information reported |
| Were baseline characteristics similar? | High risk | Some imbalance between group in the demographics of participants |
| Were incomplete outcome data adequately addressed? | High risk | Major imbalance in missing data between groups: 26.3% in the e‐learning group and 0.0% in the control group |
| Was knowledge of the allocated interventions adequately prevented during the study? | Unclear risk | No information reported |
| Was the study adequately protected against contamination? | Unclear risk | Participants were allocated within a practice and it is possible that communication between intervention and control professionals could have occurred |
| Was the study free from selective outcome reporting? | Low risk | No evidence of selective reporting of outcomes |
| Was the study free from other risks of bias (e.g. conflicts of interest)? | High risk | Indegene Inc gave assistance in developing the learning modules |
| OVERALL RISK OF BIAS | High risk | Risk of selection bias: low Risk of attrition bias: high Risk of detection bias: unclear |
| Methods | Study type: cluster‐randomised trial Study arms: 2 | |
| Participants | Participants type: healthcare providers (not otherwise specified) Number randomised (e‐learning/control): 84 clinics (385 providers, 4024 patients)/84 clinics (462 providers, 3727 patients) | |
| Interventions | E‐learning type: multicomponent website (relevant clinical guidelines, monthly summaries of pertinent peer‐review manuscripts, downloadable practice tools and patient educational materials) and pushed email cues with educational content E‐learning interactivity: high E‐learning blending: core and essential E‐learning duration: long ‐ 108 weeks Control type: clinical guidelines website and the medical letter subscription Control duration: 108 weeks Follow‐up (time from the end of the intervention to the last outcome assessment): 0 weeks (immediately after) CanMEDS framework area: medical expertise Regulation: formally accredited Setting: primary care | |
| Outcomes | Primary: 7 clinical indicators of performance improvement (5 of health professionals' behaviour, 2 of patient outcomes) Secondary: composite clinical indicator score Times the outcomes were assessed after the intervention: 1 | |
| Notes | Study dates: January 2002‐December 2008 Funding source: Veterans Affairs Health Services Research and Development Grant Declaration of interest: none declared Country: USA Topic: care after myocardial infarction | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Was the allocation sequence adequately generated? | Unclear risk | No information reported |
| Was the allocation adequately concealed? | Low risk | Unit of allocation was by team or professional and allocation performed on all units at the start of the study |
| Were baseline outcome measurements similar? | Low risk | No important differences across study groups |
| Were baseline characteristics similar? | High risk | Several imbalances between group in several participation measures (participants' providers, website visits, etc) |
| Were incomplete outcome data adequately addressed? | High risk | Missing patient data: 24.5% in the e‐learning group and 22.0% in the control group |
| Was knowledge of the allocated interventions adequately prevented during the study? | Unclear risk | No information reported |
| Was the study adequately protected against contamination? | Low risk | Allocation by clinics |
| Was the study free from selective outcome reporting? | Low risk | No evidence of selective reporting of outcomes |
| Was the study free from other risks of bias (e.g. conflicts of interest)? | Low risk | No evidence of other risk of bias |
| OVERALL RISK OF BIAS | High risk | Risk of selection bias: low Risk of attrition bias: high Risk of detection bias: unclear |
| Methods | Study type: randomised trial Study arms: 2 | |
| Participants | Participants type: nurses, physiotherapists, others health professionals Number randomised (e‐learning/control): 67/68 Lost to follow‐up (number(%); (e‐learning/control)): 24(36%)/19(28%) | |
| Interventions | E‐learning type: web‐based discussions available even by phone, DVD comprising the multimedia used in the web‐based programme, self‐directed reading and formative quizzes to interactive skills‐practice sessions with feedback opportunities E‐learning interactivity: high E‐learning blending: core and essential E‐learning duration: short ‐ 7 hours Control type: face‐to‐face intervention; copy of the presentation slides, reference to further readings, and a DVD of the assessment procedures to be covered in the seminar Control duration: 7 hours Follow‐up (time from the end of the intervention to the last outcome assessment): 1 week CanMEDS framework area: medical expertise Regulation: not specified Setting: rehabilitation | |
| Outcomes | Primary: knowledge (by an non‐validated test) Secondary: satisfaction, self‐reported change in practice Times the outcomes were assessed after the intervention: 1 | |
| Notes | Study dates: not reported Funding source: Department of Health, Victoria, Australia Declaration of interest: none declared Country: Australia Topic: falls prevention exercise | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Was the allocation sequence adequately generated? | Low risk | Computerised random number sequence |
| Was the allocation adequately concealed? | Unclear risk | No information reported |
| Were baseline outcome measurements similar? | Unclear risk | No information reported |
| Were baseline characteristics similar? | Low risk | No important differences across study groups |
| Were incomplete outcome data adequately addressed? | High risk | Missing patients data 35.8% in the e‐learning group and 27.9% in the control group |
| Was knowledge of the allocated interventions adequately prevented during the study? | Low risk | Blinded outcome assessment |
| Was the study adequately protected against contamination? | Unclear risk | No information reported |
| Was the study free from selective outcome reporting? | Low risk | No evidence of selective reporting of outcomes |
| Was the study free from other risks of bias (e.g. conflicts of interest)? | Low risk | No evidence of other risk of bias |
| OVERALL RISK OF BIAS | High risk | Risk of selection bias: low Risk of attrition bias: high Risk of detection bias: low |
| Methods | Study type: randomised trial Study arms: 3 | |
| Participants | Participants type: nurses Number randomised (e‐learning/control): 20/16 Lost to follow‐up: not reported | |
| Interventions | E‐learning type: multimedia (video clips and pictures), a short written explanation of the multimedia, links to the databases extending the amount of information if needed and questions between the content pages with correct answers presented E‐learning interactivity: high E‐learning blending: alone E‐learning duration: short ‐ 15‐30 minutes Control type: a certified trainer gave a 4‐h basic life support and defibrillation course Control duration: 240 minutes Follow‐up (time from the end of the intervention to the last outcome assessment): 2 weeks CanMEDS framework area: medical expertise Regulation: not specified Setting: secondary (hospital) care | |
| Outcomes | Primary: skills (OSCE) Secondary: none Times the outcomes were assessed after the intervention: 1 | |
| Notes | Study dates: not reported Funding source: none declared Declaration of interest: none declared Country: Finland Topic: basic life support | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Was the allocation sequence adequately generated? | Unclear risk | No information reported |
| Was the allocation adequately concealed? | Unclear risk | No information reported |
| Were baseline outcome measurements similar? | Unclear risk | No information reported |
| Were baseline characteristics similar? | Unclear risk | No information reported |
| Were incomplete outcome data adequately addressed? | Unclear risk | No information reported |
| Was knowledge of the allocated interventions adequately prevented during the study? | Low risk | Observers blinded to the educational method of the groups |
| Was the study adequately protected against contamination? | Unclear risk | No information reported |
| Was the study free from selective outcome reporting? | Low risk | No evidence of selective reporting of outcomes |
| Was the study free from other risks of bias (e.g. conflicts of interest)? | Low risk | No evidence of other risk of bias |
| OVERALL RISK OF BIAS | Unclear risk | Risk of selection bias: unclear Risk of attrition bias: unclear Risk of detection bias: low |
| Methods | Study type: randomised trial Study arms: 2 | |
| Participants | Participants type: nurses Number randomised (e‐learning/control): 25/24 | |
| Interventions | E‐learning type: e‐learning training by PowerPoint E‐learning interactivity: low E‐learning blending: alone E‐learning duration: short ‐ 40 minutes Control type: on‐site training by PowerPoint Control duration: 120 minutes Follow‐up (time from the end of the intervention to the last outcome assessment): short ‐ 0 weeks (immediately after) CanMEDS framework area: management Regulation: not specified Setting: secondary (hospital) care | |
| Outcomes | Primary: knowledge (by an non‐validated test) Secondary: none Times the outcomes were assessed after the intervention: 1 | |
| Notes | Study dates: not reported Funding source: none declared Declaration of interest: none declared Country: Brazil Topic: quality tools | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Was the allocation sequence adequately generated? | Unclear risk | No information reported |
| Was the allocation adequately concealed? | Unclear risk | No information reported |
| Were baseline outcome measurements similar? | Unclear risk | No information reported |
| Were baseline characteristics similar? | Unclear risk | No information reported |
| Were incomplete outcome data adequately addressed? | Unclear risk | No information reported |
| Was knowledge of the allocated interventions adequately prevented during the study? | Unclear risk | No information reported |
| Was the study adequately protected against contamination? | Unclear risk | No information reported |
| Was the study free from selective outcome reporting? | Low risk | No evidence of selective reporting of outcomes |
| Was the study free from other risks of bias (e.g. conflicts of interest)? | Low risk | No evidence of other risk of bias |
| OVERALL RISK OF BIAS | Unclear risk | Risk of selection bias: unclear Risk of attrition bias: unclear Risk of detection bias: unclear |
| Methods | Study type: randomised trial Study arms: 2 | |
| Participants | Participants type: physicians, nurses, students Number randomised (e‐learning/control): 1843/1889 (1255 vs 1271 without students) | |
| Interventions | E‐learning type: 4 e‐lectures and 6 interactive workshops E‐learning interactivity: high E‐learning blending: core and essential E‐learning duration: 2 days (short) Control type: conventional advanced life support Control duration: 2 days Follow‐up (time from the end of the intervention to the last outcome assessment): 0 weeks (immediately after) CanMEDS framework area: medical expertise Regulation: not specified | |
| Outcomes | Primary: skills Secondary: knowledge (by a validated test) Times the outcomes were assessed after the intervention: 1 | |
| Notes | Study dates: December 2008‐October 2010 Funding source: Resuscitation Council (UK) Declaration of interest: declared on www.apconline.org Country: UK, Australia Topic: advanced life support Other: authors provided unpublished data (Kimani 2015 [pers comm]) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Was the allocation sequence adequately generated? | Low risk | Electronic randomisation |
| Was the allocation adequately concealed? | Low risk | Centralised randomisation scheme |
| Were baseline outcome measurements similar? | Low risk | Knowledge pre‐course test better in e‐learning group. Since the final difference in knowledge is in the opposite direction (favouring traditional learning), there is no indication of a bias. |
| Were baseline characteristics similar? | Low risk | No important differences across study groups |
| Were incomplete outcome data adequately addressed? | Low risk | The proportion of missing data was unlikely to overturn the study results; the study results were analysed on an intention‐to‐treat basis |
| Was knowledge of the allocated interventions adequately prevented during the study? | Low risk | The authors were unable to ensure blinding of outcome assessment. However we judged that the outcome measurement was not likely to be influenced by lack of blinding, as the process of measurement was structured. |
| Was the study adequately protected against contamination? | Unclear risk | No information reported |
| Was the study free from selective outcome reporting? | Low risk | No evidence of selective reporting of outcomes |
| Was the study free from other risks of bias (e.g. conflicts of interest)? | Low risk | No evidence of other risk of bias |
| OVERALL RISK OF BIAS | Low risk | Risk of selection bias: low Risk of attrition bias: low Risk of detection bias: unclear (the blinding of outcome assessors is not explicitly stated) Considering the low risk of bias across most dimensions, we considered the study to be at an overall minimal risk of bias |
| Methods | Study type: randomised trial Study arms: 2 | |
| Participants | Participants type: nurses Number randomised (e‐learning/control): 22/20 Lost to follow‐up: not reported | |
| Interventions | E‐learning type: audio, video and PowerPoint presentation format E‐learning interactivity: low E‐learning blending: alone E‐learning duration: short ‐ 5.5 hours Control type: traditional in class programme Control duration: not reported Follow‐up (time from the end of the intervention to the last outcome assessment): short ‐ 0 weeks, (immediately after) CanMEDS framework area: medical expertise, communication, management, scholar Regulation: not specified Setting: secondary (hospital) care | |
| Outcomes | Primary: knowledge (by an non‐validated test) and skills in several professional dimensions Secondary: satisfaction Times the outcomes were assessed after the intervention: 1 | |
| Notes | Study dates: 2004‐2005 Funding source: Taiwan National Science Council Declaration of interest: none declared Country: Taiwan Topic: nursing care | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Was the allocation sequence adequately generated? | Low risk | Randomisation by coin flip |
| Was the allocation adequately concealed? | Low risk | Randomisation by coin flip |
| Were baseline outcome measurements similar? | Unclear risk | No information reported |
| Were baseline characteristics similar? | Low risk | No important differences across study groups |
| Were incomplete outcome data adequately addressed? | High risk | Participants who did not complete the courses were excluded and not used in data analysis |
| Was knowledge of the allocated interventions adequately prevented during the study? | High risk | Neither participants nor evaluators were blinded |
| Was the study adequately protected against contamination? | Unclear risk | No information reported |
| Was the study free from selective outcome reporting? | High risk | No result provided |
| Was the study free from other risks of bias (e.g. conflicts of interest)? | Low risk | No evidence of other risk of bias |
| OVERALL RISK OF BIAS | High risk | Risk of selection bias: low Risk of attrition bias: high Risk of detection bias:high |
| Methods | Study type: randomised trial Study arms: 2 | |
| Participants | Participants type: nurses Number randomised (e‐learning/control): 92/91 Lost to follow‐up (number(%); (e‐learning/control)): 17(18.5%)/9(9.9%) | |
| Interventions | E‐learning type: interactive online tests, hints and suggested solutions; access to a collection of tests with feedback on answers and a printout of the compendium E‐learning interactivity: high E‐learning blending: alone E‐learning duration: short ‐ 2 days Control type: conventional classroom and self‐study Control duration: 2 days Follow‐up (time from the end of the intervention to the last outcome assessment): 2‐4 weeks CanMEDS framework area: medical expertise Regulation: not specified Setting: secondary (hospital) care | |
| Outcomes | Primary: skills Secondary: certainty Times the outcomes were assessed after the intervention: 1 | |
| Notes | Study dates: September 2007‐April 2009 Funding source: South‐East Norway Health Authorities and Innlandet Hospital Trust Declaration of interest: commercial interest for one the authors Country: Norway Topic: drug dose calculation | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Was the allocation sequence adequately generated? | Low risk | Predefined computer‐generated lists |
| Was the allocation adequately concealed? | Unclear risk | No information reported |
| Were baseline outcome measurements similar? | Low risk | No important differences across study groups |
| Were baseline characteristics similar? | Low risk | No important differences across study groups |
| Were incomplete outcome data adequately addressed? | Low risk | Imbalance in missing data between groups: 18.5% in the e‐learning group and 9.9% in the control group but the proportion of missing data was unlikely to overturn the study results and the study results were analysed on an intention‐to‐treat basis |
| Was knowledge of the allocated interventions adequately prevented during the study? | Unclear risk | No information reported |
| Was the study adequately protected against contamination? | Unclear risk | No information reported |
| Was the study free from selective outcome reporting? | Low risk | No evidence of selective reporting of outcomes |
| Was the study free from other risks of bias (e.g. conflicts of interest)? | Low risk | No evidence of other risk of bias |
| OVERALL RISK OF BIAS | Unclear risk | Risk of selection bias: unclear Risk of attrition bias: low Risk of detection bias: unclear |
| Methods | Study type: randomised trial Study arms: 2 | |
| Participants | Participants type: mixed health professionals Number randomised (e‐learning/control): 25/20 Lost to follow‐up: not reported | |
| Interventions | E‐learning type: online interactive patient care scenarios E‐learning interactivity: low E‐learning blending: alone E‐learning duration: not reported Control type: instructor led training Control duration: not reported Follow‐up (time from the end of the intervention to the last outcome assessment): 0 weeks (immediately after) CanMEDS framework area: medical expertise Regulation: not specified Setting: pre‐hospital care (cardiopulmonary resuscitation) | |
| Outcomes | Primary: skills (3 outcome: correct compressions, correct ventilations, correct CPR cycles) Secondary: none Times the outcomes were assessed after the intervention: 1 | |
| Notes | Study dates: not reported Funding source: not reported Declaration of interest: not reported Country: USA Topic: Basic Life Support | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Was the allocation sequence adequately generated? | Low risk | Cards shuffling |
| Was the allocation adequately concealed? | Unclear risk | Cards shuffling |
| Were baseline outcome measurements similar? | Unclear risk | No information reported |
| Were baseline characteristics similar? | Unclear risk | Unclear differences across study groups |
| Were incomplete outcome data adequately addressed? | Unclear risk | No information reported |
| Was knowledge of the allocated interventions adequately prevented during the study? | Low risk | Outcome is not likely to be influenced by lack of blinding in this study |
| Was the study adequately protected against contamination? | Unclear risk | Contamination is unlikely |
| Was the study free from selective outcome reporting? | High risk | The results of a written exam is not reported |
| Was the study free from other risks of bias (e.g. conflicts of interest)? | Low risk | No evidence of other risk of bias |
| OVERALL RISK OF BIAS | Unclear risk | Risk of selection bias: unclear Risk of attrition bias: unclear Risk of detection bias: low |
CME: continuing medical education; OSCE: objective structured clinical examination.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Not complying with participants inclusion criteria (residents) | |
| Not complying with control inclusion criteria (e‐learning as a control) | |
| Not complying with study type inclusion criteria (no randomisation) | |
| Not complying with study type inclusion criteria (no randomisation) | |
| Not complying with control inclusion criteria (no intervention) | |
| Not complying with intervention inclusion criteria (not delivered by Internet) | |
| Not complying with participants inclusion criteria (residents) | |
| Not complying with participants inclusion criteria (residents) | |
| Not complying with participants inclusion criteria (students) | |
| Not complying with participants inclusion criteria (residents) | |
| Not complying with participants inclusion criteria (trainees) | |
| Not complying with study type inclusion criteria (no randomisation) | |
| Not complying with intervention inclusion criteria (computerised feedback system) | |
| Not complying with participants inclusion criteria (trainees) | |
| Not complying with control inclusion criteria (no intervention) | |
| Not complying with control inclusion criteria (no intervention) | |
| Not complying with control inclusion criteria (no intervention) | |
| Not complying with control inclusion criteria (no intervention) | |
| Not complying with control inclusion criteria (no intervention) | |
| Not complying with study type inclusion criteria (no randomisation) | |
| Not complying with control inclusion criteria (e‐learning as a control) | |
| Not complying with participants inclusion criteria (mixed residents and staff physicians). No answer from the authors to request of separated data (on 5 July 2015) | |
| Not complying with intervention inclusion criteria (e‐learning programmes on bio‐terrorism; focusing on non‐clinical medical topics defined as subjects different from the CanMEDS 7 physicians roles; mixed residents and staff physicians) | |
| Not complying with participants inclusion criteria (residents) | |
| Not complying with intervention inclusion criteria (computerised feedback system) | |
| Not complying with intervention inclusion criteria (e‐learning not core and essential: audit and feedback in the intervention but not in the control arm) | |
| Not complying with outcome inclusion criteria (self‐reported knowledge) | |
| Not complying with control inclusion criteria (e‐learning and usual approach vs usual approach alone) | |
| Not complying with control inclusion criteria (e‐learning as a control) | |
| Not providing data about health professionals randomised to the intervention/control groups. Authors stated their inability to provide us you with the requested information (Esche 2015 [pers comm]) | |
| Not complying with intervention inclusion criteria (e‐learning not core and essential) | |
| Not complying with intervention inclusion criteria (e‐learning not core and essential) | |
| Not complying with control inclusion criteria (no intervention) | |
| Not complying with control inclusion criteria (no intervention) | |
| Not complying with control inclusion criteria (e‐learning as a control) | |
| Not complying with control inclusion criteria (e‐learning as a control) | |
| Not complying with control inclusion criteria (e‐learning in both the arms) | |
| Not complying with study type inclusion criteria (discussion about PULSE trial). No answer from the authors to request of data (on 5 July 2015) | |
| Not complying with control inclusion criteria (no intervention). No answer from the authors to our request of explanation about control intervention (on 12 April 2015) | |
| Not complying with study type inclusion criteria (protocol). No answer from the authors to request of data (on 12 April 2015) | |
| Not complying with control inclusion criteria (no intervention) | |
| Not complying with participants inclusion criteria (trainees) | |
| Not complying with participants inclusion criteria (trainees) | |
| Not complying with study type inclusion criteria (review) | |
| Not complying with participants inclusion criteria (trainees) | |
| Not complying with participants inclusion criteria (residents) | |
| Not complying with study type inclusion criteria (no randomisation) | |
| Not complying with control inclusion criteria (no intervention) | |
| Not complying with participants inclusion criteria (residents) | |
| Not complying with control inclusion criteria (no intervention) | |
| Not complying with control inclusion criteria (no intervention) | |
| Not complying with control inclusion criteria (no intervention) (Kemper 2015 [pers comm]) | |
| Not complying with control inclusion criteria (no intervention) | |
| Not complying with control inclusion criteria (e‐learning as a control) | |
| Not complying with intervention inclusion criteria (the intervention was not distributed by the Internet) | |
| Not complying with control inclusion criteria (no intervention) | |
| Not complying with participants inclusion criteria (mixed residents and staff physicians). No answer from the authors to request of separated data (on 2 July 2015) | |
| Not complying with control inclusion criteria (same intervention as in the e‐learning group) (Kontio 2015 [pers comm]) | |
| Not complying with control inclusion criteria (same intervention as in the e‐learning group) (Kontio 2015 [pers comm]) | |
| Not complying with control inclusion criteria (same intervention as in the e‐learning group) – as in the authors email received on 17 August 2015 | |
| Not complying with control inclusion criteria (no intervention) (Lalonde 2015 [pers comm]) | |
| Not complying with control inclusion criteria (no intervention) (Liaw 2016 [pers comm]) | |
| Not complying with control inclusion criteria (no intervention) | |
| Not complying with control inclusion criteria (no intervention) | |
| Not complying with control inclusion criteria (no intervention) | |
| Not complying with participants inclusion criteria (students) | |
| Not complying with study type inclusion criteria (economic analysis) | |
| Not complying with control inclusion criteria (e‐learning intervention) | |
| Not complying with outcome inclusion criteria (satisfaction) | |
| Not complying with participants inclusion criteria (students) | |
| Not complying with control inclusion criteria (no intervention) | |
| Not complying with participants inclusion criteria (students) | |
| Not complying with control inclusion criteria (no intervention). No answer from the authors to request of data (on 31 May 2015) | |
| Not complying with study type inclusion criteria (protocol). Data still not available (answer from the authors to request of data on 09 January 2018) | |
| Not complying with participants inclusion criteria for participants (trainees) | |
| Not complying with control inclusion criteria (no intervention) | |
| Not complying with control inclusion criteria (no intervention) | |
| Not complying with control inclusion criteria (no intervention) | |
| Not complying with participants inclusion criteria (patients) | |
| Not complying with control inclusion criteria (no intervention) | |
| Not complying with participants inclusion criteria (trainees) | |
| Not complying with participants inclusion criteria (community health workersa) | |
| Not complying with participants inclusion criteria (patients) | |
| Not complying with participants inclusion criteria (patients) | |
| Not complying with study type inclusion criteria (no randomisation) | |
| Not complying with study type inclusion criteria (protocol) and with control inclusion criteria (no intervention) | |
| Not complying with participants inclusion criteria (mixed clinicians and managers). No answer from the authors to request of separated data (on 25 July 2015) | |
| Not complying with control inclusion criteria (no specific training was organised for the control group) (Pelayo‐Alvarez 2015 [pers comm]) | |
| Not complying with intervention inclusion criteria (intervention provided by audio recording) | |
| Not complying with control inclusion criteria (no intervention) | |
| Not complying with control inclusion criteria (no control group) (Pham 2016 [pers comm]) | |
| Not complying with control inclusion criteria (no intervention) | |
| Not complying with study type inclusion criteria (no randomisation) | |
| Not complying with control inclusion criteria (e‐learning group as control group): although the online tutorial was mandatory just for intervention group participants, all but 2 (out of 67) participants in the control group chose to do the tutorial. | |
| Not complying with study type inclusion criteria (protocol). No answer from the authors to request of data (on 12 April 2015) | |
| Not complying with study type inclusion criteria (no randomisation) | |
| Not complying with control inclusion criteria (no intervention as a control in the first part and e‐learning vs e‐learning in the second part) | |
| Not complying with participants inclusion criteria for participants (trainees) | |
| Not complying with outcomes inclusion criteria (self‐reported outcomes) | |
| Not complying with study type inclusion criteria (protocol) and with control inclusion criteria (no intervention) | |
| Not complying with control inclusion criteria (no intervention) | |
| Not complying with participants inclusion criteria (students) | |
| Not complying with outcome inclusion criteria (patient‐reported outcome) | |
| Not complying with control inclusion criteria (no intervention) | |
| Not complying with study type inclusion criteria (no randomisation) | |
| Not complying with participants inclusion criteria (trainees) | |
| Not complying with study type inclusion criteria (no randomisation) | |
| Not complying with intervention inclusion criteria (not delivered by Internet) | |
| Not complying with study type inclusion criteria (protocol) and with control inclusion criteria (no intervention) | |
| Not complying with control inclusion criteria (e‐learning and usual approach vs usual approach alone) | |
| Not complying with participants inclusion criteria (students) | |
| Not complying with control inclusion criteria (no intervention) | |
| Not complying with control inclusion criteria (no intervention) | |
| Not complying with control inclusion criteria (no intervention) | |
| Not complying with study type inclusion criteria (protocol). No answer from the authors to our request of data (on 28 June 2015, email) | |
| Not complying with control inclusion criteria (e‐learning as a control) | |
| Not complying with study type inclusion criteria (no randomisation) | |
| Not complying with control inclusion criteria (no intervention on the same topic) | |
| Not complying with participants inclusion criteria (trainees) | |
| Not complying with control inclusion criteria (no intervention) |
aCommunity health workers (CHW) are members of a community who are chosen by community members or organisations to provide basic health and medical care to their community.
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Not yet assessed |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Patients appropriately screened (Fordis 2005 ‐ screening for dyslipidaemia; Levine 2011 ‐ LDL measurement) Show forest plot | 2 | 6027 | Odds Ratio (M‐H, Random, 95% CI) | 0.90 [0.77, 1.06] |
| Analysis 1.1 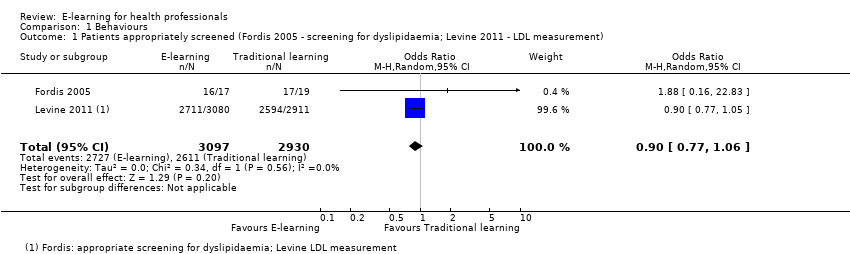 Comparison 1 Behaviours, Outcome 1 Patients appropriately screened (Fordis 2005 ‐ screening for dyslipidaemia; Levine 2011 ‐ LDL measurement). | ||||
| 2 Patients appropriately treated (Fordis 2005 ‐ treatment for dyslipidaemia; Levine 2011 ‐ statin prescription) Show forest plot | 2 | 5491 | Odds Ratio (M‐H, Random, 95% CI) | 1.15 [0.89, 1.48] |
| Analysis 1.2  Comparison 1 Behaviours, Outcome 2 Patients appropriately treated (Fordis 2005 ‐ treatment for dyslipidaemia; Levine 2011 ‐ statin prescription). | ||||
| 3 Patients appropriately screened (Fordis 2005 ‐ screening for dyslipidaemia; Levine 2011 ‐ HbA1c measurement) Show forest plot | 2 | 3056 | Odds Ratio (M‐H, Random, 95% CI) | 0.85 [0.69, 1.06] |
| Analysis 1.3 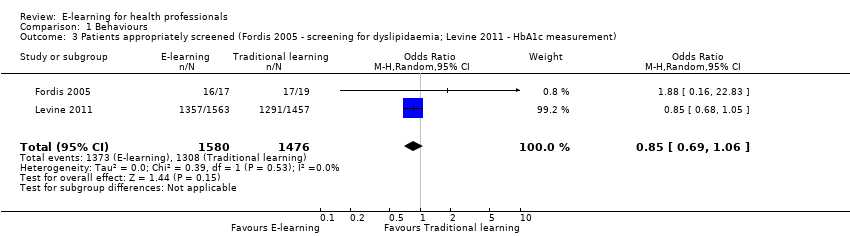 Comparison 1 Behaviours, Outcome 3 Patients appropriately screened (Fordis 2005 ‐ screening for dyslipidaemia; Levine 2011 ‐ HbA1c measurement). | ||||
| 4 Patients appropriately treated (Fordis 2005 ‐ treatment for dyslipidaemia; Levine 2011 ‐ beta‐blocker prescription) Show forest plot | 2 | 6027 | Odds Ratio (M‐H, Random, 95% CI) | 1.12 [0.97, 1.29] |
| Analysis 1.4 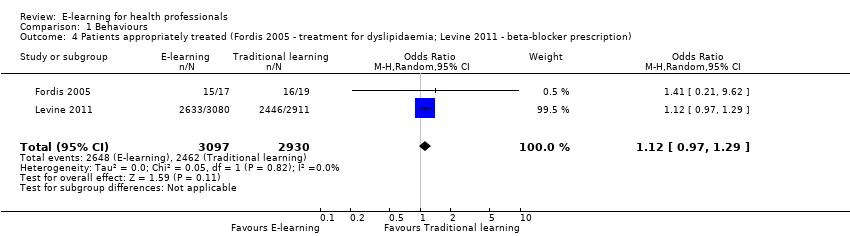 Comparison 1 Behaviours, Outcome 4 Patients appropriately treated (Fordis 2005 ‐ treatment for dyslipidaemia; Levine 2011 ‐ beta‐blocker prescription). | ||||
| 5 Patients appropriately treated (Fordis 2005 ‐ treatment for dyslipidaemia; Levine 2011 ‐ ACEI/ARB prescription) Show forest plot | 2 | 6027 | Odds Ratio (M‐H, Random, 95% CI) | 1.06 [0.94, 1.19] |
| Analysis 1.5  Comparison 1 Behaviours, Outcome 5 Patients appropriately treated (Fordis 2005 ‐ treatment for dyslipidaemia; Levine 2011 ‐ ACEI/ARB prescription). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Drug dose calculation accuracy (Simonsen 2014); ulcer classification accuracy (Bredesen 2016) Show forest plot | 2 | 201 | Std. Mean Difference (Fixed, 95% CI) | 0.03 [‐0.25, 0.31] |
| Analysis 2.1  Comparison 2 Skills, Outcome 1 Drug dose calculation accuracy (Simonsen 2014); ulcer classification accuracy (Bredesen 2016). | ||||
| 2 Cardiac arrest simulation test (CASTest) Show forest plot | 1 | 2562 | Odds Ratio (M‐H, Random, 95% CI) | 1.46 [1.22, 1.76] |
| Analysis 2.2  Comparison 2 Skills, Outcome 2 Cardiac arrest simulation test (CASTest). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 At any time (fixed‐effect) Show forest plot | 8 | 3082 | Std. Mean Difference (Fixed, 95% CI) | 0.04 [‐0.03, 0.11] |
| Analysis 3.1 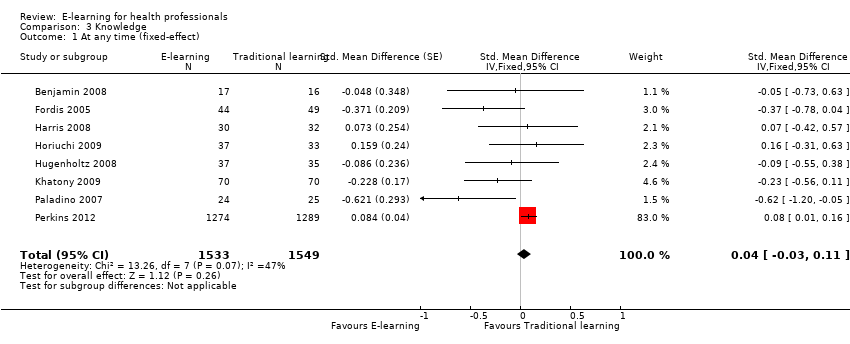 Comparison 3 Knowledge, Outcome 1 At any time (fixed‐effect). | ||||
| 2 At any time (random‐effects) Show forest plot | 8 | 3082 | Std. Mean Difference (Random, 95% CI) | ‐0.09 [‐0.27, 0.09] |
| Analysis 3.2 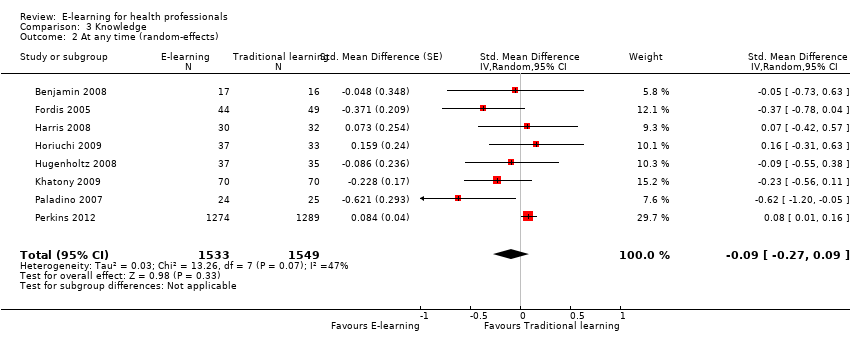 Comparison 3 Knowledge, Outcome 2 At any time (random‐effects). | ||||
| 3 Immediately after the training Show forest plot | 7 | 3012 | Std. Mean Difference (Random, 95% CI) | ‐0.10 [‐0.29, 0.08] |
| Analysis 3.3  Comparison 3 Knowledge, Outcome 3 Immediately after the training. | ||||
| 4 After 3 or more months Show forest plot | 3 | 225 | Std. Mean Difference (Random, 95% CI) | ‐0.07 [‐0.41, 0.27] |
| Analysis 3.4  Comparison 3 Knowledge, Outcome 4 After 3 or more months. | ||||
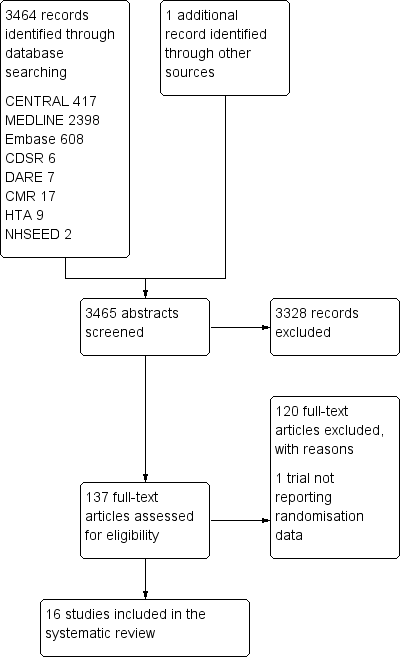
Study flow diagram
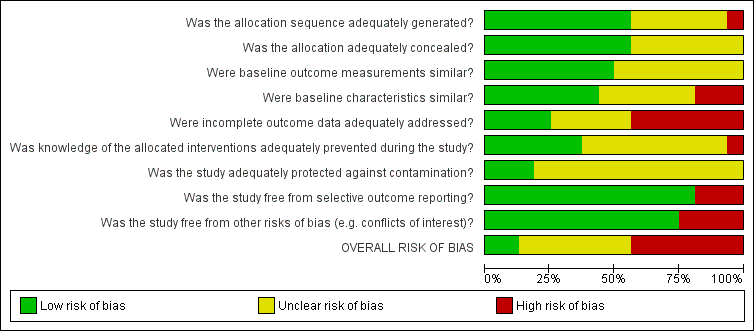
Risk of bias graph: review authors' judgments about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgments about each risk of bias item for each included study.
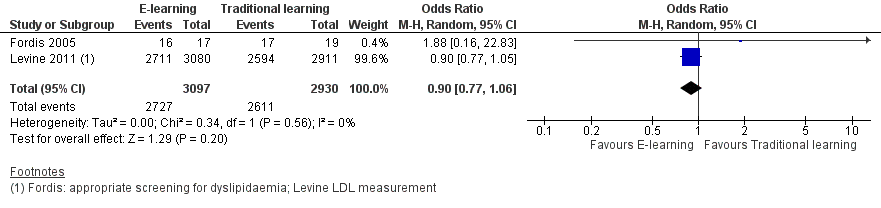
Forest plot of comparison: 1 Behaviours, outcome: 1.1 Patients appropriately screened (Fordis 2005 ‐ screening for dyslipidaemia; Levine 2011 ‐ LDL measurement).

Forest plot of comparison: 1 Behaviours, outcome: 1.2 Patients appropriately treated (Fordis 2005 ‐ treatment for dyslipidaemia; Levine 2011 ‐ statin prescription).
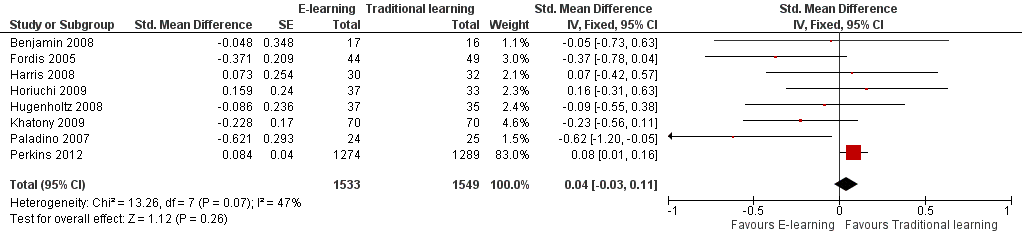
Forest plot of comparison: 3 Knowledge, outcome: 3.1 At any time (fixed‐effect).

Forest plot of comparison: 3 Knowledge, outcome: 3.2 At any time (random‐effects).

Comparison 1 Behaviours, Outcome 1 Patients appropriately screened (Fordis 2005 ‐ screening for dyslipidaemia; Levine 2011 ‐ LDL measurement).

Comparison 1 Behaviours, Outcome 2 Patients appropriately treated (Fordis 2005 ‐ treatment for dyslipidaemia; Levine 2011 ‐ statin prescription).

Comparison 1 Behaviours, Outcome 3 Patients appropriately screened (Fordis 2005 ‐ screening for dyslipidaemia; Levine 2011 ‐ HbA1c measurement).
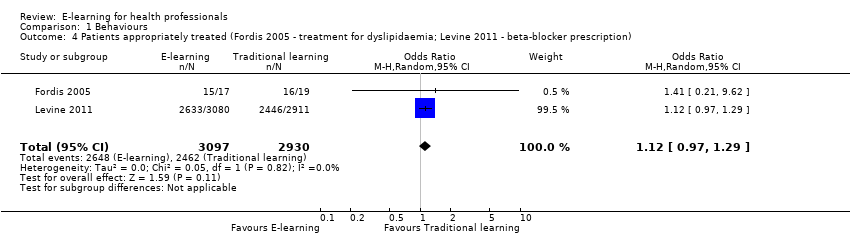
Comparison 1 Behaviours, Outcome 4 Patients appropriately treated (Fordis 2005 ‐ treatment for dyslipidaemia; Levine 2011 ‐ beta‐blocker prescription).

Comparison 1 Behaviours, Outcome 5 Patients appropriately treated (Fordis 2005 ‐ treatment for dyslipidaemia; Levine 2011 ‐ ACEI/ARB prescription).

Comparison 2 Skills, Outcome 1 Drug dose calculation accuracy (Simonsen 2014); ulcer classification accuracy (Bredesen 2016).

Comparison 2 Skills, Outcome 2 Cardiac arrest simulation test (CASTest).
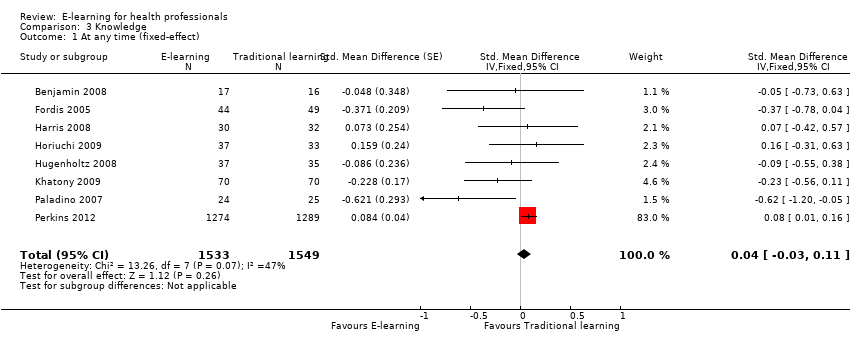
Comparison 3 Knowledge, Outcome 1 At any time (fixed‐effect).

Comparison 3 Knowledge, Outcome 2 At any time (random‐effects).
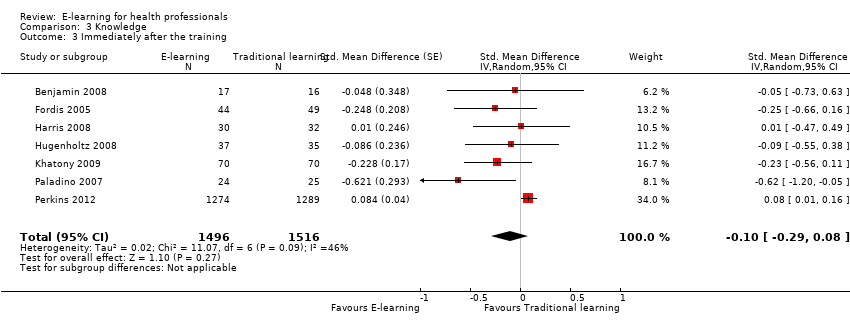
Comparison 3 Knowledge, Outcome 3 Immediately after the training.

Comparison 3 Knowledge, Outcome 4 After 3 or more months.
| E‐learning versus traditional learning for health professionals | ||||
| Patient or population: licensed health professionals (doctors, nurses and allied health professionals fully licensed to practice without supervision) Settings: postgraduate education in any setting Intervention: e‐learning (any intervention in which clinical content is distributed primarily by the Internet, Extranet or Intranet) Comparison: traditional learning (any intervention not distributed through the media mentioned above) | ||||
| Outcomes | Impact* | No of participants | Certainty of the evidence | Comments |
| Patient outcomes Follow‐up: 12 months | E‐learning may make lead to little or no difference between the groups in proportion of patients with LDL cholesterol < 100 mg/dL (adjusted difference 4.0% (95% CI −0.3 to 7.9; 6399 patients) or glycated haemoglobin level < 8% (adjusted difference 4.6%, 95% CI −1.5 to 9.8; 3114 patients) | 168 primary care clinics; 847 health professionals (1 study) | ⊕⊕⊝⊝ | — |
| Health professionals' behaviours Follow‐up: 3‐12 months | E‐learning may make little or no difference between the groups in terms of screening for dyslipidaemia (OR 0.90, 95% CI 0.77 to 1.06, 6027 patients) or treatment for dyslipidaemia (OR 1.15, 95% CI 0.89 to 1.48; 5491 patients) | 950 health professionals (2 studies) | ⊕⊕⊝⊝ | Studies reported multiple outcomes without specifying the primary outcome: to assess consistency, we explored 3 other possible combinations between the 2 study indicators. |
| Health professionals' skills Follow‐up: 0‐12 weeks | We are uncertain whether e‐learning improves or reduces health professionals' skills (SMD 0.03, 95% CI −0.25 to 0.31, I2 = 61%, 201 participants, 12 weeks' follow‐up). | 2912 health professionals (6 studies) | ⊕⊝⊝⊝ Very lowc | The results from the largest trial and 2 more trials, favouring traditional learning (2640 participants), and from one trial favouring e‐learning could not be included in the meta‐analysis. The meta‐analysis included 2 trials studying different professional skills (drug dose calculation and accuracy in pressure ulcers classification). |
| Health professionals' knowledge Any follow‐up: 0‐12 weeks | E‐learning may make little or no difference in health professionals' knowledge: 8 trials provided data to the meta‐analysis (SMD 0.04, 95% CI ‐0.03 to 0.11, I2 = 47%, 3082 participants). | 3236 health professionals (11 studies) | ⊕⊕⊝⊝ | 3 additional studies (154 participants) reported this outcome but no data were available for pooling. |
| CI: confidence interval; LDL: low‐density lipoprotein; OR: odds ratio; SD: standard deviation; SMD: standardised mean difference. *We interpreted SMDs using the following rules suggested by Higgins 2011a: < 0.40 represents a small effect size; 0.40 to 0.70, a moderate effect size; and > 0.70, a large effect size. | ||||
| GRADE Working Group grades of evidence: | ||||
| aDowngraded for study limitations (risk of bias and imprecision) and imprecision surrounding surrogate outcomes. Important benefits cannot be ruled out. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Patients appropriately screened (Fordis 2005 ‐ screening for dyslipidaemia; Levine 2011 ‐ LDL measurement) Show forest plot | 2 | 6027 | Odds Ratio (M‐H, Random, 95% CI) | 0.90 [0.77, 1.06] |
| 2 Patients appropriately treated (Fordis 2005 ‐ treatment for dyslipidaemia; Levine 2011 ‐ statin prescription) Show forest plot | 2 | 5491 | Odds Ratio (M‐H, Random, 95% CI) | 1.15 [0.89, 1.48] |
| 3 Patients appropriately screened (Fordis 2005 ‐ screening for dyslipidaemia; Levine 2011 ‐ HbA1c measurement) Show forest plot | 2 | 3056 | Odds Ratio (M‐H, Random, 95% CI) | 0.85 [0.69, 1.06] |
| 4 Patients appropriately treated (Fordis 2005 ‐ treatment for dyslipidaemia; Levine 2011 ‐ beta‐blocker prescription) Show forest plot | 2 | 6027 | Odds Ratio (M‐H, Random, 95% CI) | 1.12 [0.97, 1.29] |
| 5 Patients appropriately treated (Fordis 2005 ‐ treatment for dyslipidaemia; Levine 2011 ‐ ACEI/ARB prescription) Show forest plot | 2 | 6027 | Odds Ratio (M‐H, Random, 95% CI) | 1.06 [0.94, 1.19] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Drug dose calculation accuracy (Simonsen 2014); ulcer classification accuracy (Bredesen 2016) Show forest plot | 2 | 201 | Std. Mean Difference (Fixed, 95% CI) | 0.03 [‐0.25, 0.31] |
| 2 Cardiac arrest simulation test (CASTest) Show forest plot | 1 | 2562 | Odds Ratio (M‐H, Random, 95% CI) | 1.46 [1.22, 1.76] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 At any time (fixed‐effect) Show forest plot | 8 | 3082 | Std. Mean Difference (Fixed, 95% CI) | 0.04 [‐0.03, 0.11] |
| 2 At any time (random‐effects) Show forest plot | 8 | 3082 | Std. Mean Difference (Random, 95% CI) | ‐0.09 [‐0.27, 0.09] |
| 3 Immediately after the training Show forest plot | 7 | 3012 | Std. Mean Difference (Random, 95% CI) | ‐0.10 [‐0.29, 0.08] |
| 4 After 3 or more months Show forest plot | 3 | 225 | Std. Mean Difference (Random, 95% CI) | ‐0.07 [‐0.41, 0.27] |

