Intervenciones para las infecciones necrosantes de partes blandas en adultos
Información
- DOI:
- https://doi.org/10.1002/14651858.CD011680.pub2Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 31 mayo 2018see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Piel
- Copyright:
-
- Copyright © 2018 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
CH was the contact person with the editorial base.
CH and LLC co‐ordinated contributions from the co‐authors and wrote the final draft of the review.
CH, RB, and LLC screened papers against eligibility criteria.
CH obtained data on ongoing and unpublished studies.
CH, RB, and LLC appraised the quality of papers.
CH and RB extracted data for the review and sought additional information about papers.
CH entered data into RevMan.
CH, RB, ES, and LLC analysed and interpreted data.
LLC, ES, and CH worked on the methods sections.
CH, ES, LLC, RB, NDP, PJ, and OC drafted the clinical sections of the background and responded to the clinical comments of the referees.
LLC and ES responded to the methodology and statistics comments of the referees.
CH was the consumer co‐author and checked the review for readability and clarity, as well as ensuring outcomes are relevant to consumers.
CH is the guarantor of the update.
Disclaimer
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to the Cochrane Skin Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Sources of support
Internal sources
-
Hôpital Henri Mondor, France.
Salary support
External sources
-
aRED (Association of Dermatology Recommendations) commission of the French Society of Dermatology, France.
-
The National Institute for Health Research (NIHR), UK.
The NIHR, UK, is the largest single funder of the Cochrane Skin Group.
Declarations of interest
Camille Hua: nothing to declare.
Romain Bosc: nothing to declare.
Emilie Sbidian: nothing to declare.
Nicolas De Prost: nothing to declare.
Patricia Jabre: nothing to declare.
Olivier Chosidow: "I have received consulting fees from Cardiome Pharma Corporation for a board meeting on dalbavancin; my institution (Hôpital Henri Mondor, Department of Microbiology) has received a research grant from the French Society of Dermatology for a metagenomic study in NSTIs (masters degree)."
Laurence Le Cleach: nothing to declare.
Carolyn Hughes: nothing to declare
Acknowledgements
We thank the Information Specialist, Elizabeth Doney, for her help during the preparation of this review.
We acknowledge Judith Mathore for her help identifying the types of outcomes that we should consider for this review.
Cochrane Skin wishes to thank Urbà González, who was the Cochrane Dermatology Editor for this review; Ben Carter, who was the Statistical Editor; Esther van Zuuren, who was the Methods Editor; the clinical referees, Dennis L Stevens and Lucy Lamb; the consumer referee, Peter Smart; and the review's copy‐editor, Heather Maxwell.
Version history
| Published | Title | Stage | Authors | Version |
| 2018 May 31 | Interventions for necrotizing soft tissue infections in adults | Review | Camille Hua, Romain Bosc, Emilie Sbidian, Nicolas De Prost, Carolyn Hughes, Patricia Jabre, Olivier Chosidow, Laurence Le Cleach | |
| 2015 Apr 29 | Interventions for necrotizing soft tissue infections in adults | Protocol | Camille Hua, Romain Bosc, Emilie Sbidian, Nicolas De Prost, Patricia Jabre, Olivier Chosidow, Laurence Le Cleach | |
Differences between protocol and review
Methods > Data Collection and analysis: we added the initials of the people who did the GRADE assessments (CH, LLC) and also mention how disagreements were resolved (ES).
Objectives: we expanded the objectives for clarity.
Methods >Types of participants: we clarified that we included all adults aged 18 years and over hospitalised with a diagnosis of NSTI. This was cited in the protocol but only under Types of interventions, notTypes of participants.
Methods >Type of interventions >Surgical treatments: We revised surgical treatments as type of surgery (surgical debridement, amputation). We re‐phrased "Surgical delay less than 24 hours versus surgical delay longer than 24 hours" to "immediate versus delayed intervention".
Methods >Type of interventions >Medical treatments: we clarified our comparators.
Methods > Types of studies: we added in the Methods section that cross‐over designs were excluded because trials assessed an acute life‐threatening disease (which cannot be appropriately assessed in a cross‐over study).
Methods > Types of outcomes > Secondary outcomes: we expanded the description and assessment of 'Assessment of long‐term morbidity'. However, no study in this review measured this outcome in the way we required.
Methods >Data collection and analysis: we included all outcomes in our 'Summary of findings' tables to summarise all key information, although we only planned to include our primary outcomes.
Methods >Data extraction and management: we changed the third review author who resolved any disagreements between the two other review authors to LLC because ES was not available at the time.
Methods >Assessment of risk of bias in included studies: we changed the third review author who resolved any disagreements between the two other review authors to LLC because ES was not available at the time. We added performance bias and re‐worded the domains so they reflected the Cochrane 'Risk of bias' tool. We added the following sentence for clarification: "Studies were classified as having low risk of bias if none of the domains above were rated as high risk of bias and three or less were rated as unclear risk; moderate if one was rated as high risk of bias or none were rated as high risk of bias but four or more were rated as unclear risk, and all other cases will be assumed to pertain to high risk of bias".
Methods >Dealing with missing data: we added this sentence, "we planned to synthesise data as analysed in each study (complete cases)", but could not undertake this due to lack of studies in the review.
Methods >Assessment of reporting biases: we did not perform sensitivity analyses according to a regression‐based adjustment model because no meta‐analysis was possible since the interventions compared in the trials were not similar.
Methods >Measures of treatment effects: for survival time evaluation, we planned to use hazard ratios (HR) with 95% CI; however, the required data on survival time were not available in the three included studies.
Methods >Units of analysis issues: we planned to include cluster‐randomised trials in the meta‐analyses using the methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011); however, no clustered‐randomised trials were included in this review.
Methods >Unit of analysis issue: in cases of multi‐dose trials, we grouped together all the different dose groups as a single arm and compared them collectively with the control group.
Methods >Assessment of heterogeneity: we did not assess heterogeneity because the three included trials in this review compared different interventions. If a future update includes any new studies, heterogeneity can be assessed through forest plots and I² statistics as planned in the protocol.
Methods >Assessment of reporting bias: we planned to assess reporting bias for primary end points using funnel plots. However, the validity conditions (low heterogeneity, 10 or more studies including at least one with significant results, and a ratio of the maximal to minimal variance across studies greater than 4) for funnel plot asymmetry tests provided were not met (Ioannidis 2007). We planned to perform sensitivity analyses according to a regression‐based adjustment model; however, only three studies were included in this review, so we could not undertake any sensitivity analyses.
Methods >Data synthesis: we did not perform data synthesis because the three included trials in this review compared different interventions. Thus, we deleted all reference to pooling using a fixed‐effect model. If this review is updated, we will use the random‐effects model to pool studies in a meta‐analysis.
Methods >Subgroup analysis and investigation of heterogeneity: the planned subgroup analyses were not performed because of the low number of studies included in the review.
Methods >Sensitivity analysis: the number of included studies was insufficient to perform sensitivity analyses.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
- Amoxicillin‐Potassium Clavulanate Combination;
- Anti‐Bacterial Agents [therapeutic use];
- CD28 Antigens [therapeutic use];
- Critical Care;
- Debridement;
- Fluoroquinolones [therapeutic use];
- Immunoglobulins, Intravenous [therapeutic use];
- Moxifloxacin;
- Randomized Controlled Trials as Topic;
- Soft Tissue Infections [complications, *therapy];
Medical Subject Headings Check Words
Adult; Female; Humans; Male; Middle Aged;
PICO

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
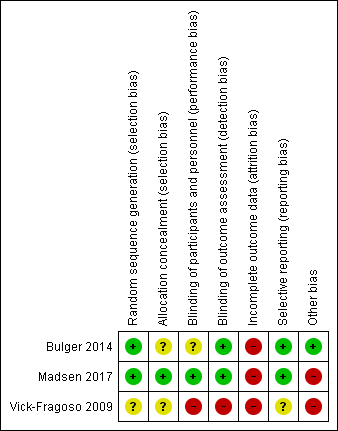
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Moxifloxacin versus amoxicillin‐clavulanate, Outcome 1 Mortality within 30 days.

Comparison 1 Moxifloxacin versus amoxicillin‐clavulanate, Outcome 2 Proportion of patients who experienced serious adverse events.
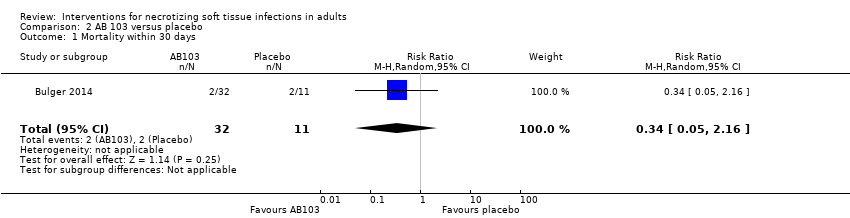
Comparison 2 AB 103 versus placebo, Outcome 1 Mortality within 30 days.
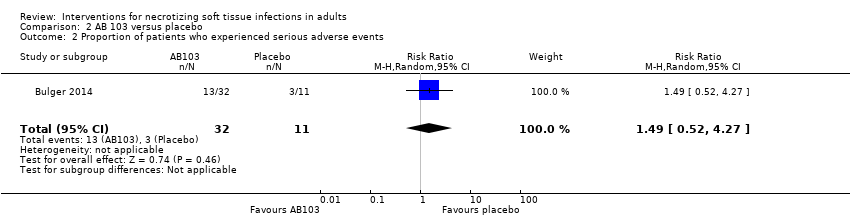
Comparison 2 AB 103 versus placebo, Outcome 2 Proportion of patients who experienced serious adverse events.

Comparison 3 IGIV versus placebo, Outcome 1 Mortality within 30 days.

Comparison 3 IGIV versus placebo, Outcome 2 Proportion of patients who experienced serious adverse events.
| Moxifloxacin compared to amoxicillin‐clavulanate for NSTI | ||||||
| Patient or population: NSTI | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality/certainty of the evidence | Comments | |
| Risk with Amoxicillin‐clavulanate | Risk with Moxifloxacin | |||||
| Mortality | Study population | RR 3.00 | 54 | ⊕⊝⊝⊝ | Data from a larger trial including several types of soft tissue infections; total number of included patients N = 804 | |
| 6 per 100 | 17 per 100 | |||||
| Serious adverse events (SAE) | Study population | RR 0.63 | 54 | ⊕⊝⊝⊝ | Description of nature of serious adverse events was not available | |
| 44 per 100 | 28 per 100 | |||||
| Survival time | — | — | — | 54 | ⊕⊝⊝⊝ | The median time of death after start of antibiotic treatment was shorter in the moxifloxacin group than in the amoxicillin‐clavulanate group (10.5 days versus 42 days) (not possible to calculate hazard ratio with the data provided) |
| Assessment of long‐term morbidity | — | — | — | — | — | Not reported |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). Assumed risk for mortality was based on data of the literature (Audureau 2017; May 2009). For serious adverse effects it was based on the results of the trial. | ||||||
| GRADE Working Group grades of evidence | ||||||
| aDowngraded by five levels to very low certainty of evidence. We downgraded two levels because of high risk of bias regarding blinding (open label trial) and high risk for attrition bias because of a high rate of withdrawal (20%). We downgraded one level for serious imprecision because of small sample size (and CI of RR included 1, where reported). We downgraded a further two levels because no clear criteria for clinical diagnosis of necrotizing fasciitis were provided and because antibiotic used as comparator is not relevant (indirectness) | ||||||
| AB103 compared to Placebo for NSTI | ||||||
| Patient or population: NSTI | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality/certainty of the evidence | Comments | |
| Risk with Placebo | Risk with AB103 | |||||
| Mortality | Study population | RR 0.34 | 43 | ⊕⊝⊝⊝ | — | |
| 23 per 100* | 6 per 100 | |||||
| Serious adverse events (SAE) | Study population | RR 1.49 | 43 | ⊕⊝⊝⊝ | There were no data about the nature of serious adverse events reported | |
| 27 per 100 | 41 per 100 | |||||
| Survival time | — | — | — | — | — | Not reported |
| Assessment of long‐term morbidity | — | — | — | — | — | Not reported |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). Assumed risk for mortality was based on data of the literature (Audureau 2017; May 2009). For serious adverse effects it was based on the results of the trial. | ||||||
| GRADE Working Group grades of evidence | ||||||
| aDowngraded by three levels: one level for high risk of attrition bias, one level for no clear clinical definition of criteria for necrotizing fasciitis diagnosis at inclusion (indirectness), and one level for serious imprecision because of small sample size and CI included no difference | ||||||
| Intravenous immunoglobulin compared to placebo for NSTI | ||||||
| Patient or population: NSTI | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality/Certainty of the evidence | Comments | |
| Risk with placebo | Risk with Intravenous immunoglobulin | |||||
| Mortality | Study population | RR 1.17 | 100 | ⊕⊕⊝⊝ | — | |
| 0 per 100 | 0 per 100 | |||||
| Moderate | ||||||
| 23 per* 100 | 21 per 100 | |||||
| Serious adverse events (SAE) | Study population | RR 0.73 | 100 | ⊕⊕⊝⊝ | Serious adverse reactions included acute kidney injury, allergic reactions, aseptic meningitis syndrome, haemolytic anaemia, thrombi, and transmissible agents | |
| 22 per 100 | 16 per 100 | |||||
| Survival time | — | — | — | 100 | ⊕⊕⊝⊝ | The median time of death was shorter in the IVIG group than in the placebo group (25 days versus 49 days) (not possible to calculate hazard ratio with the data provided) |
| Assessment of long‐term morbidity | — | — | — | — | — | Not reported |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). Assumed risk for mortality was based on data of the literature (Audureau 2017; May 2009). For serious adverse effects it was based on the results of the trial. | ||||||
| GRADE Working Group grades of evidence | ||||||
| aDowngraded by two levels: one level for high risk of attrition bias (38% lost of follow‐up); other bias: imbalance at baseline for one dose 25 IVIG received before randomisation (40% in placebo group vs 16% IVIG group). One level for indirectness as a minority of patients have an infection linked to bacteria producing toxins | ||||||
| Term used | Explanation |
| Adjuvant treatment | Treatment that is given in addition to the primary or initial therapy to improve its effectiveness |
| Empiric antimicrobial therapy | Antimicrobial therapy given before the specific bacteria causing an infection is known |
| Aseptic meningitis | Serious inflammation of the linings of the brain not caused by pyogenic bacteria |
| Empiric antibiotic therapy | Antibiotics that acts against a wide range of bacteria |
| Bullae | Blisters on the skin usually more than 5 mm in diameters |
| Cirrhosis | Advanced liver disease |
| Crepitus | Clinical signs characterised by a peculiar sound under the skin |
| Debridement | Surgery excision of necrotic tissues (medical removal of dead, damaged, or infected tissue) |
| Endotoxin | A toxin contained in bacteria that is released only when the bacteria are broken down |
| Exotoxin | A toxin that is secreted by bacteria into the surrounding medium |
| Fascia | A fibrous connective tissue that surrounds muscle and other soft tissue. Fasciae are classified according to their distinct layers and their anatomical location: superficial fascia and deep (muscle) fascia |
| Fulminant inflammatory response | Systemic inflammatory response |
| Gram‐negative bacteria | Class of bacteria gram‐negative staining |
| Haemolytic anaemia | Decrease in the total amount of red blood cells due to the abnormal breakdown of red blood cells |
| Hyperbaric oxygen therapy | Medical use of oxygen at a level higher than atmospheric pressure. This helps fight bacteria and infection |
| Hypoxia | Insufficient levels of oxygen in blood or tissue |
| Intravenous immunoglobulin (IVIG) | Administration of antibodies through the veins |
| Motricity | Strength in upper and lower extremities after disease |
| Morbidity | Disability or degree that the health condition affects the patient |
| Mortality | Death rate |
| MRSA | Methicillin‐resistant Staphylococcus aureus |
| Myonecrosis | The destruction or death of muscle tissue |
| Necrosis | Death of body tissue |
| Obliterating endarteritis | Severe proliferating endarteritis (inflammation of the inner lining of an artery) that results in an occlusion of the lumen (the space inside a tubular structure) of the smaller vessels |
| Person‐years | Unit of measurement used to estimate rate of a disease during a defined period of observation |
| Polymicrobial | Polymicrobial infection is caused by several species of micro‐organisms |
| Subcutaneous tissue | Layer of tissue below the epidermis and the dermis of the skin. It is also called the hypodermis |
| Synergistic combination | Additive effects of bacterial agents |
| Synergistic gangrenes | Necrotizing soft tissue infection caused by a mix of bacteria (usually a mix of anaerobic and aerobic micro‐organisms) |
| Systemic | Affecting the entire body |
| Third‐generation quinolones | The quinolones are a family of synthetic broad‐spectrum antibiotic drugs |
| Thrombi | A blood clot inside a blood vessel |
| Transmissible agents | Infectious pathogens that can be transmitted |
| Vasopressors | Any medication that induces vasoconstriction of blood vessels to raise reduced blood pressure |
| Vimentin | A protein, the expression of which is increased after skeletal muscle injury |
| Incidence of necrotizing fasciitis | ||||
| Authors | Period of study | Country | Pathology | Incidence |
| Kaul R et al (Kaul 1997) | 1991 | Canada | GAS NF | 0.085 per 100,000 p‐y |
| 1995 | 0.4 per 100,000 p‐y | |||
| Ellis Simonsen et al (Ellis Simonsen 2006) | January 1997 to December 2002 | United States | NF | 0.04 per 1000 p‐y |
| O'Grady et al (O'Grady 2007) | March 2002 to August 2004 | Australia | IGAS | 2.7 per 100,000 p‐y (10.9% of NF) |
| Lamagni et al (Lamagni 2008) | January 2003 to December 2004 | Europe | IGAS | 2.37 per 100,000 p‐y (8% of NF) |
| Lepoutre et al (Lepoutre 2011) | November 2006 to November 2007 | France | IGAS | 3.1 per 100,000 p‐y (18% of NF) |
| GAS NF:group A streptococcal necrotizing fasciitis; IGAS: invasive group A streptococcal disease; NF: necrotizing fasciitis; p‐y: person‐years | ||||
| Study | Contact | Requested information | Contacted | Reply (last check 23 April 2017) |
| Darenberg 2003 (awaiting classification study) | Dr Norrby‐Teglund | Outcomes in the specific subgroup of patients with NSTI: ‐Mortality at day 30, ‐Proportion of patients with serious adverse events ‐Survival time ‐Patients with alteration of 25% of Functional Impairment Scale (%) | July 28, 2015 September 07, 2015 | No response |
| Tally 1986 (awaiting classification study) | Dr Kellum | Outcomes in the specific subgroup of patients with NSTI: ‐Mortality at day 30, ‐Proportion of patients with serious adverse events ‐Survival time ‐Patients with alteration of 25% of Functional Impairment Scale (%) | July 24, 2015 September 07, 2015 | No response |
| Vick‐Fragoso 2009 (included study) | Dr Bogner, Dr Petri | Outcomes in the specific subgroup of patients with NSTI: ‐Mortality at day 30, ‐Proportion of patients with serious adverse events ‐Survival time ‐Patients with alteration of 25% of Functional Impairment Scale (%) | September 07, 2015 | Additional data to the publication provided for mortality, proportion of patients with serious adverse events and survival time. Outcome data for assessment of long term morbidity not provide |
| Bulger 2014 (included study) | Dr Bulger | Outcomes: ‐Survival time ‐Patients with alteration of 25% of Functional Impairment Scale (%) | September 07, 2015 September 09, 2015 | Outcome data not provided |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality within 30 days Show forest plot | 1 | 54 | Risk Ratio (M‐H, Random, 95% CI) | 3.0 [0.39, 23.07] |
| 2 Proportion of patients who experienced serious adverse events Show forest plot | 1 | 54 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.30, 1.31] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality within 30 days Show forest plot | 1 | 43 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.05, 2.16] |
| 2 Proportion of patients who experienced serious adverse events Show forest plot | 1 | 43 | Risk Ratio (M‐H, Random, 95% CI) | 1.49 [0.52, 4.27] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality within 30 days Show forest plot | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [0.42, 3.23] |
| 2 Proportion of patients who experienced serious adverse events Show forest plot | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.32, 1.65] |


