Intervenciones farmacológicas para la colangitis biliar primaria
Referencias
Referencias de los estudios incluidos en esta revisión
Referencias de los estudios excluidos de esta revisión
Referencias de los estudios en espera de evaluación
Referencias de los estudios en curso
Referencias adicionales
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Randomised clinical trial. | |
| Participants | Country: Italy. Number randomised: 90. Post‐randomisation dropouts: 6 (6.7%). Revised sample size: 84. Mean age: 54 years. Females: 81 (96.4%). Symptomatic participants: 84 (100%). AMA positive: not stated. Responders: not stated. Mean follow‐up period (for all groups): not stated. Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to 2 groups. Group 1: UDCA (low) + colchicine (n = 42). Further details: UDCA: 250 mg BD for 3 years + colchicine: 1 mg/day for 3 years. Group 2: UDCA (low) (n = 42). Further details: UDCA: 250 mg BD for 3 years. | |
| Outcomes | Mortality, decompensated liver disease. | |
| Notes | Reasons for post‐randomisation dropouts: adverse effects and low compliance. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Colchicine, 1 mg daily, or an indistinguishable placebo were randomly assigned to patients according to a computer‐generated list developed separately for each centre". |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomization was performed by a central study unit…". |
| Blinding of participants and personnel (performance bias) | Low risk | Comment: identical placebo used and authors stated double‐blind. |
| Blinding of outcome assessment (detection bias) | Low risk | Comment: identical placebo used and authors stated double‐blind. |
| Incomplete outcome data (attrition bias) | High risk | Comment: there were post‐randomisation dropouts. |
| Selective reporting (reporting bias) | High risk | Comment: adverse events not reported. |
| For‐profit bias | Low risk | Comment: no money received for the trial; the drug was provided by Abc Farmaceutici S.p.a (author's reply). |
| Other bias | Low risk | Comment: no other bias noted. |
| Methods | Randomised clinical trial. | |
| Participants | Country: USA. Number randomised: 155. Post‐randomisation dropouts: not stated. Revised sample size: 155. Mean age: 53 years. Females: 130 (83.9%). Symptomatic participants: not stated. AMA positive: not stated. Responders: not stated. Mean follow‐up period (for all groups): all participants followed up for 12 months. Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to 3 groups. Group 1: UDCA (low) (n = 52). Further details: UDCA: 5 mg/kg/day to 7 mg/kg/day; duration: 1 to 2 years. Group 2: UDCA (moderate) (n = 49). Further details: UDCA: 13 mg/kg/day to 15 mg/kg/day; duration: 1 to 2 years. Group 3: UDCA (high) (n = 54). Further details: UDCA: 23 mg/kg/day to 25 mg/kg/day; duration: 1 to 2 years. | |
| Outcomes | Mortality, adverse events, liver transplantation. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was carried out separately for each of the eight strata with a computer‐generated, blocked, randomized drug assignment schedule". |
| Allocation concealment (selection bias) | Low risk | Quote: "Patients were randomized by a statistician (D.W.M.), and the drug was provided by a pharmacist who was not involved in the patient's clinical evaluation or follow‐up". |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "The patients, physicians, nurses, and study coordinator were unaware throughout the study which dose was being administered. To assure blindness patients received the same number of tablets by mixing UDCA‐tablets with placebo‐tablets in a ratio defined by their assigned dose; therefore, the number of tablets taken per day according to the body weight was exactly the same regardless of the dose assigned". |
| Blinding of outcome assessment (detection bias) | Low risk | Comment: identical placebo used and authors stated double‐blind. |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: unclear whether all participants randomised were included in the analysis. |
| Selective reporting (reporting bias) | Low risk | Comment: mortality and morbidity reported. |
| For‐profit bias | Unclear risk | Comment: information not available. |
| Other bias | Low risk | Comment: no other risk of bias. |
| Methods | Randomised clinical trial. | |
| Participants | Country: USA. Number randomised: 9. Post‐randomisation dropouts: not stated. Revised sample size: 9. Mean age: not stated. Females: not stated. Symptomatic participants: 9 (100%). AMA positive: not stated. Responders: not stated. Mean follow‐up period (for all groups): all participants followed up for 5 months. Inclusion criteria
| |
| Interventions | Participants were randomly assigned to 2 groups. Group 1: UDCA (low) (n = 5). Further details: UDCA: 10 mg/kg/day for 5 months. Group 2: placebo (n = 4). | |
| Outcomes | None of the outcomes of interest reported. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: information not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: information not available. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: placebo used and authors stated double blind; however, unclear whether identical placebo used. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: placebo used and authors stated double blind; however, unclear whether identical placebo used. |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: information not available. |
| Selective reporting (reporting bias) | High risk | Comment: neither mortality nor adverse events reported. |
| For‐profit bias | Unclear risk | Comment: information not available. |
| Other bias | Low risk | Comment: no other risk of bias. |
| Methods | Randomised clinical trial. | |
| Participants | Country: USA. Number randomised: 28. Post‐randomisation dropouts: 0 (0%). Revised sample size: 28. Mean age: 54 years. Females: 26 (92.9%). Symptomatic participants: not stated. AMA positive: 27 (96.4%). Responders: not stated. Mean follow‐up period (for all groups): not stated. Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to 2 groups. Group 1: tetrathiomolybdate (n = 13). Further details: tetrathiomolybdate: 10 mg/day to 120 mg/day based on serum ceruloplasmin levels; duration: not stated. Group 2: placebo (n = 15). | |
| Outcomes | None of the outcomes of interest reported. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The patients were assigned to the placebo arm or the tetrathiomolybdate arm using a table of random numbers". |
| Allocation concealment (selection bias) | Low risk | Quote: "Central allocation by pharmacy" (author's reply). |
| Blinding of participants and personnel (performance bias) | Low risk | Comment: identical placebo used and authors stated double‐blind. |
| Blinding of outcome assessment (detection bias) | Low risk | Comment: identical placebo used and authors stated double‐blind. |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "There were not post‐randomisation drop‐outs" (author's reply). |
| Selective reporting (reporting bias) | High risk | Comment: neither mortality nor adverse events reported. |
| For‐profit bias | Low risk | Quote: "Supported by Grant FD‐02590‐02 from the U.S. Food and Drug Administration's Orphan Products Office, the General Clinical Research Center of the University of Michigan Hospitals, Grant MO1‐ RR000042 from the National Institutes of Health, and Grant Ul1‐ RR024986 Clinical and Translational Science Awards". |
| Other bias | High risk | Comment: unclear whether the participants continued to take UDCA in both groups. |
| Methods | Randomised clinical trial. | |
| Participants | Country: Italy. Number randomised: 88. Post‐randomisation dropouts: 2 (2.3%). Revised sample size: 86. Mean age: 55 years. Females: 78 (90.7%). Symptomatic participants: 86 (100%). AMA positive: 77 (89.5%). Responders: not stated. Mean follow‐up period (for all groups): minimum 6 months. Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to 2 groups. Group 1: UDCA (low) (n = 42). Further details: UDCA: 250 mg BD for 6 months. Group 2: placebo (n = 44). | |
| Outcomes | None of the outcomes of interest reported. | |
| Notes | Reasons for post‐randomisation dropouts: lost to follow‐up. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization of treatment assignments was performed separately for each centre: patients were consecutively given indistinguishable medications, which had been assigned by the central pharmacy according to a computer‐ generated list. UDCA and an identical‐appearing placebo were obtained through the courtesy of ABC Farmaceutici, Torino, Italy". |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomization of treatment assignments was performed separately for each centre: patients were consecutively given indistinguishable medications, which had been assigned by the central pharmacy according to a computer‐ generated list. UDCA and an identical‐appearing placebo were obtained through the courtesy of ABC Farmaceutici, Torino, Italy". |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "Randomization of treatment assignments was performed separately for each centre: patients were consecutively given indistinguishable medications, which had been assigned by the central pharmacy according to a computer‐ generated list. UDCA and an identical‐appearing placebo were obtained through the courtesy of ABC Farmaceutici, Torino, Italy". |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "Randomization of treatment assignments was performed separately for each centre: patients were consecutively given indistinguishable medications, which had been assigned by the central pharmacy according to a computer‐ generated list. UDCA and an identical‐appearing placebo were obtained through the courtesy of ABC Farmaceutici, Torino, Italy". |
| Incomplete outcome data (attrition bias) | High risk | Comment: there were post‐randomisation dropouts. |
| Selective reporting (reporting bias) | High risk | Comment: neither mortality nor adverse events reported. |
| For‐profit bias | Unclear risk | Comment: information not available. |
| Other bias | Low risk | Comment: no other risk of bias. |
| Methods | Randomised clinical trial. | |
| Participants | Country: Mexico. Number randomised: 40. Post‐randomisation dropouts: not stated. Revised sample size: 40. Mean age: not stated. Females: not stated. Symptomatic participants: not stated. AMA positive: not stated. Responders: not stated. Mean follow‐up period (for all groups): all participants followed up for 12 months. Inclusion criteria
| |
| Interventions | Participants were randomly assigned to 2 groups. Group 1: UDCA (moderate) + colchicine (n = 21). Further details: UDCA: 13 mg/kg/day to 15 mg/kg/day for 1 year + colchicine: 1 mg/day for 5 days in a week for 1 year. Group 2: placebo (n = 19). | |
| Outcomes | None of the outcomes of interest reported. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: information not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: information not available. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: although placebo used in double‐blind trial, unclear whether the placebo was identical. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: although placebo used in double‐blind trial, unclear whether the placebo was identical. |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: information not available. |
| Selective reporting (reporting bias) | High risk | Comment: neither mortality nor adverse events reported. |
| For‐profit bias | Unclear risk | Comment: information not available. |
| Other bias | Low risk | Comment: no other source of bias. |
| Methods | Randomised clinical trial. | |
| Participants | Country: USA. Number randomised: 57. Post‐randomisation dropouts: 10 (17.5%). Revised sample size: 47. Mean age: 52 years. Females: not stated. Symptomatic participants: 45 (95.7%). AMA positive: not stated. Responders: not stated. Mean follow‐up period (for all groups): 33 months. Inclusion criteria
| |
| Interventions | Participants were randomly assigned to 2 groups. Group 1: colchicine (n = 28). Further details: colchicine: 0.6 mg BD orally for 5 years. Group 2: placebo (n = 29). | |
| Outcomes | None of the outcomes of interest reported. | |
| Notes | Reasons for post‐randomisation dropouts: non‐compliance. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: information not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: information not available. |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "The design of our trial was that of a double‐blind, randomized evaluation of colchicine (0.6 mg) twice daily compared with an identically appearing placebo". |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "The design of our trial was that of a double‐blind, randomized evaluation of colchicine (0.6 mg) twice daily compared with an identically appearing placebo". |
| Incomplete outcome data (attrition bias) | High risk | Comment: there were post‐randomisation dropouts. |
| Selective reporting (reporting bias) | High risk | Comment: neither mortality nor adverse events reported. |
| For‐profit bias | High risk | Quote: "The colchicine and placebo tablets were prepared and generously supplied by Eli Lilly and Company, Indianapolis, Ind". |
| Other bias | Low risk | Comment: no other source of bias. |
| Methods | Randomised clinical trial. | |
| Participants | Country: multicentric; international. Number randomised: 216. Post‐randomisation dropouts: not stated. Revised sample size: 216. Mean age: 56 years. Females: 197 (91.2%). Symptomatic participants: not stated. AMA positive: not stated. Responders: not stated. Mean follow‐up period (for all groups): all participants followed up for 12 months. Inclusion criteria
| |
| Interventions | Participants were randomly assigned to 3 groups. Group 1: obeticholic acid (low) (n = 73). Further details: obeticholic acid (low): 5 mg orally for 12 months; frequency not stated. Group 2: obeticholic acid (low) (n = 73). Further details: obeticholic acid (low): 10 mg orally for 12 months; frequency not stated. Group 3: placebo (n = 70). | |
| Outcomes | None of the outcomes of interest reported. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: information not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: information not available. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: although placebo was used in double‐blind trial, unclear whether the placebo was identical. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: although placebo was used in double‐blind trial, unclear whether the placebo was identical. |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: information not available. |
| Selective reporting (reporting bias) | High risk | Comment: neither mortality nor adverse events reported. |
| For‐profit bias | High risk | Comment: Several authors had advised pharmaceutical companies or were employees of pharmaceutical company. |
| Other bias | Low risk | Comment: no other source of bias. |
| Methods | Randomised clinical trial. | |
| Participants | Country: UK. Number randomised: 21. Post‐randomisation dropouts: 8 (38.1%). Revised sample size: 13. Mean age: 55 years. Females: not stated. Symptomatic participants: not stated. AMA positive: 13 (100%). Responders: not stated. Mean follow‐up period (for all groups): all participants followed up for 12 months. Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to 2 groups. Group 1: simvastatin (n = 7). Further details: simvastatin: 20 mg/day orally for 12 months. Group 2: placebo (n = 6). | |
| Outcomes | None of the outcomes of interest reported. | |
| Notes | Reasons for post‐randomisation dropouts: adverse effects. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: information not available. |
| Allocation concealment (selection bias) | Low risk | Quote: "Patient treatment randomization and allocation was performed independently by the Department of Research Pharmacology in the Royal Victoria Hospital at the initial baseline visit". |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "The patients were blinded but the healthcare providers were not" (author's reply). |
| Blinding of outcome assessment (detection bias) | High risk | Quote: "Outcome assessors were not blinded" (author's reply). |
| Incomplete outcome data (attrition bias) | High risk | Comment: there were post‐randomisation dropouts. |
| Selective reporting (reporting bias) | High risk | Comment: neither mortality nor adverse events reported. |
| For‐profit bias | Low risk | Quote: "Financial support: The Royal Victoria Hospital Liver Support Group". |
| Other bias | High risk | Quote: "Patients were allowed to continue previous prescriptions for primary biliary cholangitis. It was not clear whether this was balanced across groups". |
| Methods | Randomised clinical trial. | |
| Participants | Country: multicentric; international. Number randomised: 248. Post‐randomisation dropouts: 63 (25.4%). Revised sample size: 185. Mean age: 55 years. Females: not stated. Symptomatic participants: not stated. AMA positive: not stated. Mean follow‐up period (for all groups): minimum 63 months. Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to 2 groups. Group 1: azathioprine (n = 98). Further details: azathioprine: escalating doses up to a maximum of 100 mg/day; duration: not stated. Group 2: placebo (n = 87). | |
| Outcomes | Mortality. | |
| Notes | Reasons for post‐randomisation dropouts: lost to follow‐up. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: information not available. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Patients were randomized to azathioprine or placebo separately for each centre and for each sex by the sealed envelope technique". Comment: further details of sealed envelope technique were not available. |
| Blinding of participants and personnel (performance bias) | Low risk | Comment: identical placebo used and authors stated double‐blind. |
| Blinding of outcome assessment (detection bias) | Low risk | Comment: identical placebo used and authors stated double‐blind. |
| Incomplete outcome data (attrition bias) | High risk | Comment: there were post‐randomisation dropouts. |
| Selective reporting (reporting bias) | High risk | Comment: adverse events not reported. |
| For‐profit bias | High risk | Quote: "This work was also supported by the Wellcome Foundation. J.N. was supported by Ciba‐Geigy Ltd". |
| Other bias | Low risk | Comment: no other source of bias. |
| Methods | Randomised clinical trial. | |
| Participants | Country: USA. Number randomised: 151. Post‐randomisation dropouts: 0 (0%). Revised sample size: 151. Mean age: 49 years. Females: 134 (88.7%). Symptomatic participants: not stated. AMA positive: not stated. Responders: not stated. Mean follow‐up period (for all groups): all participants followed up for 24 months. Inclusion criteria
Other exclusion criteria
| |
| Interventions | Participants were randomly assigned to 2 groups. Group 1: UDCA (moderate) (n = 77). Further details: UDCA: 10 mg/kg/day to 12 mg/kg/day for 2 years. Group 2: placebo (n = 74). | |
| Outcomes | Decompensated liver disease. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: information not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: information not available. |
| Blinding of participants and personnel (performance bias) | Low risk | Comment: identical placebo used in double‐blind trial. |
| Blinding of outcome assessment (detection bias) | Low risk | Comment: identical placebo used in double‐blind trial. |
| Incomplete outcome data (attrition bias) | Low risk | Comment: no post‐randomisation dropouts. |
| Selective reporting (reporting bias) | High risk | Comment: neither mortality nor adverse events reported. |
| For‐profit bias | High risk | Quote: "Supported in part by a research grant from Ciba‐Geigy". |
| Other bias | Low risk | Comment: no other risk of bias. |
| Methods | Randomised clinical trial. | |
| Participants | Country: USA. Number randomised: 265. Post‐randomisation dropouts: 0 (0%). Revised sample size: 265. Mean age: 51 years. Females: 245 (92.5%). Symptomatic participants: not stated. AMA positive: 265 (100%). Responders: not stated. Median follow‐up period (for all groups): 91 months. Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to 2 groups. Group 1: UDCA (moderate) + methotrexate (n = 132). Further details: UDCA: 15 mg/kg/day for 2 years + methotrexate: 2.5 mg orally once a week. Group 2: UDCA (moderate) (n = 133). Further details: UDCA: 15 mg/kg/day for 2 years. | |
| Outcomes | Mortality, liver transplantation, decompensated liver disease. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: information not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: information not available. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: placebo used in this double‐blind trial; however, the authors did not state whether the placebo was identical. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: placebo used in this double‐blind trial; however, the authors did not state whether the placebo was identical. |
| Incomplete outcome data (attrition bias) | Low risk | Comment: no post‐randomisation dropouts. |
| Selective reporting (reporting bias) | High risk | Comment: adverse events not reported. |
| For‐profit bias | High risk | Quote: "By provision of UDCA by Ciba‐Geigy Corporation, and subsequently Novartis; by provision of methotrexate and its placebo by Lederle Laboratories, and subsequently Wyeth‐Ayerst Laboratories". |
| Other bias | Low risk | Comment: no other source of bias. |
| Methods | Randomised clinical trial. | |
| Participants | Country: USA. Number randomised: 309. Post‐randomisation dropouts: 82 (26.5%). Revised sample size: 227. Mean age: not stated. Females: 200 (88.1%). Symptomatic participants: 182 (80.2%). AMA positive: not stated. Responders: not stated. Median follow‐up period (for all groups): 60 months. Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to 2 groups. Group 1: D‐penicillamine (n = 111). Further details: D‐penicillamine: 1000 mg/day; duration: not stated. Group 2: placebo (n = 116). | |
| Outcomes | Adverse events. | |
| Notes | Reasons for post‐randomisation dropouts: histological stages < 3; alcoholism. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomly assigned to drug or placebo groups according to a table of random numbers". |
| Allocation concealment (selection bias) | Low risk | Quote: "Penicillamine and placebo (furnished to us through the courtesy of Merck Sharp and Dohme, West Point, Pa.) were dispensed in identical yellow capsules by a central pharmacist". |
| Blinding of participants and personnel (performance bias) | Low risk | Comment: identical placebo used in double‐blind trial. |
| Blinding of outcome assessment (detection bias) | Low risk | Comment: identical placebo used in double‐blind trial. |
| Incomplete outcome data (attrition bias) | High risk | Comment: there were post‐randomisation dropouts. |
| Selective reporting (reporting bias) | High risk | Comment: mortality not reported. |
| For‐profit bias | High risk | Quote: "Penicillamine and placebo (furnished to us through the courtesy of Merck Sharp and Dohme, West Point, Pa.) were dispensed in identical yellow capsules by a central pharmacist". |
| Other bias | High risk | Comment: authors presented the results of only a subgroup of participants without explaining the reason for this. |
| Methods | Randomised clinical trial. | |
| Participants | Country: UK. Number randomised: 98. Post‐randomisation dropouts: not stated. Revised sample size: 98. Mean age: not stated. Females: not stated. Symptomatic participants: not stated. AMA positive: not stated. Responders: not stated. Mean follow‐up period (for all groups): mean: 66 months. Inclusion criteria
| |
| Interventions | Participants were randomly assigned to 2 groups. Group 1: D‐penicillamine (n = 61). Further details: D‐penicillamine: 600 mg/day to 900 mg/day for 12 months. Group 2: placebo (n = 37). | |
| Outcomes | Mortality. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: information not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: information not available. |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "The original double‐blind design of the trial was discontinued after a year because both major and minor side effects identified treated patients". |
| Blinding of outcome assessment (detection bias) | High risk | Quote: "The original double‐blind design of the trial was discontinued after a year because both major and minor side effects identified treated patients". |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: information not available. |
| Selective reporting (reporting bias) | High risk | Comment: adverse events not reported. |
| For‐profit bias | Unclear risk | Comment: information not available. |
| Other bias | Low risk | Comment: no other source of bias. |
| Methods | Randomised clinical trial. | |
| Participants | Country: Sweden. Number randomised: 116. Post‐randomisation dropouts: 15 (12.9%). Revised sample size: 101. Mean age: 57 years. Females: 99 (98%). Symptomatic participants: 39 (38.6%). AMA positive: not stated. Responders: not stated. Mean follow‐up period (for all groups): all participants followed up for 24 months. Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to 2 groups. Group 1: UDCA (low) (n = 60). Further details: UDCA: 500 mg/day for 24 months. Group 2: placebo (n = 56). | |
| Outcomes | Liver transplantation. | |
| Notes | Reasons for post‐randomisation dropouts: adverse effects, alcoholic hepatitis, liver transplantation, death. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: information not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: information not available. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: placebo used in this double‐blind trial; however, the authors did not state whether the placebo was identical. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: placebo used in this double‐blind trial; however, the authors did not state whether the placebo was identical. |
| Incomplete outcome data (attrition bias) | High risk | Comment: there were post‐randomisation dropouts. |
| Selective reporting (reporting bias) | High risk | Comment: neither mortality nor adverse events reported. |
| For‐profit bias | High risk | Quote: "We acknowledge the financial support from Meda AB, Searle AB, and the Swedish Medical Research Council (03x‐4793)". |
| Other bias | Low risk | Comment: no other source of bias. |
| Methods | Randomised clinical trial. | |
| Participants | Country: Italy. Number randomised: 30. Post‐randomisation dropouts: 0 (0%). Revised sample size: 30. Mean age: 53 years. Females: 27 (90%). Symptomatic participants: not stated. AMA positive: not stated. Responders: not stated. Mean follow‐up period (for all groups): all participants followed up for 6 months. Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to 2 groups. Group 1: TUDCA (moderate) (n = 15). Further details: TUDCA: 13 mg/day to 15 mg/day for 6 months. Group 2: UDCA (moderate) (n = 15). Further details: UDCA: 13 mg/day to 15 mg/day for 6 months. | |
| Outcomes | Adverse events. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: information not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: information not available. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: information not available. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: information not available. |
| Incomplete outcome data (attrition bias) | Low risk | Comment: adverse events, the only outcome of interest reported in this study were available from all randomised participants. |
| Selective reporting (reporting bias) | High risk | Comment: mortality not reported. |
| For‐profit bias | Unclear risk | Comment: information not available. |
| Other bias | Low risk | Comment: no other source of bias. |
| Methods | Randomised clinical trial. | |
| Participants | Country: China. Number randomised: 79. Post‐randomisation dropouts: not stated. Revised sample size: 79. Mean age: 53 years. Females: 77 (97.5%). Symptomatic participants: not stated. AMA positive: not stated. Responders: not stated. Mean follow‐up period (for all groups): not stated. Inclusion criteria
Other inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to 3 groups. Group 1: UDCA (moderate) (n = 29). Further details: UDCA: 13 mg/kg/day to 15 mg/kg/day; duration: not stated. Group 2: UDCA (moderate) + glucocorticosteroids (n = 37). Further details: UDCA: 13 mg/kg/day to 15 mg/kg/day; duration: not stated + prednisolone: 7.5 mg/day; duration: not stated. Group 3: UDCA (moderate) + azathioprine (n = 13). Further details: UDCA: 13 mg/kg/day to 15 mg/kg/day; duration: not stated + azathioprine: 1 mg/kg/day; duration: not stated. | |
| Outcomes | Adverse events, decompensated liver disease. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: information not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: information not available. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: information not available. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: information not available. |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: information not available. |
| Selective reporting (reporting bias) | High risk | Comment: mortality not reported. |
| For‐profit bias | Unclear risk | Comment: information not available. |
| Other bias | Low risk | Comment: no other source of bias. |
| Methods | Randomised clinical trial. | |
| Participants | Country: UK. Number randomised: 57. Post‐randomisation dropouts: not stated. Revised sample size: 57. Mean age: not stated. Females: not stated. Symptomatic participants: 30 (52.6%). AMA positive: not stated. Responders: not stated. Mean follow‐up period (for all groups): 15 months. Inclusion criteria
| |
| Interventions | Participants were randomly assigned to 4 groups. Group 1: UDCA (low) (n = not stated). Further details: UDCA: 10 mg/kg/day; duration: not stated. Group 2: colchicine (n = not stated). Further details: colchicine: 1 mg/day; duration: not stated. Group 3: UDCA (low) + colchicine (n = not stated). Further details: UDCA: 10 mg/kg/day; duration: not stated + colchicine: 1 mg/day; duration: not stated. Group 4: placebo (n = not stated). | |
| Outcomes | None of the outcomes of interest reported. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: information not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: information not available. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: placebo used; however, the authors did not mention blinding. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: placebo used; however, the authors did not mention blinding. |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: information not available. |
| Selective reporting (reporting bias) | High risk | Comment: neither mortality nor adverse events reported. |
| For‐profit bias | Unclear risk | Comment: information not available. |
| Other bias | Low risk | Comment: no other source of bias. |
| Methods | Randomised clinical trial. | |
| Participants | Country: Chile. Number randomised: 25. Post‐randomisation dropouts: not stated. Revised sample size: 25. Mean age: 50 years. Females: 25 (100%). Symptomatic participants: not stated. AMA positive: not stated. Responders: not stated. Mean follow‐up period (for all groups): all participants followed up for 11 months. Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to 2 groups. Group 1: UDCA (low) + methotrexate (n = 13). Further details: UDCA: 250 mg BD for 48 weeks + methotrexate: 10 mg/week given over 48 hours each week for 48 months. Group 2: UDCA (low) (n = 12). Further details: UDCA: 250 mg BD for 48 weeks. | |
| Outcomes | Mortality, adverse events. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: information not available. |
| Allocation concealment (selection bias) | Low risk | Quote: "A physician who was blinded to the treatment, followed them up clinically, evaluated clinical symptoms, adverse side effects, complications and compliance". |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: placebo used in this double‐blind trial; however, the authors did not state whether the placebo was identical. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: placebo used in this double‐blind trial; however, the authors did not state whether the placebo was identical. |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: information not available. |
| Selective reporting (reporting bias) | Low risk | Comment: mortality and morbidity reported. |
| For‐profit bias | Unclear risk | Comment: information not available. |
| Other bias | Low risk | Comment: no other source of bias. |
| Methods | Randomised clinical trial. | |
| Participants | Country: UK. Number randomised: 45. Post‐randomisation dropouts: 6 (13.3%). Revised sample size: 39. Mean age: 51 years. Females: not stated. Symptomatic participants: 39 (100%). AMA positive: not stated. Responders: not stated. Mean follow‐up period (for all groups): not stated. Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to 2 groups. Group 1: azathioprine (n = 19). Further details: azathioprine: 2 mg/kg; frequency and duration: not stated. Group 2: control (n = 20). | |
| Outcomes | Mortality, cirrhosis. | |
| Notes | Reasons for post‐randomisation dropouts: adverse events, wrong diagnosis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: information not available. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Patients were randomly allocated to the treatment or control group, using the sealed envelope technique". Comment: further details of sealed envelope technique not available. |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "No placebo was given to the control patients". |
| Blinding of outcome assessment (detection bias) | High risk | Quote: "No placebo was given to the control patients". |
| Incomplete outcome data (attrition bias) | High risk | Comment: there were post‐randomisation dropouts. |
| Selective reporting (reporting bias) | High risk | Comment: adverse events not reported. |
| For‐profit bias | Low risk | Quote: "This work was supported by the Medical Research Council and the Ingram Fund". |
| Other bias | Low risk | Comment: no other source of bias. |
| Methods | Randomised clinical trial. | |
| Participants | Country: Canada. Number randomised: 222. Post‐randomisation dropouts: not stated. Revised sample size: 222. Mean age: 56 years. Females: 206 (92.8%). Symptomatic participants: not stated. AMA positive: not stated. Responders: not stated. Mean follow‐up period (for all groups): all participants followed up for 24 months. Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to 2 groups. Group 1: UDCA (moderate) (n = 111). Further details: UDCA: 14 mg/kg/day for 2 years. Group 2: placebo (n = 111). | |
| Outcomes | Mortality, liver transplantation. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: information not available. |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomization was done separately at each centre by the study pharmacist using consecutive identification numbers, and patients were stratified according to whether they were symptomatic or asymptomatic". |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "Once informed consent was obtained from the patients, double‐blind randomization to UDCA or an identical placebo (1 : 1) was conducted". |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "Once informed consent was obtained from the patients, double‐blind randomization to UDCA or an identical placebo (1 : 1) was conducted". |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: unclear whether the authors have reported the outcomes on all randomised participants. |
| Selective reporting (reporting bias) | High risk | Comment: adverse events not reported. |
| For‐profit bias | High risk | Quote: "Study medications kindly provided by Interfalk Canada and Jouveinal Inc., Quebec, Canada". |
| Other bias | Low risk | Comment: no other source of bias. |
| Methods | Randomised clinical trial. | |
| Participants | Country: UK. Number randomised: 60. Post‐randomisation dropouts: not stated. Revised sample size: 60. Mean age: 57 years. Females: 55 (91.7%). Symptomatic participants: 57 (95%). AMA positive: 51 (85%). Responders: not stated. Mean follow‐up period (for all groups): minimum 68 months. Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to 2 groups. Group 1: methotrexate (n = 30). Further details: methotrexate: 2.5 mg 3 times weekly for 6 years. Group 2: placebo (n = 30). Further details: placebo: 3 times weekly for 6 years. | |
| Outcomes | Mortality, liver transplantation. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomized in groups of 10 by a random‐number technique, operated by the hospital pharmacy, to receive 2.5 mg MTX [methotrexate] or identical placebo tablets, both supplied by Lederle Laboratories, on Friday, Saturday, and Sunday of each week for up to 6 years". |
| Allocation concealment (selection bias) | Low risk | Quote: "Patients were randomized in groups of 10 by a random‐number technique, operated by the hospital pharmacy, to receive 2.5 mg MTX or identical placebo tablets, both supplied by Lederle Laboratories, on Friday, Saturday, and Sunday of each week for up to 6 years". |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: placebo used in this double‐blind trial; however, the authors did not state whether the placebo was identical. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: placebo used in this double‐blind trial; however, the authors did not state whether the placebo was identical. |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: information not available. |
| Selective reporting (reporting bias) | High risk | Comment: neither mortality nor morbidity reported. |
| For‐profit bias | Unclear risk | Comment: information not available. |
| Other bias | Low risk | Comment: no other source of bias. |
| Methods | Randomised clinical trial. | |
| Participants | Country: multicentric; international. Number randomised: 165. Post‐randomisation dropouts: 0 (0%). Revised sample size: 165. Mean age: 55 years. Females: 157 (95.2%). Symptomatic participants: not stated. AMA positive: 134 (81.2%). Responders: 0 (0%). Mean follow‐up period (for all groups): all participants followed up for 3 months. Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to 3 groups. Group 1: obeticholic acid (low) (n = 38). Further details: obeticholic acid (low): 10 mg for 85 days; frequency not stated. Group 2: obeticholic acid (moderate) (n = 48). Further details: obeticholic acid (moderate): 25 mg for 85 days; frequency not stated. Group 3: obeticholic acid (high) (n = 41). Further details: obeticholic acid (high): 50 mg for 85 days; frequency not stated. Group 4: placebo (n = 38). | |
| Outcomes | Adverse events. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The computerized randomization schedule used a block size of 4 at each center". |
| Allocation concealment (selection bias) | Unclear risk | Comment: information not available. |
| Blinding of participants and personnel (performance bias) | Low risk | Comment: identical placebo used in this double‐blind trial. |
| Blinding of outcome assessment (detection bias) | Low risk | Comment: identical placebo used in this double‐blind trial. |
| Incomplete outcome data (attrition bias) | Low risk | Comment: no post‐randomisation dropouts. |
| Selective reporting (reporting bias) | High risk | Comment: mortality not reported. |
| For‐profit bias | Unclear risk | Comment: information not available. |
| Other bias | High risk | Quote: "Patients received varying doses of UDCA". |
| Methods | Randomised clinical trial. | |
| Participants | Country: multicentric; international. Number randomised: 24. Post‐randomisation dropouts: 0 (0%). Revised sample size: 24. Mean age: 47 years. Females: 23 (95.8%). Symptomatic participants: 24 (100%). AMA positive: 22 (91.7%). Responders: not stated. Mean follow‐up period (for all groups): 52 months. Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to 2 groups. Group 1: chlorambucil (n = 13). Further details: chlorambucil: 2 mg OD; duration: not stated. Group 2: control (n = 11). | |
| Outcomes | Mortality, adverse events. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomized by random numbers (generated by pharmacy) to either chlorambucil or no therapy. Pre‐computer age. They were kept in envelopes" (author's reply). |
| Allocation concealment (selection bias) | Low risk | Quote: "Patients were randomized by random numbers (generated by pharmacy) to either chlorambucil or no therapy. Pre‐computer age. They were kept in envelopes" (author's reply). |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "Not a blinded study" (author's reply). |
| Blinding of outcome assessment (detection bias) | High risk | Quote: "The outcomes were not blinded" (author's reply). |
| Incomplete outcome data (attrition bias) | Low risk | Comment: no post‐randomisation dropouts. |
| Selective reporting (reporting bias) | Low risk | Comment: mortality and morbidity reported. |
| For‐profit bias | Low risk | Quote: "The study was funded by the NIH intramural program" (author's reply). |
| Other bias | Low risk | Comment: no other source of bias. |
| Methods | Randomised clinical trial. | |
| Participants | Country: Japan. Number randomised: 27. Post‐randomisation dropouts: 0 (0%). Revised sample size: 27. Mean age: 64 years. Females: 22 (81.5%). Symptomatic participants: not stated. AMA positive: not stated. Responders: 0 (0%). Mean follow‐up period (for all groups): minimum: 96 months. Inclusion criteria
Other inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to 2 groups. Group 1: UDCA (moderate) + bezafibrate (n = 13). Further details: UDCA: 12 mg/kg/day to 15 mg/kg/day; duration: not stated + bezafibrate: 400 mg/day; duration: not stated. Group 2: UDCA (moderate) (n = 14). Further details: UDCA: 12 mg/kg/day to 15 mg/kg/day; duration: not stated. | |
| Outcomes | Mortality, adverse events, liver transplantation. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Sealed opaque envelopes" (author's reply). |
| Allocation concealment (selection bias) | Low risk | Quote: "These patients were randomly allocated to treatment with either UDCA alone (the UDCA group) or with the combination therapy (the UDCA+BF [bezafibrate] group), according to sequential sealed envelopes in blocks of four to ensure equal randomization for the duration of the study". |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "However, our study was an unblinded, open trial and was therefore not free from bias". |
| Blinding of outcome assessment (detection bias) | High risk | Quote: "However, our study was an unblinded, open trial and was therefore not free from bias". |
| Incomplete outcome data (attrition bias) | Low risk | Comment: no post‐randomisation dropouts. |
| Selective reporting (reporting bias) | Low risk | Comment: mortality and adverse events reported. |
| For‐profit bias | Low risk | Quote: "This study was supported by the authors' own research funds". |
| Other bias | Low risk | Comment: no other source of bias. |
| Methods | Randomised clinical trial. | |
| Participants | Country: Japan. Number randomised: 22. Post‐randomisation dropouts: 0 (0%). Revised sample size: 22. Mean age: 61 years. Females: 19 (86.4%). Symptomatic participants: 7 (31.8%). AMA positive: 22 (100%). Responders: not stated. Mean follow‐up period (for all groups): all participants followed up for 24 months. Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to 2 groups. Group 1: UDCA (moderate) + colchicine (n = 10). Further details: UDCA: 9 mg/kg/day to 15 mg/kg/day for 2 years + colchicine: 1 mg/day for 2 years. Group 2: UDCA (moderate) (n = 12). Further details: UDCA: 9 mg/kg/day to 15 mg/kg/day for 2 years. | |
| Outcomes | Adverse events. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: information not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: information not available. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: information not available. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: information not available. |
| Incomplete outcome data (attrition bias) | Low risk | Comment: no post‐randomisation dropouts. |
| Selective reporting (reporting bias) | High risk | Comment: mortality not reported. |
| For‐profit bias | Unclear risk | Quote: "Part of the present study was supported by a grant from the Intractable Liver Diseases Research Committee, the Ministry of Health and Welfare, Japan". Comment: unclear how the remaining part of the funds were obtained. |
| Other bias | High risk | Comment: dose range for UDCA was very wide. |
| Methods | Randomised clinical trial. | |
| Participants | Country: Japan. Number randomised: 45. Post‐randomisation dropouts: not stated. Revised sample size: 45. Mean age: 56 years. Females: 37 (82.2%). Symptomatic participants: not stated. AMA positive: not stated. Responders: not stated. Mean follow‐up period (for all groups): all participants followed up for 12 months. Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to 2 groups. Group 1: bezafibrate (n = 20). Further details: bezafibrate: 400 mg/day for 52 weeks. Group 2: UDCA (low) (n = 25). Further details: UDCA: 600 mg/day for 52 weeks. | |
| Outcomes | None of the outcomes of interest reported. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: information not available. |
| Allocation concealment (selection bias) | Low risk | Quote: "Consecutive patients from these hospitals were randomized centrally at the Kanagawa Dental University and were enrolled into the study if they met the following criteria". |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "A randomized, open study design was used because there was no suitable placebo for bezafibrate available". |
| Blinding of outcome assessment (detection bias) | High risk | Quote: "A randomized, open study design was used because there was no suitable placebo for bezafibrate available". |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: information not available. |
| Selective reporting (reporting bias) | High risk | Comment: neither mortality nor adverse events reported. |
| For‐profit bias | Low risk | Quote: "The Ministry of Health, Labour and Welfare of Japan supported this study from 2002 to 2004 with a Health Science Research Grant on a Specific Disease (Study of Intractable Liver Diseases) to chief scientist Gotaro Toda". |
| Other bias | Low risk | Comment: no other bias. |
| Methods | Randomised clinical trial. | |
| Participants | Country: Japan. Number randomised: 22. Post‐randomisation dropouts: not stated. Revised sample size: 22. Mean age: 54 years. Females: 19 (86.4%). Symptomatic participants: not stated. AMA positive: not stated. Responders: 0 (0%). Mean follow‐up period (for all groups): all participants followed up for 12 months. Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to 2 groups. Group 1: UDCA (low) + bezafibrate (n = 10). Further details: UDCA: 600 mg/day for 52 weeks + bezafibrate: 400 mg/day for 52 weeks. Group 2: UDCA (low) (n = 12). Further details: UDCA: 600 mg/day for 52 weeks. | |
| Outcomes | None of the outcomes of interest reported. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: information not available. |
| Allocation concealment (selection bias) | Low risk | Quote: "Consecutive patients from these hospitals were randomized centrally at the Kanagawa Dental University and were enrolled into the study if they met the following criteria". |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "A randomized, open study design was used because there was no suitable placebo for bezafibrate available". |
| Blinding of outcome assessment (detection bias) | High risk | Quote: "A randomized, open study design was used because there was no suitable placebo for bezafibrate available". |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: information not available. |
| Selective reporting (reporting bias) | High risk | Comment: neither mortality nor adverse events reported. |
| For‐profit bias | Low risk | Quote: "The Ministry of Health, Labour and Welfare of Japan supported this study from 2002 to 2004 with a Health Science Research Grant on a Specific Disease (Study of Intractable Liver Diseases) to chief scientist Gotaro Toda". |
| Other bias | Low risk | Comment: no other bias. |
| Methods | Randomised clinical trial. | |
| Participants | Country: Japan. Number randomised: 22. Post‐randomisation dropouts: 0 (0%). Revised sample size: 22. Mean age: 56 years. Females: 19 (86.4%). Symptomatic participants: not stated. AMA positive: not stated. Responders: 0 (0%). Mean follow‐up period (for all groups): minimum 7 months. Inclusion criteria
Other inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to 2 groups. Group 1: UDCA (low) + bezafibrate (n = 11). Further details: UDCA: 600 mg/day for 6 months + bezafibrate: 400 mg/day for 52 weeks. Group 2: UDCA (low) (n = 11). Further details: UDCA: 600 mg/day for 6 months. | |
| Outcomes | Adverse events. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: information not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: information not available. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: information not available. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: information not available. |
| Incomplete outcome data (attrition bias) | Low risk | Comment: no post‐randomisation dropouts. |
| Selective reporting (reporting bias) | High risk | Comment: mortality was not reported. |
| For‐profit bias | Unclear risk | Comment: information not available. |
| Other bias | Low risk | Comment: no other bias. |
| Methods | Randomised clinical trial. | |
| Participants | Country: USA. Number randomised: 60. Post‐randomisation dropouts: 3 (5%). Revised sample size: 57. Mean age: not stated. Females: 57 (100%). Symptomatic participants: 45 (78.9%). AMA positive: not stated. Responders: not stated. Mean follow‐up period (for all groups): all participants followed up for 24 months. Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to 2 groups. Group 1: colchicine (n = 28). Further details: colchicine: 0.6 mg BD for ≥ 2 years. Group 2: placebo (n = 29). | |
| Outcomes | Mortality. | |
| Notes | Reasons for post‐randomisation dropouts: adverse effects, despondent about treatment. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: information not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: information not available. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: authors stated that this was a double‐blind trial and used a placebo; however, they did not state whether the placebo was identical. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: authors stated that this was a double‐blind trial and used a placebo; however, they did not state whether the placebo was identical. |
| Incomplete outcome data (attrition bias) | High risk | Comment: there were post‐randomisation dropouts. |
| Selective reporting (reporting bias) | High risk | Comment: adverse events not reported. |
| For‐profit bias | Unclear risk | Comment: information not available. |
| Other bias | Low risk | Comment: no other bias. |
| Methods | Randomised clinical trial. | |
| Participants | Country: USA. Number randomised: 87. Post‐randomisation dropouts: 2 (2.3%). Revised sample size: 85. Mean age: 51 years. Females: 82 (96.5%). Symptomatic participants: 71 (83.5%). AMA positive: 77 (90.6%). Responders: not stated. Mean follow‐up period (for all groups): minimum 24 months. Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to 2 groups. Group 1: colchicine (n = 43). Further details: colchicine: 0.6 mg BD for 2 years. Group 2: methotrexate (n = 42). Further details: methotrexate: 15 mg/week orally. | |
| Outcomes | None of the outcomes of interest reported. | |
| Notes | Reasons for post‐randomisation dropouts: withdrawal from study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: information not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: information not available. |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "Both the patients and investigators were blinded to the treatment assignments". |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "Both the patients and investigators were blinded to the treatment assignments". Comment: authors stated that this was a double‐blind trial and used a placebo; however, they did not state whether the placebo was identical. |
| Incomplete outcome data (attrition bias) | High risk | Comment: there were post‐randomisation dropouts. |
| Selective reporting (reporting bias) | High risk | Comment: neither mortality nor adverse events reported. |
| For‐profit bias | Unclear risk | Comment: information not available. |
| Other bias | Low risk | Comment: no other bias. |
| Methods | Randomised clinical trial. | |
| Participants | Country: multicentric; international. Number randomised: 59. Post‐randomisation dropouts: not stated. Revised sample size: 59. Mean age: 55 years. Females: 50 (84.7%). Symptomatic participants: not stated. AMA positive: not stated. Responders: not stated. Mean follow‐up period (for all groups): not stated. Inclusion criteria
| |
| Interventions | Participants were randomly assigned to 3 groups. Group 1: obeticholic acid (low) (n = 20). Further details: obeticholic acid (low): 10 mg OD for 12 weeks. Group 2: obeticholic acid (high) (n = 16). Further details: obeticholic acid (high): 50 mg OD for 12 weeks. Group 3: placebo (n = 23). | |
| Outcomes | None of the outcomes of interest reported. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: information not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: information not available. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: although this was a double‐blind trial and placebo was used, unclear whether the placebo was identical. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: although this was a double‐blind trial and placebo was used, unclear whether the placebo was identical. |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: information not available. |
| Selective reporting (reporting bias) | High risk | Comment: neither mortality nor adverse events reported. |
| For‐profit bias | High risk | Comment: some of the coauthors were from the pharmaceutical industry. |
| Other bias | Low risk | Comment: no other source of bias. |
| Methods | Randomised clinical trial. | |
| Participants | Country: Japan. Number randomised: 24. Post‐randomisation dropouts: not stated. Revised sample size: 24. Mean age: 60 years. Females: 23 (95.8%). Symptomatic participants: not stated. AMA positive: not stated. Responders: not stated. Mean follow‐up period (for all groups): not stated. Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to 2 groups. Group 1: bezafibrate (n = 12). Further details: bezafibrate: 400 mg/day for 1 year. Group 2: UDCA (low) (n = 12). Further details: UDCA: 600 mg/day for 1 year. | |
| Outcomes | Adverse events. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: information not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: information not available. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: information not available. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: information not available. |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: information not available. |
| Selective reporting (reporting bias) | High risk | Comment: mortality not reported. |
| For‐profit bias | Unclear risk | Comment: information not available. |
| Other bias | Low risk | Comment: no other bias. |
| Methods | Randomised clinical trial. | |
| Participants | Country: Germany. Number randomised: 20. Post‐randomisation dropouts: 2 (10%). Revised sample size: 18. Mean age: not stated. Females: 18 (100%). Symptomatic participants: not stated. AMA positive: not stated. Responders: not stated. Mean follow‐up period (for all groups): all participants followed up for 12 months. Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to 2 groups. Group 1: UDCA (low) (n = 10). Further details: UDCA: 10 mg/kg/day for 9 months. Group 2: placebo (n = 8). | |
| Outcomes | Mortality, adverse events. | |
| Notes | Reasons for post‐randomisation dropouts: not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: information not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: information not available. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: although placebo was used in this double‐blind trial, unclear whether identical placebo used. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: although placebo was used in this double‐blind trial, unclear whether identical placebo used. |
| Incomplete outcome data (attrition bias) | High risk | Comment: there were post‐randomisation dropouts. |
| Selective reporting (reporting bias) | Low risk | Comment: mortality and morbidity reported. |
| For‐profit bias | Unclear risk | Comment: information not available. |
| Other bias | Low risk | Comment: no other source of bias. |
| Methods | Randomised clinical trial. | |
| Participants | Country: Germany. Number randomised: 40. Post‐randomisation dropouts: 1 (2.5%). Revised sample size: 39. Mean age: 58 years. Females: 37 (94.9%). Symptomatic participants: not stated. AMA positive: not stated. Responders: not stated. Mean follow‐up period (for all groups): all participants followed up for 24 months. Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to 2 groups. Group 1: UDCA (moderate) + corticosteroids (n = 20). Further details: UDCA: 10 mg/kg/day to 15 mg/kg/day for 2 years + budesonide: 3 mg 3 times daily for 2 years. Group 2: UDCA (moderate) (n = 19). | |
| Outcomes | None of the outcomes of interest reported. | |
| Notes | Reasons for post‐randomisation dropouts: personal reasons. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Complete randomization was done according to Rancode + (version 3.1; IDV‐Co., Marsaglia and Bray, Gauting, Germany)". |
| Allocation concealment (selection bias) | Unclear risk | Comment: information not available. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: although placebo was used in this double‐blind trial, unclear whether identical placebo used. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: although placebo was used in this double‐blind trial, unclear whether identical placebo used. |
| Incomplete outcome data (attrition bias) | High risk | Comment: there were post‐randomisation dropouts. |
| Selective reporting (reporting bias) | High risk | Comment: neither mortality nor adverse events reported. |
| For‐profit bias | High risk | Quote: "UDCA, budesonide, and placebo were provided by Dr. Falk Pharma GmbH (Freiburg, Germany)". |
| Other bias | Low risk | Comment: no other source of bias. |
| Methods | Randomised clinical trial. | |
| Participants | Country: Greece. Number randomised: 10. Post‐randomisation dropouts: not stated. Revised sample size: 10. Mean age: 57 years. Females: 8 (80%). Symptomatic participants: not stated. AMA positive: not stated. Responders: 0 (0%). Mean follow‐up period (for all groups): not stated. Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to 2 groups. Group 1: UDCA (low) + fenofibrate (n = 6). Further details: UDCA: 600 mg/day for 8 weeks + fenofibrate: 200 mg/day for 8 weeks. Group 2: UDCA (low) (n = 4). Further details: UDCA: 600 mg/day for 8 weeks. | |
| Outcomes | None of the outcomes of interest reported. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: information not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: information not available. |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "Continue open‐label UDCA". |
| Blinding of outcome assessment (detection bias) | High risk | Quote: "Continue open‐label UDCA". |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: information not available. |
| Selective reporting (reporting bias) | High risk | Comment: neither mortality nor adverse events reported. |
| For‐profit bias | Unclear risk | Quote: "This study was conducted independently; no company or institution supported it financially. Some of the authors have given talks, attended conferences and participated in trials and advisory boards sponsored by various pharmaceutical companies". |
| Other bias | Low risk | Comment: no other source of bias. |
| Methods | Randomised clinical trial. | |
| Participants | Country: UK. Number randomised: 32. Post‐randomisation dropouts: not stated. Revised sample size: 32. Mean age: not stated. Females: not stated. Symptomatic participants: not stated. AMA positive: not stated. Responders: not stated. Mean follow‐up period (for all groups): not stated. Inclusion criteria
| |
| Interventions | Participants were randomly assigned to 2 groups. Group 1: UDCA (moderate) (n = not stated). Further details: UDCA: 10 mg/kg/day to 12 mg/kg/day; duration: not stated. Group 2: placebo (n = not stated). | |
| Outcomes | None of the outcomes of interest reported. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: information not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: information not available. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: although a placebo was used in this double‐blind trial, unclear whether the placebo was identical. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: although a placebo was used in this double‐blind trial, unclear whether the placebo was identical. |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: information not available. |
| Selective reporting (reporting bias) | High risk | Comment: neither mortality nor adverse events reported. |
| For‐profit bias | Unclear risk | Comment: information not available. |
| Other bias | Low risk | Comment: no other source of bias. |
| Methods | Randomised clinical trial. | |
| Participants | Country: USA. Number randomised: 180. Post‐randomisation dropouts: 10 (5.6%). Revised sample size: 170. Mean age: 53 years. Females: 160 (94.1%). Symptomatic participants: not stated. AMA positive: not stated. Responders: not stated. Mean follow‐up period (for all groups): all participants followed up for 24 months. Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to 2 groups. Group 1: UDCA (moderate) (n = 86). Further details: UDCA: 13 mg/kg/day to 15 mg/kg/day; duration: not stated. Group 2: placebo (n = 84). | |
| Outcomes | Mortality, adverse events, liver transplantation. | |
| Notes | Reasons for post‐randomisation dropouts: not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: information not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: information not available. |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "The patients, physicians, nurses, and study coordinators were blinded as to whether active drug or placebo was being administered". |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "The patients, physicians, nurses, and study coordinators were blinded as to whether active drug or placebo was being administered". |
| Incomplete outcome data (attrition bias) | High risk | Comment: there were post‐randomisation dropouts. |
| Selective reporting (reporting bias) | Low risk | Comment: mortality and morbidity reported. |
| For‐profit bias | High risk | Quote: "Supported by Falk Pharma and Interfalk". |
| Other bias | Low risk | Comment: no other risk of bias. |
| Methods | Randomised clinical trial. | |
| Participants | Country: USA. Number randomised: 150. Post‐randomisation dropouts: not stated. Revised sample size: 150. Mean age: not stated. Females: not stated. Symptomatic participants: not stated. AMA positive: not stated. Responders: not stated. Mean follow‐up period (for all groups): all participants followed up for 12 months. Inclusion criteria
| |
| Interventions | Participants were randomly assigned to 3 groups. Group 1: UDCA (low) (n = not stated). Further details: UDCA: 5 mg/kg/day to 7 mg/kg/day; duration: not stated. Group 2: UDCA (moderate) (n = not stated). Further details: UDCA: 13 mg/kg/day to 15 mg/kg/day; duration: not stated. Group 3: UDCA (high) (n = not stated). Further details: UDCA: 22 mg/kg/day to 25 mg/kg/day; duration: not stated. | |
| Outcomes | None of the outcomes of interest reported. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: information not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: information not available. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: information not available. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: information not available. |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: information not available. |
| Selective reporting (reporting bias) | High risk | Comment: neither mortality nor adverse events reported. |
| For‐profit bias | Unclear risk | Comment: information not available. |
| Other bias | Low risk | Comment: no other risk of bias. |
| Methods | Randomised clinical trial. | |
| Participants | Country: multicentric; international. Number randomised: 349. Post‐randomisation dropouts: 0 (0%). Revised sample size: 349. Mean age: 54 years. Females: 298 (85.4%). Symptomatic participants: not stated. AMA positive: not stated. Responders: not stated. Median follow‐up period (for all groups): 31 months. Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to 2 groups. Group 1: ciclosporin (n = 176). Further details: ciclosporin: 3 mg/kg/day to maintain levels at 150 ng/mL by polyclonal radioimmunoassay or 75 ng/mL by monoclonal radioimmunoassay. Group 2: placebo (n = 173). | |
| Outcomes | Mortality, adverse events, liver transplantation. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: information not available. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Sealed envelopes" (author's reply). |
| Blinding of participants and personnel (performance bias) | Low risk | Comment: identical placebo used in this double‐blind trial. |
| Blinding of outcome assessment (detection bias) | Low risk | Comment: identical placebo used in this double‐blind trial. |
| Incomplete outcome data (attrition bias) | Low risk | Comment: no post‐randomisation dropouts. |
| Selective reporting (reporting bias) | Low risk | Comment: mortality and morbidity reported. |
| For‐profit bias | High risk | Quote: "The authors are grateful to Sandoz Pharmaceuticals, Basle, Switzerland and their international sub‐offices for supplying Sandimmune and placebo for this study and for their support throughout. The authors are grateful to Sandoz Pharmaceuticals, Basle, Zerland and their international sub‐offices for supplying Sandimmune and placebo for this study and for their support throughout". |
| Other bias | Low risk | Comment: no other source of bias. |
| Methods | Randomised clinical trial. | |
| Participants | Country: China. Number randomised: 199. Post‐randomisation dropouts: 8 (4.0%). Revised sample size: 191. Mean age: 51 years. Females: 167 (83.9%). Symptomatic participants: 38 (19.9%). AMA positive: 187 (97.9%). Responders: not stated. Mean follow‐up period (for all groups): all participants: 6 months. Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to 2 groups. Group 1: UDCA (moderate) (n = 66). Further details: UDCA: 250 mg 3 times daily for 24 weeks. Group 2: TUDCA (moderate) (n = 125). Further details: TUDCA: 250 mg 3 times daily for 24 weeks. | |
| Outcomes | Adverse events. | |
| Notes | Reason for drop‐outs: not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A centralized telecommunication‐based interactive voice response system was used for patient randomization after patient eligibility was determined through clinical and laboratory screening assessments". |
| Allocation concealment (selection bias) | Low risk | Quote: "A centralized telecommunication‐based interactive voice response system was used for patient randomization after patient eligibility was determined through clinical and laboratory screening assessments". |
| Blinding of participants and personnel (performance bias) | Low risk | Comment: identical placebo used in this double‐blind trial. |
| Blinding of outcome assessment (detection bias) | Low risk | Comment: identical placebo used in this double‐blind trial. |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: unclear whether all participants were included in the analysis. |
| Selective reporting (reporting bias) | High risk | Comment: mortality not reported. |
| For‐profit bias | High risk | Quote: "This study was sponsored by the Beijing Trendful Kangjian Medical Information Consulting Co., Ltd. and the Major Science and Technology Special Project of China Twelfth Five‐year Plan (2012ZX10002003). Registration Number: NCT01829698". |
| Other bias | Low risk | Comment: no other source of bias. |
| Methods | Randomised clinical trial. | |
| Participants | Country: UK. Number randomised: 60. Post‐randomisation dropouts: 0 (0%). Revised sample size: 60. Mean age: not stated. Females: not stated. Symptomatic participants: not stated. AMA positive: not stated. Responders: not stated. Mean follow‐up period (for all groups): 37 months. Inclusion criteria
| |
| Interventions | Participants were randomly assigned to 2 groups. Group 1: D‐penicillamine (n = 41). Further details: D‐penicillamine: 250 mg/day or 1 g/day; duration: not stated. Group 2: placebo (n = 19). | |
| Outcomes | Mortality, adverse events. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: information not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: information not available. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: although placebo was used, there is no mention of blinding. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: although placebo was used, there is no mention of blinding. |
| Incomplete outcome data (attrition bias) | Low risk | Comment: no post‐randomisation dropouts. |
| Selective reporting (reporting bias) | Low risk | Comment: mortality and morbidity reported. |
| For‐profit bias | Unclear risk | Comment: information not available. |
| Other bias | Low risk | Comment: no other bias. |
| Methods | Randomised clinical trial. | |
| Participants | Country: Italy. Number randomised: 32. Post‐randomisation dropouts: not stated. Revised sample size: 32. Mean age: not stated. Females: not stated. Symptomatic participants: not stated. AMA positive: not stated. Responders: not stated. Mean follow‐up period (for all groups): all participants followed up for 1 month. Inclusion criteria
| |
| Interventions | Participants were randomly assigned to 2 groups. Group 1: SAMe (n = 16). Further details: SAMe: 800 mg/day IV for 2 weeks. Group 2: placebo (n = 16). | |
| Outcomes | None of the outcomes of interest reported. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: information not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: information not available. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: although placebo was used, there was no mention of blinding. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: although placebo was used, there was no mention of blinding. |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: information not available. |
| Selective reporting (reporting bias) | High risk | Comment: neither mortality nor adverse events reported. |
| For‐profit bias | Unclear risk | Comment: information not available. |
| Other bias | Low risk | Comment: no other bias. |
| Methods | Randomised clinical trial. | |
| Participants | Country: Italy. Number randomised: 6. Post‐randomisation dropouts: not stated. Revised sample size: 6. Mean age: not stated. Females: not stated. Symptomatic participants: not stated. AMA positive: not stated. Responders: not stated. Mean follow‐up period (for all groups): all participants followed up for 2 months. Inclusion criteria
| |
| Interventions | Participants were randomly assigned to 2 groups. Group 1: SAMe (n = 3). Further details: SAMe: 1600 mg/day orally for 8 weeks. Group 2: placebo (n = 3). | |
| Outcomes | None of the outcomes of interest reported. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: information not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: information not available. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: although placebo was used, there was no mention of blinding. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: although placebo was used, there was no mention of blinding. |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: information not available. |
| Selective reporting (reporting bias) | High risk | Comment: neither mortality nor adverse events reported. |
| For‐profit bias | Unclear risk | Comment: information not available. |
| Other bias | Low risk | Comment: no other bias. |
| Methods | Randomised clinical trial. | |
| Participants | Country: multicentric; international. Number randomised: 59. Post‐randomisation dropouts: 0 (0%). Revised sample size: 59. Mean age: 56 years. Females: 58 (98.3%). Symptomatic participants: not stated. AMA positive: 59 (100%). Responders: 0 (0%). Mean follow‐up period (for all groups): all participants followed up for 6 months. Inclusion criteria
Other exclusion criteria
| |
| Interventions | Participants were randomly assigned to 2 groups. Group 1: lamivudine + zidovudine + UDCA (moderate) (n = 30). Further details: lamivudine: 150 mg BD for 6 months + zidovudine: 300 mg BD for 6 months + UDCA: 13 mg/kg/day to 15 mg/kg/day for 6 months. Group 2: UDCA (moderate) (n = 29). Further details: UDCA: 13 mg/kg/day to 15 mg/kg/day for 6 months. | |
| Outcomes | Adverse events. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomisation was performed centrally at the University of Alberta by a dynamic randomization" (author's reply). |
| Allocation concealment (selection bias) | Low risk | Quote: "Sealed opaque envelopes" (author's reply). |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "Dilan Clinical Packaging Ltd (Mississauga, ON, Canada) coded samples ensuring that the investigators and patients were blinded to the treatment". |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "Dilan Clinical Packaging Ltd (Mississauga, ON, Canada) coded samples ensuring that the investigators and patients were blinded to the treatment". |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: information not available. |
| Selective reporting (reporting bias) | High risk | Comment: mortality not reported. |
| For‐profit bias | High risk | Quote: "This study was funded in full by GlaxoSmithKline and Axcan Pharma". |
| Other bias | Low risk | Comment: no other bias. |
| Methods | Randomised clinical trial. | |
| Participants | Country: USA. Number randomised: 52. Post‐randomisation dropouts: 0 (0%). Revised sample size: 52. Mean age: 52 years. Females: 48 (92.3%). Symptomatic participants: not stated. AMA positive: 42 (80.8%). Responders: not stated. Mean follow‐up period (for all groups): minimum 24 months. Inclusion criteria
| |
| Interventions | Participants were randomly assigned to 2 groups. Group 1: D‐penicillamine (n = 26). Further details: D‐penicillamine: 1 g/day; duration: not stated. Group 2: placebo (n = 26). | |
| Outcomes | Mortality, adverse events. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: information not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: information not available. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: although placebo was used in this double‐blind trial, there was no mention about identical placebo. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: although placebo was used in this double‐blind trial, there was no mention about identical placebo. |
| Incomplete outcome data (attrition bias) | Low risk | Comment: no post‐randomisation dropouts. |
| Selective reporting (reporting bias) | Low risk | Comment: mortality and morbidity reported. |
| For‐profit bias | High risk | Quote: "We are indebted to Merck, Sharp and Dohme Research Laboratories for providing the D‐penicillamine and placebo tablets". |
| Other bias | Low risk | Comment: no other bias. |
| Methods | Randomised clinical trial. | |
| Participants | Country: multicentric; international. Number randomised: 45. Post‐randomisation dropouts: 3 (6.7%). Revised sample size: 42. Mean age: 56 years. Females: 38 (90.5%). Symptomatic participants: not stated. AMA positive: not stated. Responders: not stated. Mean follow‐up period (for all groups): not stated. Inclusion criteria
| |
| Interventions | Participants were randomly assigned to 2 groups. Group 1: NGM282 (n = 27). Further details: NGM282: 0.3 mg/day or 3 mg/day SC for 28 days. Group 2: placebo (n = 15). | |
| Outcomes | None of the outcomes of interest reported. | |
| Notes | Reasons for post‐randomisation dropouts: not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: information not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: information not available. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: although placebo was used in this double‐blind trial, unclear whether the placebo was identical. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: although placebo was used in this double‐blind trial, unclear whether the placebo was identical. |
| Incomplete outcome data (attrition bias) | High risk | Comment: there were post‐randomisation dropouts. |
| Selective reporting (reporting bias) | High risk | Comment: neither mortality nor adverse events reported. |
| For‐profit bias | High risk | Quote: "Grant/Research Support: Intercept, Salix, NGM, Lumena, Gilead". |
| Other bias | Low risk | Comment: no other bias. |
| Methods | Randomised clinical trial. | |
| Participants | Country: Italy. Number randomised: 42. Post‐randomisation dropouts: not stated. Revised sample size: 42. Mean age: not stated. Females: 37 (88.1%). Symptomatic participants: not stated. AMA positive: not stated. Responders: not stated. Mean follow‐up period (for all groups): all participants followed up for 72 months. Inclusion criteria
| |
| Interventions | Participants were randomly assigned to 2 groups. Group 1: UDCA (high) (n = 21). Further details: UDCA (high): 30 mg/kg/day for 6 years. Group 2: UDCA (moderate) (n = 21). Further details: UDCA (moderate): 10.5 mg/kg/day for 6 years. | |
| Outcomes | None of the outcomes of interest reported. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: information not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: information not available. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: information not available. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: information not available. |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: information not available. |
| Selective reporting (reporting bias) | High risk | Comment: neither mortality nor adverse events reported. |
| For‐profit bias | Unclear risk | Comment: information not available. |
| Other bias | Low risk | Comment: no other bias. |
| Methods | Randomised clinical trial. | |
| Participants | Country: UK. Number randomised: 18. Post‐randomisation dropouts: 0 (0%). Revised sample size: 18. Mean age: 60 years. Females: 14 (77.8%). Symptomatic participants: not stated. AMA positive: not stated. Responders: not stated. Mean follow‐up period (for all groups): not stated. Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to 2 groups. Group 1: thalidomide (n = 10). Further details: thalidomide: 100 mg/day for 6 months. Group 2: placebo (n = 8). | |
| Outcomes | None of the outcomes of interest reported. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: information not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: information not available. |
| Blinding of participants and personnel (performance bias) | Low risk | Comment: identical placebo used in double‐blind trial. |
| Blinding of outcome assessment (detection bias) | Low risk | Comment: identical placebo used in double‐blind trial. |
| Incomplete outcome data (attrition bias) | Low risk | Comment: no post‐randomisation dropouts. |
| Selective reporting (reporting bias) | High risk | Comment: neither mortality nor adverse events reported. |
| For‐profit bias | High risk | Quote: "Thalidomide and identical placebo tablets were supplied by Penn Pharmaceuticals Ltd". |
| Other bias | Low risk | Comment: no other bias. |
| Methods | Randomised clinical trial. | |
| Participants | Country: Canada. Number randomised: 12. Post‐randomisation dropouts: 0 (0%). Revised sample size: 12. Mean age: 55 years. Females: 11 (91.7%). Symptomatic participants: not stated. AMA positive: not stated. Responders: not stated. Mean follow‐up period (for all groups): not stated. Inclusion criteria
| |
| Interventions | Participants were randomly assigned to 2 groups. Group 1: ciclosporin (n = 6). Further details: ciclosporin: maintain serum radioimmunoassay dosage between 100 ng/mL and 200 ng/mL for 12 months. Group 2: placebo (n = 6). | |
| Outcomes | Mortality, adverse events. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: information not available. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "…patients were randomized by sealed envelope to receive either cyclosporin A or placebo". |
| Blinding of participants and personnel (performance bias) | Low risk | Comment: identical placebo used in this double‐blind trial. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: although a placebo was used, there was no mention about blinding. |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: although a placebo was used, there was no mention about blinding. |
| Selective reporting (reporting bias) | Low risk | Comment: mortality and morbidity reported. |
| For‐profit bias | High risk | Comment: the drugs were provided by the pharmaceutical company. |
| Other bias | Low risk | Comment: no other bias. |
| Methods | Randomised clinical trial. | |
| Participants | Country: UK. Number randomised: 36. Post‐randomisation dropouts: 0 (0%). Revised sample size: 36. Mean age: 55 years. Females: 33 (91.7%). Symptomatic participants: 35 (97.2%). AMA positive: not stated. Responders: not stated. Mean follow‐up period (for all groups): all participants followed up for 36 months. Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to 2 groups. Group 1: glucocorticosteroids (n = 19). Further details: prednisolone: 10 mg/day for 36 months (loading dose was used). Group 2: placebo (n = 17). | |
| Outcomes | Mortality. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were paired according to the presence or absence of cirrhosis, their age by decade, menopausal status (for women) and their serum bilirubin (greater or less than 30 µmoles per litre)". Comment: minimisation used. |
| Allocation concealment (selection bias) | Low risk | Quote: "Patients were paired according to the presence or absence of cirrhosis, their age by decade, menopausal status (for women) and their serum bilirubin (greater or less than 30 µmoles per litre)". Comment: minimisation used. |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "Study was double‐blind for the first year, single blind thereafter (patients were blinded)". |
| Blinding of outcome assessment (detection bias) | High risk | Quote: "Study was double‐blind for the first year, single blind thereafter (patients were blinded)". |
| Incomplete outcome data (attrition bias) | Low risk | Comment: no post‐randomisation dropouts. |
| Selective reporting (reporting bias) | High risk | Comment: adverse events not reported. |
| For‐profit bias | Unclear risk | Comment: information not available. |
| Other bias | Low risk | Comment: no other bias. |
| Methods | Randomised clinical trial. | |
| Participants | Country: multicentric; international. Number randomised: 104. Post‐randomisation dropouts: 3 (2.9%). Revised sample size: 101. Mean age: 54 years. Females: 93 (92.1%). Symptomatic participants: 101 (100%). AMA positive: not stated. Responders: not stated. Median follow‐up period (for all groups): 25 months. Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to 2 groups. Group 1: malotilate (n = 52). Further details: malotilate: 500 mg 3 times daily; mean duration: 23 months. Group 2: placebo (n = 49). | |
| Outcomes | Mortality, adverse events. | |
| Notes | Reasons for post‐randomisation dropouts: elementary data not available. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Random sequence was generated by the trial statistician with tables with random numbers" (author's reply). |
| Allocation concealment (selection bias) | Low risk | Quote: "Sequentially numbered identical containers" (author's reply). |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "Both patients and doctors were unaware of the nature of the tablets". |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "Both patients and doctors were unaware of the nature of the tablets". |
| Incomplete outcome data (attrition bias) | High risk | Comment: there were post‐randomisation dropouts. |
| Selective reporting (reporting bias) | Low risk | Comment: mortality and morbidity reported. |
| For‐profit bias | High risk | Quote: "The study was supported in part by Zyma S.A., Nyon, Switzerland, and by Nihon Nohyaku, Tokyo, Japan". |
| Other bias | Low risk | Comment: no other source of bias. |
| Methods | Randomised clinical trial. | |
| Participants | Country: Japan. Number randomised: 23. Post‐randomisation dropouts: not stated. Revised sample size: 23. Mean age: 57 years. Females: not stated. Symptomatic participants: not stated. AMA positive: not stated. Responders: not stated. Mean follow‐up period (for all groups): all participants followed up for 12 months. Inclusion criteria
| |
| Interventions | Participants were randomly assigned to 2 groups. Group 1: UDCA (low) + bezafibrate (n = 13). Further details: UDCA: 600 mg/day; duration: not stated + bezafibrate: 400 mg/day; duration: not stated. Group 2: UDCA (low) (n = 10). Further details: UDCA: 600 mg/day; duration: not stated. | |
| Outcomes | None of the outcomes of interest reported. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: information not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: information not available. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: information not available. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: information not available. |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: information not available. |
| Selective reporting (reporting bias) | High risk | Comment: neither mortality nor adverse events reported. |
| For‐profit bias | Low risk | Quote: "This work was supported by a Grant‐in‐Aid for Scientific Research from the Ministry of Education, Science and Culture of Japan". |
| Other bias | Low risk | Comment: no other source of bias. |
| Methods | Randomised clinical trial. | |
| Participants | Country: multicentric; international. Number randomised: 189. Post‐randomisation dropouts: not stated. Revised sample size: 189. Mean age: not stated. Females: 174 (92.1%). Symptomatic participants: 172 (91%). AMA positive: 163 (86.2%). Responders: not stated. Mean follow‐up period (for all groups): not stated. Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to 2 groups. Group 1: D‐penicillamine (n = 98). Further details: D‐penicillamine: 1200 mg/day; duration: not stated. Group 2: placebo (n = 91). | |
| Outcomes | Mortality, liver transplantation. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: information not available. |
| Allocation concealment (selection bias) | Low risk | Quote: "Opaque sealed envelopes" (author's reply). |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "Double blind trial, identical appearing placebo" (author's reply). |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "Assessors were blinded, identical placebo" (author's reply). |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: information not available. |
| Selective reporting (reporting bias) | High risk | Comment: adverse events not reported. |
| For‐profit bias | Unclear risk | Quote: "Not pharmaceutical funding" (author's reply). |
| Other bias | Low risk | Comment: no other source of bias. |
| Methods | Randomised clinical trial. | |
| Participants | Country: multicentric; international. Number randomised: 217. Post‐randomisation dropouts: 1. Revised sample size: 216. Mean age: 56 years. Females: 196 (90.7%). Symptomatic participants: not stated. AMA positive: not stated. Responders: 0 (0%). Mean follow‐up period (for all groups): all participants followed up for 12 months. Inclusion criteria
| |
| Interventions | Participants were randomly assigned to 2 groups. Group 1: obeticholic acid (low) + UDCA (moderate) (n = 143). Further details: obeticholic acid: 5 mg to 10 mg for 1 year + UDCA: 13 mg/kg/day to 15 mg/kg/day for 1 year. Group 2: UDCA (moderate) (n = 73). Further details: UDCA: 13 mg/kg/day to 15 mg/kg/day for 1 year. | |
| Outcomes | Mortality, adverse events. | |
| Notes | Reasons for post‐randomisation dropouts: withdrawal from study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "On a predefined randomization code (generated by the Sponsor or designee) using an IWRS". |
| Allocation concealment (selection bias) | Low risk | Quote: "The randomization number will be recorded in the CRF and will serve for patient identification and for assignment of appropriate study medication and bottle number(s) by the IWRS". |
| Blinding of participants and personnel (performance bias) | Low risk | Comment: identical placebo used and authors stated double‐blind. |
| Blinding of outcome assessment (detection bias) | Low risk | Comment: identical placebo used and authors stated double‐blind. |
| Incomplete outcome data (attrition bias) | High risk | Comment: there were post‐randomisation dropouts. |
| Selective reporting (reporting bias) | Low risk | Comment: mortality and morbidity reported. |
| For‐profit bias | High risk | Quote: "Supported by Intercept Pharmaceuticals". |
| Other bias | Low risk | Comment: no other source of bias. |
| Methods | Randomised clinical trial. | |
| Participants | Country: Japan. Number randomised: 52. Post‐randomisation dropouts: 7 (13.5%). Revised sample size: 45. Mean age: 59 years. Females: 41 (91.1%). Symptomatic participants: 17 (37.8%). AMA positive: 41 (91.1%). Responders: not stated. Mean follow‐up period (for all groups): not stated. Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to 2 groups. Group 1: UDCA (low) (n = 22). Further details: UDCA: 600 mg/day for 24 weeks. Group 2: placebo (n = 23). | |
| Outcomes | None of the outcomes of interest reported. | |
| Notes | Reasons for post‐randomisation dropouts: worsening liver disease, lack of compliance. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: information not available. |
| Allocation concealment (selection bias) | Low risk | Quote: "The patients were allocated to two groups, a UDCA group and a placebo group, by a single monitor according to a randomization scheme in which the number of patients allocated to two groups tended to be equal". |
| Blinding of participants and personnel (performance bias) | Low risk | Comment: identical placebo used in this double‐blind trial. |
| Blinding of outcome assessment (detection bias) | Low risk | Comment: identical placebo used in this double‐blind trial. |
| Incomplete outcome data (attrition bias) | High risk | Comment: there were post‐randomisation dropouts. |
| Selective reporting (reporting bias) | High risk | Comment: neither mortality nor adverse events reported. |
| For‐profit bias | High risk | Quote: "UDCA and placebo tablets were generously furnished by Tokyo Tanabe Pharmaceutical Company". |
| Other bias | Low risk | Comment: no other source of bias. |
| Methods | Randomised clinical trial. | |
| Participants | Country: Greece. Number randomised: 92. Post‐randomisation dropouts: 6 (6.5%). Revised sample size: 86. Mean age: 54 years. Females: 77 (89.5%). Symptomatic participants: 86 (100%). AMA positive: not stated. Responders: not stated. Mean follow‐up period (for all groups): 89 months. Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to 2 groups. Group 1: UDCA (moderate) (n = 43). Further details: UDCA: 12 mg/kg//day to 15 mg/kg//day for ≥ 2 years. Group 2: control (n = 43). | |
| Outcomes | Mortality, liver transplantation, decompensated liver disease. | |
| Notes | Reasons for post‐randomisation dropouts: not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was carried out by serially numbered sealed envelopes containing random table numbers 14 patients crossed over from placebo to UDCA". |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomization was carried out by serially numbered sealed envelopes containing random table numbers 14 patients crossed over from placebo to UDCA". |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "Patients and healthcare providers were not blinded" (author's reply). |
| Blinding of outcome assessment (detection bias) | High risk | Quote: "The outcome assessors were not blinded" (author's reply). |
| Incomplete outcome data (attrition bias) | High risk | Comment: there were post‐randomisation dropouts in the initial report. |
| Selective reporting (reporting bias) | High risk | Comment: adverse events not reported. |
| For‐profit bias | High risk | Quote: "Support for this work was provided during the first 2 years of the study by a research grant the pharmaceutical company Galenica Hellas and by the Greek Ministry of Health and Welfare". |
| Other bias | High risk | Comment: 14 participants crossed over from placebo to UDCA. |
| Methods | Randomised clinical trial. | |
| Participants | Country: Spain. Number randomised: 192. Post‐randomisation dropouts: 0 (0%). Revised sample size: 192. Mean age: 54 years. Females: 179 (93.2%). Symptomatic participants: not stated. AMA positive: 172 (89.6%). Responders: not stated. Median follow‐up period (for all groups): 41 months. Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to 2 groups. Group 1: UDCA (moderate) (n = 99). Further details: UDCA 14 mg/kg/day to 16 mg/kg/day; duration: 25 to 73 months. Group 2: placebo (n = 93). | |
| Outcomes | Mortality, adverse events. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: information not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: information not available. |
| Blinding of participants and personnel (performance bias) | Low risk | Comment: identical placebo used in this double‐blind trial. |
| Blinding of outcome assessment (detection bias) | Low risk | Comment: identical placebo used in this double‐blind trial. |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: unclear whether all participants were included in the analysis. |
| Selective reporting (reporting bias) | Low risk | Comment: mortality and morbidity reported. |
| For‐profit bias | High risk | Quote: "We are indebted to Zambon S. A., Laboratorio Farmaceutico for supplying the UDCA and placebo capsules, and for the invaluable administrative support". |
| Other bias | Low risk | Comment: no other risk of bias. |
| Methods | Randomised clinical trial. | |
| Participants | Country: France. Number randomised: 149. Post‐randomisation dropouts: 3 (2%). Revised sample size: 146. Mean age: 56 years. Females: 134 (91.8%). Symptomatic participants: not stated. AMA positive: not stated. Responders: not stated. Mean follow‐up period (for all groups): not stated. Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to 2 groups. Group 1: UDCA (moderate) (n = 73). Further details: UDCA: 13 mg/kg/day to 15 mg/kg/day for 2 years. Group 2: placebo (n = 73). | |
| Outcomes | None of the outcomes of interest reported. | |
| Notes | Reasons for post‐randomisation dropouts: bilirubin > 300 μmol/L, ascites, other coexisting disease. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: information not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: information not available. |
| Blinding of participants and personnel (performance bias) | Low risk | Comment: identical placebo used in this double‐blind trial. |
| Blinding of outcome assessment (detection bias) | Low risk | Comment: identical placebo used in this double‐blind trial. |
| Incomplete outcome data (attrition bias) | High risk | Comment: there were post‐randomisation dropouts. |
| Selective reporting (reporting bias) | High risk | Comment: neither mortality nor adverse events reported. |
| For‐profit bias | High risk | Quote: "This work was supported in part by Synthélabo‐Recherche and in Canada by Jouveinal and Interfalk". |
| Other bias | Low risk | Comment: no other source of bias. |
| Methods | Randomised clinical trial. | |
| Participants | Country: France. Number randomised: 74. Post‐randomisation dropouts: not stated. Revised sample size: 74. Mean age: 54 years. Females: 63 (85.1%). Symptomatic participants: not stated. AMA positive: not stated. Responders: not stated. Mean follow‐up period (for all groups): all participants followed up for 24 months. Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to 2 groups. Group 1: UDCA (moderate) + colchicine (n = 37). Further details: UDCA: 13 mg/kg/day to 15 mg/kg/day for 2 years + colchicine: 1 mg/day for 5 days in a week for 2 years. Group 2: UDCA (moderate) (n = 37). Further details: UDCA: 13 mg/kg/day to 15 mg/kg/day for 2 years. | |
| Outcomes | Mortality, adverse events. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: information not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: information not available. |
| Blinding of participants and personnel (performance bias) | Low risk | Comment: identical placebo used in this double‐blind trial. |
| Blinding of outcome assessment (detection bias) | Low risk | Comment: identical placebo used in this double‐blind trial. |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: unclear whether all randomised participants were included for analysis. |
| Selective reporting (reporting bias) | Low risk | Comment: mortality and morbidity reported. |
| For‐profit bias | High risk | Quote: "Supported in part by Laboratoires Houde (France) and Jouveinal (Canada)". |
| Other bias | Low risk | Comment: no other source of bias. |
| Methods | Randomised clinical trial. | |
| Participants | Country: Germany. Number randomised: 28. Post‐randomisation dropouts: 8 (28.6%). Revised sample size: 20. Mean age: 54 years. Females: 20 (100%). Symptomatic participants: not stated. AMA positive: not stated. Responders: not stated. Mean follow‐up period (for all groups): all participants followed up for 24 months. Inclusion criteria
| |
| Interventions | Participants were randomly assigned to 2 groups. Group 1: UDCA (moderate) + colchicine (n = 8). Further details: UDCA: 10 mg/kg/day to 12 mg/kg/day for 24 months + colchicine: 1 mg/day for 24 months. Group 2: UDCA (moderate) (n = 12). Further details: UDCA: 10 mg/kg/day to 12 mg/kg/day for 24 months. | |
| Outcomes | Adverse events. | |
| Notes | Reasons for post‐randomisation dropouts: adverse events, lost to follow‐up. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: information not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: information not available. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: although a placebo used in this double‐blind trial, unclear whether the placebo was identical. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: although a placebo used in this double‐blind trial, unclear whether the placebo was identical. |
| Incomplete outcome data (attrition bias) | High risk | Comment: there were post‐randomisation dropouts. |
| Selective reporting (reporting bias) | High risk | Comment: mortality not reported. |
| For‐profit bias | Unclear risk | Comment: information not available. |
| Other bias | Low risk | Comment: no other source of bias. |
| Methods | Randomised clinical trial. | |
| Participants | Country: Finland. Number randomised: 77. Post‐randomisation dropouts: 8 (10.4%). Revised sample size: 69. Mean age: 53 years. Females: 60 (87%). Symptomatic participants: not stated. AMA positive: not stated. Responders: not stated. Mean follow‐up period (for all groups): all participants followed up for 36 months. Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to 2 groups. Group 1: UDCA (moderate) + glucocorticosteroids (n = 37). Further details: UDCA: 15 mg/kg/day for 3 years + budesonide: 6 mg/day for 3 years. Group 2: UDCA (moderate) (n = 32). Further details: UDCA: 15 mg/kg/day for 3 years. | |
| Outcomes | Adverse events. | |
| Notes | Reasons for post‐randomisation dropouts: adverse effects, death, refused follow‐up biopsy. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: information not available. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Randomization was done centrally at Helsinki University Hospital with sealed envelopes in a block of 10". Comment: further details of sealed envelope technique not available. |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "Study design was randomized but open because placebo for budesonide was not available for us". |
| Blinding of outcome assessment (detection bias) | High risk | Quote: "Study design was randomized but open because placebo for budesonide was not available for us". |
| Incomplete outcome data (attrition bias) | High risk | Comment: there were post‐randomisation dropouts. |
| Selective reporting (reporting bias) | High risk | Comment: mortality not reported. |
| For‐profit bias | High risk | Quote: "Medication was supplied free of charge by AstraZeneca Finland (budesonide, Entocort) and Leiras Finland (UDCA, Adursal)". |
| Other bias | Low risk | Comment: no other source of bias. |
| Methods | Randomised clinical trial. | |
| Participants | Country: USA. Number randomised: 20. Post‐randomisation dropouts: 1 (5%). Revised sample size: 19. Mean age: not stated. Females: not stated. Symptomatic participants: not stated. AMA positive: not stated. Responders: not stated. Mean follow‐up period (for all groups): all participants followed up for 18 months. Inclusion criteria
| |
| Interventions | Participants were randomly assigned to 2 groups. Group 1: UDCA (low) (n = 9). Further details: UDCA (low): 8 mg/kg/day to 12 mg/kg/day for 6 months. Group 2: placebo (n = 10). | |
| Outcomes | None of the outcomes of interest reported. | |
| Notes | Reasons for post‐randomisation dropouts: had coexisting gallstones. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: information not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: information not available. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: although a placebo used in this double‐blind trial, unclear whether the placebo was identical. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: although a placebo used in this double‐blind trial, unclear whether the placebo was identical. |
| Incomplete outcome data (attrition bias) | High risk | Comment: there were post‐randomisation dropouts. |
| Selective reporting (reporting bias) | High risk | Comment: neither mortality nor adverse events reported. |
| For‐profit bias | High risk | Quote: "and ursodiol supplies provided by Ciba‐Geigy Corporation". |
| Other bias | Low risk | Comment: no other source of bias. |
| Methods | Randomised clinical trial. | |
| Participants | Country: UK. Number randomised: 20. Post‐randomisation dropouts: not stated. Revised sample size: 20. Mean age: not stated. Females: not stated. Symptomatic participants: not stated. AMA positive: not stated. Responders: not stated. Mean follow‐up period (for all groups): not stated. Inclusion criteria
| |
| Interventions | Participants were randomly assigned to 2 groups. Group 1: antioxidants (n = not stated). Further details: antioxidant: cocktail of vitamin E 100 mg, zinc 135 mg, and selenium 100 μg daily; duration: not stated. Group 2: placebo (n = not stated). | |
| Outcomes | None of the outcomes of interest reported. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: information not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: information not available. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: although a placebo was used, no mention of blinding made. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: although a placebo was used, no mention of blinding made. |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: information not available. |
| Selective reporting (reporting bias) | High risk | Comment: neither mortality nor adverse events reported. |
| For‐profit bias | Unclear risk | Comment: information not available. |
| Other bias | Low risk | Comment: no other source of bias. |
| Methods | Randomised clinical trial. | |
| Participants | Country: Belgium. Number randomised: 14. Post‐randomisation dropouts: not stated. Revised sample size: 14. Mean age: 51 years. Females: 12 (85.7%). Symptomatic participants: not stated. AMA positive: 13 (92.9%). Responders: not stated. Mean follow‐up period (for all groups): all participants followed up for 24 months. Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to 2 groups. Group 1: UDCA (low) + methotrexate (n = 8). Further details: UDCA: 500 mg/day; duration: not stated + methotrexate: 15 mg/week; duration: not stated. Group 2: control (n = 6). | |
| Outcomes | None of the outcomes of interest reported. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Using a random number table…". |
| Allocation concealment (selection bias) | Unclear risk | Comment: information not available. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: information not available. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: information not available. |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: information not available. |
| Selective reporting (reporting bias) | High risk | Comment: neither mortality nor adverse events reported. |
| For‐profit bias | Unclear risk | Comment: information not available. |
| Other bias | Low risk | Comment: no other source of bias. |
| Methods | Randomised clinical trial. | |
| Participants | Country: Netherlands. Number randomised: 24. Post‐randomisation dropouts: not stated. Revised sample size: 24. Mean age: 49 years. Females: 23 (95.8%). Symptomatic participants: 24 (100%). AMA positive: not stated. Responders: not stated. Mean follow‐up period (for all groups): all participants followed up for 18 months. Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to 2 groups. Group 1: D‐penicillamine (n = 11). Further details: D‐penicillamine: 250 mg/day to 1000 mg/day (escalating dose) and then 500 mg/day: total duration: 1 year. Group 2: placebo (n = 13). | |
| Outcomes | Mortality, adverse events, decompensated liver disease. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: information not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: information not available. |
| Blinding of participants and personnel (performance bias) | Low risk | Comment: identical placebo used in this double‐blind trial. |
| Blinding of outcome assessment (detection bias) | Low risk | Comment: identical placebo used in this double‐blind trial. |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: information not available. |
| Selective reporting (reporting bias) | Low risk | Comment: mortality and adverse events reported. |
| For‐profit bias | Unclear risk | Comment: information not available. |
| Other bias | Low risk | Comment: no other source of bias. |
| Methods | Randomised clinical trial. | |
| Participants | Country: UK. Number randomised: 35. Post‐randomisation dropouts: not stated. Revised sample size: 35. Mean age: not stated. Females: not stated. Symptomatic participants: not stated. AMA positive: not stated. Responders: not stated. Mean follow‐up period (for all groups): not stated. Inclusion criteria
| |
| Interventions | Participants were randomly assigned to 2 groups. Group 1: D‐penicillamine (n = not stated). Further details: D‐penicillamine: 250 mg to 875 mg (escalating dose). Group 2: placebo (n = not stated). | |
| Outcomes | Mortality. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: information not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: information not available. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: in this double‐blind trial, unclear whether the placebo was identical to active treatment. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: in this double‐blind trial, unclear whether the placebo was identical to active treatment. |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: information not available. |
| Selective reporting (reporting bias) | High risk | Comment: adverse events not reported. |
| For‐profit bias | High risk | Quote: "The UDCA and placebo tablets were generously donated by Thames Laboratories, Wrexham, Wales". |
| Other bias | Low risk | Comment: no other source of bias. |
| Methods | Randomised clinical trial. | |
| Participants | Country: UK. Number randomised: 46. Post‐randomisation dropouts: 0 (0%). Revised sample size: 46. Mean age: 58 years. Females: 44 (95.7%). Symptomatic participants: not stated. AMA positive: not stated. Responders: not stated. Mean follow‐up period (for all groups): all participants followed up for 24 months. Inclusion criteria
| |
| Interventions | Participants were randomly assigned to 2 groups. Group 1: UDCA (low) (n = 22). Further details: UDCA: 10 mg/kg/day for 2 years. Group 2: placebo (n = 24). | |
| Outcomes | Mortality, liver transplantation, cirrhosis. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: information not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: information not available. |
| Blinding of participants and personnel (performance bias) | Low risk | Comment: identical placebo used in this double‐blind trial. |
| Blinding of outcome assessment (detection bias) | Low risk | Comment: identical placebo used in this double‐blind trial. |
| Incomplete outcome data (attrition bias) | Low risk | Comment: no post‐randomisation dropouts. |
| Selective reporting (reporting bias) | High risk | Comment: adverse events not reported. |
| For‐profit bias | Unclear risk | Comment: information not available. |
| Other bias | Low risk | Comment: no other source of bias. |
| Methods | Randomised clinical trial. | |
| Participants | Country: Japan. Number randomised: 20. Post‐randomisation dropouts: not stated. Revised sample size: 20. Mean age: not stated. Females: 16 (80%). Symptomatic participants: not stated. AMA positive: not stated. Responders: 0 (0%). Mean follow‐up period (for all groups): not stated. Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to 2 groups. Group 1: lamivudine (n = not stated). Further details: lamivudine: 100 mg/day for 3 months. Group 2: placebo (n = not stated). | |
| Outcomes | None of the outcomes of interest reported. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: information not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: information not available. |
| Blinding of participants and personnel (performance bias) | Low risk | Comment: identical placebo used in this double‐blind trial. |
| Blinding of outcome assessment (detection bias) | Low risk | Comment: identical placebo used in this double‐blind trial. |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: information not available. |
| Selective reporting (reporting bias) | High risk | Comment: neither mortality nor adverse events reported. |
| For‐profit bias | Unclear risk | Comment: information not available. |
| Other bias | Low risk | Comment: no other source of bias. |
| Methods | Randomised clinical trial. | |
| Participants | Country: Netherlands. Number randomised: 61. Post‐randomisation dropouts: 2 (3.3%). Revised sample size: 59. Mean age: 57 years. Females: 55 (93.2%). Symptomatic participants: not stated. AMA positive: not stated. Responders: 0 (0%). Mean follow‐up period (for all groups): not stated. Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to 2 groups. Group 1: UDCA (low) (n = 32). Further details: UDCA (low): 10 mg/kg/day for 6 months. Group 2: UDCA (moderate) (n = 27). Further details: UDCA (moderate): 20 mg/kg/day for 6 months. | |
| Outcomes | Adverse events. | |
| Notes | Reasons for post‐randomisation dropouts: developed liver failure, lost to follow‐up. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Tables with random numbers" (author's reply). |
| Allocation concealment (selection bias) | Low risk | Quote: "Opaque closed envelopes" (author's reply). |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "randomised open controlled trial". |
| Blinding of outcome assessment (detection bias) | High risk | Quote: "randomised open controlled trial". |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: information not available. |
| Selective reporting (reporting bias) | High risk | Comment: mortality not reported. |
| For‐profit bias | High risk | Quote: "This study was supported in part by Zambon Nederland BV, Amersfoort, the Netherlands". |
| Other bias | Low risk | Comment: no other source of bias. |
| Methods | Randomised clinical trial. | |
| Participants | Country: UK. Number randomised: 64. Post‐randomisation dropouts: not stated. Revised sample size: 64. Mean age: not stated. Females: not stated. Symptomatic participants: not stated. AMA positive: 64 (100%). Responders: not stated. Median follow‐up period (for all groups): 19 months. Inclusion criteria
| |
| Interventions | Participants were randomly assigned to 2 groups. Group 1: colchicine (n = 34). Further details: colchicine: 0.5 mg BD; duration: not stated. Group 2: placebo (n = 30). | |
| Outcomes | Mortality, adverse events. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "To ensure that treatment groups were comparable, patients were stratified according to serum bilirubin level at entry (A, < 19/µmol/1; B, 20‐34/ µmol/L; C, 35‐102/µmol/L; D, >102/µmol/1). The first patient in any pair was allocated by the staff pharmacist to the treatment or placebo group by reference to random tables. The pair was completed when another patient, in the same bilirubin group and with an age within 5 years of the first patient, was entered into the study. The second member of the pair was allocated to the alternative treatment group. The study was double‐blind". |
| Allocation concealment (selection bias) | Low risk | Quote: "To ensure that treatment groups were comparable, patients were stratified according to serum bilirubin level at entry (A, < 19/µmol/1; B, 20‐34/ µmol/L; C, 35‐102/µmol/L; D, >102/µmol/1). The first patient in any pair was allocated by the staff pharmacist to the treatment or placebo group by reference to random tables. The pair was completed when another patient, in the same bilirubin group and with an age within 5 years of the first patient, was entered into the study. The second member of the pair was allocated to the alternative treatment group. The study was double‐blind". |
| Blinding of participants and personnel (performance bias) | Low risk | Comment: identical placebo used in this double‐blind trial. |
| Blinding of outcome assessment (detection bias) | Low risk | Comment: identical placebo used in this double‐blind trial. |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: information not available. |
| Selective reporting (reporting bias) | Low risk | Comment: mortality and morbidity reported. |
| For‐profit bias | Unclear risk | Comment: information not available. |
| Other bias | Low risk | Comment: no other source of bias. |
| Methods | Randomised clinical trial. | |
| Participants | Country: USA. Number randomised: 40. Post‐randomisation dropouts: 11 (27.5%). Revised sample size: 29. Mean age: 46 years. Females: 28 (96.6%). Symptomatic participants: not stated. AMA positive: not stated. Responders: not stated. Median follow‐up period (for all groups): 35 months. Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to 2 groups. Group 1: ciclosporin (n = 19). Further details: ciclosporin: 4 mg/kg/day. Group 2: placebo (n = 10). | |
| Outcomes | Mortality, adverse events, liver transplantation. | |
| Notes | Reasons for post‐randomisation dropouts: follow‐up < 1 year. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: information not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: information not available. |
| Blinding of participants and personnel (performance bias) | Low risk | Comment: identical placebo used in this double‐blind trial. |
| Blinding of outcome assessment (detection bias) | Low risk | Comment: identical placebo used in this double‐blind trial. |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: information not available. |
| Selective reporting (reporting bias) | Low risk | Comment: mortality and morbidity reported. |
| For‐profit bias | High risk | Quote: "Supported by a grant from Sandoz and by the Mayo foundation". |
| Other bias | Low risk | Comment: no other source of bias. |
| Methods | Randomised clinical trial. | |
| Participants | Country: Netherlands. Number randomised: 50. Post‐randomisation dropouts: not stated. Revised sample size: 50. Mean age: 52 years. Females: 45 (90%). Symptomatic participants: not stated. AMA positive: not stated. Responders: 0 (0%). Mean follow‐up period (for all groups): all participants followed up for 12 months. Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to 2 groups. Group 1: UDCA (moderate) + azathioprine + glucocorticosteroids (n = 26). Further details: UDCA: 10 mg/kg/day for 6 months + azathioprine: 50 mg/day for 6 months + prednisolone: 10 mg/day for 6 months. Group 2: UDCA (moderate) (n = 24). Further details: UDCA: 10 mg/kg/day for 6 months. | |
| Outcomes | Adverse events, cirrhosis. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Tables with random numbers" (author's reply). |
| Allocation concealment (selection bias) | Low risk | Quote: "Opaque closed envelopes" (author's reply). |
| Blinding of participants and personnel (performance bias) | Low risk | Comment: identical placebo used in this double‐blind trial. |
| Blinding of outcome assessment (detection bias) | Low risk | Comment: identical placebo used in this double‐blind trial. |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: information not available. |
| Selective reporting (reporting bias) | High risk | Comment: mortality not reported. |
| For‐profit bias | High risk | Quote: "Supported by…Zambon Nederland B.v. and Glaxo Wellcome Research and Development Ltd". |
| Other bias | Low risk | Comment: no other source of bias. |
| Methods | Randomised clinical trial. | |
| Participants | Country: Japan. Number randomised: 11. Post‐randomisation dropouts: not stated. Revised sample size: 11. Mean age: 54 years. Females: 9 (81.8%). Symptomatic participants: 11 (100%). AMA positive: not stated. Responders: not stated. Mean follow‐up period (for all groups): not stated. Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to 2 groups. Group 1: UDCA (low) + colestilan (n = 5). Further details: UDCA: 600 mg/day for 8 weeks + colestilan: 6.42 mg/day for 4 weeks. Group 2: UDCA (low) (n = 6). Further details: UDCA: 600 mg/day for 8 weeks. | |
| Outcomes | Adverse events. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: information not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: information not available. |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "open‐label". |
| Blinding of outcome assessment (detection bias) | High risk | Quote: "open‐label". |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: information not available. |
| Selective reporting (reporting bias) | High risk | Comment: mortality not reported. |
| For‐profit bias | Unclear risk | Comment: information not available. |
| Other bias | Low risk | Comment: no other source of bias. |
AMA: antimitochondrial antibody; BD: twice daily; IV: intravenous; OD: once daily; SAMe: S‐adenosyl methionine; SC: subcutaneous; TUDCA: taurodeoxycholic acid; UDCA: ursodeoxycholic acid.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Long‐term follow‐up of participants included in an included trial (Lindor 1994), but the randomisation was not maintained. | |
| Long‐term follow‐up of participants included in an included trial (Lindor 1994), but the randomisation was not maintained. | |
| Comparison of different administration schedules of the same dose of UDCA. | |
| Not in people with primary biliary cholangitis. | |
| Not a randomised clinical trial. | |
| Not a randomised clinical trial. | |
| Not a randomised clinical trial. | |
| Not a randomised clinical trial. | |
| Not a randomised clinical trial. | |
| Not a randomised clinical trial. | |
| Not a randomised clinical trial. | |
| Not a primary study (editorial). | |
| Cross‐over RCT; no results presented before cross‐over. | |
| Long‐term follow‐up of Nevens 2016, but excluded because randomisation not maintained. | |
| Not a randomised clinical trial. | |
| Not a primary study (letter to editor). | |
| Not a primary study (editorial). | |
| Not a primary study (editorial). | |
| Long‐term follow‐up of participants in an included RCT (Combes 1995a); however, all participants received the intervention after the end of the initial study. | |
| Not a randomised clinical trial. | |
| Long‐term follow‐up of an included trial (Poupon 1991a); however, all participants received the active intervention at the end of the trial period. | |
| Not a primary study (editorial). | |
| Cross‐over RCT; no outcomes of interest reported before cross‐over. | |
| In this RCT of different doses of TUDCA, participants who were intolerant to the drug were replaced. This affected the randomisation. | |
| No separate data on people who were randomised (included non‐randomised participants in the results). | |
| Long‐term follow‐up of an included trial (Poupon 1991a); however, all participants received the active intervention at the end of the trial period. | |
| No separate data on people who were randomised (included non‐randomised participants in the results). | |
| Long‐term follow‐up of an included trial (Combes 1995a); however, all participants received the active intervention at the end of the trial period. | |
| Not a randomised clinical trial. | |
| Not a randomised clinical trial. | |
| Not a randomised clinical trial. | |
| Not a primary study. | |
| Not a randomised clinical trial. | |
| Not a randomised clinical trial. | |
| Not a randomised clinical trial. | |
| Cross‐over RCT; none of the outcomes of interest reported prior to cross‐over. | |
| Cross‐over RCT, no results presented before cross‐over. | |
| Not a primary study. | |
| Not a primary study. | |
| Not a randomised clinical trial. | |
| Not a primary study (letter to editor). | |
| Long‐term follow‐up of an included trial (Lindor 1994); however, all participants received the active intervention at the end of the trial period. | |
| Not a primary study. | |
| Not a primary study (editorial). | |
| Not a primary study (letter to editor). | |
| Long‐term follow‐up of an included trial (Kaplan 1999), but the treatment was changed at the completion of the RCT. | |
| Not a primary study (letter to editor). | |
| Quasi‐randomised study (allocation by case numbers). | |
| Quasi‐randomised study (allocation by case numbers). | |
| Not a randomised clinical trial. | |
| Not a randomised clinical trial. | |
| Long‐term follow‐up of Kowdley 2011, but excluded because randomisation was not maintained. | |
| Not a primary study (commentary). | |
| Not a randomised clinical trial. | |
| Not a randomised clinical trial. | |
| Cross‐over RCT; no results presented before cross‐over. | |
| Not a randomised clinical trial. | |
| Long‐term follow‐up of a subgroup of participants in an included trial (Kaplan 1999), where additional interventions were added after completion of the trial period. | |
| Long‐term follow‐up of a subgroup of participants in an included trial (Kaplan 1999), where additional interventions were added after completion of the trial period. | |
| Not a primary study (review). | |
| Not a primary study (review). | |
| Quasi‐randomised study (allocation by alternation). | |
| Quasi‐randomised study (allocation by alternation). | |
| Quasi‐randomised study (allocation by alternation). | |
| Not a primary study (review). | |
| Not a primary study (review). | |
| Not a primary study (editorial). | |
| Not a primary study (letter to editor). | |
| Not a randomised clinical trial. | |
| Not a randomised clinical trial | |
| Long‐term follow‐up an included RCT (Lindor 1994); however, all participants received the intervention after the completion of the RCT. | |
| Not a randomised clinical trial. | |
| Not a primary study (review). | |
| Long‐term follow‐up an included RCT (Lindor 1994); however, all participants received the intervention after the completion of the RCT. | |
| Not a primary study (letter to editor). | |
| Not a primary study (review). | |
| Not a primary study (review). | |
| Cross‐over RCT; no outcomes reported prior to cross‐over. | |
| Not a randomised clinical trial | |
| Quasi‐randomised study (allocation by case numbers). | |
| Quasi‐randomised study (allocation by case numbers). | |
| Only 4 participants in this trial had primary biliary cholangitis and separate data not available for these 4 participants. | |
| Only 5 participants had primary biliary cholangitis and only 1 of them received placebo. Separate data not available on these participants. | |
| Not a randomised clinical trial. | |
| Not a randomised clinical trial. | |
| Only 5 participants had primary biliary cholangitis. Separate data not available for these participants. | |
| Not a randomised clinical trial. | |
| Cross‐over study of different doses of UDCA; outcomes not reported at the end of first treatment. | |
| Not a primary study (review). | |
| Not a primary study (review). | |
| Not a primary study (commentary). | |
| Long‐term follow‐up of an included trial (Poupon 1991a); however, all participants received the active intervention at the end of the trial period. | |
| Not a primary study. | |
| Not a randomised clinical trial. | |
| Not a primary study. | |
| Not a randomised clinical trial. | |
| Not a primary study (editorial). | |
| Not a randomised clinical trial. | |
| Not a randomised clinical trial. | |
| Unclear whether this was a randomised clinical trial. | |
| Comparison of 2 doses of D‐penicillamine with no other treatment as comparator. | |
| In this RCT of different doses of TUDCA, participants who were intolerant to the drug were replaced. This affected the randomisation. | |
| In this RCT of different doses of TUDCA, participants who were intolerant to the drug were replaced. This affected the randomisation. | |
| Not a randomised clinical trial. | |
| Not a randomised clinical trial. | |
| Not a pharmacological agent. | |
| Not a pharmacological agent. | |
| Cross‐over RCT; no results presented before cross‐over. | |
| Not a primary study (review). | |
| Not a randomised clinical trial. | |
| Quasi‐randomised study (allocation by case numbers). | |
| Quasi‐randomised study (allocation by case numbers). | |
| Not a randomised clinical trial. | |
| Not a randomised clinical trial. | |
| Not a primary study (letter to editor). | |
| Not a randomised clinical trial. | |
| Symptomatic treatment of dyslipidaemia associated with primary biliary cholangitis. |
TUDCA: taurodeoxycholic acid; UDCA: ursodeoxycholic acid.
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | Full text not available. |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | Full text not available. |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Biochemical Response of PBC‐AIH Overlap Syndrome Induced by Ursodeoxycholic Acid Only or Combination Therapy of Immunosuppressive Agents |
| Methods | Randomised parallel clinical trial |
| Participants | People with primary biliary cholangitis and autoimmune hepatitis overlap syndrome. |
| Interventions | Group 1: UDCA + immunosuppression Further details: not provided. Group 2: UDCA. Further details: not provided. |
| Outcomes | Adverse events |
| Starting date | Not stated. |
| Contact information | |
| Notes | Status: recruiting. |
| Trial name or title | A 12‐Week, Double‐Blind, Randomized, Placebo‐Controlled, Phase 2 Study to Evaluate the Effects of Two Doses of MBX‐8025 in Subjects with Primary Biliary Cirrhosis (PBC) and an Inadequate Response to Ursodeoxycholic Acid (UDCA). |
| Methods | Randomised, placebo‐controlled, double‐blind clinical trial. |
| Participants | People with primary biliary cholangitis (non‐responders). |
| Interventions | Group 1: MBX‐8025. Further details: not provided. Group 2: placebo. |
| Outcomes | None of the outcomes of interest for this review measured in this trial. |
| Starting date | Not stated. |
| Contact information | |
| Notes | Status: recruiting. |
| Trial name or title | A Phase 3b, Double‐Blind, Randomized, Placebo‐Controlled, Multicenter Study Evaluating the Effect of Obeticholic Acid on Clinical Outcomes in Patients With Primary Biliary Cirrhosis |
| Methods | Phase 3, double‐blind, randomised, placebo‐controlled, multicentre study. |
| Participants | People with primary biliary cholangitis. |
| Interventions | Group 1: obeticholic acid. Further details: obeticholic acid 5 mg to 10 mg tablets once daily for the duration of the study based on tolerability at 3 months. Group 2: placebo. Further details: 1 tablet daily for the remainder of the study. |
| Outcomes | Mortality, liver transplantation, liver decompensation, hepatocellular carcinoma. |
| Starting date | December 2014. |
| Contact information | |
| Notes | Status: recruiting. |
| Trial name or title | The Effect of Bezafibrate on Cholestatic Itch |
| Methods | Double‐blind, randomised, placebo‐controlled clinical trial. |
| Participants | People with primary biliary cholangitis. |
| Interventions | Group 1: bezafibrate. Further details: bezafibrate 400 mg/day. Group 2: placebo. |
| Outcomes | None of the outcomes of interest for this review are measured in this trial. |
| Starting date | February 2016. |
| Contact information | |
| Notes | Status: recruiting. |
| Trial name or title | Fenofibrate in Combination with Ursodeoxycholic Acid in Primary Biliary Cirrhosis: a Randomized Control Study |
| Methods | Phase 3, open‐label, randomised clinical trial. |
| Participants | People with primary biliary cholangitis. |
| Interventions | Group 1: UDCA + fenofibrate. Further details: not provided. Group 2: UDCA. Further details: not provided. |
| Outcomes | None of the outcomes of interest for this review are measured in this trial. |
| Starting date | January 2016. |
| Contact information | |
| Notes | Status: recruiting. |
| Trial name or title | Fenofibrate for Patients with Primary Biliary Cirrhosis who had an Inadequate Response to Ursodeoxycholic Acid |
| Methods | Phase 3, open‐label, randomised clinical trial. |
| Participants | People with primary biliary cholangitis. |
| Interventions | Group 1: UDCA + fenofibrate Further details: not provided. Group 2: UDCA. Further details: not provided. |
| Outcomes | None of the outcomes of interest for this review measured in this trial. |
| Starting date | January 2016. |
| Contact information | |
| Notes | Status: recruiting. May be the same as NCT02823353. |
| Trial name or title | Efficacy and Security of Bezafibrate in Patients with Primary Biliary Cirrhosis without Biochemical Response to Ursodeoxycholic Acid: a Randomized, Double‐blind, Placebo‐controlled Trial |
| Methods | Randomised, double‐blind, placebo‐controlled clinical trial. |
| Participants | People with primary biliary cholangitis (non‐responders). |
| Interventions | Group 1: UDCA + bezafibrate. Further details: bezafibrate 200 mg capsule every 12 hours + UDCA 13 mg/kg/day to 15 mg/kg/day for 12 months. Group 2: UDCA + placebo. Further details: placebo capsule (for bezafibrate 200 mg capsule) every 12 hours + UDCA 13 mg/kg/day to 15 mg/kg/day for 12 months. |
| Outcomes | Quality of life. |
| Starting date | October 2016. |
| Contact information | |
| Notes | Status: recruiting. |
| Trial name or title | A Phase 2, Randomized, Double‐Blind, Placebo Controlled Study Evaluating the Safety, Tolerability, and Efficacy of GS‐9674 in Subjects with Primary Biliary Cholangitis without Cirrhosis |
| Methods | Randomised, double‐blind, placebo‐controlled clinical trial. |
| Participants | People with primary biliary cholangitis. |
| Interventions | Group 1: GS‐9674. Further details: GS‐9674 30 mg for 12 weeks. Group 2: placebo. |
| Outcomes | Adverse events. |
| Starting date | December 2016. |
| Contact information | GS‐US‐427‐[email protected] |
| Notes | Status: recruiting. |
| Trial name or title | A Randomized Controlled Clinical Trial on the Efficacy and Safety of Fenofibrate Combined with Ursodeoxycholic Acid in PBC Patients with an Incomplete Biochemical Response to UDCA |
| Methods | Open‐label, randomised clinical trial. |
| Participants | People with primary biliary cholangitis. |
| Interventions | Group 1: fenofibrate + UDCA. Further details: UDCA 13 mg/kg/day to 15 mg/kg/day + fenofibrate 200 mg once daily for 12 months. Group 2: UDCA. Further details: UDCA 13 mg/kg/day to 15 mg/kg/day. |
| Outcomes | None of the outcomes of interest for this review measured in this trial. |
| Starting date | January 2016. |
| Contact information | |
| Notes | Status: recruiting. |
UDCA: ursodeoxycholic acid.
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality at maximal follow‐up Show forest plot | 28 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.1  Comparison 1 Main analysis, Outcome 1 Mortality at maximal follow‐up. | ||||
| 1.1 Azathioprine versus no intervention | 2 | 224 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.32, 0.98] |
| 1.2 Chlorambucil versus no intervention | 1 | 24 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.01, 3.28] |
| 1.3 Colchicine versus no intervention | 2 | 122 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.32, 1.85] |
| 1.4 Cyclosporin versus no intervention | 3 | 390 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.51, 1.50] |
| 1.5 D‐Penicillamine versus no intervention | 5 | 423 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.57, 1.44] |
| 1.6 Glucocorticosteroids versus no intervention | 1 | 36 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.14, 2.92] |
| 1.7 Malotilate versus no intervention | 1 | 101 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.47, 8.48] |
| 1.8 Methotrexate versus no intervention | 1 | 60 | Odds Ratio (M‐H, Fixed, 95% CI) | 8.83 [1.01, 76.96] |
| 1.9 UDCA versus no intervention | 6 | 734 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.60, 1.64] |
| 1.10 Bezafibrate plus UDCA versus UDCA | 1 | 27 | Odds Ratio (M‐H, Fixed, 95% CI) | 9.67 [0.45, 207.78] |
| 1.11 Colchicine plus UDCA versus UDCA | 2 | 158 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.84 [0.38, 8.91] |
| 1.12 Methotrexate plus UDCA versus UDCA | 2 | 290 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.55, 2.51] |
| 1.13 Obeticholic acid plus UDCA versus UDCA | 1 | 216 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.55 [0.06, 38.46] |
| 2 Mortality (< 1 year) Show forest plot | 8 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.2 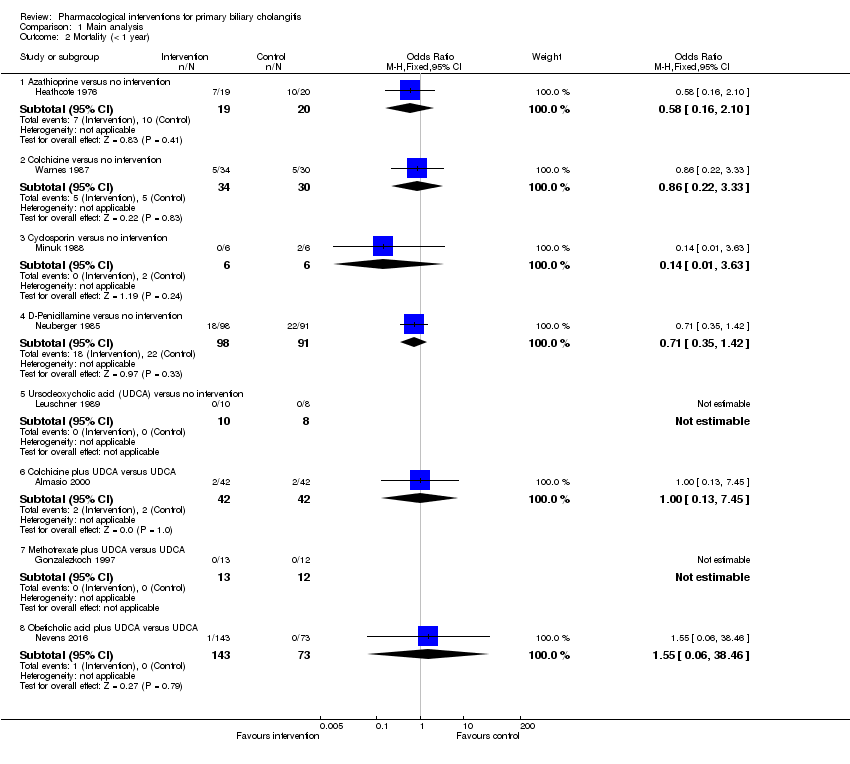 Comparison 1 Main analysis, Outcome 2 Mortality (< 1 year). | ||||
| 2.1 Azathioprine versus no intervention | 1 | 39 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.16, 2.10] |
| 2.2 Colchicine versus no intervention | 1 | 64 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.22, 3.33] |
| 2.3 Cyclosporin versus no intervention | 1 | 12 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.01, 3.63] |
| 2.4 D‐Penicillamine versus no intervention | 1 | 189 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.35, 1.42] |
| 2.5 Ursodeoxycholic acid (UDCA) versus no intervention | 1 | 18 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.6 Colchicine plus UDCA versus UDCA | 1 | 84 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.13, 7.45] |
| 2.7 Methotrexate plus UDCA versus UDCA | 1 | 25 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.8 Obeticholic acid plus UDCA versus UDCA | 1 | 216 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.55 [0.06, 38.46] |
| 3 Mortality (1 to 5 years) Show forest plot | 20 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.3  Comparison 1 Main analysis, Outcome 3 Mortality (1 to 5 years). | ||||
| 3.1 Azathioprine versus no intervention | 1 | 185 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.30, 1.04] |
| 3.2 Chlorambucil versus no intervention | 1 | 24 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.01, 3.28] |
| 3.3 Colchicine versus no intervention | 1 | 58 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.22, 2.25] |
| 3.4 Cyclosporin versus no intervention | 2 | 378 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.54, 1.64] |
| 3.5 D‐Penicillamine versus no intervention | 4 | 234 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.59, 2.08] |
| 3.6 Glucocorticosteroids versus no intervention | 1 | 36 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.14, 2.92] |
| 3.7 Malotilate versus no intervention | 1 | 101 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.47, 8.48] |
| 3.8 Methotrexate versus no intervention | 1 | 60 | Odds Ratio (M‐H, Fixed, 95% CI) | 8.83 [1.01, 76.96] |
| 3.9 UDCA versus no intervention | 5 | 716 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.60, 1.64] |
| 3.10 Bezafibrate plus UDCA versus UDCA | 1 | 27 | Odds Ratio (M‐H, Fixed, 95% CI) | 9.67 [0.45, 207.78] |
| 3.11 Colchicine plus UDCA versus UDCA | 1 | 74 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.28 [0.24, 113.87] |
| 3.12 Methotrexate plus UDCA versus UDCA | 1 | 265 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.55, 2.51] |
| 4 Serious adverse events (proportion) Show forest plot | 11 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.4 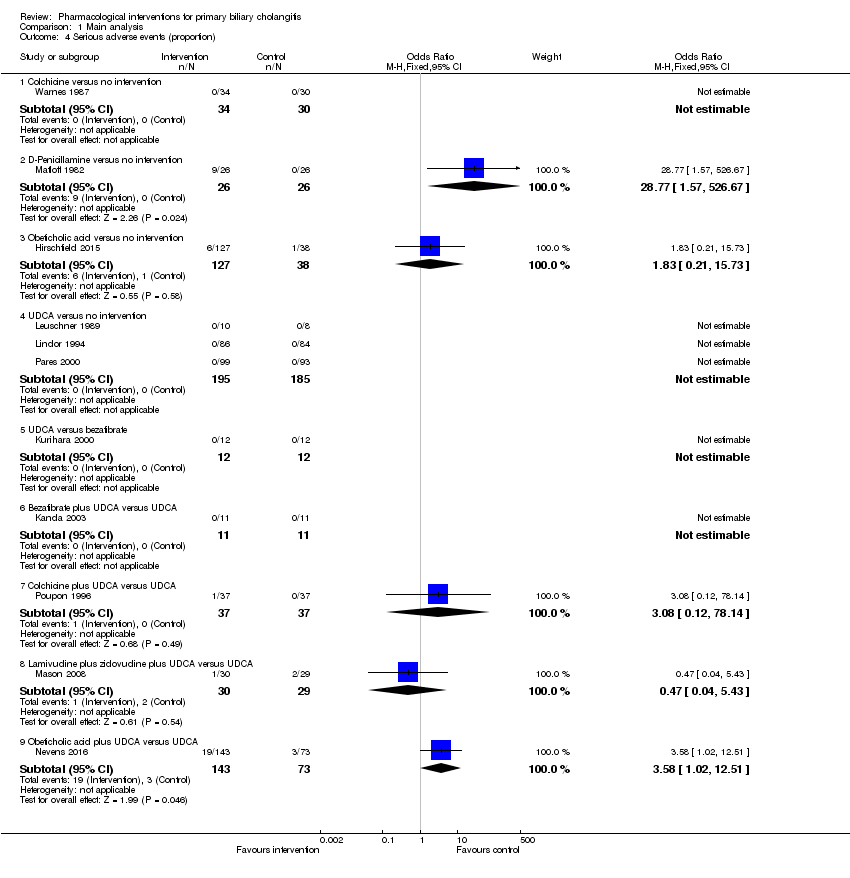 Comparison 1 Main analysis, Outcome 4 Serious adverse events (proportion). | ||||
| 4.1 Colchicine versus no intervention | 1 | 64 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 D‐Penicillamine versus no intervention | 1 | 52 | Odds Ratio (M‐H, Fixed, 95% CI) | 28.77 [1.57, 526.67] |
| 4.3 Obeticholic acid versus no intervention | 1 | 165 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.83 [0.21, 15.73] |
| 4.4 UDCA versus no intervention | 3 | 380 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.5 UDCA versus bezafibrate | 1 | 24 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.6 Bezafibrate plus UDCA versus UDCA | 1 | 22 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.7 Colchicine plus UDCA versus UDCA | 1 | 74 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.08 [0.12, 78.14] |
| 4.8 Lamivudine plus zidovudine plus UDCA versus UDCA | 1 | 59 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.04, 5.43] |
| 4.9 Obeticholic acid plus UDCA versus UDCA | 1 | 216 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.58 [1.02, 12.51] |
| 5 Serious adverse events (number of events) Show forest plot | 1 | Rate Ratio (Fixed, 95% CI) | Subtotals only | |
| Analysis 1.5  Comparison 1 Main analysis, Outcome 5 Serious adverse events (number of events). | ||||
| 5.1 Obeticholic acid plus UDCA versus UDCA | 1 | 216 | Rate Ratio (Fixed, 95% CI) | 1.66 [0.75, 3.66] |
| 6 Adverse events (proportion) Show forest plot | 19 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.6 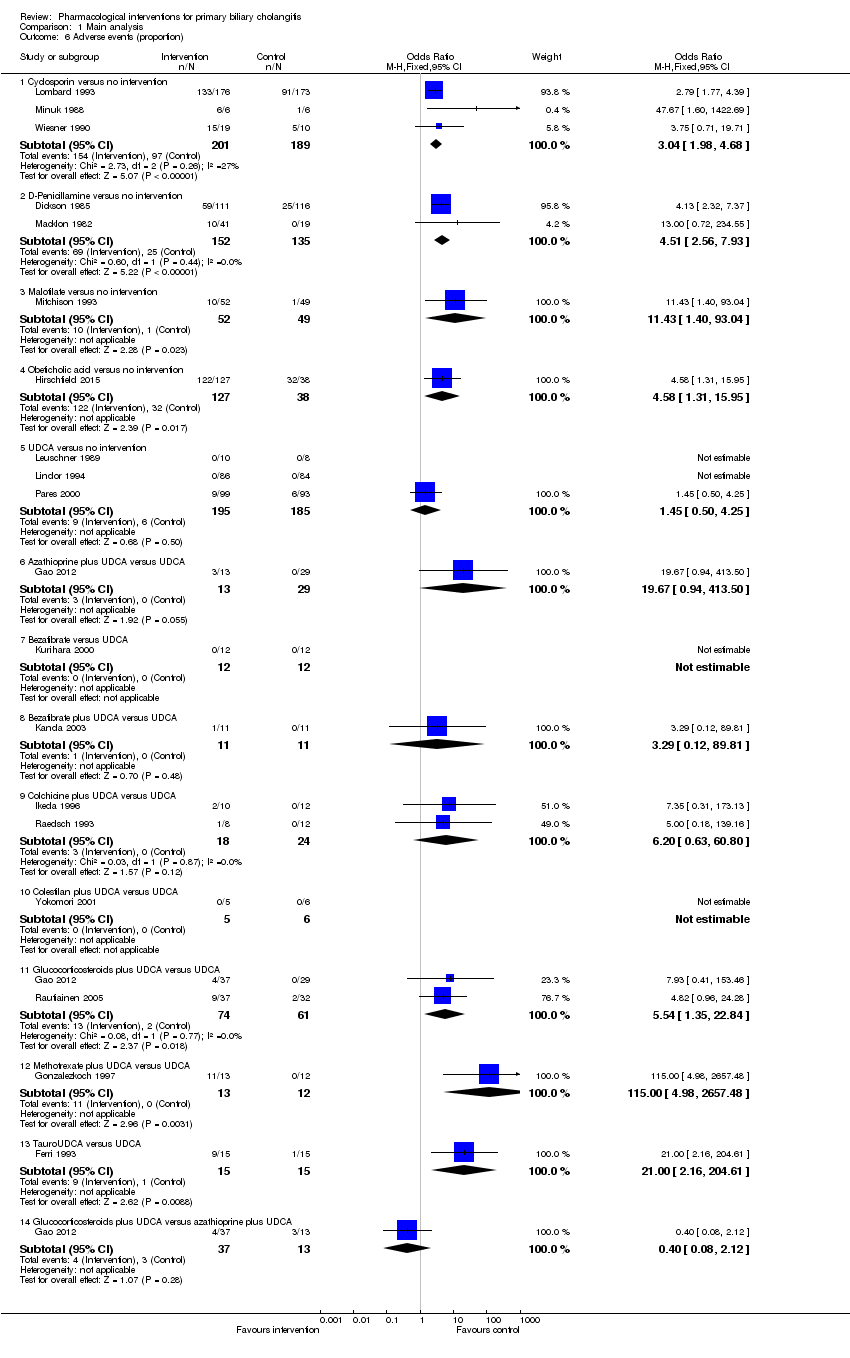 Comparison 1 Main analysis, Outcome 6 Adverse events (proportion). | ||||
| 6.1 Cyclosporin versus no intervention | 3 | 390 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.04 [1.98, 4.68] |
| 6.2 D‐Penicillamine versus no intervention | 2 | 287 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.51 [2.56, 7.93] |
| 6.3 Malotilate versus no intervention | 1 | 101 | Odds Ratio (M‐H, Fixed, 95% CI) | 11.43 [1.40, 93.04] |
| 6.4 Obeticholic acid versus no intervention | 1 | 165 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.58 [1.31, 15.95] |
| 6.5 UDCA versus no intervention | 3 | 380 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.45 [0.50, 4.25] |
| 6.6 Azathioprine plus UDCA versus UDCA | 1 | 42 | Odds Ratio (M‐H, Fixed, 95% CI) | 19.67 [0.94, 413.50] |
| 6.7 Bezafibrate versus UDCA | 1 | 24 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.8 Bezafibrate plus UDCA versus UDCA | 1 | 22 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.29 [0.12, 89.81] |
| 6.9 Colchicine plus UDCA versus UDCA | 2 | 42 | Odds Ratio (M‐H, Fixed, 95% CI) | 6.20 [0.63, 60.80] |
| 6.10 Colestilan plus UDCA versus UDCA | 1 | 11 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.11 Glucocorticosteroids plus UDCA versus UDCA | 2 | 135 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.54 [1.35, 22.84] |
| 6.12 Methotrexate plus UDCA versus UDCA | 1 | 25 | Odds Ratio (M‐H, Fixed, 95% CI) | 115.0 [4.98, 2657.48] |
| 6.13 TauroUDCA versus UDCA | 1 | 30 | Odds Ratio (M‐H, Fixed, 95% CI) | 21.0 [2.16, 204.61] |
| 6.14 Glucocorticosteroids plus UDCA versus azathioprine plus UDCA | 1 | 50 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.08, 2.12] |
| 7 Adverse events (number) Show forest plot | 14 | Rate Ratio (Random, 95% CI) | Subtotals only | |
| Analysis 1.7  Comparison 1 Main analysis, Outcome 7 Adverse events (number). | ||||
| 7.1 Chlorambucil versus no intervention | 1 | 24 | Rate Ratio (Random, 95% CI) | 3.67 [1.04, 12.87] |
| 7.2 Cyclosporin versus no intervention | 3 | 390 | Rate Ratio (Random, 95% CI) | 2.58 [1.26, 5.31] |
| 7.3 D‐Penicillamine versus no intervention | 3 | 303 | Rate Ratio (Random, 95% CI) | 2.99 [1.04, 8.63] |
| 7.4 Malotilate versus no intervention | 1 | 101 | Rate Ratio (Random, 95% CI) | 6.13 [1.38, 27.14] |
| 7.5 Obeticholic acid versus no intervention | 1 | 76 | Rate Ratio (Random, 95% CI) | 1.41 [1.13, 1.75] |
| 7.6 Azathioprine plus glucocorticosteroids plus UDCA versus UDCA | 1 | 50 | Rate Ratio (Random, 95% CI) | 1.32 [0.88, 1.97] |
| 7.7 Bezafibrate plus UDCA versus UDCA | 1 | 29 | Rate Ratio (Random, 95% CI) | 11.79 [0.65, 213.14] |
| 7.8 Colchicine plus UDCA versus UDCA | 1 | 24 | Rate Ratio (Random, 95% CI) | 5.91 [0.28, 123.08] |
| 7.9 Methotrexate plus UDCA versus UDCA | 1 | 27 | Rate Ratio (Random, 95% CI) | 30.64 [1.84, 510.76] |
| 7.10 TauroUDCA versus UDCA | 1 | 191 | Rate Ratio (Random, 95% CI) | 1.17 [0.81, 1.71] |
| 8 Liver transplantation Show forest plot | 11 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.8  Comparison 1 Main analysis, Outcome 8 Liver transplantation. | ||||
| 8.1 Cyclosporin versus no intervention | 2 | 378 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.43, 1.72] |
| 8.2 D‐Penicillamine versus no intervention | 1 | 189 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.06, 15.05] |
| 8.3 Methotrexate versus no intervention | 1 | 60 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.17 [0.02, 1.58] |
| 8.4 UDCA versus no intervention | 5 | 640 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.48, 1.68] |
| 8.5 Bezafibrate plus UDCA versus UDCA | 1 | 27 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.6 Methotrexate plus UDCA versus UDCA | 1 | 265 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.35, 1.39] |
| 9 Decompensated liver disease Show forest plot | 7 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.9 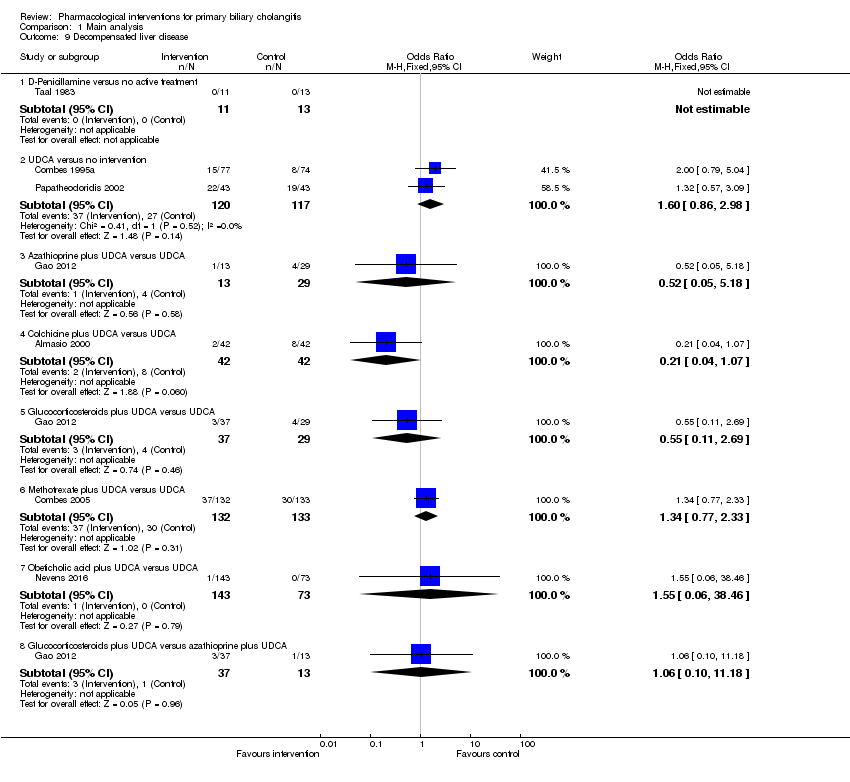 Comparison 1 Main analysis, Outcome 9 Decompensated liver disease. | ||||
| 9.1 D‐Penicillamine versus no active treatment | 1 | 24 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.2 UDCA versus no intervention | 2 | 237 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.60 [0.86, 2.98] |
| 9.3 Azathioprine plus UDCA versus UDCA | 1 | 42 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.05, 5.18] |
| 9.4 Colchicine plus UDCA versus UDCA | 1 | 84 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.04, 1.07] |
| 9.5 Glucocorticosteroids plus UDCA versus UDCA | 1 | 66 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.11, 2.69] |
| 9.6 Methotrexate plus UDCA versus UDCA | 1 | 265 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.34 [0.77, 2.33] |
| 9.7 Obeticholic acid plus UDCA versus UDCA | 1 | 216 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.55 [0.06, 38.46] |
| 9.8 Glucocorticosteroids plus UDCA versus azathioprine plus UDCA | 1 | 50 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.10, 11.18] |
| 10 Cirrhosis Show forest plot | 3 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.10 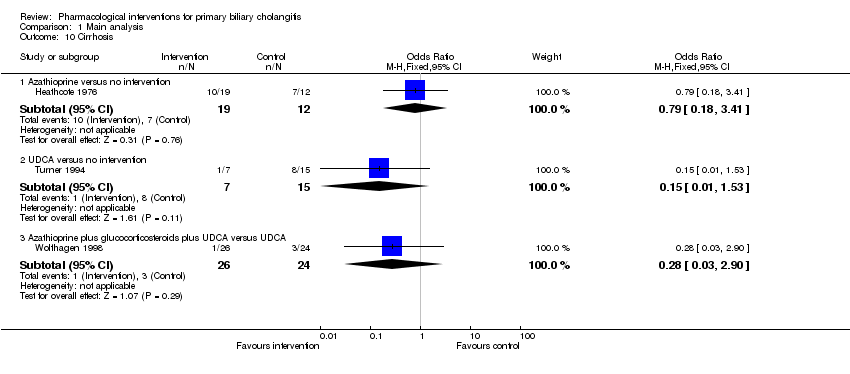 Comparison 1 Main analysis, Outcome 10 Cirrhosis. | ||||
| 10.1 Azathioprine versus no intervention | 1 | 31 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.18, 3.41] |
| 10.2 UDCA versus no intervention | 1 | 22 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.15 [0.01, 1.53] |
| 10.3 Azathioprine plus glucocorticosteroids plus UDCA versus UDCA | 1 | 50 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.28 [0.03, 2.90] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality at maximal follow‐up Show forest plot | 29 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.1 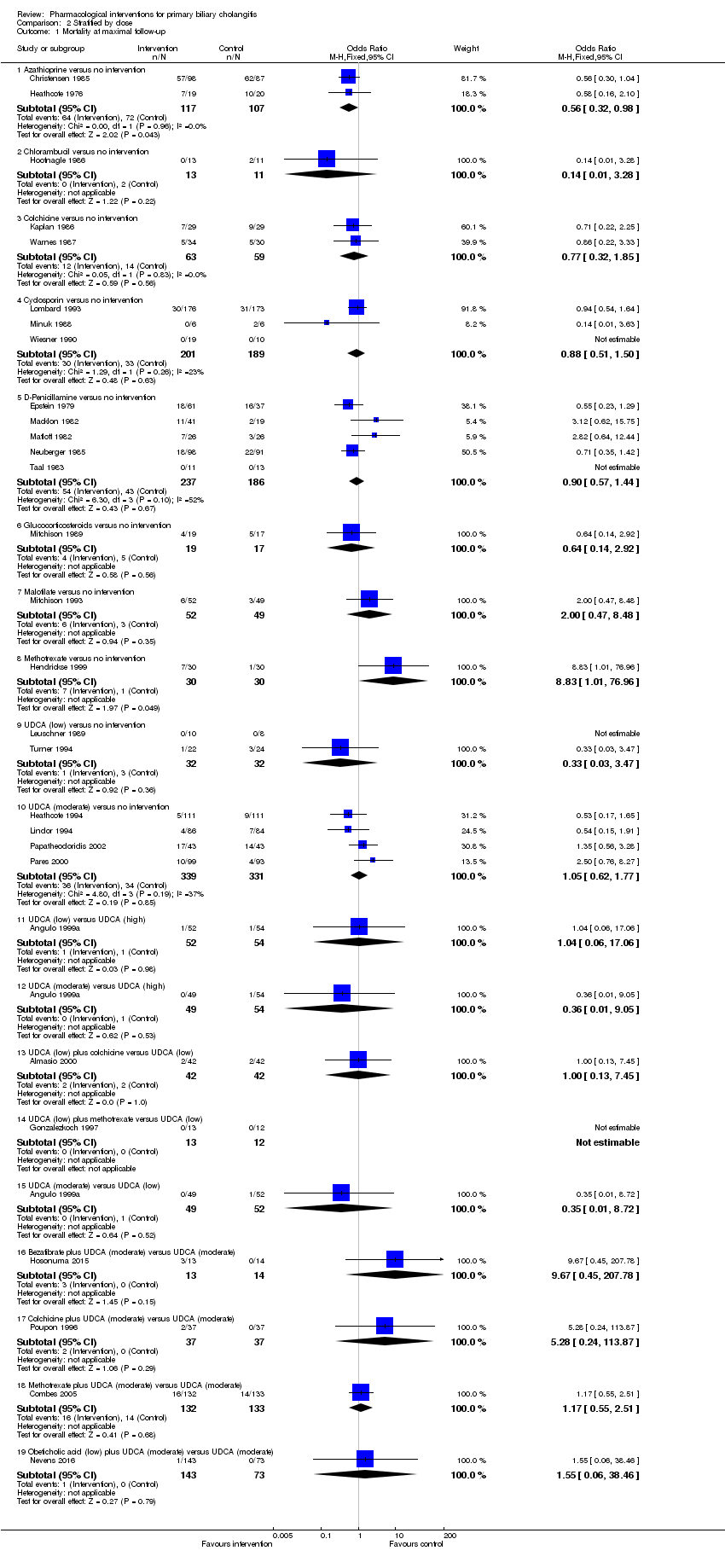 Comparison 2 Stratified by dose, Outcome 1 Mortality at maximal follow‐up. | ||||
| 1.1 Azathioprine versus no intervention | 2 | 224 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.32, 0.98] |
| 1.2 Chlorambucil versus no intervention | 1 | 24 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.01, 3.28] |
| 1.3 Colchicine versus no intervention | 2 | 122 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.32, 1.85] |
| 1.4 Cyclosporin versus no intervention | 3 | 390 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.51, 1.50] |
| 1.5 D‐Penicillamine versus no intervention | 5 | 423 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.57, 1.44] |
| 1.6 Glucocorticosteroids versus no intervention | 1 | 36 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.14, 2.92] |
| 1.7 Malotilate versus no intervention | 1 | 101 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.47, 8.48] |
| 1.8 Methotrexate versus no intervention | 1 | 60 | Odds Ratio (M‐H, Fixed, 95% CI) | 8.83 [1.01, 76.96] |
| 1.9 UDCA (low) versus no intervention | 2 | 64 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.03, 3.47] |
| 1.10 UDCA (moderate) versus no intervention | 4 | 670 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.62, 1.77] |
| 1.11 UDCA (low) versus UDCA (high) | 1 | 106 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.06, 17.06] |
| 1.12 UDCA (moderate) versus UDCA (high) | 1 | 103 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.01, 9.05] |
| 1.13 UDCA (low) plus colchicine versus UDCA (low) | 1 | 84 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.13, 7.45] |
| 1.14 UDCA (low) plus methotrexate versus UDCA (low) | 1 | 25 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.15 UDCA (moderate) versus UDCA (low) | 1 | 101 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.01, 8.72] |
| 1.16 Bezafibrate plus UDCA (moderate) versus UDCA (moderate) | 1 | 27 | Odds Ratio (M‐H, Fixed, 95% CI) | 9.67 [0.45, 207.78] |
| 1.17 Colchicine plus UDCA (moderate) versus UDCA (moderate) | 1 | 74 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.28 [0.24, 113.87] |
| 1.18 Methotrexate plus UDCA (moderate) versus UDCA (moderate) | 1 | 265 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.55, 2.51] |
| 1.19 Obeticholic acid (low) plus UDCA (moderate) versus UDCA (moderate) | 1 | 216 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.55 [0.06, 38.46] |
| 2 Mortality (< 1 year) Show forest plot | 9 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.2  Comparison 2 Stratified by dose, Outcome 2 Mortality (< 1 year). | ||||
| 2.1 Azathioprine versus no intervention | 1 | 39 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.16, 2.10] |
| 2.2 Colchicine versus no intervention | 1 | 64 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.22, 3.33] |
| 2.3 Cyclosporin versus no intervention | 1 | 12 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.01, 3.63] |
| 2.4 D‐Penicillamine versus no intervention | 1 | 189 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.35, 1.42] |
| 2.5 UDCA (low) versus no intervention | 1 | 18 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.6 UDCA (low) versus UDCA (high) | 1 | 106 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.06, 17.06] |
| 2.7 UDCA (moderate) versus UDCA (high) | 1 | 103 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.01, 9.05] |
| 2.8 Obeticholic acid (low) plus UDCA (moderate) versus UDCA (moderate) | 1 | 216 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.55 [0.06, 38.46] |
| 2.9 UDCA (low) plus colchicine versus UDCA (low) | 1 | 84 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.13, 7.45] |
| 2.10 UDCA (low) plus methotrexate versus UDCA (low) | 1 | 25 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.11 UDCA (moderate) versus UDCA (low) | 1 | 101 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.01, 8.72] |
| 3 Mortality (1 to 5 years) Show forest plot | 20 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.3  Comparison 2 Stratified by dose, Outcome 3 Mortality (1 to 5 years). | ||||
| 3.1 Azathioprine versus no intervention | 1 | 185 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.30, 1.04] |
| 3.2 Chlorambucil versus no intervention | 1 | 24 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.01, 3.28] |
| 3.3 Colchicine versus no intervention | 1 | 58 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.22, 2.25] |
| 3.4 Cyclosporin versus no intervention | 2 | 378 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.54, 1.64] |
| 3.5 D‐Penicillamine versus no intervention | 4 | 234 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.59, 2.08] |
| 3.6 Glucocorticosteroids versus no intervention | 1 | 36 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.14, 2.92] |
| 3.7 Malotilate versus no intervention | 1 | 101 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.47, 8.48] |
| 3.8 Methotrexate versus no intervention | 1 | 60 | Odds Ratio (M‐H, Fixed, 95% CI) | 8.83 [1.01, 76.96] |
| 3.9 UDCA (low) versus no intervention | 1 | 46 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.03, 3.47] |
| 3.10 UDCA (moderate) versus no intervention | 4 | 670 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.62, 1.77] |
| 3.11 Bezafibrate plus UDCA (moderate) versus UDCA (moderate) | 1 | 27 | Odds Ratio (M‐H, Fixed, 95% CI) | 9.67 [0.45, 207.78] |
| 3.12 Colchicine plus UDCA (moderate) versus UDCA (moderate) | 1 | 74 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.28 [0.24, 113.87] |
| 3.13 Methotrexate plus UDCA (moderate) versus UDCA (moderate) | 1 | 265 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.55, 2.51] |
| 4 Serious adverse events (proportion) Show forest plot | 12 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.4 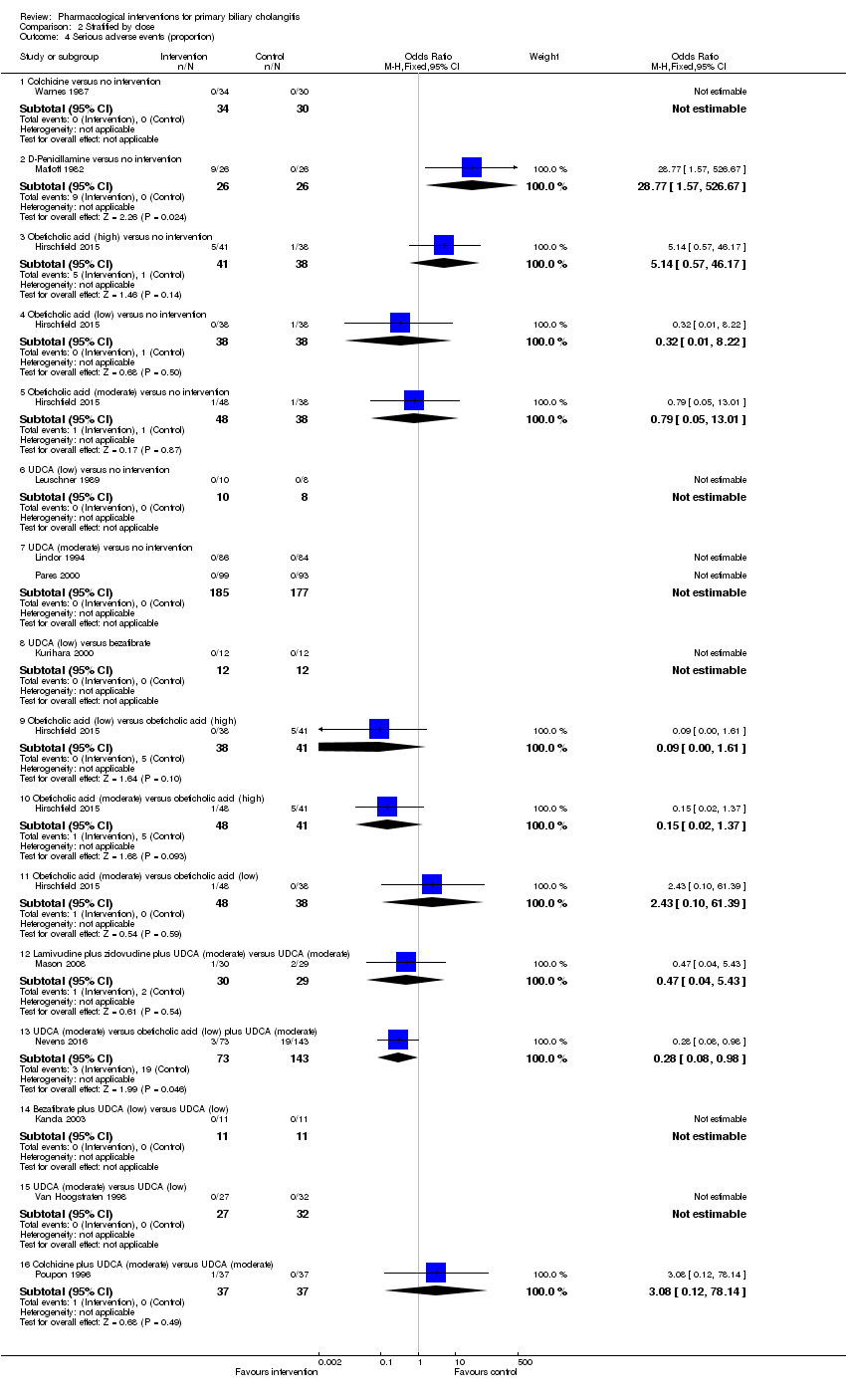 Comparison 2 Stratified by dose, Outcome 4 Serious adverse events (proportion). | ||||
| 4.1 Colchicine versus no intervention | 1 | 64 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 D‐Penicillamine versus no intervention | 1 | 52 | Odds Ratio (M‐H, Fixed, 95% CI) | 28.77 [1.57, 526.67] |
| 4.3 Obeticholic acid (high) versus no intervention | 1 | 79 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.14 [0.57, 46.17] |
| 4.4 Obeticholic acid (low) versus no intervention | 1 | 76 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.01, 8.22] |
| 4.5 Obeticholic acid (moderate) versus no intervention | 1 | 86 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.05, 13.01] |
| 4.6 UDCA (low) versus no intervention | 1 | 18 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.7 UDCA (moderate) versus no intervention | 2 | 362 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.8 UDCA (low) versus bezafibrate | 1 | 24 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.9 Obeticholic acid (low) versus obeticholic acid (high) | 1 | 79 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.09 [0.00, 1.61] |
| 4.10 Obeticholic acid (moderate) versus obeticholic acid (high) | 1 | 89 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.15 [0.02, 1.37] |
| 4.11 Obeticholic acid (moderate) versus obeticholic acid (low) | 1 | 86 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.43 [0.10, 61.39] |
| 4.12 Lamivudine plus zidovudine plus UDCA (moderate) versus UDCA (moderate) | 1 | 59 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.04, 5.43] |
| 4.13 UDCA (moderate) versus obeticholic acid (low) plus UDCA (moderate) | 1 | 216 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.28 [0.08, 0.98] |
| 4.14 Bezafibrate plus UDCA (low) versus UDCA (low) | 1 | 22 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.15 UDCA (moderate) versus UDCA (low) | 1 | 59 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.16 Colchicine plus UDCA (moderate) versus UDCA (moderate) | 1 | 74 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.08 [0.12, 78.14] |
| 5 Serious adverse events (number of events) Show forest plot | 1 | Rate Ratio (Fixed, 95% CI) | Subtotals only | |
| Analysis 2.5  Comparison 2 Stratified by dose, Outcome 5 Serious adverse events (number of events). | ||||
| 5.1 Obeticholic acid (low) plus UDCA (moderate) versus UDCA (moderate) | 1 | Rate Ratio (Fixed, 95% CI) | 1.66 [0.75, 3.66] | |
| 6 Adverse events (proportion) Show forest plot | 20 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.6 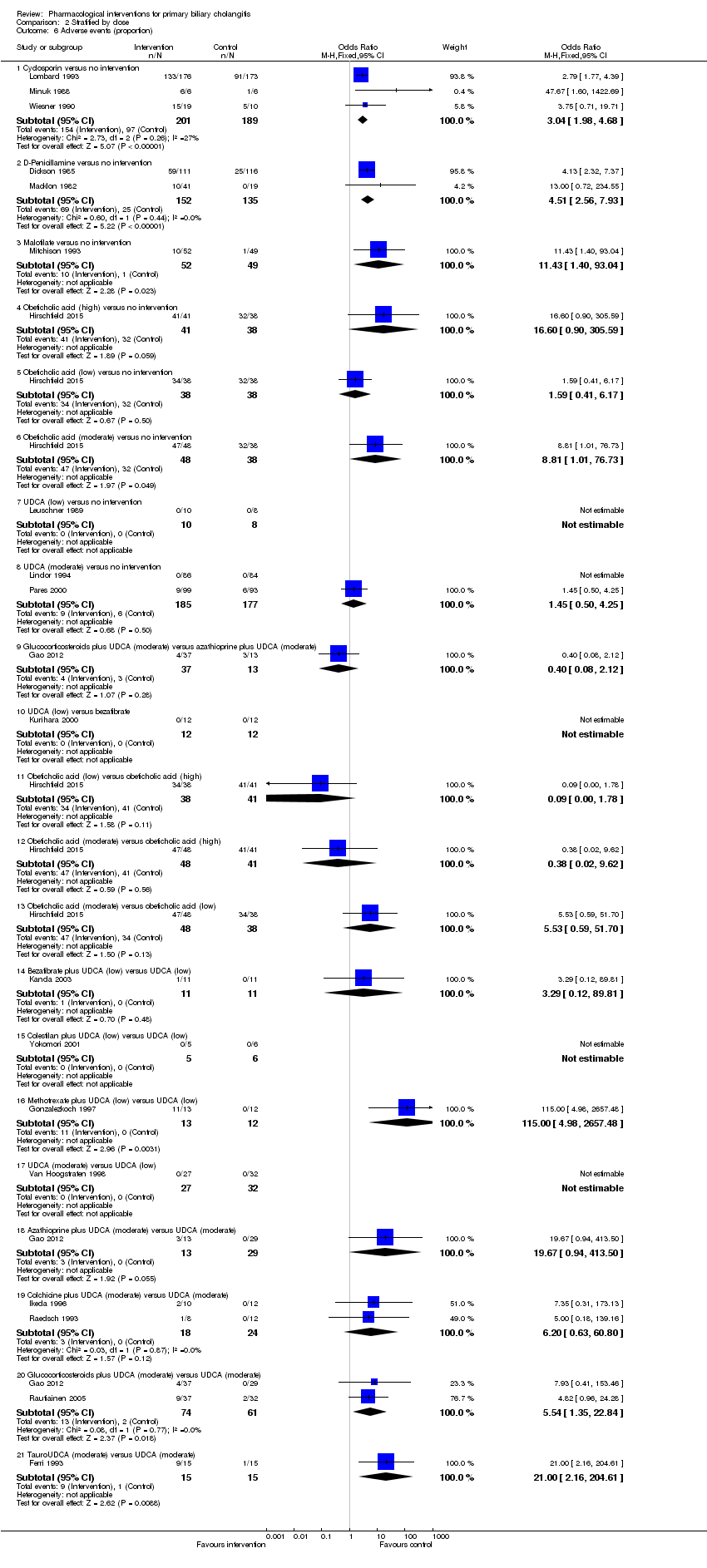 Comparison 2 Stratified by dose, Outcome 6 Adverse events (proportion). | ||||
| 6.1 Cyclosporin versus no intervention | 3 | 390 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.04 [1.98, 4.68] |
| 6.2 D‐Penicillamine versus no intervention | 2 | 287 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.51 [2.56, 7.93] |
| 6.3 Malotilate versus no intervention | 1 | 101 | Odds Ratio (M‐H, Fixed, 95% CI) | 11.43 [1.40, 93.04] |
| 6.4 Obeticholic acid (high) versus no intervention | 1 | 79 | Odds Ratio (M‐H, Fixed, 95% CI) | 16.6 [0.90, 305.59] |
| 6.5 Obeticholic acid (low) versus no intervention | 1 | 76 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.59 [0.41, 6.17] |
| 6.6 Obeticholic acid (moderate) versus no intervention | 1 | 86 | Odds Ratio (M‐H, Fixed, 95% CI) | 8.81 [1.01, 76.73] |
| 6.7 UDCA (low) versus no intervention | 1 | 18 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.8 UDCA (moderate) versus no intervention | 2 | 362 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.45 [0.50, 4.25] |
| 6.9 Glucocorticosteroids plus UDCA (moderate) versus azathioprine plus UDCA (moderate) | 1 | 50 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.08, 2.12] |
| 6.10 UDCA (low) versus bezafibrate | 1 | 24 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.11 Obeticholic acid (low) versus obeticholic acid (high) | 1 | 79 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.09 [0.00, 1.78] |
| 6.12 Obeticholic acid (moderate) versus obeticholic acid (high) | 1 | 89 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.02, 9.62] |
| 6.13 Obeticholic acid (moderate) versus obeticholic acid (low) | 1 | 86 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.53 [0.59, 51.70] |
| 6.14 Bezafibrate plus UDCA (low) versus UDCA (low) | 1 | 22 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.29 [0.12, 89.81] |
| 6.15 Colestilan plus UDCA (low) versus UDCA (low) | 1 | 11 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.16 Methotrexate plus UDCA (low) versus UDCA (low) | 1 | 25 | Odds Ratio (M‐H, Fixed, 95% CI) | 115.0 [4.98, 2657.48] |
| 6.17 UDCA (moderate) versus UDCA (low) | 1 | 59 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.18 Azathioprine plus UDCA (moderate) versus UDCA (moderate) | 1 | 42 | Odds Ratio (M‐H, Fixed, 95% CI) | 19.67 [0.94, 413.50] |
| 6.19 Colchicine plus UDCA (moderate) versus UDCA (moderate) | 2 | 42 | Odds Ratio (M‐H, Fixed, 95% CI) | 6.20 [0.63, 60.80] |
| 6.20 Glucocorticosteroids plus UDCA (moderate) versus UDCA (moderate) | 2 | 135 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.54 [1.35, 22.84] |
| 6.21 TauroUDCA (moderate) versus UDCA (moderate) | 1 | 30 | Odds Ratio (M‐H, Fixed, 95% CI) | 21.0 [2.16, 204.61] |
| 7 Adverse events (number) Show forest plot | 15 | Rate Ratio (Fixed, 95% CI) | Subtotals only | |
| Analysis 2.7  Comparison 2 Stratified by dose, Outcome 7 Adverse events (number). | ||||
| 7.1 Chlorambucil versus no intervention | 1 | Rate Ratio (Fixed, 95% CI) | 3.67 [1.04, 12.87] | |
| 7.2 Cyclosporin versus no intervention | 3 | Rate Ratio (Fixed, 95% CI) | 1.87 [1.51, 2.32] | |
| 7.3 D‐Penicillamine versus no intervention | 3 | Rate Ratio (Fixed, 95% CI) | 2.64 [1.78, 3.91] | |
| 7.4 Malotilate versus no intervention | 1 | Rate Ratio (Fixed, 95% CI) | 6.13 [1.38, 27.14] | |
| 7.5 Obeticholic acid (high) versus no intervention | 1 | Rate Ratio (Fixed, 95% CI) | 1.91 [1.50, 2.44] | |
| 7.6 Obeticholic acid (low) versus no intervention | 1 | Rate Ratio (Fixed, 95% CI) | 1.05 [0.80, 1.39] | |
| 7.7 Obeticholic acid (moderate) versus no intervention | 1 | Rate Ratio (Fixed, 95% CI) | 1.25 [0.97, 1.62] | |
| 7.8 Obeticholic acid (low) versus obeticholic acid (high) | 1 | Rate Ratio (Fixed, 95% CI) | 0.55 [0.43, 0.70] | |
| 7.9 Obeticholic acid (moderate) versus obeticholic acid (high) | 1 | Rate Ratio (Fixed, 95% CI) | 0.66 [0.53, 0.81] | |
| 7.10 Obeticholic acid (moderate) versus obeticholic acid (low) | 1 | Rate Ratio (Fixed, 95% CI) | 1.19 [0.93, 1.53] | |
| 7.11 UDCA (low) versus UDCA (high) | 1 | Rate Ratio (Fixed, 95% CI) | 2.08 [0.78, 5.53] | |
| 7.12 UDCA (moderate) versus UDCA (high) | 1 | Rate Ratio (Fixed, 95% CI) | 0.73 [0.21, 2.60] | |
| 7.13 UDCA (low) plus methotrexate versus UDCA (low) | 1 | Rate Ratio (Fixed, 95% CI) | 30.64 [1.84, 510.76] | |
| 7.14 UDCA (moderate) versus UDCA (low) | 1 | Rate Ratio (Fixed, 95% CI) | 0.35 [0.11, 1.10] | |
| 7.15 Azathioprine plus glucocorticosteroids plus UDCA (moderate) versus UDCA (moderate) | 1 | Rate Ratio (Fixed, 95% CI) | 1.32 [0.88, 1.97] | |
| 7.16 Bezafibrate plus UDCA (moderate) versus UDCA (moderate) | 1 | Rate Ratio (Fixed, 95% CI) | 11.79 [0.65, 213.14] | |
| 7.17 Colchicine plus UDCA (moderate) versus UDCA (moderate) | 1 | Rate Ratio (Fixed, 95% CI) | 5.91 [0.28, 123.08] | |
| 7.18 TauroUDCA (moderate) versus UDCA (moderate) | 1 | Rate Ratio (Fixed, 95% CI) | 1.17 [0.81, 1.71] | |
| 8 Liver transplantation Show forest plot | 12 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.8  Comparison 2 Stratified by dose, Outcome 8 Liver transplantation. | ||||
| 8.1 Cyclosporin versus no intervention | 2 | 378 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.43, 1.72] |
| 8.2 D‐Penicillamine versus no intervention | 1 | 189 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.06, 15.05] |
| 8.3 Methotrexate versus no intervention | 1 | 60 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.17 [0.02, 1.58] |
| 8.4 UDCA (low) versus no intervention | 2 | 162 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.24, 4.06] |
| 8.5 UDCA (moderate) versus no intervention | 3 | 478 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.44, 1.76] |
| 8.6 UDCA (low) versus UDCA (high) | 1 | 106 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.17 [0.13, 79.71] |
| 8.7 UDCA (moderate) versus UDCA (high) | 1 | 103 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.37 [0.13, 84.70] |
| 8.8 UDCA (moderate) versus UDCA (low) | 1 | 101 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.06, 17.47] |
| 8.9 Bezafibrate plus UDCA (moderate) versus UDCA (moderate) | 1 | 27 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.10 Methotrexate plus UDCA (moderate) versus UDCA (moderate) | 1 | 265 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.35, 1.39] |
| 9 Decompensated liver disease Show forest plot | 7 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.9 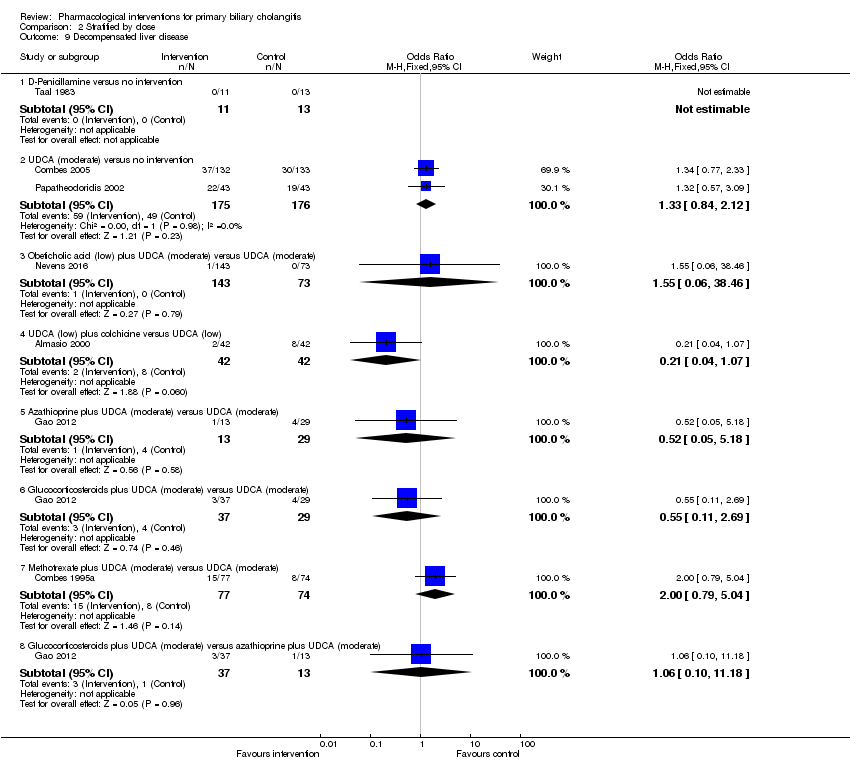 Comparison 2 Stratified by dose, Outcome 9 Decompensated liver disease. | ||||
| 9.1 D‐Penicillamine versus no intervention | 1 | 24 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.2 UDCA (moderate) versus no intervention | 2 | 351 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.84, 2.12] |
| 9.3 Obeticholic acid (low) plus UDCA (moderate) versus UDCA (moderate) | 1 | 216 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.55 [0.06, 38.46] |
| 9.4 UDCA (low) plus colchicine versus UDCA (low) | 1 | 84 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.04, 1.07] |
| 9.5 Azathioprine plus UDCA (moderate) versus UDCA (moderate) | 1 | 42 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.05, 5.18] |
| 9.6 Glucocorticosteroids plus UDCA (moderate) versus UDCA (moderate) | 1 | 66 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.11, 2.69] |
| 9.7 Methotrexate plus UDCA (moderate) versus UDCA (moderate) | 1 | 151 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.00 [0.79, 5.04] |
| 9.8 Glucocorticosteroids plus UDCA (moderate) versus azathioprine plus UDCA (moderate) | 1 | 50 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.10, 11.18] |
| 10 Cirrhosis Show forest plot | 3 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.10  Comparison 2 Stratified by dose, Outcome 10 Cirrhosis. | ||||
| 10.1 Azathioprine versus no intervention | 1 | 31 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.18, 3.41] |
| 10.2 UDCA (low) versus no intervention | 1 | 22 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.15 [0.01, 1.53] |
| 10.3 Azathioprine plus glucocorticosteroids plus UDCA (moderate) versus UDCA (moderate) | 1 | 50 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.28 [0.03, 2.90] |
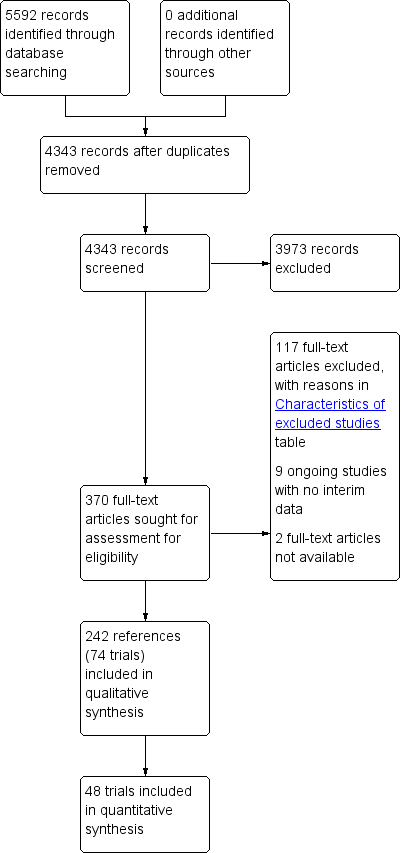
Study flow diagram.
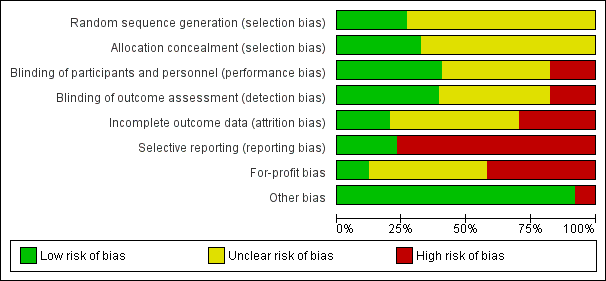
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
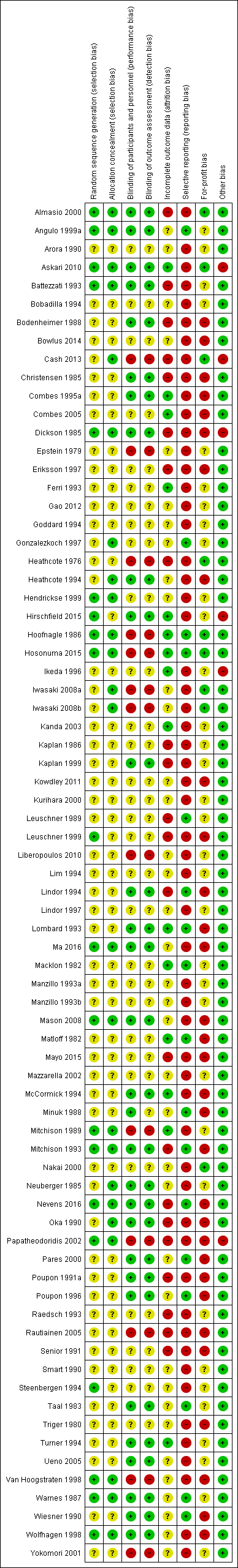
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
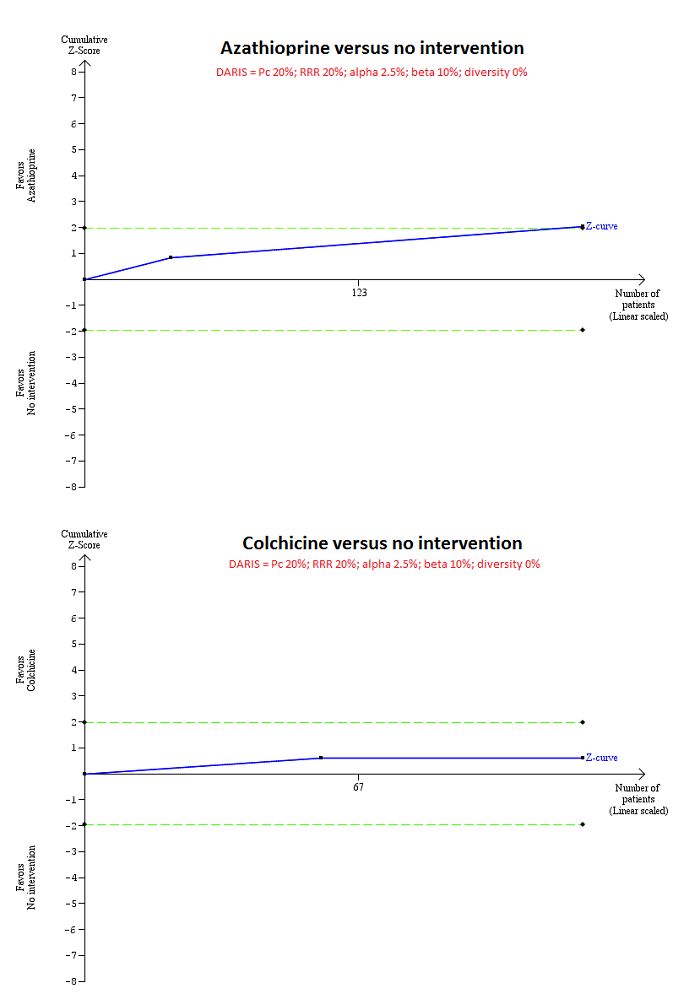
Trial Sequential Analysis of mortality at maximal follow‐up: azathioprine versus no intervention and colchicine versus no intervention.
Based on an alpha error of 2.5%, power of 90% (beta error of 10%), a relative risk reduction (RRR) of 20%, a control group proportion observed in the trials (Pc = 20%), and diversity observed in the analyses (0%), the accrued sample size (224 for azathioprine versus intervention and 122 for colchicine versus no intervention) was only a small fraction of the diversity adjusted required information size (DARIS) (4580 for both comparisons); therefore, the trial sequential monitoring boundaries were not drawn. The Z‐curve (blue line) crossed the conventional boundaries (dotted green line) favouring azathioprine for azathioprine versus no intervention, but did not cross the conventional boundaries for colchicine versus no intervention. This indicates that there is a high risk of random errors in both these comparisons.
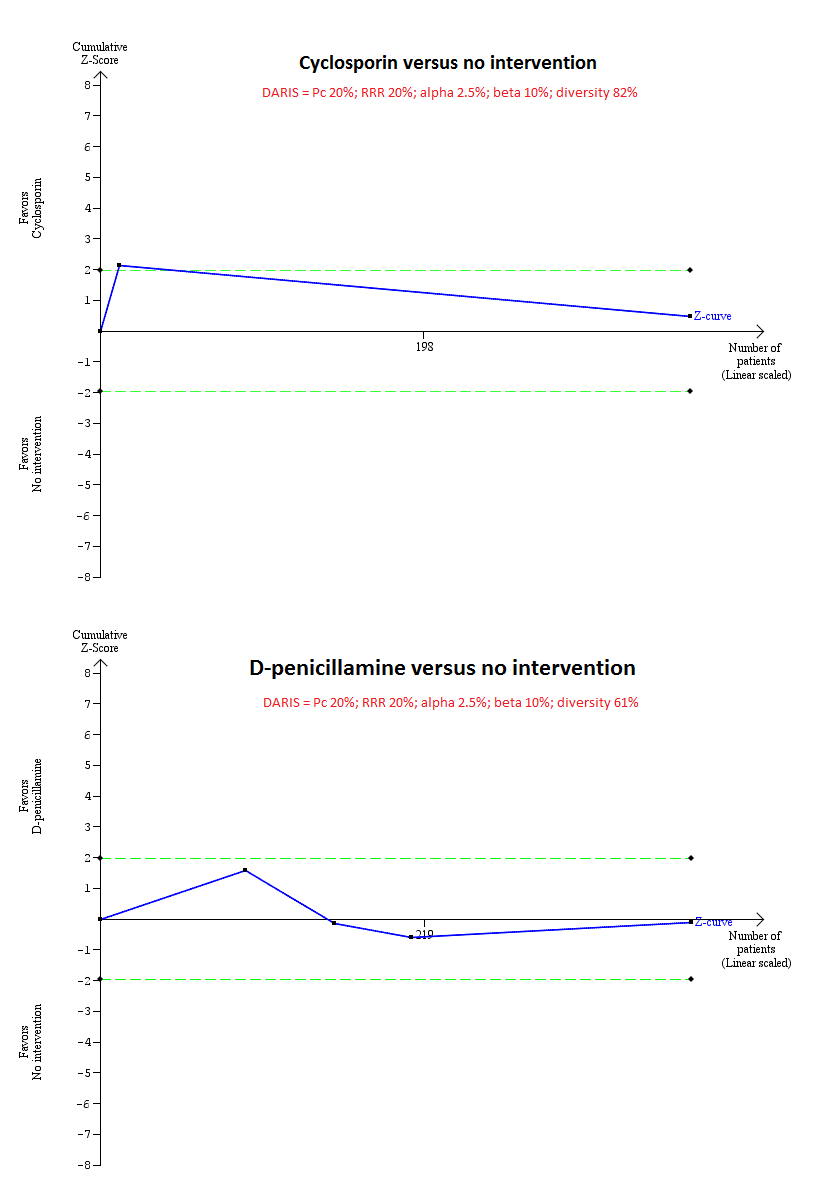
Trial Sequential Analysis of mortality at maximal follow‐up: ciclosporin versus no intervention and D‐penicillamine versus no intervention.
Based on an alpha error of 2.5%, power of 90% (beta error of 10%), a relative risk reduction (RRR) of 20%, a control group proportion observed in the trials (Pc = 20%), and diversity observed in the analyses (82% for ciclosporin versus no intervention and 61% for D‐penicillamine versus no intervention), the accrued sample size (394 for ciclosporin versus no intervention and 423 for D‐penicillamine versus no intervention) was only a small fraction of the diversity adjusted required information size (DARIS) (25,098 for ciclosporin versus no intervention and 11,623 for D‐penicillamine versus no intervention); therefore, the trial sequential monitoring boundaries were not drawn. The Z‐curve (blue line) crossed the conventional boundaries (dotted green line) favouring ciclosporin for ciclosporin versus no intervention, but did not cross the conventional boundaries for D‐penicillamine versus no intervention. This indicates that there is a high risk of random errors in both these comparisons.
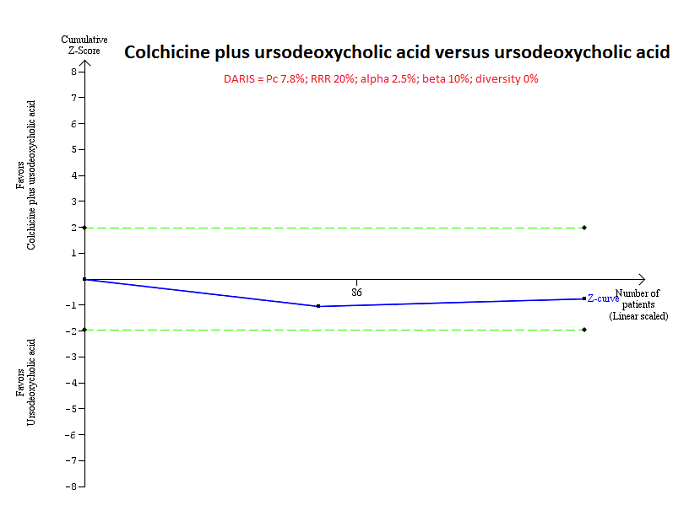
Trial Sequential Analysis of mortality at maximal follow‐up: colchicine plus UDCA versus UDCA.
Based on an alpha error of 2.5%, power of 90% (beta error of 10%), a relative risk reduction (RRR) of 20%, a control group proportion observed in the trials (Pc = 7.8%), and diversity observed in the analyses (0%), the accrued sample size (160 participants) was only a small fraction of the diversity adjusted required information size (DARIS) (13,316); therefore, the trial sequential monitoring boundaries were not drawn. The Z‐curve (blue line) did not cross the conventional boundaries (green dotted line). This indicates that there is a high risk of random errors in both this comparison.

Comparison 1 Main analysis, Outcome 1 Mortality at maximal follow‐up.
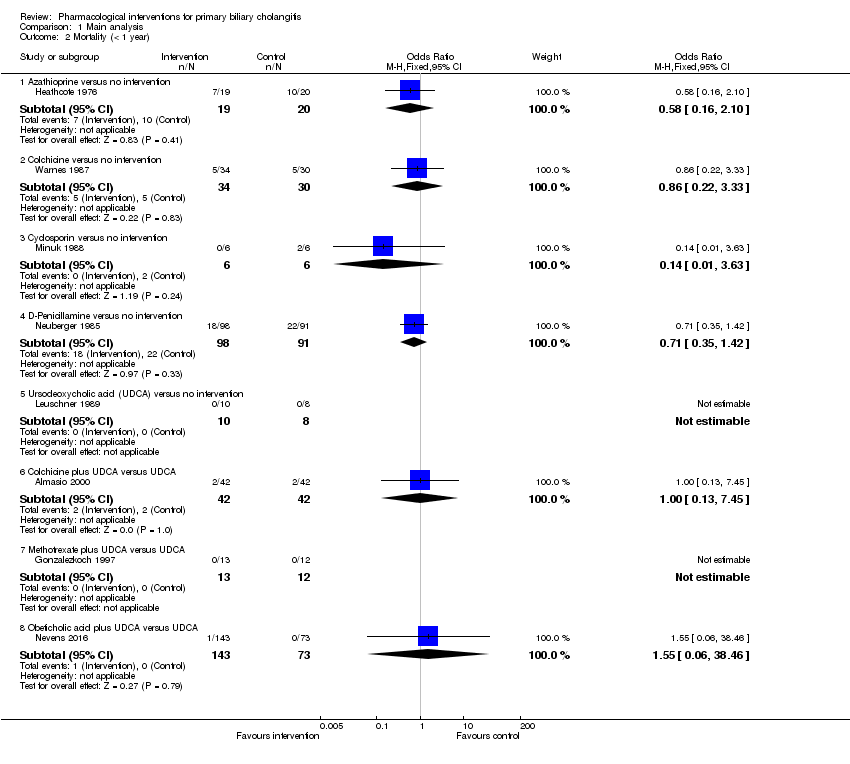
Comparison 1 Main analysis, Outcome 2 Mortality (< 1 year).

Comparison 1 Main analysis, Outcome 3 Mortality (1 to 5 years).
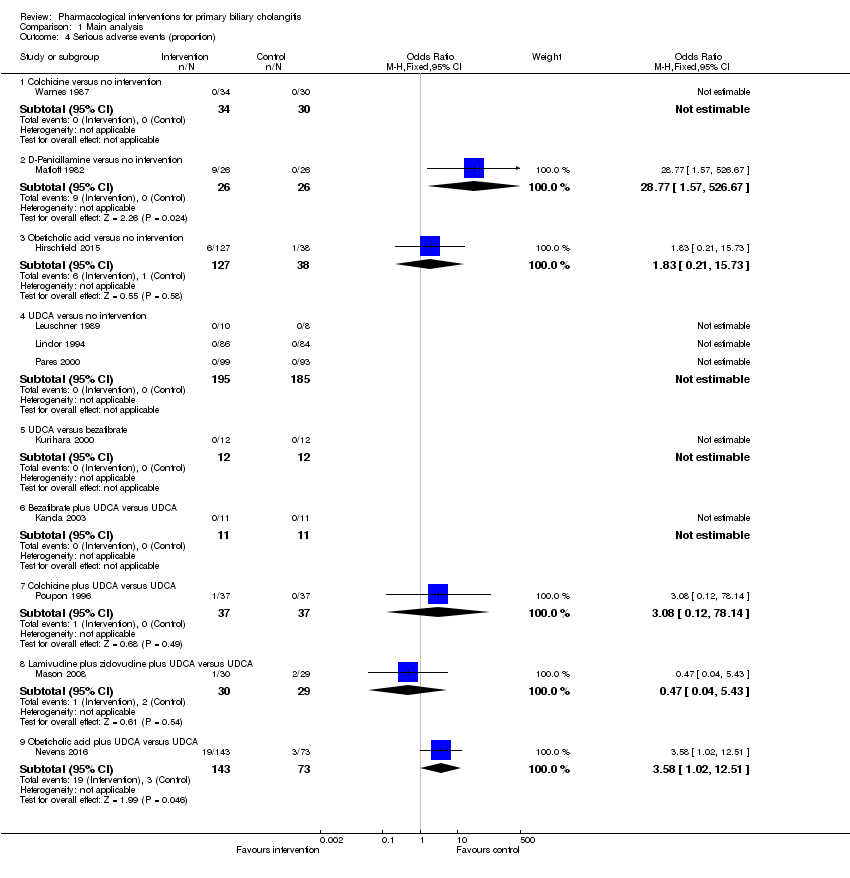
Comparison 1 Main analysis, Outcome 4 Serious adverse events (proportion).

Comparison 1 Main analysis, Outcome 5 Serious adverse events (number of events).

Comparison 1 Main analysis, Outcome 6 Adverse events (proportion).

Comparison 1 Main analysis, Outcome 7 Adverse events (number).
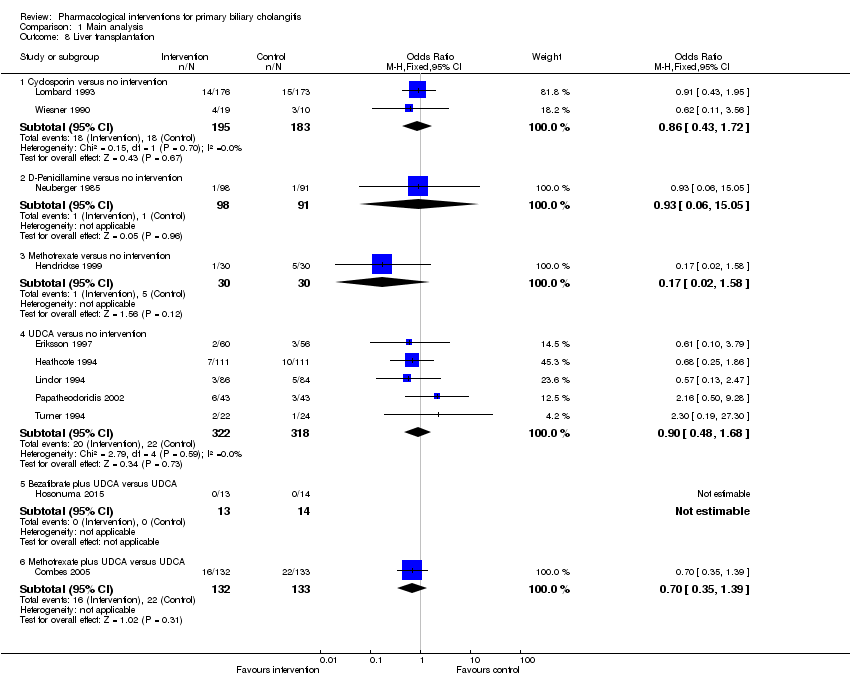
Comparison 1 Main analysis, Outcome 8 Liver transplantation.

Comparison 1 Main analysis, Outcome 9 Decompensated liver disease.
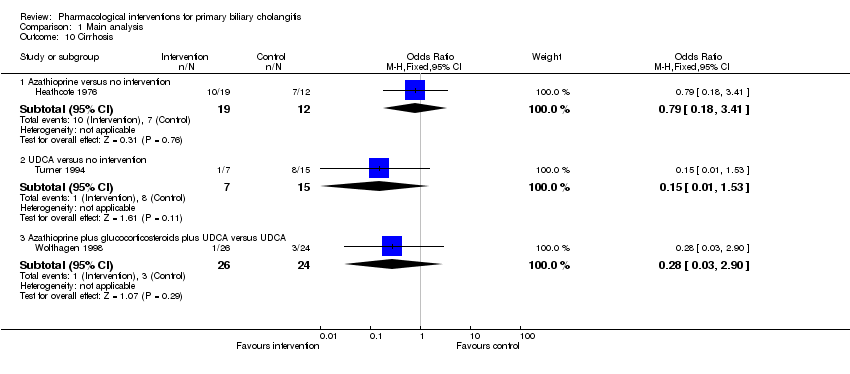
Comparison 1 Main analysis, Outcome 10 Cirrhosis.
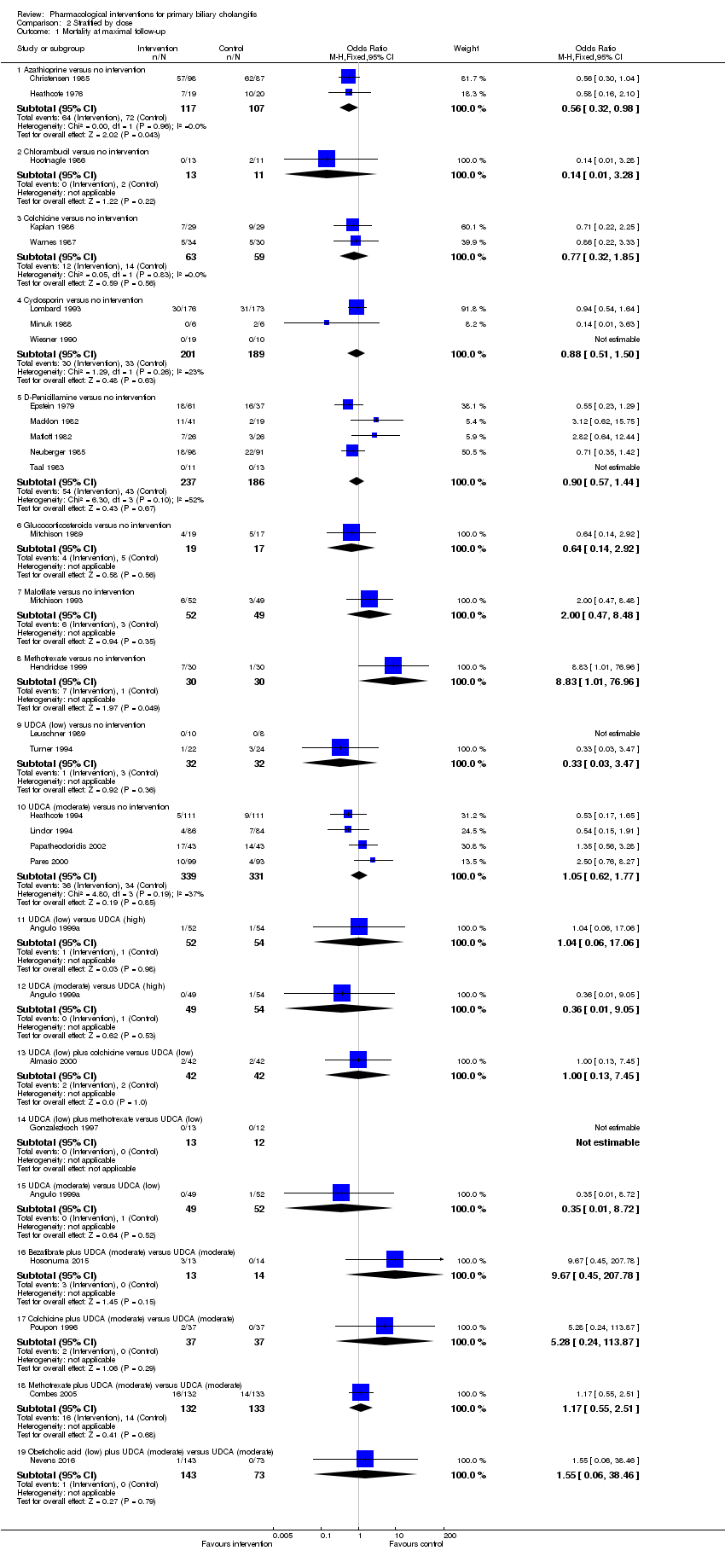
Comparison 2 Stratified by dose, Outcome 1 Mortality at maximal follow‐up.

Comparison 2 Stratified by dose, Outcome 2 Mortality (< 1 year).

Comparison 2 Stratified by dose, Outcome 3 Mortality (1 to 5 years).

Comparison 2 Stratified by dose, Outcome 4 Serious adverse events (proportion).
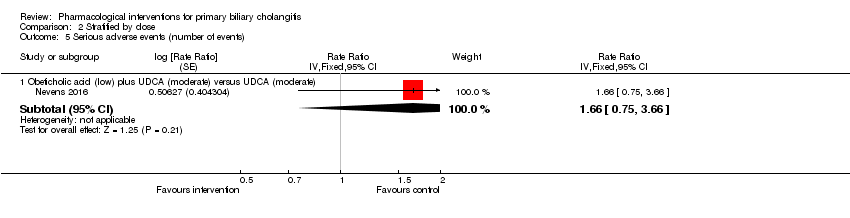
Comparison 2 Stratified by dose, Outcome 5 Serious adverse events (number of events).
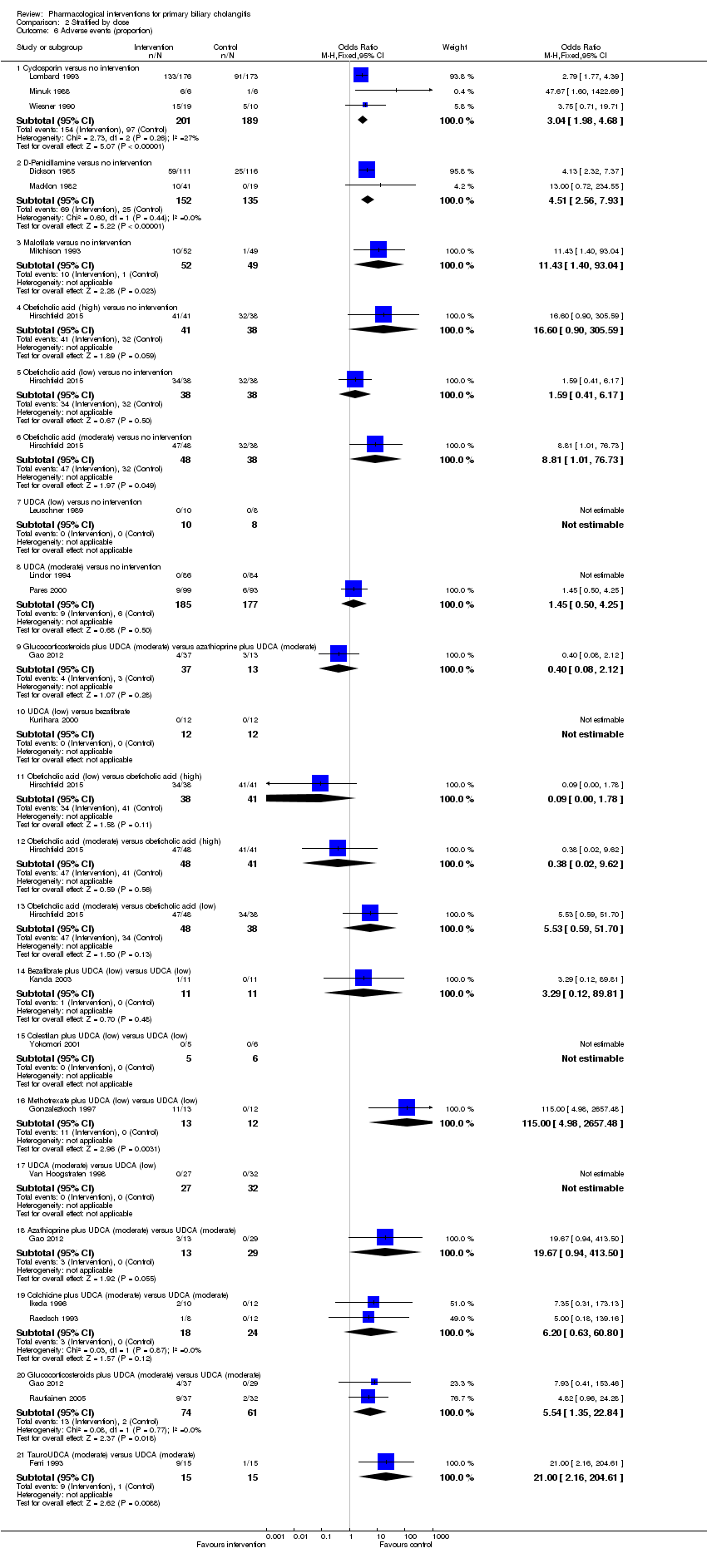
Comparison 2 Stratified by dose, Outcome 6 Adverse events (proportion).

Comparison 2 Stratified by dose, Outcome 7 Adverse events (number).

Comparison 2 Stratified by dose, Outcome 8 Liver transplantation.

Comparison 2 Stratified by dose, Outcome 9 Decompensated liver disease.

Comparison 2 Stratified by dose, Outcome 10 Cirrhosis.
| UDCA versus no intervention for primary biliary cholangitis | |||||
| Patient or population: people with primary biliary cholangitis Settings: secondary or tertiary care Intervention: UDCA Comparison: no intervention | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | |
| Assumed risk | Corresponding risk | ||||
| No intervention | UDCA | ||||
| Mortality at maximal follow‐up Follow‐up: 12 to 89 months | 208 per 1000 | 206 per 1000 | OR 0.99 | 734 | ⊕⊝⊝⊝ |
| Serious adverse events (proportion) Follow‐up: 12 to 41 months | There were no events in either group | 380 | ⊕⊝⊝⊝ | ||
| Serious adverse events (number of events) | None of the trials reported this outcome. | ||||
| Health‐related quality of life | None of the trials reported this outcome. | ||||
| *The basis for the assumed risk is the mean control group proportion across all the trials. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| 1 Risk of bias in the trial(s) was high (downgraded by two levels). 3 There was moderate heterogeneity (downgraded by one level). | |||||
| Azathioprine versus no intervention for primary biliary cholangitis | |||||
| Patient or population: people with primary biliary cholangitis Settings: secondary or tertiary care Intervention: azathioprine Comparison: no intervention | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | |
| Assumed risk | Corresponding risk | ||||
| No intervention | Azathioprine | ||||
| Mortality at maximal follow‐up Follow‐up: 63 months in 1 trial and not stated in 1 trial | 208 per 1000 | 128 per 1000 | OR 0.56 | 224 | ⊕⊝⊝⊝ |
| Serious adverse events (proportion) | None of the trials reported this outcome. | ||||
| Serious adverse events (number of events) | None of the trials reported this outcome. | ||||
| Health‐related quality of life | None of the trials reported this outcome. | ||||
| *The basis for the assumed risk is the mean control group proportion across all the trials. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| 1 Risk of bias in the trial(s) was high (downgraded by two levels). | |||||
| Colchicine versus no intervention for primary biliary cholangitis | |||||
| Patient or population: people with primary biliary cholangitis Settings: secondary or tertiary care Intervention: colchicine Comparison: no intervention | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | |
| Assumed risk | Corresponding risk | ||||
| No intervention | Colchicine | ||||
| Mortality at maximal follow‐up Follow‐up: 12 to 24 months | 208 per 1000 | 168 per 1000 | OR 0.77 | 122 | ⊕⊝⊝⊝ |
| Serious adverse events (proportion) Follow‐up: 12 months | There were no events in either group | 64 | ⊕⊝⊝⊝ | ||
| Serious adverse events (number of events) | None of the trials reported this outcome. | ||||
| Health‐related quality of life | None of the trials reported this outcome. | ||||
| *The basis for the assumed risk is the mean control group proportion across all the trials. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| 1 Risk of bias in the trial(s) was high (downgraded by two levels). 3 There was moderate heterogeneity (downgraded by one level). | |||||
| Ciclosporin versus no intervention for primary biliary cholangitis | |||||
| Patient or population: people with primary biliary cholangitis Settings: secondary or tertiary care Intervention: ciclosporin Comparison: no intervention | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | |
| Assumed risk | Corresponding risk | ||||
| No intervention | Ciclosporin | ||||
| Mortality at maximal follow‐up Follow‐up: 31 to 35 months | 208 per 1000 | 188 per 1000 | OR 0.88 | 390 | ⊕⊝⊝⊝ |
| Serious adverse events (proportion) | None of the trials reported this outcome. | ||||
| Serious adverse events (number of events) | None of the trials reported this outcome. | ||||
| Health‐related quality of life | None of the trials reported this outcome. | ||||
| *The basis for the assumed risk is the mean control group proportion across all the trials. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| 1 Risk of bias in the trial(s) was high (downgraded by two levels). | |||||
| D‐Penicillamine versus no intervention for primary biliary cholangitis | |||||
| Patient or population: people with primary biliary cholangitis Settings: secondary or tertiary care Intervention: D‐penicillamine Comparison: no intervention | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | |
| Assumed risk | Corresponding risk | ||||
| No intervention | D‐Penicillamine | ||||
| Mortality at maximal follow‐up (Follow‐up 24 to 66 months) | 208 per 1000 | 191 per 1000 | OR 0.90 | 423 | ⊕⊝⊝⊝ |
| Serious adverse events (proportion) (Follow‐up 24 months) | 4 per 1000 | 104 per 1000 | OR 28.77 | 52 | ⊕⊝⊝⊝ |
| Serious adverse events (number of events) | None of the trials reported this outcome. | ||||
| Health‐related quality of life | None of the trials reported this outcome. | ||||
| *The basis for the assumed risk is the mean control group proportion across all the trials. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| 1 Risk of bias in the trial(s) was high (downgraded by two levels). 3 There was moderate heterogeneity (downgraded by one level). | |||||
| Colchicine plus UDCA versus UDCA for primary biliary cholangitis | |||||
| Patient or population: people with primary biliary cholangitis Settings: secondary or tertiary care Intervention: colchicine + UDCA Comparison: UDCA | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | |
| Assumed risk | Corresponding risk | ||||
| UDCA | Colchicine + UDCA | ||||
| Mortality at maximal follow‐up Follow‐up: 24 months in 1 trial; not reported in 1 trial | 110 per 1000 | 185 per 1000 | OR 1.84 | 158 | ⊕⊝⊝⊝ |
| Serious adverse events (proportion) Follow‐up: not stated | 14 per 1000 | 42 per 1000 | OR 3.08 | 74 | ⊕⊝⊝⊝ |
| Serious adverse events (number of events) | None of the trials reported this outcome. | ||||
| Health‐related quality of life | None of the trials reported this outcome. | ||||
| *The basis for the assumed risk is the mean control group proportion across all the trials. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| 1 Risk of bias in the trial(s) was high (downgraded by two levels). 3 There was moderate heterogeneity (downgraded by one level). | |||||
| Methotrexate plus UDCA versus UDCA for primary biliary cholangitis | |||||
| Patient or population: people with primary biliary cholangitis Settings: secondary or tertiary care Intervention: methotrexate + UDCA Comparison: UDCA | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | |
| Assumed risk | Corresponding risk | ||||
| UDCA | Methotrexate + UDCA | ||||
| Mortality at maximal follow‐up Follow‐up: 11 to 91 months | 110 per 1000 | 126 per 1000 | OR 1.17 | 290 | ⊕⊝⊝⊝ |
| Serious adverse events (proportion) | None of the trials reported this outcome. | ||||
| Serious adverse events (number of events) | None of the trials reported this outcome. | ||||
| Health‐related quality of life | None of the trials reported this outcome. | ||||
| *The basis for the assumed risk is the mean control group proportion across all the trials. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| 1 Risk of bias in the trial(s) was high (downgraded by two levels). 3 There was moderate heterogeneity (downgraded by one level). | |||||
| Study name | No participants randomised | Post‐randomisation dropouts | No participants for whom outcome was reported | Intervention(s) | Control | Mean follow‐up period (months) |
| 20 | Not stated | 20 | Antioxidants | No intervention | Not stated | |
| 248 | 63 | 185 | Azathioprine | No intervention | 63 | |
| 45 | 6 | 39 | Azathioprine | No intervention | Not stated | |
| 24 | 0 | 24 | Chlorambucil | No intervention | 52 | |
| 57 | 10 | 47 | Colchicine | No intervention | 33 | |
| 60 | 3 | 57 | Colchicine | No intervention | 24 | |
| 64 | Not stated | 64* | Colchicine | No intervention | 19 (median) | |
| 40 | Not stated | 40 | Colchicine + UDCA | No intervention | 12 | |
| 349 | 0 | 349 | Ciclosporin | No intervention | 31 (median) | |
| 12 | 0 | 12 | Ciclosporin | No intervention | Not stated | |
| 40 | 11 | 29 | Ciclosporin | No intervention | 35 (median) | |
| 309 | 82 | 227 | D‐Penicillamine | No intervention | 60 (median) | |
| 98 | Not stated | 98 | D‐Penicillamine | No intervention | 66 | |
| 60 | 0 | 60 | D‐Penicillamine | No intervention | 37 | |
| 52 | 0 | 52 | D‐Penicillamine | No intervention | 24 | |
| 189 | Not stated | 189 | D‐Penicillamine | No intervention | Not stated | |
| 24 | Not stated | 24 | D‐Penicillamine | No intervention | 18 | |
| 35 | Not stated | 35 | D‐Penicillamine | No intervention | Not stated | |
| 36 | 0 | 36 | Glucocorticosteroids | No intervention | 36 | |
| 20 | Not stated | 20 | Lamivudine | No intervention | Not stated | |
| 104 | 3 | 101 | Malotilate | No intervention | 25 (median) | |
| 60 | Not stated | 60 | Methotrexate | No intervention | 68 | |
| 14 | Not stated | 14 | Methotrexate + UDCA | No intervention | 24 | |
| 45 | 3 | 42 | NGM282 | No intervention | Not stated | |
| 216 | Not stated | 216 | Obeticholic acid | No intervention | 12 | |
| 165 | 0 | 165 | Obeticholic acid | No intervention | 3 | |
| 59 | Not stated | 59 | Obeticholic acid | No intervention | Not stated | |
| 32 | Not stated | 32 | S‐Adenosyl methionine | No intervention | 1 | |
| 6 | Not stated | 6 | S‐Adenosyl methionine | No intervention | 2 | |
| 21 | 8 | 13 | Simvastatin | No intervention | 12 | |
| 28 | 0 | 28 | Tetrathiomolybdate | No intervention | Not stated | |
| 18 | 0 | 18 | Thalidomide | No intervention | Not stated | |
| 9 | Not stated | 9 | UDCA | No intervention | 5 | |
| 88 | 2 | 86 | UDCA | No intervention | 6 | |
| 151 | 0 | 151 | UDCA | No intervention | 24 | |
| 116 | 15 | 101 | UDCA | No intervention | 24 | |
| 222 | Not stated | 222 | UDCA | No intervention | 24 | |
| 20 | 0 | 18 | UDCA | No intervention | 12 | |
| 32 | Not stated | 32 | UDCA | No intervention | Not stated | |
| 180 | 10 | 170 | UDCA | No intervention | 24 | |
| 52 | 7 | 45 | UDCA | No intervention | Not stated | |
| 92 | 6 | 86 | UDCA | No intervention | 89 | |
| 192 | 0 | 192 | UDCA | No intervention | 41 (median) | |
| 149 | 3 | 146 | UDCA | No intervention | Not stated | |
| 20 | 1 | 19 | UDCA | No intervention | 18 | |
| 46 | 0 | 46 | UDCA | No intervention | 24 | |
| 57 | Not stated | 57 | Intervention 1: UDCA | No intervention | 15 | |
| 50 | Not stated | 50 | Azathioprine + glucocorticosteroids + UDCA | UDCA | 12 | |
| 45 | Not stated | 45 | Bezafibrate | UDCA | 12 | |
| 24 | Not stated | 24 | Bezafibrate | UDCA | Not stated | |
| 27 | 0 | 27 | Bezafibrate + UDCA | UDCA | 96 | |
| 22 | Not stated | 22 | Bezafibrate + UDCA | UDCA | 12 | |
| 22 | 0 | 22 | Bezafibrate + UDCA | UDCA | 7 | |
| 23 | Not stated | 23 | Bezafibrate + UDCA | UDCA | 12 | |
| 90 | 6 | 84 | Colchicine + UDCA | UDCA | Not stated | |
| 22 | 0 | 22 | Colchicine + UDCA | UDCA | 24 | |
| 74 | Not stated | 74 | Colchicine + UDCA | UDCA | 24 | |
| 28 | 8 | 20 | Colchicine + UDCA | UDCA | 24 | |
| 11 | Not stated | 11 | Colestilan + UDCA | UDCA | Not stated | |
| 10 | Not stated | 10 | Fenofibrate + UDCA | UDCA | Not stated | |
| 40 | 0 | 39 | Glucocorticosteroids + UDCA | UDCA | 24 | |
| 77 | 8 | 69 | Glucocorticosteroids + UDCA | UDCA | 36 | |
| 79 | Not stated | 79 | Intervention 1: glucocorticosteroids + UDCA | UDCA | Not stated | |
| 59 | 0 | 59 | Lamivudine + zidovudine + UDCA | UDCA | 6 | |
| 265 | 0 | 265 | Methotrexate + UDCA | UDCA | 91 (median) | |
| 25 | Not stated | 25 | Methotrexate + UDCA | UDCA | 11 | |
| 217 | Not stated | 216 | Obeticholic acid + UDCA | UDCA | 12 | |
| 30 | 0 | 30 | TUDCA | UDCA | 6 | |
| 199 | 8 | 191 | TUDCA | UDCA | 6 | |
| 87 | 2 | 85 | Colchicine | Methotrexate | 24 | |
| Comparison of doses | ||||||
| 150 | Not stated | 150 | Intervention 1: UDCA (high) Intervention 2: UDCA (moderate) | UDCA (low) | 12 | |
| 155 | Not stated | 155 | Intervention 1: UDCA (high) Intervention 2: UDCA (moderate) | UDCA (low) | 12 | |
| 61 | 2 | 59 | UDCA (moderate) | UDCA (low) | Not stated | |
| 42 | Not stated | 42 | UDCA (high) | UDCA (moderate) | 72 | |
| TUDCA: taurodeoxycholic acid; UDCA: ursodeoxycholic acid. | ||||||
| Name of studies | Intervention(s) | Control | Random sequence generation | Allocation concealment | Blinding of participants and health professionals | Blinding of outcome assessors | Missing outcome bias | Selective outcome reporting | For‐profit bias | Other bias |
| Antioxidants | No intervention | Unclear | Unclear | Unclear | Unclear | Unclear | High | Unclear | Low | |
| Azathioprine | No intervention | Unclear | Unclear | Low | Low | High | High | High | Low | |
| Azathioprine | No intervention | Unclear | Unclear | High | High | High | High | Low | Low | |
| Chlorambucil | No intervention | Low | Low | High | High | Low | Low | Low | Low | |
| Colchicine | No intervention | Unclear | Unclear | Low | Low | High | High | High | Low | |
| Colchicine | No intervention | Unclear | Unclear | Unclear | Unclear | High | High | Unclear | Low | |
| Colchicine | No intervention | Low | Low | Low | Low | Unclear | Low | Unclear | Low | |
| Colchicine + UDCA | No intervention | Unclear | Unclear | Unclear | Unclear | Unclear | High | Unclear | Low | |
| Ciclosporin | No intervention | Unclear | Unclear | Low | Low | Low | Low | High | Low | |
| Ciclosporin | No intervention | Unclear | Unclear | Low | Unclear | Unclear | Low | High | Low | |
| Ciclosporin | No intervention | Unclear | Unclear | Low | Low | Unclear | Low | High | Low | |
| D‐Penicillamine | No intervention | Low | Low | Low | Low | High | High | High | High | |
| D‐Penicillamine | No intervention | Unclear | Unclear | High | High | Unclear | High | Unclear | Low | |
| D‐Penicillamine | No intervention | Unclear | Unclear | Unclear | Unclear | Low | Low | Unclear | Low | |
| D‐Penicillamine | No intervention | Unclear | Unclear | Unclear | Unclear | Low | Low | High | Low | |
| D‐Penicillamine | No intervention | Unclear | Low | Low | Low | Unclear | High | Unclear | Low | |
| D‐Penicillamine | No intervention | Unclear | Unclear | Low | Low | Unclear | Low | Unclear | Low | |
| D‐Penicillamine | No intervention | Unclear | Unclear | Unclear | Unclear | Unclear | High | High | Low | |
| Glucocorticosteroids | No intervention | Low | Low | High | High | Low | High | Unclear | Low | |
| Lamivudine | No intervention | Unclear | Unclear | Low | Low | Unclear | High | Unclear | Low | |
| Malotilate | No intervention | Low | Low | Low | Low | High | Low | High | Low | |
| Methotrexate | No intervention | Low | Low | Unclear | Unclear | Unclear | High | Unclear | Low | |
| Methotrexate + UDCA | No intervention | Low | Unclear | Unclear | Unclear | Unclear | High | Unclear | Low | |
| NGM282 | No intervention | Unclear | Unclear | Unclear | Unclear | High | High | High | Low | |
| Obeticholic acid | No intervention | Unclear | Unclear | Unclear | Unclear | Unclear | High | High | Low | |
| Obeticholic acid | No intervention | Low | Unclear | Low | Low | Low | High | Unclear | High | |
| Obeticholic acid | No intervention | Unclear | Unclear | Unclear | Unclear | Unclear | High | High | Low | |
| S‐Adenosyl methionine | No intervention | Unclear | Unclear | Unclear | Unclear | Unclear | High | Unclear | Low | |
| S‐Adenosyl methionine | No intervention | Unclear | Unclear | Unclear | Unclear | Unclear | High | Unclear | Low | |
| Simvastatin | No intervention | Unclear | Low | High | High | High | High | Low | High | |
| Tetrathiomolybdate | No intervention | Low | Low | Low | Low | Low | High | Low | High | |
| Thalidomide | No intervention | Unclear | Unclear | Low | Low | Low | High | High | Low | |
| UDCA | No intervention | Unclear | Unclear | Unclear | Unclear | Unclear | High | Unclear | Low | |
| UDCA | No intervention | Low | Low | Low | Low | High | High | Unclear | Low | |
| UDCA | No intervention | Unclear | Unclear | Low | Low | Low | High | High | Low | |
| UDCA | No intervention | Unclear | Unclear | Unclear | Unclear | High | High | High | Low | |
| UDCA | No intervention | Unclear | Low | Low | Low | Unclear | High | High | Low | |
| UDCA | No intervention | Unclear | Unclear | Unclear | Unclear | High | Low | Unclear | Low | |
| UDCA | No intervention | Unclear | Unclear | Unclear | Unclear | Unclear | High | Unclear | Low | |
| UDCA | No intervention | Unclear | Unclear | Low | Low | High | Low | High | Low | |
| UDCA | No intervention | Unclear | Low | Low | Low | High | High | High | Low | |
| UDCA | No intervention | Low | Low | High | High | High | High | High | High | |
| UDCA | No intervention | Unclear | Unclear | Low | Low | Unclear | Low | High | Low | |
| UDCA | No intervention | Unclear | Unclear | Low | Low | High | High | High | Low | |
| UDCA | No intervention | Unclear | Unclear | Unclear | Unclear | High | High | High | Low | |
| UDCA | No intervention | Unclear | Unclear | Low | Low | Low | High | Unclear | Low | |
| Intervention 1: UDCA | No intervention | Unclear | Unclear | Unclear | Unclear | Unclear | High | Unclear | Low | |
| Azathioprine + glucocorticosteroids + UDCA | UDCA | Low | Low | Low | Low | Unclear | High | High | Low | |
| Bezafibrate | UDCA | Unclear | Low | High | High | Unclear | High | Low | Low | |
| Bezafibrate | UDCA | Unclear | Unclear | Unclear | Unclear | Unclear | High | Unclear | Low | |
| Bezafibrate + UDCA | UDCA | Low | Low | High | High | Low | Low | Low | Low | |
| Bezafibrate + UDCA | UDCA | Unclear | Low | High | High | Unclear | High | Low | Low | |
| Bezafibrate + UDCA | UDCA | Unclear | Unclear | Unclear | Unclear | Low | High | Unclear | Low | |
| Bezafibrate + UDCA | UDCA | Unclear | Unclear | Unclear | Unclear | Unclear | High | Low | Low | |
| Colchicine + UDCA | UDCA | Low | Low | Low | Low | High | High | Low | Low | |
| Colchicine + UDCA | UDCA | Unclear | Unclear | Unclear | Unclear | Low | High | Unclear | High | |
| Colchicine + UDCA | UDCA | Unclear | Unclear | Low | Low | Unclear | Low | High | Low | |
| Colchicine + UDCA | UDCA | Unclear | Unclear | Unclear | Unclear | High | High | Unclear | Low | |
| Colestilan + UDCA | UDCA | Unclear | Unclear | High | High | Unclear | High | Unclear | Low | |
| Fenofibrate + UDCA | UDCA | Unclear | Unclear | High | High | Unclear | High | Unclear | Low | |
| Glucocorticosteroids + UDCA | UDCA | Low | Unclear | Unclear | Unclear | High | High | High | Low | |
| Glucocorticosteroids + UDCA | UDCA | Unclear | Unclear | High | High | High | High | High | Low | |
| Glucocorticosteroids + UDCA | UDCA | Unclear | Unclear | Unclear | Unclear | Unclear | High | Unclear | Low | |
| Lamivudine + zidovudine + UDCA | UDCA | Low | Low | Low | Low | Unclear | High | High | Low | |
| Methotrexate + UDCA | UDCA | Unclear | Unclear | Unclear | Unclear | Low | High | High | Low | |
| Methotrexate + UDCA | UDCA | Unclear | Low | Unclear | Unclear | Unclear | Low | Unclear | Low | |
| Obeticholic acid + UDCA | UDCA | Low | Low | Low | Low | High | Low | High | Low | |
| TUDCA | UDCA | Unclear | Unclear | Unclear | Unclear | Low | High | Unclear | Low | |
| TUDCA | UDCA | Low | Low | Low | Low | Unclear | High | High | Low | |
| Colchicine | Methotrexate | Unclear | Unclear | Low | Low | High | High | Unclear | Low | |
| Comparison of doses | ||||||||||
| Intervention 1: UDCA (high) Intervention 2: UDCA (moderate) | UDCA (low) | Unclear | Unclear | Unclear | Unclear | Unclear | High | Unclear | Low | |
| Intervention 1: UDCA (high) Intervention 2: UDCA (moderate) | UDCA (low) | Low | Low | Low | Low | Unclear | Low | Unclear | Low | |
| UDCA (moderate) | UDCA (low) | Low | Low | High | High | Unclear | High | High | Low | |
| UDCA (high) | UDCA (moderate) | Unclear | Unclear | Unclear | Unclear | Unclear | High | Unclear | Low | |
| TUDCA: taurodeoxycholic acid; UDCA: ursodeoxycholic acid. | ||||||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality at maximal follow‐up Show forest plot | 28 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Azathioprine versus no intervention | 2 | 224 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.32, 0.98] |
| 1.2 Chlorambucil versus no intervention | 1 | 24 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.01, 3.28] |
| 1.3 Colchicine versus no intervention | 2 | 122 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.32, 1.85] |
| 1.4 Cyclosporin versus no intervention | 3 | 390 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.51, 1.50] |
| 1.5 D‐Penicillamine versus no intervention | 5 | 423 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.57, 1.44] |
| 1.6 Glucocorticosteroids versus no intervention | 1 | 36 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.14, 2.92] |
| 1.7 Malotilate versus no intervention | 1 | 101 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.47, 8.48] |
| 1.8 Methotrexate versus no intervention | 1 | 60 | Odds Ratio (M‐H, Fixed, 95% CI) | 8.83 [1.01, 76.96] |
| 1.9 UDCA versus no intervention | 6 | 734 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.60, 1.64] |
| 1.10 Bezafibrate plus UDCA versus UDCA | 1 | 27 | Odds Ratio (M‐H, Fixed, 95% CI) | 9.67 [0.45, 207.78] |
| 1.11 Colchicine plus UDCA versus UDCA | 2 | 158 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.84 [0.38, 8.91] |
| 1.12 Methotrexate plus UDCA versus UDCA | 2 | 290 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.55, 2.51] |
| 1.13 Obeticholic acid plus UDCA versus UDCA | 1 | 216 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.55 [0.06, 38.46] |
| 2 Mortality (< 1 year) Show forest plot | 8 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Azathioprine versus no intervention | 1 | 39 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.16, 2.10] |
| 2.2 Colchicine versus no intervention | 1 | 64 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.22, 3.33] |
| 2.3 Cyclosporin versus no intervention | 1 | 12 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.01, 3.63] |
| 2.4 D‐Penicillamine versus no intervention | 1 | 189 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.35, 1.42] |
| 2.5 Ursodeoxycholic acid (UDCA) versus no intervention | 1 | 18 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.6 Colchicine plus UDCA versus UDCA | 1 | 84 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.13, 7.45] |
| 2.7 Methotrexate plus UDCA versus UDCA | 1 | 25 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.8 Obeticholic acid plus UDCA versus UDCA | 1 | 216 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.55 [0.06, 38.46] |
| 3 Mortality (1 to 5 years) Show forest plot | 20 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Azathioprine versus no intervention | 1 | 185 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.30, 1.04] |
| 3.2 Chlorambucil versus no intervention | 1 | 24 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.01, 3.28] |
| 3.3 Colchicine versus no intervention | 1 | 58 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.22, 2.25] |
| 3.4 Cyclosporin versus no intervention | 2 | 378 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.54, 1.64] |
| 3.5 D‐Penicillamine versus no intervention | 4 | 234 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.59, 2.08] |
| 3.6 Glucocorticosteroids versus no intervention | 1 | 36 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.14, 2.92] |
| 3.7 Malotilate versus no intervention | 1 | 101 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.47, 8.48] |
| 3.8 Methotrexate versus no intervention | 1 | 60 | Odds Ratio (M‐H, Fixed, 95% CI) | 8.83 [1.01, 76.96] |
| 3.9 UDCA versus no intervention | 5 | 716 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.60, 1.64] |
| 3.10 Bezafibrate plus UDCA versus UDCA | 1 | 27 | Odds Ratio (M‐H, Fixed, 95% CI) | 9.67 [0.45, 207.78] |
| 3.11 Colchicine plus UDCA versus UDCA | 1 | 74 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.28 [0.24, 113.87] |
| 3.12 Methotrexate plus UDCA versus UDCA | 1 | 265 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.55, 2.51] |
| 4 Serious adverse events (proportion) Show forest plot | 11 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Colchicine versus no intervention | 1 | 64 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 D‐Penicillamine versus no intervention | 1 | 52 | Odds Ratio (M‐H, Fixed, 95% CI) | 28.77 [1.57, 526.67] |
| 4.3 Obeticholic acid versus no intervention | 1 | 165 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.83 [0.21, 15.73] |
| 4.4 UDCA versus no intervention | 3 | 380 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.5 UDCA versus bezafibrate | 1 | 24 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.6 Bezafibrate plus UDCA versus UDCA | 1 | 22 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.7 Colchicine plus UDCA versus UDCA | 1 | 74 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.08 [0.12, 78.14] |
| 4.8 Lamivudine plus zidovudine plus UDCA versus UDCA | 1 | 59 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.04, 5.43] |
| 4.9 Obeticholic acid plus UDCA versus UDCA | 1 | 216 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.58 [1.02, 12.51] |
| 5 Serious adverse events (number of events) Show forest plot | 1 | Rate Ratio (Fixed, 95% CI) | Subtotals only | |
| 5.1 Obeticholic acid plus UDCA versus UDCA | 1 | 216 | Rate Ratio (Fixed, 95% CI) | 1.66 [0.75, 3.66] |
| 6 Adverse events (proportion) Show forest plot | 19 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 Cyclosporin versus no intervention | 3 | 390 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.04 [1.98, 4.68] |
| 6.2 D‐Penicillamine versus no intervention | 2 | 287 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.51 [2.56, 7.93] |
| 6.3 Malotilate versus no intervention | 1 | 101 | Odds Ratio (M‐H, Fixed, 95% CI) | 11.43 [1.40, 93.04] |
| 6.4 Obeticholic acid versus no intervention | 1 | 165 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.58 [1.31, 15.95] |
| 6.5 UDCA versus no intervention | 3 | 380 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.45 [0.50, 4.25] |
| 6.6 Azathioprine plus UDCA versus UDCA | 1 | 42 | Odds Ratio (M‐H, Fixed, 95% CI) | 19.67 [0.94, 413.50] |
| 6.7 Bezafibrate versus UDCA | 1 | 24 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.8 Bezafibrate plus UDCA versus UDCA | 1 | 22 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.29 [0.12, 89.81] |
| 6.9 Colchicine plus UDCA versus UDCA | 2 | 42 | Odds Ratio (M‐H, Fixed, 95% CI) | 6.20 [0.63, 60.80] |
| 6.10 Colestilan plus UDCA versus UDCA | 1 | 11 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.11 Glucocorticosteroids plus UDCA versus UDCA | 2 | 135 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.54 [1.35, 22.84] |
| 6.12 Methotrexate plus UDCA versus UDCA | 1 | 25 | Odds Ratio (M‐H, Fixed, 95% CI) | 115.0 [4.98, 2657.48] |
| 6.13 TauroUDCA versus UDCA | 1 | 30 | Odds Ratio (M‐H, Fixed, 95% CI) | 21.0 [2.16, 204.61] |
| 6.14 Glucocorticosteroids plus UDCA versus azathioprine plus UDCA | 1 | 50 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.08, 2.12] |
| 7 Adverse events (number) Show forest plot | 14 | Rate Ratio (Random, 95% CI) | Subtotals only | |
| 7.1 Chlorambucil versus no intervention | 1 | 24 | Rate Ratio (Random, 95% CI) | 3.67 [1.04, 12.87] |
| 7.2 Cyclosporin versus no intervention | 3 | 390 | Rate Ratio (Random, 95% CI) | 2.58 [1.26, 5.31] |
| 7.3 D‐Penicillamine versus no intervention | 3 | 303 | Rate Ratio (Random, 95% CI) | 2.99 [1.04, 8.63] |
| 7.4 Malotilate versus no intervention | 1 | 101 | Rate Ratio (Random, 95% CI) | 6.13 [1.38, 27.14] |
| 7.5 Obeticholic acid versus no intervention | 1 | 76 | Rate Ratio (Random, 95% CI) | 1.41 [1.13, 1.75] |
| 7.6 Azathioprine plus glucocorticosteroids plus UDCA versus UDCA | 1 | 50 | Rate Ratio (Random, 95% CI) | 1.32 [0.88, 1.97] |
| 7.7 Bezafibrate plus UDCA versus UDCA | 1 | 29 | Rate Ratio (Random, 95% CI) | 11.79 [0.65, 213.14] |
| 7.8 Colchicine plus UDCA versus UDCA | 1 | 24 | Rate Ratio (Random, 95% CI) | 5.91 [0.28, 123.08] |
| 7.9 Methotrexate plus UDCA versus UDCA | 1 | 27 | Rate Ratio (Random, 95% CI) | 30.64 [1.84, 510.76] |
| 7.10 TauroUDCA versus UDCA | 1 | 191 | Rate Ratio (Random, 95% CI) | 1.17 [0.81, 1.71] |
| 8 Liver transplantation Show forest plot | 11 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 Cyclosporin versus no intervention | 2 | 378 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.43, 1.72] |
| 8.2 D‐Penicillamine versus no intervention | 1 | 189 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.06, 15.05] |
| 8.3 Methotrexate versus no intervention | 1 | 60 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.17 [0.02, 1.58] |
| 8.4 UDCA versus no intervention | 5 | 640 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.48, 1.68] |
| 8.5 Bezafibrate plus UDCA versus UDCA | 1 | 27 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.6 Methotrexate plus UDCA versus UDCA | 1 | 265 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.35, 1.39] |
| 9 Decompensated liver disease Show forest plot | 7 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9.1 D‐Penicillamine versus no active treatment | 1 | 24 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.2 UDCA versus no intervention | 2 | 237 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.60 [0.86, 2.98] |
| 9.3 Azathioprine plus UDCA versus UDCA | 1 | 42 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.05, 5.18] |
| 9.4 Colchicine plus UDCA versus UDCA | 1 | 84 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.04, 1.07] |
| 9.5 Glucocorticosteroids plus UDCA versus UDCA | 1 | 66 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.11, 2.69] |
| 9.6 Methotrexate plus UDCA versus UDCA | 1 | 265 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.34 [0.77, 2.33] |
| 9.7 Obeticholic acid plus UDCA versus UDCA | 1 | 216 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.55 [0.06, 38.46] |
| 9.8 Glucocorticosteroids plus UDCA versus azathioprine plus UDCA | 1 | 50 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.10, 11.18] |
| 10 Cirrhosis Show forest plot | 3 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 10.1 Azathioprine versus no intervention | 1 | 31 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.18, 3.41] |
| 10.2 UDCA versus no intervention | 1 | 22 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.15 [0.01, 1.53] |
| 10.3 Azathioprine plus glucocorticosteroids plus UDCA versus UDCA | 1 | 50 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.28 [0.03, 2.90] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality at maximal follow‐up Show forest plot | 29 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Azathioprine versus no intervention | 2 | 224 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.32, 0.98] |
| 1.2 Chlorambucil versus no intervention | 1 | 24 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.01, 3.28] |
| 1.3 Colchicine versus no intervention | 2 | 122 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.32, 1.85] |
| 1.4 Cyclosporin versus no intervention | 3 | 390 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.51, 1.50] |
| 1.5 D‐Penicillamine versus no intervention | 5 | 423 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.57, 1.44] |
| 1.6 Glucocorticosteroids versus no intervention | 1 | 36 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.14, 2.92] |
| 1.7 Malotilate versus no intervention | 1 | 101 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.47, 8.48] |
| 1.8 Methotrexate versus no intervention | 1 | 60 | Odds Ratio (M‐H, Fixed, 95% CI) | 8.83 [1.01, 76.96] |
| 1.9 UDCA (low) versus no intervention | 2 | 64 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.03, 3.47] |
| 1.10 UDCA (moderate) versus no intervention | 4 | 670 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.62, 1.77] |
| 1.11 UDCA (low) versus UDCA (high) | 1 | 106 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.06, 17.06] |
| 1.12 UDCA (moderate) versus UDCA (high) | 1 | 103 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.01, 9.05] |
| 1.13 UDCA (low) plus colchicine versus UDCA (low) | 1 | 84 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.13, 7.45] |
| 1.14 UDCA (low) plus methotrexate versus UDCA (low) | 1 | 25 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.15 UDCA (moderate) versus UDCA (low) | 1 | 101 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.01, 8.72] |
| 1.16 Bezafibrate plus UDCA (moderate) versus UDCA (moderate) | 1 | 27 | Odds Ratio (M‐H, Fixed, 95% CI) | 9.67 [0.45, 207.78] |
| 1.17 Colchicine plus UDCA (moderate) versus UDCA (moderate) | 1 | 74 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.28 [0.24, 113.87] |
| 1.18 Methotrexate plus UDCA (moderate) versus UDCA (moderate) | 1 | 265 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.55, 2.51] |
| 1.19 Obeticholic acid (low) plus UDCA (moderate) versus UDCA (moderate) | 1 | 216 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.55 [0.06, 38.46] |
| 2 Mortality (< 1 year) Show forest plot | 9 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Azathioprine versus no intervention | 1 | 39 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.16, 2.10] |
| 2.2 Colchicine versus no intervention | 1 | 64 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.22, 3.33] |
| 2.3 Cyclosporin versus no intervention | 1 | 12 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.01, 3.63] |
| 2.4 D‐Penicillamine versus no intervention | 1 | 189 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.35, 1.42] |
| 2.5 UDCA (low) versus no intervention | 1 | 18 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.6 UDCA (low) versus UDCA (high) | 1 | 106 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.06, 17.06] |
| 2.7 UDCA (moderate) versus UDCA (high) | 1 | 103 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.01, 9.05] |
| 2.8 Obeticholic acid (low) plus UDCA (moderate) versus UDCA (moderate) | 1 | 216 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.55 [0.06, 38.46] |
| 2.9 UDCA (low) plus colchicine versus UDCA (low) | 1 | 84 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.13, 7.45] |
| 2.10 UDCA (low) plus methotrexate versus UDCA (low) | 1 | 25 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.11 UDCA (moderate) versus UDCA (low) | 1 | 101 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.01, 8.72] |
| 3 Mortality (1 to 5 years) Show forest plot | 20 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Azathioprine versus no intervention | 1 | 185 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.30, 1.04] |
| 3.2 Chlorambucil versus no intervention | 1 | 24 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.01, 3.28] |
| 3.3 Colchicine versus no intervention | 1 | 58 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.22, 2.25] |
| 3.4 Cyclosporin versus no intervention | 2 | 378 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.54, 1.64] |
| 3.5 D‐Penicillamine versus no intervention | 4 | 234 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.59, 2.08] |
| 3.6 Glucocorticosteroids versus no intervention | 1 | 36 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.14, 2.92] |
| 3.7 Malotilate versus no intervention | 1 | 101 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.47, 8.48] |
| 3.8 Methotrexate versus no intervention | 1 | 60 | Odds Ratio (M‐H, Fixed, 95% CI) | 8.83 [1.01, 76.96] |
| 3.9 UDCA (low) versus no intervention | 1 | 46 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.03, 3.47] |
| 3.10 UDCA (moderate) versus no intervention | 4 | 670 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.62, 1.77] |
| 3.11 Bezafibrate plus UDCA (moderate) versus UDCA (moderate) | 1 | 27 | Odds Ratio (M‐H, Fixed, 95% CI) | 9.67 [0.45, 207.78] |
| 3.12 Colchicine plus UDCA (moderate) versus UDCA (moderate) | 1 | 74 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.28 [0.24, 113.87] |
| 3.13 Methotrexate plus UDCA (moderate) versus UDCA (moderate) | 1 | 265 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.55, 2.51] |
| 4 Serious adverse events (proportion) Show forest plot | 12 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Colchicine versus no intervention | 1 | 64 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 D‐Penicillamine versus no intervention | 1 | 52 | Odds Ratio (M‐H, Fixed, 95% CI) | 28.77 [1.57, 526.67] |
| 4.3 Obeticholic acid (high) versus no intervention | 1 | 79 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.14 [0.57, 46.17] |
| 4.4 Obeticholic acid (low) versus no intervention | 1 | 76 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.01, 8.22] |
| 4.5 Obeticholic acid (moderate) versus no intervention | 1 | 86 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.05, 13.01] |
| 4.6 UDCA (low) versus no intervention | 1 | 18 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.7 UDCA (moderate) versus no intervention | 2 | 362 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.8 UDCA (low) versus bezafibrate | 1 | 24 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.9 Obeticholic acid (low) versus obeticholic acid (high) | 1 | 79 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.09 [0.00, 1.61] |
| 4.10 Obeticholic acid (moderate) versus obeticholic acid (high) | 1 | 89 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.15 [0.02, 1.37] |
| 4.11 Obeticholic acid (moderate) versus obeticholic acid (low) | 1 | 86 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.43 [0.10, 61.39] |
| 4.12 Lamivudine plus zidovudine plus UDCA (moderate) versus UDCA (moderate) | 1 | 59 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.04, 5.43] |
| 4.13 UDCA (moderate) versus obeticholic acid (low) plus UDCA (moderate) | 1 | 216 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.28 [0.08, 0.98] |
| 4.14 Bezafibrate plus UDCA (low) versus UDCA (low) | 1 | 22 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.15 UDCA (moderate) versus UDCA (low) | 1 | 59 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.16 Colchicine plus UDCA (moderate) versus UDCA (moderate) | 1 | 74 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.08 [0.12, 78.14] |
| 5 Serious adverse events (number of events) Show forest plot | 1 | Rate Ratio (Fixed, 95% CI) | Subtotals only | |
| 5.1 Obeticholic acid (low) plus UDCA (moderate) versus UDCA (moderate) | 1 | Rate Ratio (Fixed, 95% CI) | 1.66 [0.75, 3.66] | |
| 6 Adverse events (proportion) Show forest plot | 20 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 Cyclosporin versus no intervention | 3 | 390 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.04 [1.98, 4.68] |
| 6.2 D‐Penicillamine versus no intervention | 2 | 287 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.51 [2.56, 7.93] |
| 6.3 Malotilate versus no intervention | 1 | 101 | Odds Ratio (M‐H, Fixed, 95% CI) | 11.43 [1.40, 93.04] |
| 6.4 Obeticholic acid (high) versus no intervention | 1 | 79 | Odds Ratio (M‐H, Fixed, 95% CI) | 16.6 [0.90, 305.59] |
| 6.5 Obeticholic acid (low) versus no intervention | 1 | 76 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.59 [0.41, 6.17] |
| 6.6 Obeticholic acid (moderate) versus no intervention | 1 | 86 | Odds Ratio (M‐H, Fixed, 95% CI) | 8.81 [1.01, 76.73] |
| 6.7 UDCA (low) versus no intervention | 1 | 18 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.8 UDCA (moderate) versus no intervention | 2 | 362 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.45 [0.50, 4.25] |
| 6.9 Glucocorticosteroids plus UDCA (moderate) versus azathioprine plus UDCA (moderate) | 1 | 50 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.08, 2.12] |
| 6.10 UDCA (low) versus bezafibrate | 1 | 24 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.11 Obeticholic acid (low) versus obeticholic acid (high) | 1 | 79 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.09 [0.00, 1.78] |
| 6.12 Obeticholic acid (moderate) versus obeticholic acid (high) | 1 | 89 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.02, 9.62] |
| 6.13 Obeticholic acid (moderate) versus obeticholic acid (low) | 1 | 86 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.53 [0.59, 51.70] |
| 6.14 Bezafibrate plus UDCA (low) versus UDCA (low) | 1 | 22 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.29 [0.12, 89.81] |
| 6.15 Colestilan plus UDCA (low) versus UDCA (low) | 1 | 11 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.16 Methotrexate plus UDCA (low) versus UDCA (low) | 1 | 25 | Odds Ratio (M‐H, Fixed, 95% CI) | 115.0 [4.98, 2657.48] |
| 6.17 UDCA (moderate) versus UDCA (low) | 1 | 59 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.18 Azathioprine plus UDCA (moderate) versus UDCA (moderate) | 1 | 42 | Odds Ratio (M‐H, Fixed, 95% CI) | 19.67 [0.94, 413.50] |
| 6.19 Colchicine plus UDCA (moderate) versus UDCA (moderate) | 2 | 42 | Odds Ratio (M‐H, Fixed, 95% CI) | 6.20 [0.63, 60.80] |
| 6.20 Glucocorticosteroids plus UDCA (moderate) versus UDCA (moderate) | 2 | 135 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.54 [1.35, 22.84] |
| 6.21 TauroUDCA (moderate) versus UDCA (moderate) | 1 | 30 | Odds Ratio (M‐H, Fixed, 95% CI) | 21.0 [2.16, 204.61] |
| 7 Adverse events (number) Show forest plot | 15 | Rate Ratio (Fixed, 95% CI) | Subtotals only | |
| 7.1 Chlorambucil versus no intervention | 1 | Rate Ratio (Fixed, 95% CI) | 3.67 [1.04, 12.87] | |
| 7.2 Cyclosporin versus no intervention | 3 | Rate Ratio (Fixed, 95% CI) | 1.87 [1.51, 2.32] | |
| 7.3 D‐Penicillamine versus no intervention | 3 | Rate Ratio (Fixed, 95% CI) | 2.64 [1.78, 3.91] | |
| 7.4 Malotilate versus no intervention | 1 | Rate Ratio (Fixed, 95% CI) | 6.13 [1.38, 27.14] | |
| 7.5 Obeticholic acid (high) versus no intervention | 1 | Rate Ratio (Fixed, 95% CI) | 1.91 [1.50, 2.44] | |
| 7.6 Obeticholic acid (low) versus no intervention | 1 | Rate Ratio (Fixed, 95% CI) | 1.05 [0.80, 1.39] | |
| 7.7 Obeticholic acid (moderate) versus no intervention | 1 | Rate Ratio (Fixed, 95% CI) | 1.25 [0.97, 1.62] | |
| 7.8 Obeticholic acid (low) versus obeticholic acid (high) | 1 | Rate Ratio (Fixed, 95% CI) | 0.55 [0.43, 0.70] | |
| 7.9 Obeticholic acid (moderate) versus obeticholic acid (high) | 1 | Rate Ratio (Fixed, 95% CI) | 0.66 [0.53, 0.81] | |
| 7.10 Obeticholic acid (moderate) versus obeticholic acid (low) | 1 | Rate Ratio (Fixed, 95% CI) | 1.19 [0.93, 1.53] | |
| 7.11 UDCA (low) versus UDCA (high) | 1 | Rate Ratio (Fixed, 95% CI) | 2.08 [0.78, 5.53] | |
| 7.12 UDCA (moderate) versus UDCA (high) | 1 | Rate Ratio (Fixed, 95% CI) | 0.73 [0.21, 2.60] | |
| 7.13 UDCA (low) plus methotrexate versus UDCA (low) | 1 | Rate Ratio (Fixed, 95% CI) | 30.64 [1.84, 510.76] | |
| 7.14 UDCA (moderate) versus UDCA (low) | 1 | Rate Ratio (Fixed, 95% CI) | 0.35 [0.11, 1.10] | |
| 7.15 Azathioprine plus glucocorticosteroids plus UDCA (moderate) versus UDCA (moderate) | 1 | Rate Ratio (Fixed, 95% CI) | 1.32 [0.88, 1.97] | |
| 7.16 Bezafibrate plus UDCA (moderate) versus UDCA (moderate) | 1 | Rate Ratio (Fixed, 95% CI) | 11.79 [0.65, 213.14] | |
| 7.17 Colchicine plus UDCA (moderate) versus UDCA (moderate) | 1 | Rate Ratio (Fixed, 95% CI) | 5.91 [0.28, 123.08] | |
| 7.18 TauroUDCA (moderate) versus UDCA (moderate) | 1 | Rate Ratio (Fixed, 95% CI) | 1.17 [0.81, 1.71] | |
| 8 Liver transplantation Show forest plot | 12 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 Cyclosporin versus no intervention | 2 | 378 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.43, 1.72] |
| 8.2 D‐Penicillamine versus no intervention | 1 | 189 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.06, 15.05] |
| 8.3 Methotrexate versus no intervention | 1 | 60 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.17 [0.02, 1.58] |
| 8.4 UDCA (low) versus no intervention | 2 | 162 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.24, 4.06] |
| 8.5 UDCA (moderate) versus no intervention | 3 | 478 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.44, 1.76] |
| 8.6 UDCA (low) versus UDCA (high) | 1 | 106 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.17 [0.13, 79.71] |
| 8.7 UDCA (moderate) versus UDCA (high) | 1 | 103 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.37 [0.13, 84.70] |
| 8.8 UDCA (moderate) versus UDCA (low) | 1 | 101 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.06, 17.47] |
| 8.9 Bezafibrate plus UDCA (moderate) versus UDCA (moderate) | 1 | 27 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.10 Methotrexate plus UDCA (moderate) versus UDCA (moderate) | 1 | 265 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.35, 1.39] |
| 9 Decompensated liver disease Show forest plot | 7 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9.1 D‐Penicillamine versus no intervention | 1 | 24 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.2 UDCA (moderate) versus no intervention | 2 | 351 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.84, 2.12] |
| 9.3 Obeticholic acid (low) plus UDCA (moderate) versus UDCA (moderate) | 1 | 216 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.55 [0.06, 38.46] |
| 9.4 UDCA (low) plus colchicine versus UDCA (low) | 1 | 84 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.04, 1.07] |
| 9.5 Azathioprine plus UDCA (moderate) versus UDCA (moderate) | 1 | 42 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.05, 5.18] |
| 9.6 Glucocorticosteroids plus UDCA (moderate) versus UDCA (moderate) | 1 | 66 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.11, 2.69] |
| 9.7 Methotrexate plus UDCA (moderate) versus UDCA (moderate) | 1 | 151 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.00 [0.79, 5.04] |
| 9.8 Glucocorticosteroids plus UDCA (moderate) versus azathioprine plus UDCA (moderate) | 1 | 50 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.10, 11.18] |
| 10 Cirrhosis Show forest plot | 3 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 10.1 Azathioprine versus no intervention | 1 | 31 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.18, 3.41] |
| 10.2 UDCA (low) versus no intervention | 1 | 22 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.15 [0.01, 1.53] |
| 10.3 Azathioprine plus glucocorticosteroids plus UDCA (moderate) versus UDCA (moderate) | 1 | 50 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.28 [0.03, 2.90] |

